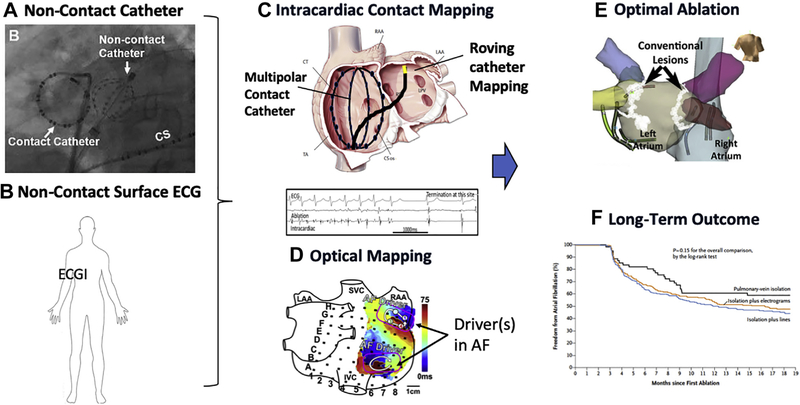
Ablation of atrial fibrillation (AF) remains a big clinical challenge, in large part because of a lack of understanding of the mechanisms that trigger and maintain AF. Isolation of pulmonary veins (PVs) has become a cornerstone of AF ablation because the PV antra are a common source of triggers for initiation of AF but also likely harbor nontriggering substrates that help to maintain AF once it is initiated (1). Although PV isolation may be associated with 1-year success rates of ≥75% in patients with paroxysmal AF, outcomes for persistent AF are substantially lower. Previous clinical trials have suggested that addition of fractionated electrographic and/or linear ablation do not add to clinical success beyond PV isolation alone (2,3). However, newer techniques of mapping during AF have identified stable focal, rotational, or repetitive activation patterns during human AF, and ablation of these targets has demonstrated some clinical success (4–6). Over the last few years, a number of technologies have been developed to facilitate mapping of AF (Figure 1), including whole-chamber basket mapping (e.g., FIRM [Focal impulse and Rotor Mapping]), contact-based unipolar mapping using serial applications of a multipolar catheter (7), or noncontact mapping using the inverse solution to re-construct unipolar electrograms (electrocardiographic imaging, dipole density mapping). Regardless of the technology used, confidence in using such systems to guide ablation can only be achieved by validating the system against a gold standard, such as by voltage-sensitive dye optical mapping (Figure 1D) (8) or if clinical outcomes improve because of ablating activations identified by the mapping system(s) (Figure 1F).
FIGURE 1. Incorporating Mapping Into Ablation for AF.
Noncontact mapping can be used to acquire signals, using (A) intracardiac small basket catheters (9) or (B) body surface electrodes (4–6). Such approaches are typically compared with (C) contact mapping, which may be panoramic with a larger basket, or serial mapping with a multipolar catheter. (D) Validation of mapping can be performed using voltage-sensitive, near-infrared optical mapping (8). All methods have limitations. Important considerations are (E) which lesions sets to apply and (F) clinical trials of ablation outcomes. AF = atrial fibrillation; ECG = electrocardiography.
In this context, the study by Shi et al. (9) in this issue of JACC: Clinical Electrophysiology attempted to validate the noncontact-based dipole density mapping system (AcQMap, Acutus Medical, Carlsbad, California) against simultaneous contact-based recordings in the left atrium (collected with a standard circular mapping catheter) in both sinus rhythm and during AF. The AcQMap system consists of a 25-mm basket with 6 splines, each of which has 8 electrodes and 8 ultrasound transducers (Figure 1A). The system reconstructs 3-dimensional anatomy using ultrasound and also derives reconstructed electrograms from the raw, unipolar electrograms sensed by the noncontact electrodes. Using an inverse solution and corrections to estimate charge, as opposed to voltage alone, the system mathematically reconstructs post-processed, voltage-based electrograms across the entire left atrial surface that are then projected onto the ultrasound-based anatomy. The study found that there was generally good correlation between reconstructed and contact-based electrograms as long as the center of the basket catheter was no more than 40 mm away from the circular catheter. In sinus rhythm, morphology correlation was 0.87, and the timing difference between contact and noncontact electrograms was 5.7 ms. In AF, the correlation was weaker (0.81), and the timing difference increased to 12.3 ms. Once the distance between basket and circular catheter was >40 mm, both the morphology correlations and the timing differences between contact and noncontact electrograms were worse (correlation: 0.67; timing difference: 28.3 ms). It was interesting that this parallels earlier work that compared inverse solution electrograms derived by the Ensite 3000 system (St. Jude Medical, Abbott Laboratories, Abbott Park, Illinois) to contact-based electrograms (10,11). Correlation coefficients of AF morphology (in the range of 0.79) and timing differences (approximately 14.4 ms) were similar, and the error rate also increased when the tissue surface was >40 mm away from the mapping catheter (correlation of 0.81 vs. correlation of 0.67).
The investigators should be commended on this rigorous validation of a novel mapping approach. More work needs to be done in this area to ensure confidence that AF drivers mapped by different systems actually correlate to one another and that such drivers are not specific to 1 technology. It is reassuring that findings from both contact- and noncontact-based mapping systems do seem to be converging. Both focal and rotational activations can now be identified by a variety of systems with similar spatial distributions (4–6). The work by Shi et al. (9) certainly provides validation data to support this convergence of findings.
However, there are some important limitations that need to be considered. First, the study only examined short segments of AF ranging from 300 to 600 ms, although 30 s of data were acquired at each point. This is important because longer segments of AF (≥15 s) may be required to conclude whether a focal or rotational activation is spatially and temporally stable (12,13). Second, errors introduced by the system, although small in absolute terms, have the potential to drastically affect AF maps and their interpretation, especially when compounded by mathematical assumptions. For example, in AF, Shi et al. (9) found that the median timing difference was only 12.3 ms, but similar errors in marking activation times might convert a rotational activation into a focal, partial rotational, or no discernable pattern at all (14). This error might further be compounded in regions where electrograms are very low amplitude, highly fractionated, or indicate slow conduction (14).
Ultimately, any mapping system should try to faithfully represent true electrical activation. Data like that found in the study by Shi et al. (9) represent an excellent start. In the future, further studies should compare dipole density mapping with other panoramic mapping systems, such as FIRM, stochastic trajectory analysis of ranked signals mapping (STAR) (7), electrocardiographic imaging, and 4-dimensional contact-based mapping. Comparison of all of these technologies with ex vivo optical mapping using voltage-sensitive dyes is another step that may be taken, as has been performed for some other tools (Figure 1D) (8).
However, mapping is only 1 step in the equation, and ultimately, any system must be judged by the long-term outcomes of freedom from AF at 1 year and beyond. The problem is that outcome can be affected by so many factors other than mapping accuracy. Even if a system can faithfully and reliably represent activation patterns during AF, how should these patterns be ablated? Do we ablate in the center of the pattern, cut across it with a line, or both? What power and duration are required for optimal energy delivery? Do we need to eliminate the pattern only, or should we completely eliminate all local electrograms? And even if the patterns are eliminated immediately, will they recur later because of non-transmural lesions and tissue reconnection? We are making progress. Energy delivery and consistency is improving with better catheters, optimal power and duration settings, ablation indexes, and even newer energy sources (e.g., pulsed field ablation). As long-term outcome studies are performed using the various AF driver mapping systems, we will also learn about optimal lesion sets (Figure 1E) and hierarchies of targets to be ablated. Ultimately, large-scale randomized trials, such as STAR AF 3 (Substrate and Trigger Ablation for Reduction of Atrial Fibrillation 3), will determine the true value of adjuvant ablation strategies beyond PV antral isolation. Thus, there remains a tremendous amount of work to be done before we ultimately get in close contact with the mechanisms driving AF or not.
Acknowledgments
Dr. Narayan is supported in part by grants from the National Institutes of Health (R01 HL83359 and K24 HL103800). Dr. Verma has been a consultant for the American College of Cardiology; has been a member of the Advisory Board for Bayer, Biosense Webster, Medtronic, Vytronus, and Thermedical; and has received grants from Bayer, Biotronik, Biosense Webster, and Bristol-Myers Squibb. Dr. Narayan has been a consultant for Beyond Limits.ai, TDK, UpToDate, Abbott Laboratories, and the American College of Cardiology Foundation; and holds intellectual property rights from University of California Regents and Stanford University.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Clinical Electrophysiology author instructions page.
Editorials published in the JACC: Clinical Electrophysiology reflect the views of the authors and do not necessarily represent the views of JACC: Clinical Electrophysiology or the American College of Cardiology.
REFERENCES
- 1.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20: e1–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 3.Clarnette JA, Brooks AG, Mahajan R, et al. Outcomes of persistent and long-standing persistent atrial fibrillation ablation: a systematic review and meta-analysis. Europace 2018;20:f366–76. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez FD, Birnie DH, Nair GM, et al. Efficacy and safety of driver-guided catheter ablation for atrial fibrillation: a systematic review and meta-analysis. J Cardiovasc Electrophysiol 2017;28: 1371–8. [DOI] [PubMed] [Google Scholar]
- 5.Baykaner T, Rogers AJ, Meckler GL, et al. Clinical implications of ablation of drivers for atrial fibrillation: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol 2018;11:e006119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin CY, Lin YJ, Narayan SM, et al. Comparison of phase mapping and electrogram-based driver mapping for catheter ablation in atrial fibrillation. Pacing Clin Electrophysiol 2019;42:216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honarbakhsh S, Hunter RJ, Finlay M, et al. Development, in vitro validation and human application of a novel method to identify arrhythmia mechanisms: the stochastic trajectory analysis of ranked signals mapping method. J Cardiovasc Electrophysiol 2019;30:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen BJ, Zhao J, Li N, et al. Human atrial fibrillation drivers resolved with integrated functional and structural imaging to benefit clinical mapping. J Am Coll Cardiol EP 2018;4:1501–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi R, Parikh P, Chen Z, et al. Validation of dipole density mapping during atrial fibrillation and sinus rhythm in human left atrium. J Am Coll Cardiol EP 2020;6:171–81. [DOI] [PubMed] [Google Scholar]
- 10.Schilling RJ, Kadish AH, Peters NS, Goldberger J, Davies DW. Endocardial mapping of atrial fibrillation in the human right atrium using a non-contact catheter. Eur Heart J 2000;21:550–64. [DOI] [PubMed] [Google Scholar]
- 11.Earley M, Abrams D, Sporton S, Schilling R. Validation of the non-contact mapping system in the left atrium during permanent atrial fibrillation and sinus rhythm. J Am Coll Cardiol 2006;48: 485–91. [DOI] [PubMed] [Google Scholar]
- 12.Verma A, Sarkozy A, Skanes A, et al. Characterization and significance of localized sources identified by a novel automated algorithm during mapping of human persistent atrial fibrillation. J Cardiovasc Electrophysiol 2018;29:1480–8. [DOI] [PubMed] [Google Scholar]
- 13.Kowalewski CAB, Shenasa F, Rodrigo M, et al. Interaction of localized drivers and disorganized activation in persistent atrial fibrillation: reconciling putative mechanisms using multiple mapping techniques. Circ Arrhythm Electrophysiol 2018;11:e005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaman JAB, Sauer WH, Al-Husseini MI, et al. Identification and characterization of sites where persistent atrial fibrillation is terminated by localized ablation. Circ Arrhythm Electrophysiol 2018;11:e005258. [DOI] [PMC free article] [PubMed] [Google Scholar]