Abstract
Background:
Circular external fixation for limb-lengthening is associated with frequent and numerous complications. Intramedullary lengthening devices represent a potential advance in limb-lengthening. The purpose of this study was to compare the outcomes of femoral lengthening in pediatric patients treated by either circular external fixation or a motorized intramedullary nail.
Methods:
All patients with a diagnosis of congenital femoral deficiency who had undergone femoral lengthening with either circular external fixation or a motorized intramedullary nail were identified. The motorized intramedullary nail (FITBONE) was used with approval of the U.S. Food and Drug Administration on an individual compassionate-use basis.
Results:
Fourteen skeletally mature patients underwent fourteen femoral lengthening sessions using circular external fixation, and thirteen patients underwent fifteen lengthening sessions using the motorized nail. The amount lengthened was similar, with a mean of 4.8 cm (range, 1.0 to 7.4 cm) in the circular fixation group and 4.4 cm (range, 1.5 to 7.0 cm) in the motorized nail group. Complications occurred in all lengthening sessions in all fourteen patients managed with the circular external fixation and in 73% of fifteen lengthening sessions in the thirteen patients managed with the motorized nail. The circular external fixation group averaged 2.36 complications per lengthening session compared with 1.2 per session in the motorized nail group. Twenty-nine percent of the circular fixation group failed to achieve a lengthening goal of at least 4 cm compared with 27% of the motorized nail group who failed to reach the goal. Eight patients had undergone eleven femoral lengthening sessions with circular external fixation prior to undergoing ten lengthening sessions by motorized nail. These patients had a comparable rate of complications with both types of lengthening, but the total number of complications averaged 2.6 per lengthening session with circular external fixation compared with 1.6 per lengthening session with the motorized nail.
Conclusions:
A decreased number of complications was noted with use of a motorized intramedullary nail compared with circular external fixation in pediatric patients undergoing femoral lengthening for congenital femoral deficiency.
Level of Evidence:
Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
Circular external fixation and gradual distraction of an osteotomy-induced fracture callus is a well-recognized method of limb-lengthening. However, limb-lengthening using external fixation is associated with numerous complications, with rates reported to range from 24% to 117%1-11. Prolonged treatment duration, regular pin care, and restriction of joint mobility may lead to substantial disability, resulting in psychological stress and a delay in return to normal activity12.
Combined external and internal fixation techniques can reduce the time spent in an external fixator and the risk of regenerate fracture. These techniques have also been reported to decrease the number of complications7. However, complications related to pin fixation remain. Implantable devices capable of both fixation and lengthening, obviating the need for external fixation altogether13-18, have thus generated strong interest19,20.
Comparison of the outcomes in patients undergoing limb-lengthening is challenged by the lack of universally accepted, comprehensive, and objective complication classification systems. Reports have often involved patients with heterogeneous diagnoses2,4,5,21. Congenital femoral deficiency has been reported to occur in one of every 52,000 live births22-28. Patients with congenital femoral deficiency have been reported to incur a higher rate of complications associated with femoral lengthening than those without the disorder2,6,29.
The purpose of the present study was to compare the outcomes and complications of femoral lengthening in pediatric patients with congenital femoral deficiency treated with either circular external fixation or a fully implantable, motorized, intramedullary lengthening nail, using an outcome-specific complication classification system.
Materials and Methods
The institutional review board at our institution approved this study. All patients who underwent femoral lengthening between October 21, 1996, and October 31, 2013, were identified. Only patients with a diagnosis of congenital femoral deficiency who were skeletally mature at the time of femoral lengthening and were managed with circular external fixation (Ilizarov apparatus [Smith & Nephew, Memphis, Tennessee] or TrueLok [Orthofix, Lewisville, Texas]) or a fully implantable, intramedullary lengthening nail (FITBONE Telescope Active Actuator [TAA] nail; Wittenstein Intens, Igersheim, Germany), inserted in a retrograde femoral fashion, were included in this study.
FITBONE is not currently approved by the U.S. Food and Drug Administration (FDA). This nail was used at our institution with individual, institutional administrative, institutional review board, and FDA approval on a compassionate-use basis. Patients so treated are restricted to standard-of-care treatment and investigations (other than use of the device itself) and cannot be enrolled in a research study. Their medical and radiographic records may, however, be evaluated in a retrospective manner with institutional review board approval. All patients who were petitioned for the use of this device had a substantial shortening, had refused shortening of the contralateral limb, had refused initial or repeat lengthening with circular fixation, were skeletally mature, and had a femur of appropriate size and shape to allow installation of the device. All patients and their families were made aware of the experimental status of this device with the FDA and consented to its use. Per institutional review board and FDA stipulations, the present investigation is a strictly retrospective chart and radiographic review, which is a considerable limitation to the study. Both circular external fixation devices used for the patients in this study were approved by the FDA.
The FITBONE TAA femoral nail is a stainless steel, straight nail capable of 40 to 80 mm of distraction, depending on the specific model used. A radiofrequency wave transmitter activates the electric spindle motor housed in the nail by transmitting waves to a subcutaneous antenna-receiver, which converts the waves into small electrical impulses. Twenty-seven individual activations (divided into nine pulses sent three times daily) result in a millimeter of distraction. The rate of distraction can be modified by altering the number of activations. All FITBONE devices were inserted in a retrograde femoral fashion after careful preoperative analysis of the deformity, using the reverse planning method of Baumgart30 (Figs. 1-A through 1-D). After complete osseous consolidation, the implants were removed, per the manufacturer’s recommendation.
Figs. 1-A through 1-D Illustrative case of a patient treated with a FITBONE TAA motorized intramedullary nail inserted in a retrograde femoral fashion. The patient experienced no complications during treatment.
Fig. 1-A.
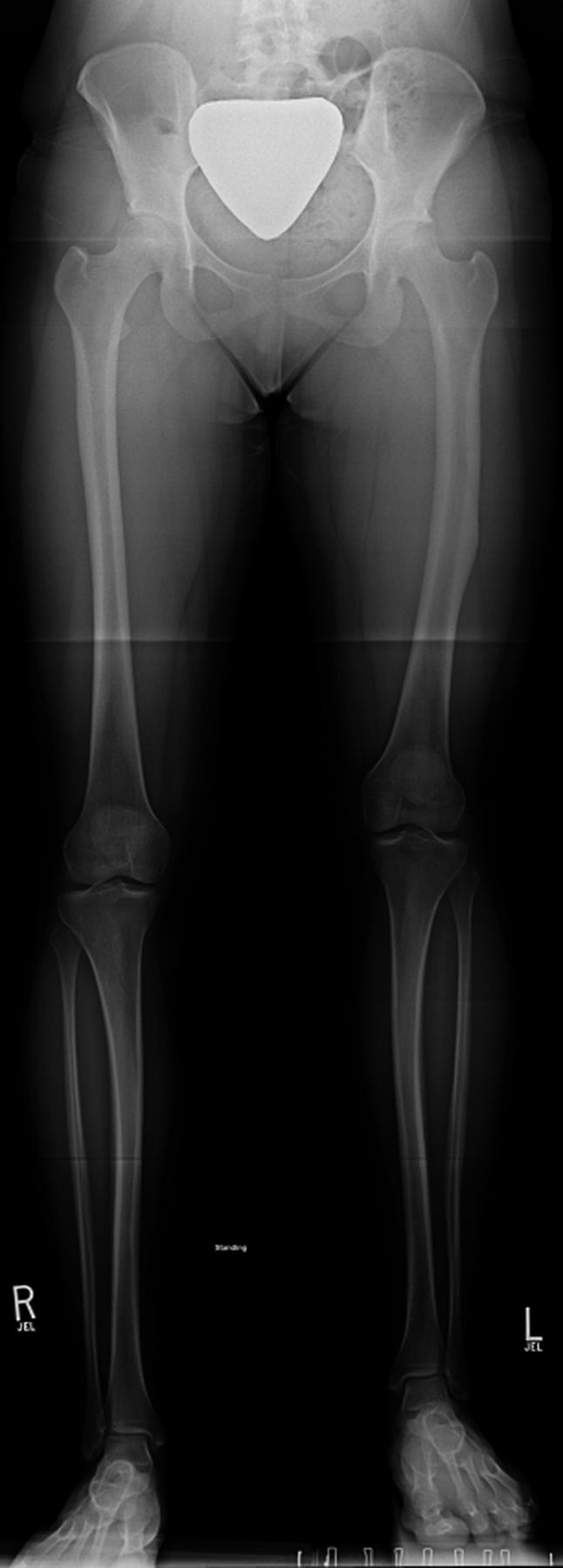
Preoperative anteroposterior radiograph of the lower extremities made with the patient standing on a 4.5-cm block under the left foot. Note the midshaft femoral varus deformity and the midshaft tibial valgus deformity.
Fig. 1-B.
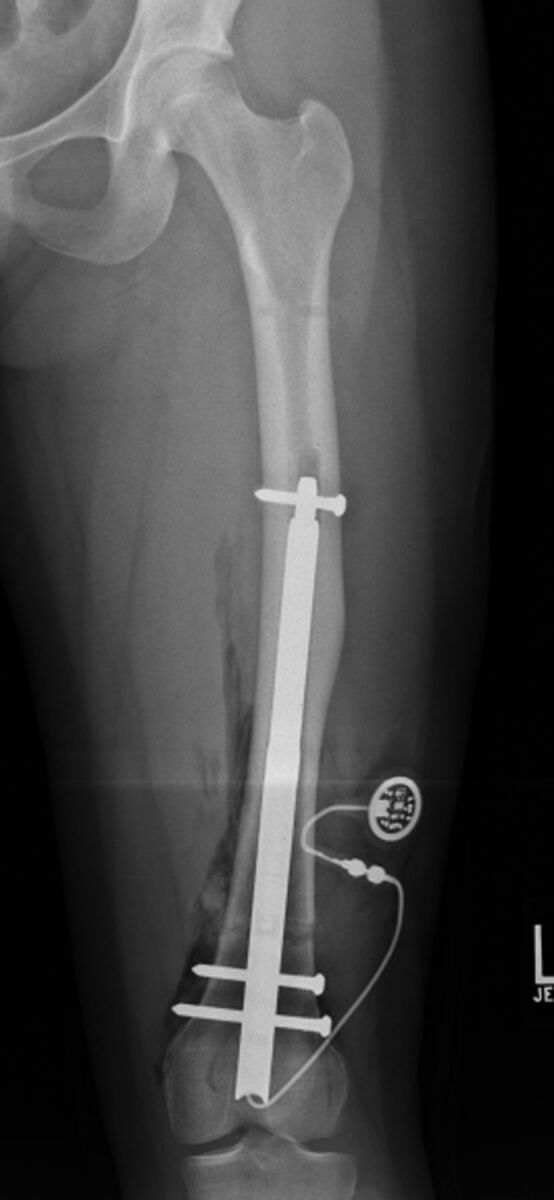
Anteroposterior radiograph of the femur made at the end of surgery. Note the retrograde insertion, proximal and distal locking bolts, and the cable leading from the base of the nail through a drill-hole in the lateral femoral condyle. This cable is connected at the time of surgery to the subcutaneously implanted radiofrequency receiver. To effect lengthening, radiofrequency waves are transmitted transcutaneously to the receiver, which converts them to electrical impulses discharged to the electric motor housed within the nail.
Fig. 1-C.
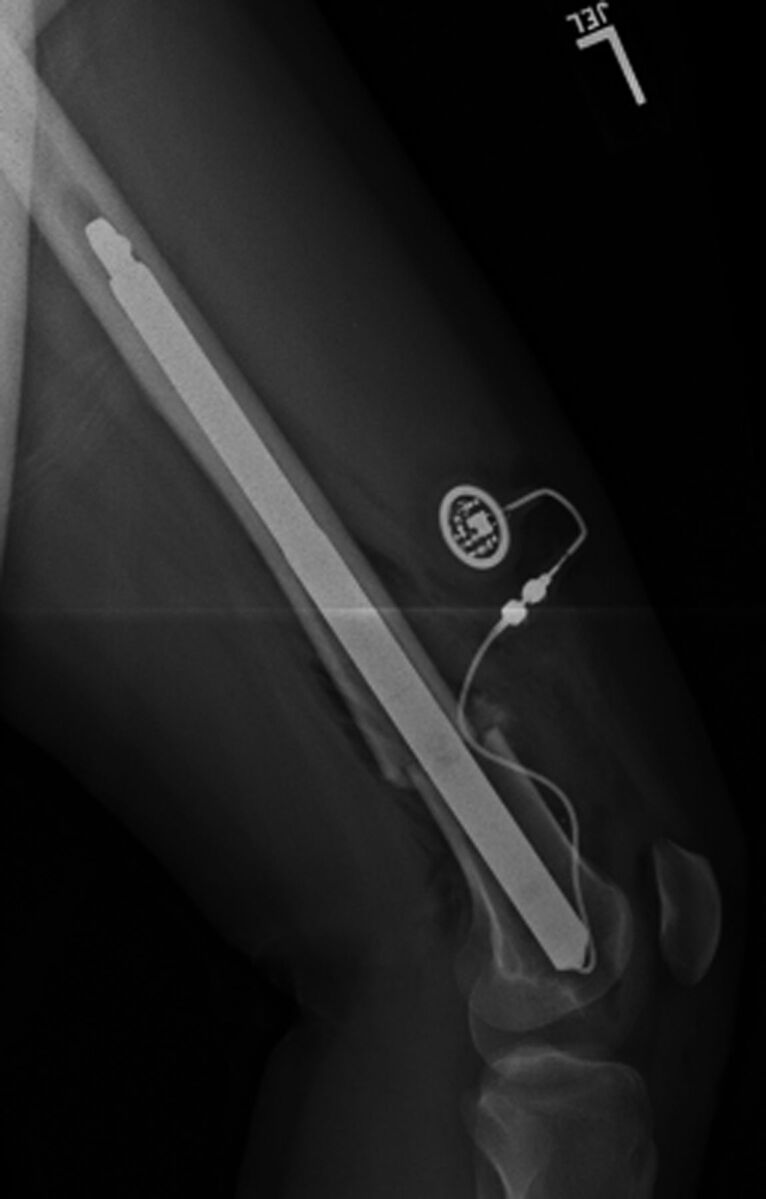
Lateral radiograph of the femur made at the end of surgery. Note the straight nail. During preoperative planning and intraoperative canal preparation by reaming, the diameter, length, and straight contour of the nail must be taken into consideration.
Fig. 1-D.
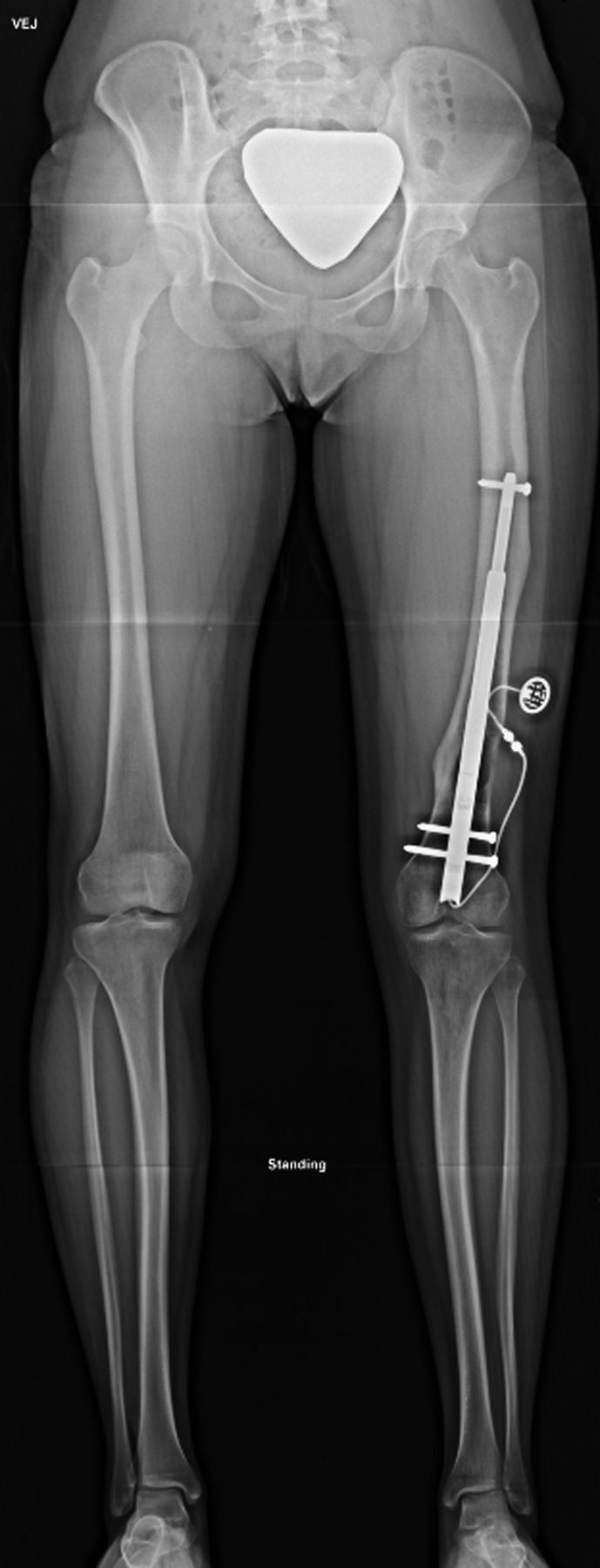
Anteroposterior radiograph of the patient made six months after surgery. The limb lengths were equal. The patient had excellent clinical and radiographic alignment of the legs and had returned to full function. The nail was removed, per manufacturer’s recommendation, eighteen months after installation.
The present retrospective chart review was undertaken to identify patient demographics, length of hospitalization, time to full weight-bearing, and any complications associated with lengthening. Radiographic review was done to determine the femoral limb-length discrepancy, amount lengthened, and any other complications. Patients being treated with the motorized nail were instructed to bear partial weight (≤20 kg) with two crutches until consolidation was deemed adequate to allow full weight-bearing. Patients treated with circular external fixation were allowed to bear full weight during treatment; however, many did not do so because of pain and discomfort. The time to full weight-bearing was determined as the interval between surgery and the postoperative clinic visit at which the patient was permitted full weight-bearing.
For the purposes of this study, the treatment goal was to achieve at least 4 cm of lengthening without incurring a new abnormality. Most patients with congenital femoral deficiency had distal femoral valgus and external rotational deformities that were usually corrected either gradually or acutely as part of their treatment. However, in each case, the primary goal of treatment was limb-lengthening.
All complications were categorized according to the classification system developed at our institution (Table I)31. Categories are based on both the magnitude of the treatment necessary and the impact on the final outcome. Complications are graded retrospectively because their impact can only be determined after treatment is complete. A Category-I complication is one that required minimal or no change to the treatment plan, with the treatment goals still achieved. Examples include a pin-track infection treated with oral antibiotics or joint stiffness resolved by physical therapy. A Category-II complication is one that results in a substantial change in the treatment plan, but the goal of treatment is still achieved. Examples include delayed consolidation requiring additional intervention, device revision, or an unplanned return to surgery. A Category-III complication is defined as one that causes a failure to achieve the treatment goal or the development of a new pathology or permanent sequelae and is divided into two subtypes. A Category-IIIA complication is one in which the treatment goal is not achieved, but there is no new pathology. Examples include a lengthening that is aborted for any reason. A Category-IIIB complication is one in which a new pathology is created, with or without achievement of the treatment goal. Examples include function-impairing joint stiffness, joint subluxation, a regenerate fracture with shortening and deformity, or a deep infection requiring systemic and/or surgical treatment. Any patient could incur more than one complication and could have more than one category of complication.
TABLE I.
Complication Classification
| Category | Definition | Examples |
| I | Minimal intervention required; treatment goal still achieved | Pin-track infection and mild joint contractures |
| II | Substantial change in treatment plan; treatment goal still achieved | Unplanned return to operating room, delayed consolidation requiring additional intervention, and device problem needing revision |
| IIIA | Failure to achieve treatment goal; no new pathology or permanent sequelae | Premature consolidation with aborted lengthening, inability to tolerate lengthening, and fracture at fixation site or regenerate bone |
| IIIB | Failure to achieve treatment goal and/or new pathology or permanent sequelae developed | Joint subluxation, regenerate fracture with deformity, and deep infection |
Means and standard deviations, medians, and ranges were used to describe continuous variables, and the nonparametric Mann-Whitney test was used to compare the circular external fixator and motorized nail groups. A chi-square test was used to compare categorical variables between the groups. When the sample was small, a Fisher exact test was performed. Significance was set at p < 0.05.
Source of Funding
There was no external source of funding for this study.
Results
Fourteen skeletally mature patients underwent fourteen femoral lengthening sessions with circular external fixation. Thirteen patients underwent fifteen retrograde femoral lengthening sessions using the motorized nail.
Data from our chart and radiographic review are summarized in Table II. The average age of the patients was 15.8 years (range, 12.3 to 18.8 years) in the circular external fixation group and 18.2 years (range, 15.5 to 21.2 years) in the motorized nail group. The average femoral discrepancy at the time of surgery was 8.4 cm (range, 4.5 to 17 cm) in the circular fixation group and 6.8 cm (range, 4 to 12 cm) in the motorized nail group. The average amount lengthened was 4.8 cm (range, 1.0 to 7.4 cm) in the circular fixator group and 4.4 cm (range, 1.5 to 7.0 cm) in the motorized nail group. The goal of at least 4 cm of lengthening was achieved in 71% of the circular external fixation group and in 73% of the motorized nail group.
TABLE II.
Comparison of Demographic, Clinical, and Radiographic Data Between the Treatment Methods
| Variable | Circular Fixation* | Motorized Nail* | P Value |
| Age† (yr) | 15.8 ± 1.9 (16.3; 12.3-18.8) | 18.2 ± 1.7 (18.6; 15.5-21.2) | <0.01 |
| Male patients (no. [%]) | 7 (50) | 7 (54) | 0.84 |
| Limb-length discrepancy at time of operation† (cm) | 8.4 ± 3.9 (7; 4.5-17) | 6.8 ± 2.5 (6; 4-12) | 0.30 |
| Lengthening amount† (cm) | 4.8 ± 1.7 (5.0; 1-7.4) | 4.4 ± 1.6 (4.5; 1.5-7) | 0.63 |
| Length of hospitalization for all patients† (days) | 47.9 ± 46.0 (28.5; 7-134) | 34.2 ± 63.0 (8; 4-224) | 0.04 |
| Length of hospitalization for patients who chose not to extend stay†‡ (days) | 19.3 ± 25.3 (9.5; 7-81) | 17.1 ± 35.0 (7; 4-133) | 0.12 |
| No. (%) of lengthening sessions for patients who extended hospitalization by choice | 6 (43) | 2 (13) | 0.11 |
| Time to full weight-bearing† (mo) | 8.8 ± 2.3 (9.1; 4.8-13) | 7.7 ± 2.2 (7.4; 4.4-11.7) | 0.27 |
| Length of follow-up† (yr) | 3.6 ± 2.0 (2.8; 1.1-7.6) | 3.0 ± 1.5 (2.7; 1.1-5.8) | 0.60 |
The circular fixation group included fourteen lengthening sessions in fourteen patients, and the motorized nail group included fifteen lengthening sessions in thirteen patients.
The values are given as the mean and the standard deviation, with the median and the range in parentheses.
Length of hospitalization after removal of data on patients who chose to extend their hospitalization for monitoring and/or therapy.
As a charitable institution, we were able to provide patients the option of an extended hospital stay to supervise the lengthening process. The circular external fixator group had six extended stays among the fourteen lengthening procedures, whereas the motorized nail group had two extended stays among the fifteen procedures (p = 0.11). There was one unplanned readmission for physical therapy and pain management in each group. Overall, the median duration of hospitalization was 28.5 days (range, seven to 134 days) for patients treated with circular external fixation compared with eight days (range, four to 224 days) for the motorized nail group (p = 0.04). Excluding the patients who elected an extended hospital stay, the median duration of hospitalization was 9.5 days (range, seven to eighty-one days) for the circular external fixation group compared with seven days (range, four to 133 days) for the motorized nail group (p = 0.12). The average time to full weight-bearing was 8.8 months (range, 4.8 to 13.0 months) in the circular fixation group and 7.7 months (range, 4.4 to 11.7 months) in the motorized nail group (p = 0.27). The average length of follow-up was 3.6 years in the circular fixation group and 3.0 years in the motorized nail group.
The severity and rate of complications for both groups are recorded in Tables III and IV. Since patients could have more than one complication and more than one category of complication, we analyzed the complication rate in two ways: the number of patients incurring specific categories of complications per lengthening session (Table III) and the total number of complications by category per lengthening session (Table IV). Complications of varying severity occurred in all fourteen lengthening sessions (all fourteen patients) in the group that had circular external fixation and in eleven (73%) of fifteen lengthening sessions (thirteen patients) in the group treated with the motorized nail (p = 0.10) (Table III). Eleven of fourteen lengthening sessions (fourteen patients) in the circular fixation group were associated with Category-I complications compared with five (33%) of the fifteen lengthening sessions (thirteen patients) in the motorized nail group (p = 0.03). No significant difference was detected between the circular fixation and motorized nail groups with respect to the percentage of Category-II complications, with 43% (six complications) and 40% (six complications), respectively (p = 0.88), or with respect to the Category-IIIA complications, with 29% (four complications) and 20% (three complications) (p = 0.68). The rate of Category-IIIB complications in the motorized nail group (three; 20%) was lower than that in the circular fixation group (five; 36%); however, the difference was not significant (p = 0.43).
TABLE III.
Comparison of Circular Fixation and Motorized Nail Groups with Regard to Lengthening Sessions Affected by Complications
| Circular Fixation* |
Motorized Nail* |
||||
| Complication Category | No. of Complications (N = 14) | No. (%) of Lengthening Sessions Affected by Complications | No. of Complications (N = 15) | No. (%) of Lengthening Sessions Affected by Complications | P Value |
| I | 15 | 11 (79) | 7 | 5 (33) | 0.03 |
| II | 8 | 6 (43) | 6 | 6 (40) | 0.88 |
| IIIA | 4 | 4 (29) | 4 | 3 (20) | 0.68 |
| IIIB | 6 | 5 (36) | 3 | 3 (20) | 0.43 |
| Any complication | 33 | 14 (100) | 20 | 11 (73) | 0.10 |
The circular fixation group included fourteen lengthening sessions in fourteen patients, and the motorized nail group included fifteen lengthening sessions in thirteen patients.
TABLE IV.
Comparison of the Number of Complications per Lengthening Session with Circular Fixation or Motorized Nail
| Complication Category | Circular Fixation* | Motorized Nail* | P Value |
| I | 1.1 ± 0.8 (1; 0-3) | 0.4 ± 0.6 (0; 0-2) | 0.02 |
| II | 0.6 ± 0.9 (0; 0-3) | 0.4 ± 0.5 (0; 0-1) | 0.78 |
| IIIA | 0.3 ± 0.5 (0; 0-1) | 0.2 ± 0.4 (0; 0-1) | 0.62 |
| IIIB | 0.4 ± 0.7 (0; 0-2) | 0.2 ± 0.4 (0; 0-1) | 0.33 |
| Total complications | 2.4 ± 1.3 (2; 1-5) | 1.2 ± 1.1 (1; 0-4) | 0.02 |
The values are given as the mean and the standard deviation, with the median and the range in parentheses. The circular fixation group included fourteen lengthening sessions in fourteen patients, and the motorized nail group included fifteen lengthening sessions in thirteen patients.
The average number of complications by category per lengthening session for both groups is summarized in Table IV. The average number of Category-I complications per lengthening session was 1.1 in the circular external fixation group compared with 0.4 in the motorized nail group (p = 0.02). The number of Category-II, IIIA, and IIIB complications per lengthening session was not significantly different between the treatment groups. The average total number of complications was 2.4 per lengthening session in the circular external fixation group compared with 1.2 per session in the motorized nail group (p = 0.02) (Table IV).
Eight patients had undergone eleven femoral lengthening sessions with circular external fixation prior to subsequent lengthening using the motorized nail (ten lengthening sessions). The rate of complications by category and the number of lengthening sessions associated with any complication (eleven of eleven sessions with circular external fixation and nine of ten sessions with the motorized nail) were comparable (Table V). The total number of complications averaged 2.6 during lengthening with circular external fixation compared with 1.6 during subsequent lengthening with the motorized nail (p = 0.03) (Table VI).
TABLE V.
Comparison of Lengthening Sessions Affected by Complications in Eight Patients Managed with Circular Fixation with Subsequent Lengthening with Motorized Nail
| Complication Category | Circular Fixation* (no. [%] of sessions) | Motorized Nail* (no. [%] of sessions) | P Value |
| I | 10 (91) | 5 (50) | 0.06 |
| II | 4 (36) | 5 (50) | 0.67 |
| IIIA | 3 (27) | 2 (20) | >0.99 |
| IIIB | 7 (64) | 3 (30) | 0.20 |
| Any complication | 11 (100) | 9 (90) | 0.48 |
The circular fixation group included eleven lengthening sessions in eight patients, and the motorized nail group included ten lengthening sessions in eight patients.
TABLE VI.
Comparison of the Number of Complications per Lengthening Session in Patients Managed with Circular Fixation with Subsequent Lengthening with Motorized Nail
| Complication Category | Circular Fixation* | Motorized Nail* | P Value |
| I | 1.3 ± 0.8 (1; 0-3) | 0.6 ± 0.7 (0.5; 0-2) | 0.05 |
| II | 0.4 ± 0.5 (0; 0-1) | 0.5 ± 0.5 (0.5; 0-1) | 0.57 |
| IIIA | 0.3 ± 0.5 (0; 0-1) | 0.2 ± 0.4 (0; 0-1) | 0.74 |
| IIIB | 0.7 ± 0.7 (1; 0-2) | 0.3 ± 0.5 (0; 0-1) | 0.12 |
| Total complications | 2.6 ± 1.1 (2; 1-4) | 1.6 ± 1.1 (1.5; 0-4) | 0.03 |
The values are given as the mean and the standard deviation, with the median and the range in parentheses. The circular fixation group included eleven lengthening sessions in eight patients, and the motorized nail group included ten lengthening sessions in eight patients.
In the circular external fixation group, there were four Category-IIIA complications, including premature consolidation with cessation of lengthening (one patient), an inability to tolerate the lengthening (two patients), and deformity of the regenerate bone (one patient). In the motorized nail group, there were three Category-IIIA complications, including an unrecognized settling of the telescopic rod that resulted in a decrease of the 4-cm lengthening that had been achieved to a final length increase of 2.5 cm in one patient. Another patient had a shift of the rod within the medullary canal that resulted in the need to abort the procedure after 1.5 cm of lengthening (Figs. 2-A and 2-B). A third patient, who was noncompliant with the partial weight-bearing protocol, fell shortly after achieving the desired lengthening goal (4.5 cm) and incurred a segmental fracture with shortening. The motorized nail was replaced with a retrograde trauma nail, and shortening to a 2.2-cm length increase was accepted (Figs. 3-A and 3-B). None of these complications was device-specific.
Figs. 2-A and 2-B A patient who had failure to achieve the lengthening goal (a Category-IIIA complication).
Fig. 2-A.
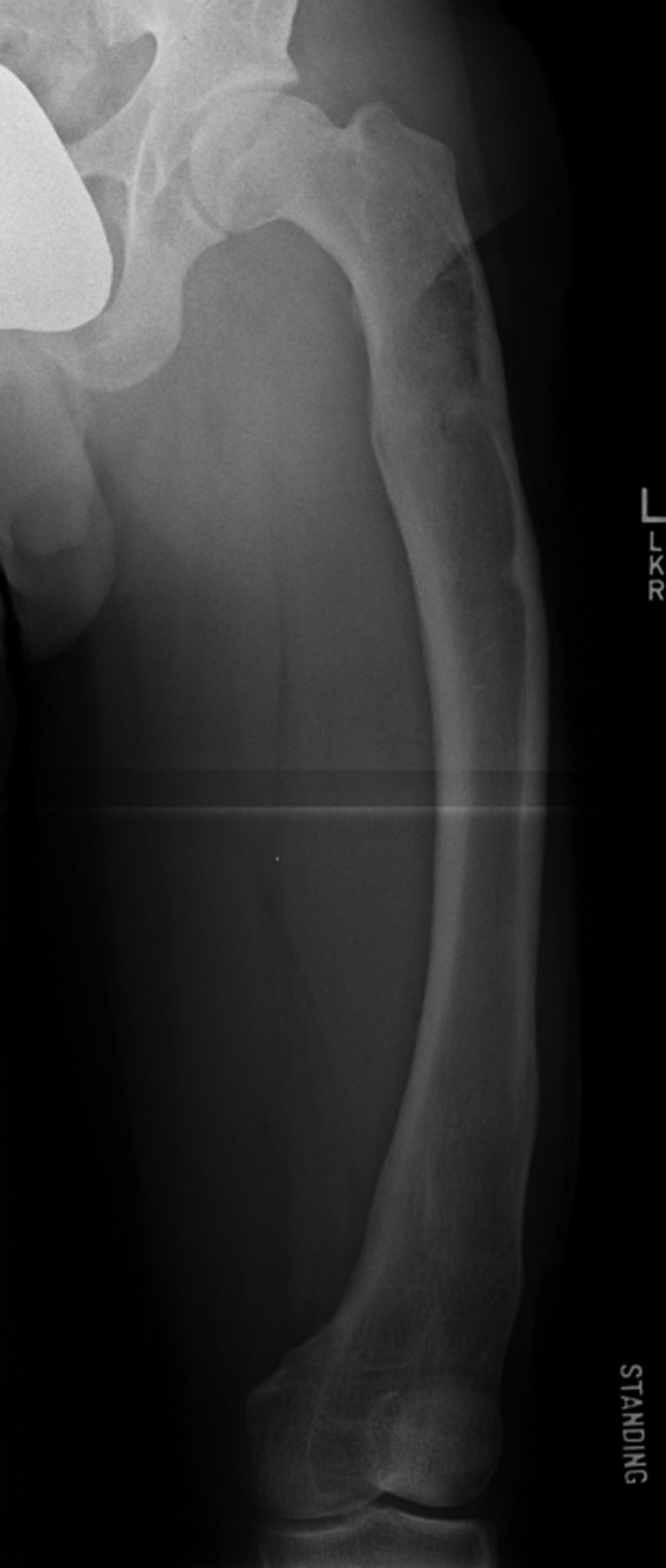
Anteroposterior radiograph of the left femur demonstrating substantial varus deformity. The patient had two prior femoral lengthening procedures using a circular external fixator.
Fig. 2-B.
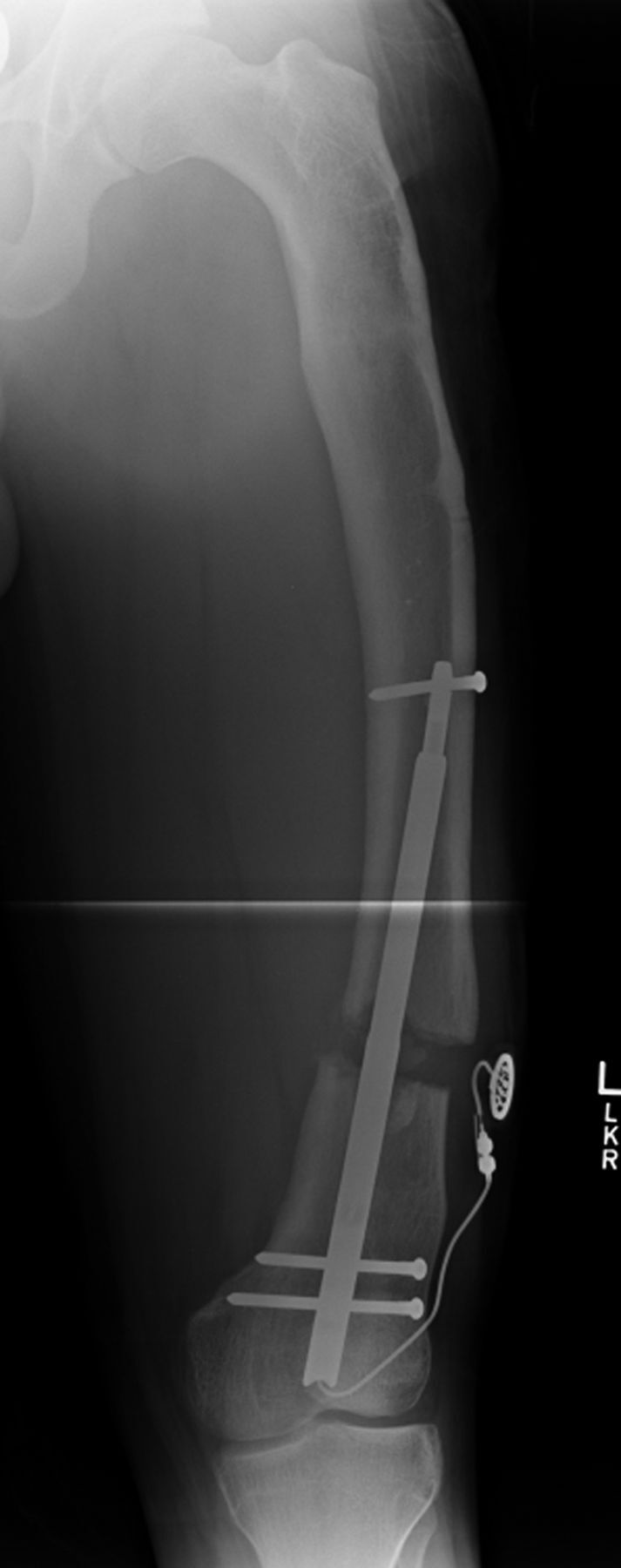
At three weeks postoperatively, the motorized nail had shifted within the patulous medullary canal, with bending of the proximal locking bolt. Lengthening was ceased at this point.
Figs. 3-A and 3-B A patient with congenital femoral deficiency who had femoral lengthening with a motorized intramedullary nail.
Fig. 3-A.
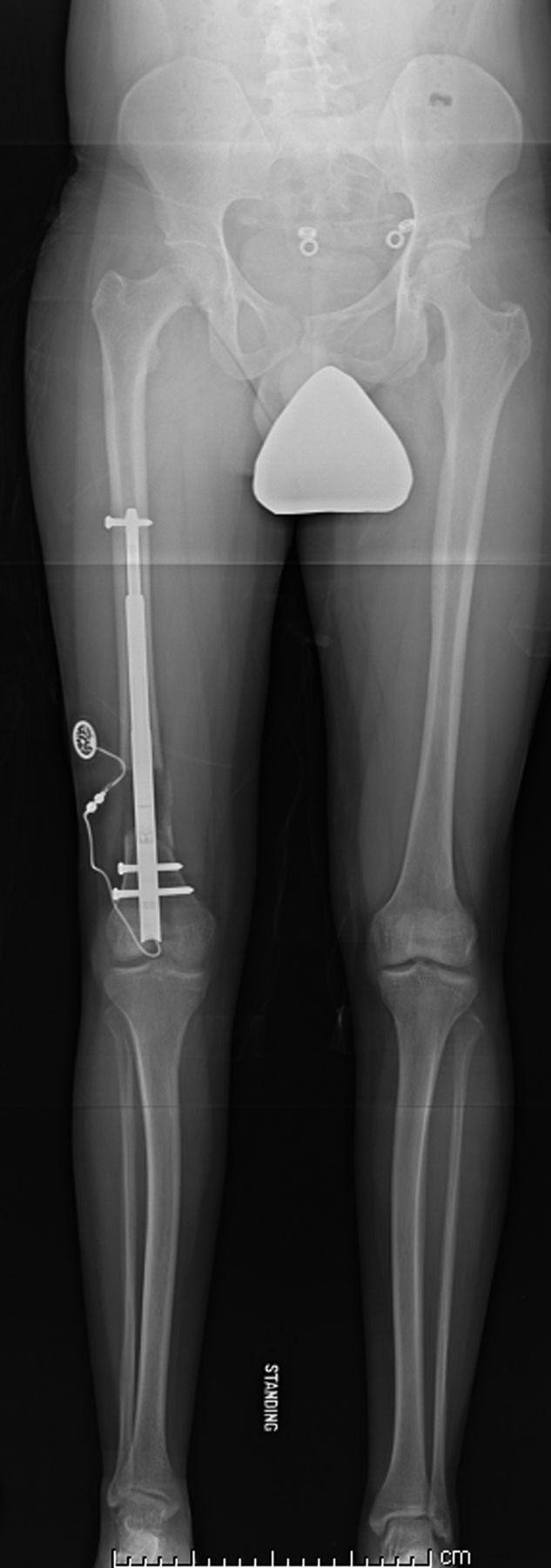
Standing anteroposterior radiograph, made at the end of lengthening, demonstrates full correction of the limb-length inequality and excellent limb alignment.
Fig. 3-B.
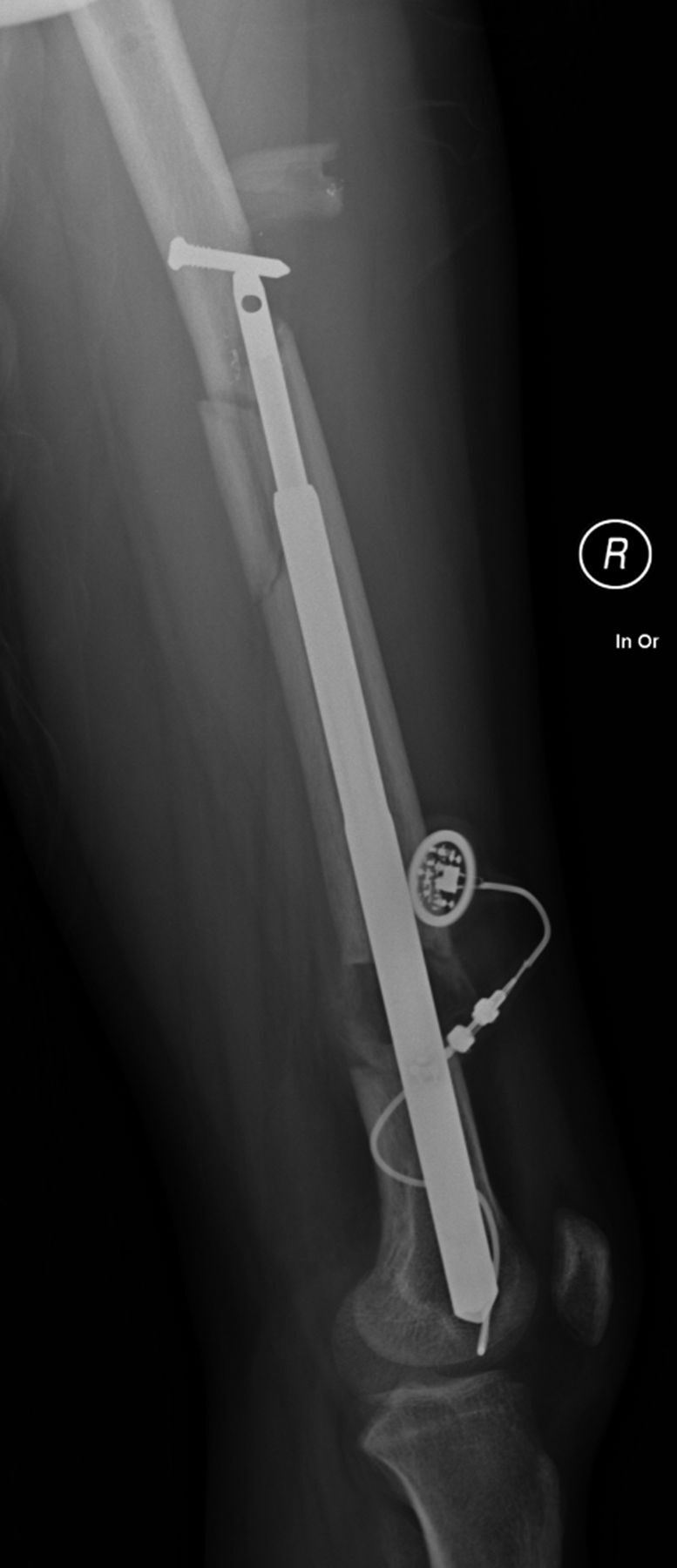
After weight-bearing without crutches against medical advice, and then falling, the patient sustained a segmental fracture of the proximal part of the femur through the proximal locking bolt site, with telescoping of the lengthened segment. The patient underwent revision to a retrograde femoral trauma nail, with some loss of previously achieved length.
In the circular fixation group, six Category-IIIB complications occurred in five patients during five lengthening sessions and included a regenerate fracture treated with open reduction and internal fixation (one patient); quadriceps contracture, with or without obligate patellar dislocation, requiring a quadricepsplasty (three patients); and a deep infection (two patients). Patients in the circular external fixation group with Category-IIIB complications had an average lengthening of 5.7 cm (range, 3.0 to 7.4 cm). The three Category-IIIB complications in the motorized nail group included the need for quadricepsplasty (two patients) and a deep intramedullary infection requiring serial debridement and antibiotic suppression during lengthening (one patient). All three of these patients achieved lengthening of >5.0 cm.
Discussion
The use of circular external fixation and gradual distraction of a fracture callus after osteotomy has extended the indications for limb-lengthening. Prolonged immobilization, difficulty with pin care, and a high rate of complications, however, have stimulated interest in improving limb-lengthening techniques. Lengthening with external fixation over an intramedullary nail, with locking of the nail at the end of the distraction period and removal of the external fixator, was popularized by Paley et al.7. They reported the results for twenty-nine patients who had lengthening with this technique compared with thirty-one who had lengthening using an Ilizarov circular fixator. They found that the group that had lengthening over a nail had reduced time in circular fixation, a decreased number of complications, fewer subsequent fractures, and earlier rehabilitation, although the overall final results were similar between the two groups.
The first fully implantable intramedullary lengthening device is attributed to Bliskunov32. Reports of totally implantable intramedullary lengthening devices include the Albizzia nail (DePuy, Villerubanne, France)18,20,33, the intramedullary skeletal kinetic distractor (ISKD; Orthofix, Lewisville, Texas)34,35, FITBONE nails (Wittenstein Intens)17,18,36, and PRECICE nails (Ellipse Technologies, Irvine, California)37-39. The Albizzia and the ISKD nails rely on limb rotation to effect lengthening. The FITBONE and PRECICE are the only motorized intramedullary lengthening devices; the latter device has the added benefit of being reversible. The overall complication rate for intramedullary devices has been reported to range from 10% to 47%17,20,33,34,37-42. Those studies included small numbers of patients, heterogeneous diagnoses, and both femoral and tibial lengthening sessions, and they lacked matched patients treated with external fixation.
Reporting of complications associated with limb-lengthening has been affected by heterogeneous patient populations and a lack of standardized categorization of complications. Donnan et al. utilized a complication classification system in a review of acute correction of lower limb deformity and limb-lengthening using circular fixation4. A grade-I complication had no long-term functional or anatomic importance and did not require surgery or anesthesia. A grade-II complication had no long-term importance, but surgery or anesthesia was needed to correct it. A grade-III complication had functional or anatomic impact but either resolved spontaneously or was correctable by surgery. A grade-IV complication was unresolvable by any standard treatment. Dahl et al. used a system in which complications were graded as minor, serious, and severe2. Paley used the terms problems (grade 1), obstacles (grade 2), and complications (grade 3), with problems indicating no operative intervention was required, obstacles meaning operative intervention was required, and complications indicating intraoperative injuries or issues had occurred and had not been resolved before the end of treatment. The complications were subdivided into major and minor complications6. The absence of a standardized complication classification limits the ability to compare complication rates across studies5,21.
It must be noted that the observations noted in our study are substantially impeded by the fact that the study is purely a retrospective chart and radiographic review, and many of those observations must be considered subjective in nature. In addition, the only clinically important and significant differences in the rate of complications were of the minor (Category-I) type (primarily pin-track infections). Our study included only skeletally mature patients with the diagnosis of congenital femoral deficiency who were treated under the same lengthening protocols at one institution and were carefully evaluated using a critical and comprehensive complication classification system. The patients treated with the motorized nail in our study were, on average, an extremely challenging patient group, with the majority having undergone one or more previous lengthening sessions by circular external fixation. Nevertheless, the patients treated with a motorized nail had a lower rate of complications than those treated with circular external fixation, and the patients who had previous circular fixator lengthening sessions had a lower complication rate after treatment with the motorized nail. Furthermore, the patients treated with a motorized nail had a shorter hospitalization, on average, and fewer required prolonged hospitalizations to supervise the lengthening procedure. The obligate retrospective nature of this study made evaluation of potentially confounding variables, such as individual patient selection, surgeon factors, and socioeconomic differences among patients, not feasible, and thus the findings must be considered as subjective observations only.
We had the impression that the patients treated with the motorized nail had, on average, substantially less pain after the initial postoperative period, required less intensive physical therapy supervision and intervention, and incurred less disruption in the activities of daily living than did those treated with circular external fixation. These observations, however, cannot be accurately quantified in a strictly retrospective chart and radiographic review and are purely subjective in nature. Furthermore, we had not employed other femoral lengthening methods (such as those involving monolateral or hexapod external fixators, or combined external and internal fixation) and thus cannot make direct comparison between outcomes of patients so treated and our patient population.
Using our complication classification scheme, a homogeneous pediatric population with congenital femoral deficiency treated with circular fixation and the motorized nail were compared in terms of outcomes and complications. Lengthening with the motorized nail decreased the number of complications compared with circular external fixation. This classification system can be used to quantify and qualify complications in limb-lengthening. A decreased rate of complications and improved outcomes may be realized with the use of motorized intramedullary lengthening devices in motivated patients with congenital femoral deficiency who require femoral lengthening. Although the specific intramedullary lengthening nail used in these patients did not have FDA approval, the overall positive experience encouraged us to continue to use FDA-approved intramedullary lengthening nails for the same indications.
Acknowledgments
Note: The authors thank Professor Rainer Baumgart for his excellent clinical instruction in the proper application of intramedullary lengthening techniques. They also thank Mr. Bruce Foster from Adelaide, South Australia, Australia, for his friendship and guidance.
Footnotes
Investigation performed at the Department of Orthopedics, Texas Scottish Rite Hospital for Children, Dallas, Texas
Disclosure: None of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of any aspect of this work. One or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. In addition, one or more of the authors has a patent or patents, planned, pending, or issued, that is broadly relevant to the work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Aldegheri R, Dall’Oca C. Limb lengthening in short stature patients. J Pediatr Orthop B. 2001. July;10(3):238-47. [PubMed] [Google Scholar]
- 2.Dahl MT, Gulli B, Berg T. Complications of limb lengthening. A learning curve. Clin Orthop Relat Res. 1994. April;301:10-8. [PubMed] [Google Scholar]
- 3.Faber FW, Keessen W, van Roermund PM. Complications of leg lengthening. 46 procedures in 28 patients. Acta Orthop Scand. 1991. August;62(4):327-32. [DOI] [PubMed] [Google Scholar]
- 4.Donnan LT, Saleh M, Rigby AS. Acute correction of lower limb deformity and simultaneous lengthening with a monolateral fixator. J Bone Joint Surg Br. 2003. March;85(2):254-60. [DOI] [PubMed] [Google Scholar]
- 5.Noonan KJ, Leyes M, Forriol F, Cañadell J. Distraction osteogenesis of the lower extremity with use of monolateral external fixation. A study of two hundred and sixty-one femora and tibiae. J Bone Joint Surg Am. 1998. June;80(6):793-806. [DOI] [PubMed] [Google Scholar]
- 6.Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res. 1990. January;250:81-104. [PubMed] [Google Scholar]
- 7.Paley D, Herzenberg JE, Paremain G, Bhave A. Femoral lengthening over an intramedullary nail. A matched-case comparison with Ilizarov femoral lengthening. J Bone Joint Surg Am. 1997. October;79(10):1464-80. [DOI] [PubMed] [Google Scholar]
- 8.Tjernström B, Olerud S, Rehnberg L. Limb lengthening by callus distraction. Complications in 53 cases operated 1980-1991. Acta Orthop Scand. 1994. August;65(4):447-55. [DOI] [PubMed] [Google Scholar]
- 9.Young N, Bell DF, Anthony A. Pediatric pain patterns during Ilizarov treatment of limb length discrepancy and angular deformity. J Pediatr Orthop. 1994. May-Jun;14(3):352-7. [DOI] [PubMed] [Google Scholar]
- 10.Herzenberg JE, Scheufele LL, Paley D, Bechtel R, Tepper S. Knee range of motion in isolated femoral lengthening. Clin Orthop Relat Res. 1994. April;301:49-54. [PubMed] [Google Scholar]
- 11.Danziger MB, Kumar A, DeWeese J. Fractures after femoral lengthening using the Ilizarov method. J Pediatr Orthop. 1995. Mar-Apr;15(2):220-3. [PubMed] [Google Scholar]
- 12.Ramaker RR, Lagro SWJ, van Roermund PM, Sinnema G. The psychological and social functioning of 14 children and 12 adolescents after Ilizarov leg lengthening. Acta Orthop Scand. 2000. February;71(1):55-9. [DOI] [PubMed] [Google Scholar]
- 13.Baumann F, Harms J. [The extension nail. A new method for lengthening of the femur and tibia (author’s transl)]. Arch Orthop Unfallchir. 1977. December 9;90(2):139-46. German. [DOI] [PubMed] [Google Scholar]
- 14.Götz J, Schellmann WD. [Continuous lengthening of the femur with intramedullary stabilisation (author’s transl)]. Arch Orthop Unfallchir. 1975. July 28;82(4):305-10. German. [DOI] [PubMed] [Google Scholar]
- 15.Witt AN, Jäger M, Bruns H, Küsswetter W. [An implantable femur distractor for operative leg lengthening (author’s transl)]. Arch Orthop Trauma Surg. 1978. October 27;92(4):291-6. German. [DOI] [PubMed] [Google Scholar]
- 16.Betz A, Baumgart R, Schweiberer L. [First fully implantable intramedullary system for callus distraction—intramedullary nail with programmable drive for leg lengthening and segment displacement. Principles and initial clinical results]. Chirurg. 1990. August;61(8):605-9. German. [PubMed] [Google Scholar]
- 17.Cole JD, Justin D, Kasparis T, DeVlught D, Knobloch C. The intramedullary skeletal kinetic distractor (ISKD): first clinical results of a new intramedullary nail for lengthening of the femur and tibia. Injury. 2001. December;32(Suppl 4):SD129-39. [DOI] [PubMed] [Google Scholar]
- 18.Guichet JM, Casar RS. Mechanical characterization of a totally intramedullary gradual elongation nail. Clin Orthop Relat Res. 1997. April;337:281-90. [DOI] [PubMed] [Google Scholar]
- 19.Krieg AH, Speth BM, Foster BK. Leg lengthening with a motorized nail in adolescents: an alternative to external fixators? Clin Orthop Relat Res. 2008. January;466(1):189-97. Epub 2008 Jan 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guichet JM, Deromedis B, Donnan LT, Peretti G, Lascombes P, Bado F. Gradual femoral lengthening with the Albizzia intramedullary nail. J Bone Joint Surg Am. 2003. May;85(5):838-48. [DOI] [PubMed] [Google Scholar]
- 21.Velazquez RJ, Bell DF, Armstrong PF, Babyn P, Tibshirani R. Complications of use of the Ilizarov technique in the correction of limb deformities in children. J Bone Joint Surg Am. 1993. August;75(8):1148-56. [DOI] [PubMed] [Google Scholar]
- 22.Gillespie R, Torode IP. Classification and management of congenital abnormalities of the femur. J Bone Joint Surg Br. 1983. November;65(5):557-68. [DOI] [PubMed] [Google Scholar]
- 23.Kalamchi A, Cowell HR, Kim KI. Congenital deficiency of the femur. J Pediatr Orthop. 1985. Mar-Apr;5(2):129-34. [PubMed] [Google Scholar]
- 24.Pappas AM. Congenital abnormalities of the femur and related lower extremity malformations: classification and treatment. J Pediatr Orthop. 1983. February;3(1):45-60. [DOI] [PubMed] [Google Scholar]
- 25.Bell DF, Boyer MI, Armstrong PF. The use of the Ilizarov technique in the correction of limb deformities associated with skeletal dysplasia. J Pediatr Orthop. 1992. May-Jun;12(3):283-90. [DOI] [PubMed] [Google Scholar]
- 26.Karger C, Guille JT, Bowen JR. Lengthening of congenital lower limb deficiencies. Clin Orthop Relat Res. 1993. June;291:236-45. [PubMed] [Google Scholar]
- 27.Maffuli N, Fixsen JA. Distraction osteogenesis in congenital limb length discrepancy: a review. J R Coll Surg Edinb. 1996. August;41(4):258-64. [PubMed] [Google Scholar]
- 28.Tsuchiya H, Uehara K, Abdel-Wanis ME, Sakurakichi K, Kabata T, Tomita K. Deformity correction followed by lengthening with the Ilizarov method. Clin Orthop Relat Res. 2002. September;402:176-83. [DOI] [PubMed] [Google Scholar]
- 29.Herzenberg JE, Branfoot T, Paley D, Violante FH. Femoral nailing to treat fractures after lengthening for congenital femoral deficiency in young children. J Pediatr Orthop B. 2010. March;19(2):150-4. [DOI] [PubMed] [Google Scholar]
- 30.Baumgart R. The reverse planning method for lengthening of the lower limb using a straight intramedullary nail with or without deformity correction. A new method. Oper Orthop Traumatol. 2009. June;21(2):221-33. [DOI] [PubMed] [Google Scholar]
- 31.Cherkashin AM, Samchukov ML, Birch JG, Da Cunha AL. Evaluation of complications of treatment of severe Blount’s disease by circular external fixation using a novel classification scheme. J Pediatr Orthop B. 2015. March;24(2):123-30. [DOI] [PubMed] [Google Scholar]
- 32.Bliskunov AI. [Implantable devices for lengthening the femur without external drive mechanisms]. Med Tekh. 1984. Mar-Apr;(2):44-9. Russian. [PubMed] [Google Scholar]
- 33.García-Cimbrelo E, Curto de la Mano A, García-Rey E, Cordero J, Marti-Ciruelos R. The intramedullary elongation nail for femoral lengthening. J Bone Joint Surg Br. 2002. September;84(7):971-7. [DOI] [PubMed] [Google Scholar]
- 34.Lee DH, Ryu KJ, Song HR, Han SH. Complications of the Intramedullary Skeletal Kinetic Distractor (ISKD) in distraction osteogenesis. Clin Orthop Relat Res. 2014. December;472(12):3852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hankemeier S, Pape HC, Gosling T, Hufner T, Richter M, Krettek C. Improved comfort in lower limb lengthening with the intramedullary skeletal kinetic distractor. Principles and preliminary clinical experiences. Arch Orthop Trauma Surg. 2004. March;124(2):129-33. Epub 2004 Jan 27. [DOI] [PubMed] [Google Scholar]
- 36.Baumgart R, Betz A, Schweiberer L. A fully implantable motorized intramedullary nail for limb lengthening and bone transport. Clin Orthop Relat Res. 1997. October;343:135-43. [PubMed] [Google Scholar]
- 37.Al-Sayyad MJ. Lower limb lengthening and deformity correction using the Fitbone motorized nail system in the adolescent patient. J Pediatr Orthop B. 2012. March;21(2):131-6. [DOI] [PubMed] [Google Scholar]
- 38.Kirane YM, Fragomen AT, Rozbruch SR. Precision of the PRECICE internal bone lengthening nail. Clin Orthop Relat Res. 2014. December;472(12):3869-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shabtai L, Specht SC, Standard SC, Herzenberg JE. Internal lengthening device for congenital femoral deficiency and fibular hemimelia. Clin Orthop Relat Res. 2014. December;472(12):3860-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leidinger B, Winkelmann W, Roedl R. [Limb lengthening with a fully implantable mechanical distraction intramedullary nail]. Z Orthop Ihre Grenzgeb. 2006. Jul-Aug;144(4):419-26. German. [DOI] [PubMed] [Google Scholar]
- 41.Krieg AH, Lenze U, Speth BM, Hasler CC. Intramedullary leg lengthening with a motorized nail. Acta Orthop. 2011. June;82(3):344-50. Epub 2011 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baumgart R, Thaller P, Hinterwimmer S, Krammer M, Hierl T, Mutschler W. A fully implantable, programmable distraction nail (Fitbone): new perspectives for corrective and reconstructive limb survey. In: Leung KS, Taglang G, Schnettler R, editors. Practice of intramedullary locked nails. 3rd ed Heidelberg, Germany: Springer Berlin; 2006. p 189-198. [Google Scholar]


