Abstract
Purpose
To evaluate the effect of postoperative adjuvant transarterial chemoembolization (PA-TACE) on the prognosis of hepatocellular carcinoma (HCC) with macroscopic bile duct tumor thrombus (BDTT).
Patients and Methods
This study included 109 patients who underwent R0 resection for HCC with BDTT between January 2008 and December 2017: non-TACE (48) and PA-TACE (61). Propensity-score matching (PSM) was conducted in a 1:1 ratio. Recurrence and overall survival (OS) rates were analyzed using the Kaplan–Meier method. Independent risk factors were identified by univariate and multivariate Cox regression analyses. Subgroup analysis was performed by risk-factor stratification.
Results
The recurrence rates in the non-TACE and PA-TACE groups were different at 6 months (50.9% vs 26.9%, P=0.03) before PSM and at 6 months (59.3% vs 26.5%, P=0.02) and 12 months (81.4% vs 37.5%, P=0.022) after PSM. OS rates of the non-TACE and PA-TACE groups were different at 6 months (74.0% vs 91.6%, P<0.001) and 12 months (61.1% vs 77.6%, P=0.01) before PSM and at 6 months (73.0% vs 96.8%, P=0.01), 12 months (52.1% vs 89.6%, P=0.001), and 18 months (33.8% vs 64.4%, P=0.034) after PSM. PA-TACE was an independent prognostic factor for both recurrence and OS before and after PSM. Subgroup analysis showed that patients with no HBV infection, tumors >5 cm, macrovascular invasion, alpha-fetoprotein (AFP) >400 ng/mL, or gamma-glutamyl transferase (GGT) >150 U/L benefited significantly from PA-TACE in terms of recurrence rates (all P<0.05). Patients with no HBV infection, multiple tumors, tumors >5 cm, macrovascular invasion, or AFP >400 ng/mL benefited significantly from PA-TACE in terms of OS (all P<0.05).
Conclusion
PA-TACE could prolong the short-term prognosis of HCC with macroscopic BDTT and should be recommended for patients with no HBV infection, multiple tumors, tumors >5 cm, poor differentiation, macrovascular invasion, AFP >400 ng/mL, or GGT >150 U/L.
Keywords: hepatocellular carcinoma, bile duct tumor thrombus, transarterial chemoembolization, recurrence, survival
Introduction
Bile duct tumor thrombus (BDTT) involves invasion of hepatocellular carcinoma (HCC) into the biliary tree. The incidence rate of HCC with macroscopic BDTT has been reported to be 0.45–12.9%.1–3 Although the incidence is low, an increasing amount of research has focused on BDTT in recent years. Compared with patients without BDTT, patients with BDTT had a shorter survival time and a higher recurrence rate. This has been proven by different retrospective studies and meta-analyses.2,4–10 The median survival of patients with BDTT is 1.6–4.3 months with conservative management.11,12
Surgical resection is the most commonly used management strategy, which allows radical cure of patients with resectable HCC with BDTT. However, the high recurrence rate after resection is a barrier to long-term survival. The 1-year recurrence rate of patients with HCC with BDTT remains between 42.9 and 70.3% even after resection.10,13 Therefore, postoperative adjuvant treatment for such patients cannot be ignored.
Postoperative adjuvant transarterial chemoembolization (PA-TACE) has been applied clinically for decades as an anti-recurrence therapy. Some randomized clinical trials have proven that HCC with microvascular invasion or portal vein tumor thrombus can benefit from PA-TACE.14–16 However, whether HCC with BDTT after R0 resection could benefit from PA-TACE remains unknown.
Thus, in this study, we aimed to evaluate the short-term effect of PA-TACE on the prognosis of patients after R0 resection.
Patients and Methods
Patient Selection
The study was conducted according to the Declaration of Helsinki and the Ethical Guidelines for Clinical Studies, and was approved by the institutional research ethics committee of Mengchao Hepatobiliary Hospital of Fujian Medical University (Approval Number:2020_077_01). All patients signed an informed consent form before surgery. As some elderly patients cannot read, they sign the informed consent by hand print instead of signature, and their direct relatives sign the informed consent at the same time. Patients who underwent R0 resection for HCC with BDTT between January 2008 and December 2017 at primary liver cancer big data were enrolled for in this study.17 Data including baseline and clinical characteristics and information on follow-up were extracted and censored on December 31, 2019.
The inclusion criteria for the study were as follows: patients (1) who underwent R0 resection with curative intent for HCC and (2) in whom HCC and macroscopic BDTT were confirmed by histopathological analysis. The exclusion criteria for the study were as follows: patients (1) aged <18 years; (2) with other accompanying cancers; (3) with recurrent or metastatic HCC; (4) with a diagnosis of combined hepatocellular carcinoma-intrahepatic cholangiocarcinoma (cHCC-ICC); and (5) who received anti-cancer therapy before surgery. R0 resection was defined as removal of all macroscopic tumors with a microscopically negative margin and no recurrence within two months after surgery.
Surgery
The scope of liver resection was determined on the basis of the location and size of the tumor, liver function, and residual liver volume. Hepatectomy includes partial resection, subsegmentectomy, segmentectomy, lobectomy, and extended hepatectomy. The objective of the operation was to completely remove the tumor to achieve R0 resection. When the tumor invaded the extrahepatic bile duct or the contralateral hepatic duct, the extrahepatic bile duct was removed or choledochotomy was performed, and intraoperative choledochoscopy was performed to ensure complete thrombectomy.
PA-TACE
PA-TACE is recommended per the personal experience and judgment of the physician. Initial PA-TACE was performed within 1–2 months after resection. A 5-F catheter or microcatheter was inserted into an appropriate hepatic artery using the Seldinger technique. Hepatic angiography was performed to ascertain the distribution of the arteries. Chemotherapeutic agents, including cisplatin (10–30 mg), doxorubicin hydrochloride (10 mg), and pharmorubicin (20–40 mg) were slowly injected, followed by an emulsion of lipiodol (5–10 mL) (Lipiodol Ultrafluide, Guerbet, AulnaySousBois, France). Individualized dosages of chemotherapeutic agents and lipiodol were determined on the basis of the remaining liver volume and body surface.
Postoperative Follow-Up
All patients were regularly followed up after discharge from the hospital. Follow-up visits were scheduled once every 2–3 months in the first 2 years, once every 6 months from 2 to 5 years, and once every year after 5 years. Routine follow-up tests included a liver function test, a test to determine the serum AFP level, and abdominal ultrasound. Contrast-enhanced computed tomography or magnetic resonance imaging was performed when recurrence was suspected. Recurrence or metastasis was defined as the appearance of new lesions with radiologic features of HCC, and further treatment was immediately initiated when recurrence was confirmed.
Primary Endpoint
The primary endpoints were the recurrence rate and overall survival (OS) rate. Time to recurrence was defined as the time between the date of resection to the date of recurrence or the date of the latest follow-up, whichever occurred first. OS was calculated from the time of resection to the date of either death or the latest follow-up.
Baseline Characteristics and Clinical Variables of Patients
Baseline characteristics and clinical variables included age, sex, cirrhosis, number of tumors, maximum tumor size, macroscopic and microscopic vascular invasion, presence of satellites and tumor differentiation, preoperative serum HBV DNA, serum hepatitis B surface antigen, gamma-glutamyltransferase (GGT), alkaline phosphatase, total bilirubin, prealbumin, alpha-fetoprotein (AFP), platelet, prothrombin time, Child–Pugh grade, and Fibrosis-4 index. HBV infection was defined as HBV DNA-positive or serum hepatitis B surface antigen positive. Cirrhosis was confirmed histopathologically or via clinical diagnosis. Tumor differentiation was classified according to the Edmonson–Steiner grade.18 BDTT was classified using Satoh typing19 as follows: Type I, BDTT is located in the first branch of the hepatic duct and does not reach the confluence of the right and left hepatic ducts; Type II, BDTT extends across the confluence of the right and left hepatic ducts; Type III, BDTT separates from the primary tumor and is located in the common bile ducts.
Propensity-Score Matching
Propensity-score matching (PSM) was adopted to minimize selection bias. Potentially confounding factors either unbalanced in the baseline table or independent in the multivariable Cox model were matched one-to-one using the nearest neighbor method with a caliber of 0.2.
Statistical Analysis
Continuous variables with a normal distribution were expressed as medians with interquartile ranges (IQR) and compared using the Mann–Whitney U-test. Fisher’s exact test or the chi-squared test was used for comparison of categorical variables, as appropriate. Comparison of recurrence and OS curves between the non-TACE and PA-TACE groups was performed using the Kaplan-Meier method and tested using the Log rank test. Univariable and multivariable Cox regression analyses were performed to identify the independent risk factors associated with recurrence and OS. Hazard ratios (HRs) and their 95% confidence intervals (95% CI) were estimated using univariable and multivariable Cox regression analyses. Potentially relevant variables (with P < 0.2 in the univariable Cox regression analysis) were considered for generating the multivariable Cox model using the stepwise backward method. The forest plot of the subgroup analysis was described with estimated HRs and 95% CIs. Data were analyzed via Rstudio using “Table 1,” “survminer,” “survival,” “forestplot,” “MacthIt” packages. Statistical significance was set at P < 0.05.
Table 1.
Clinicopathological Characteristics of Non-TACE Group Compare with PA-TACE Group Before and After PSM
| Parameters | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| Non-TACE | TACE | P | Non-TACE | TACE | P | |
| (n=48) | (n=61) | (n=31) | (n=31) | |||
| Age(years) | ||||||
| Mean (IQR) | 51.0 [46.0, 56.0] | 49.0 [45.0, 55.0] | 0.564 | 54.0 [48.0, 59.0] | 51.0 [45.5, 59.0] | 0.306 |
| Sex | ||||||
| Female | 6 (12.5%) | 11 (18.0%) | 0.6 | 4 (12.9%) | 3 (9.7%) | 1 |
| Male | 42 (87.5%) | 50 (82.0%) | 27 (87.1%) | 28 (90.3%) | ||
| HBV | ||||||
| No | 13 (27.1%) | 16 (26.2%) | 1 | 9 (29.0%) | 9 (29.0%) | 1 |
| Yes | 35 (72.9%) | 45 (73.8%) | 22 (71.0%) | 22 (71.0%) | ||
| Cirrhosis | ||||||
| No | 6 (12.5%) | 16 (26.2%) | 0.125 | 2 (6.5%) | 7 (22.6%) | 0.149 |
| Yes | 42 (87.5%) | 45 (73.8%) | 29 (93.5%) | 24 (77.4%) | ||
| Tumor Number | ||||||
| Single | 41 (85.4%) | 44 (72.1%) | 0.153 | 27 (87.1%) | 24 (77.4%) | 0.506 |
| Multiple | 7 (14.6%) | 17 (27.9%) | 4 (12.9%) | 7 (22.6%) | ||
| Tumor Size(cm) | ||||||
| ≤5cm | 30 (62.5%) | 16 (26.2%) | <0.001 | 13 (41.9%) | 13 (41.9%) | 1 |
| >5cm | 18 (37.5%) | 45 (73.8%) | 18 (58.1%) | 18 (58.1%) | ||
| Satellite Nodules | ||||||
| No | 24 (50.0%) | 21 (34.4%) | 0.149 | 17 (54.8%) | 10 (32.3%) | 0.124 |
| Yes | 24 (50.0%) | 40 (65.6%) | 14 (45.2%) | 21 (67.7%) | ||
| Differentiation | ||||||
| I+II | 9 (18.8%) | 13 (21.3%) | 0.928 | 3 (9.7%) | 7 (22.6%) | 0.3 |
| III+IV | 39 (81.2%) | 48 (78.7%) | 28 (90.3%) | 24 (77.4%) | ||
| Capsule | ||||||
| No | 26 (54.2%) | 24 (39.3%) | 0.178 | 14 (45.2%) | 10 (32.3%) | 0.434 |
| Yes | 22 (45.8%) | 37 (60.7%) | 17 (54.8%) | 21 (67.7%) | ||
| MVI | ||||||
| No | 9 (18.8%) | 11 (18.0%) | 1 | 5 (16.1%) | 5 (16.1%) | 1 |
| Yes | 39 (81.2%) | 50 (82.0%) | 26 (83.9%) | 26 (83.9%) | ||
| MaVI | ||||||
| No | 41 (85.4%) | 41 (67.2%) | 0.0498 | 24 (77.4%) | 24 (77.4%) | 1 |
| Yes | 7 (14.6%) | 20 (32.8%) | 7 (22.6%) | 7 (22.6%) | ||
| Satoh | ||||||
| I | 26 (54.2%) | 30 (49.2%) | 0.746 | 16 (51.6%) | 15 (48.4%) | 1 |
| II+III | 22 (45.8%) | 31 (50.8%) | 15 (48.4%) | 16 (51.6%) | ||
| AFP(ng/mL) | ||||||
| ≤400 | 24 (50.0%) | 29 (47.5%) | 0.951 | 10 (32.3%) | 17 (54.8%) | 0.124 |
| >400 | 24 (50.0%) | 32 (52.5%) | 21 (67.7%) | 14 (45.2%) | ||
| TBIL(umol/L) | ||||||
| ≤34.2 | 31 (64.6%) | 40 (65.6%) | 1 | 22 (71.0%) | 21 (67.7%) | 1 |
| >34.2 | 17 (35.4%) | 21 (34.4%) | 9 (29.0%) | 10 (32.3%) | ||
| ALP(U/L) | ||||||
| ≤150 | 22 (45.8%) | 23 (37.7%) | 0.509 | 14 (45.2%) | 14 (45.2%) | 1 |
| >150 | 26 (54.2%) | 38 (62.3%) | 17 (54.8%) | 17 (54.8%) | ||
| GGT(U/L) | ||||||
| ≤150 | 11 (22.9%) | 9 (14.8%) | 0.399 | 8 (25.8%) | 4 (12.9%) | 0.335 |
| >150 | 37 (77.1%) | 52 (85.2%) | 23 (74.2%) | 27 (87.1%) | ||
| PALB(mg/L) | ||||||
| ≤170 | 17 (35.4%) | 20 (32.8%) | 0.933 | 11 (35.5%) | 11 (35.5%) | 1 |
| >170 | 31 (64.6%) | 41 (67.2%) | 20 (64.5%) | 20 (64.5%) | ||
| PLT(×10^9/L) | ||||||
| <100 | 4 (8.3%) | 4 (6.6%) | 1 | 2 (6.5%) | 3 (9.7%) | 1 |
| ≥100 | 44 (91.7%) | 57 (93.4%) | 29 (93.5%) | 28 (90.3%) | ||
| PT(s) | ||||||
| ≤13 | 28 (58.3%) | 41 (67.2%) | 0.45 | 16 (51.6%) | 20 (64.5%) | 0.44 |
| >13 | 20 (41.7%) | 20 (32.8%) | 15 (48.4%) | 11 (35.5%) | ||
| Child-Pugh | ||||||
| A | 43 (89.6%) | 55 (90.2%) | 1 | 29 (93.5%) | 27 (87.1%) | 0.668 |
| B | 5 (10.4%) | 6 (9.8%) | 2 (6.5%) | 4 (12.9%) | ||
| FIB-4 | ||||||
| ≤3.25 | 27 (56.2%) | 38 (62.3%) | 0.659 | 15 (48.4%) | 16 (51.6%) | 1 |
| >3.25 | 21 (43.8%) | 23 (37.7%) | 16 (51.6%) | 15 (48.4%) | ||
Note: The bold text means P<0.05.
Abbreviations: AFP, alpha-fetoprotein; ALP, alkaline phosphatase; FIB-4, Fibrosis-4 index; GGT, gamma-glutamyltransferase; HBV, hepatitis B virus; IQR, interquartile range; MaVI, macrovascular invasion; MVI, microvascular invasion; PA-TACE, postoperative adjuvant transarterial chemoembolization; PALB, prealbumin; PLT, platelet; PSM, propensity-score matching; PT, prothrombin time; TBIL, total bilirubin.
Results
Patients Characteristics
A total of 109 patients were enrolled in this study: 48 in the non-TACE group and 61 in the PA-TACE group. Patients with tumor size > 5 cm and macrovascular invasion were more likely to receive PA-TACE; the difference between the two groups was balanced after 1:1 PSM (Table 1).
Recurrence and OS Rates
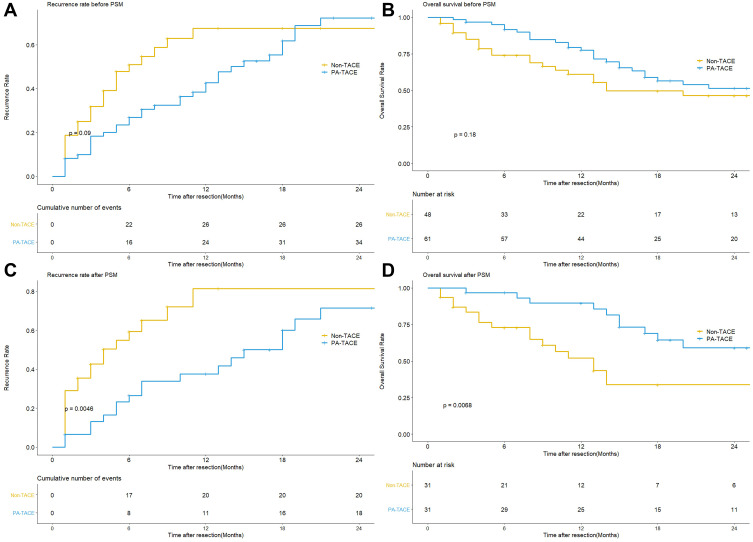
The median follow-up period of the whole cohort was 14.0 (7.0–27.0) months. Before PSM, the median recurrence time of the non-TACE and PA-TACE groups was 4.0 (2.0–7.0) and 11 (5.0–17.0) months, respectively (P=0.09) (Figure 1A). The recurrence rates in the non-TACE and PA-TACE groups were different at 6 months (50.9% vs 26.9%, P=0.03) (Table 2). After PSM, the median recurrence time of the two groups was 3.0 (1.0–6.0) and 12.0 (5.5–18.0) months, respectively (P=0.0046) (Figure 1C). The recurrence rates in the two groups were different at 6 months (59.3% vs 26.5%, P=0.02) and 12 months (81.4% vs 37.5%, P=0.022) (Table 2).
Figure 1.
(A) Comparison of recurrence rate between the PA-TACE and non-TACE groups before PSM. (B) Comparison of overall survival rate between the PA-TACE and non-TACE groups before PSM. (C) Comparison of recurrence rate between the PA-TACE and non-TACE groups after PSM. (D) Comparison of overall survival rate between the PA-TACE and non-TACE groups after PSM.
Table 2.
Recurrence Rate and Overall Survival of Non-TACE Group Compare with PA-TACE Groups Before and After PSM
| Time | Before PSM | After PSM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recurrence Rate | Overall Survival Rate | Recurrence Rate | Overall Survival Rate | |||||||||
| Non-TACE | PA-TACE | P | Non-TACE | PA-TACE | P | Non-TACE | PA-TACE | P | Non-TACE | PA-TACE | P | |
| 6 mo | 50.9% | 26.9% | 0.03 | 74.0% | 91.6% | <0.001 | 59.3% | 26.5% | 0.02 | 73.0% | 96.8% | 0.01 |
| 12 mo | 67.5% | 42.7% | 0.12 | 61.1% | 77.6% | 0.01 | 81.4% | 37.5% | 0.022 | 52.1% | 89.6% | 0.001 |
| 18 mo | 67.5% | 61.8% | 0.73 | 49.7% | 56.6% | 0.55 | 81.4% | 60.0% | 0.303 | 33.8% | 64.4% | 0.034 |
| 24 mo | 67.5% | 72.2% | 0.87 | 46.4% | 51.5% | 0.52 | 81.4% | 71.4% | 0.602 | 33.8% | 59.0% | 0.155 |
Note: The bold text means P<0.05.
Before PSM, the median OS time of the non-TACE and PA-TACE groups was 10.5 (4.0–24.25) and 15 (11.0–27.0) months, respectively (P=0.18) (Figure 1B). The OS rates of the non-TACE and PA-TACE groups were different at 6 months (74.0% vs 91.6%, P < 0.001) and 12 months (61.1% vs 77.6%, P=0.01) (Table 2). After PSM, the OS time of the two groups was 9.0 (4.0–14.0) and 17.0 (12.0–31.5) months, respectively (P=0.0068) (Figure 1D). The OS rate in the two groups was different at 6 months (73.0% vs 96.8%, P=0.01), 12 months (52.1% vs 89.6%, P=0.001), and 18 months, respectively (33.8% vs 64.4%, P=0.034) (Table 2).
Risk Factors Associated with Recurrence and OS
Multivariate analysis showed that GGT > 150 U/L and PA-TACE were independent prognostic factors for recurrence both before and after PSM; GGT > 150 U/L (HR=3.233, 95% CI=1.353–7.723, P=0.008 before PSM and HR=2.908, 95% CI=1.071–7.9, P=0.036 after PSM) and PA-TACE (HR=0.537, 95% CI=0.313–0.921, P=0.024 before PSM and HR=0.319, 95% CI=0.156–0.655, P=0.002) (Tables 3 and 4).
Table 3.
Univariate and Multivariate Analysis of Recurrence and Overall Survival for HCC with BDTT Before PSM
| Parameters | Recurrence | Overall Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| HR | CI95% | P | HR | CI95% | P | HR | CI95% | P | HR | CI95% | P | |
| Age, >50 years | 1.238 | 0.745–2.057 | 0.41 | 1.504 | 0.847–2.67 | 0.164 | ||||||
| Sex, male | 1.698 | 0.804–3.584 | 0.165 | 1.424 | 0.637–3.186 | 0.389 | ||||||
| HBV, yes | 1.09 | 0.614–1.935 | 0.769 | 1.453 | 0.721–2.926 | 0.296 | ||||||
| Cirrhosis, yes | 2.301 | 1.125–4.704 | 0.022 | 1.662 | 0.744–3.713 | 0.215 | ||||||
| Tumor Number, multiple | 0.986 | 0.512–1.898 | 0.966 | 1.922 | 0.995–3.711 | 0.052 | 3.319 | 1.54–7.156 | 0.002 | |||
| Tumor Size, >5cm | 0.777 | 0.467–1.29 | 0.329 | 1.035 | 0.58–1.846 | 0.907 | ||||||
| Satellite Nodules, yes | 1.201 | 0.717–2.012 | 0.487 | 1.372 | 0.761–2.472 | 0.293 | ||||||
| Differentiation, III+IV | 2.122 | 1.04–4.329 | 0.039 | 1.49 | 0.695–3.192 | 0.306 | ||||||
| Capsule, yes | 1.303 | 0.778–2.182 | 0.315 | 0.692 | 0.389–1.231 | 0.21 | ||||||
| MVI, yes | 1.418 | 0.718–2.802 | 0.314 | 1.424 | 0.638–3.181 | 0.388 | ||||||
| MaVI, yes | 1.485 | 0.824–2.676 | 0.189 | 2.489 | 1.337–4.636 | 0.004 | 4.318 | 2.099–8.885 | <0.001 | |||
| BDTT type, II+III | 1.053 | 0.634–1.749 | 0.842 | 1.582 | 0.886–2.823 | 0.121 | ||||||
| AFP, >400ug/L | 0.925 | 0.557–1.537 | 0.764 | 2.221 | 1.223–4.035 | 0.009 | 2.389 | 1.297–4.4 | 0.005 | |||
| ALP, >150U/L | 1.065 | 0.636–1.783 | 0.812 | 1.763 | 0.954–3.257 | 0.07 | ||||||
| GGT, >150U/L | 2.985 | 1.269–7.023 | 0.012 | 3.233 | 1.353–7.723 | 0.008 | 2.848 | 1.02–7.951 | 0.046 | |||
| TBIL, >34.2umol/L | 0.813 | 0.463–1.429 | 0.473 | 1.516 | 0.839–2.738 | 0.168 | ||||||
| PALB, >170mg/L | 1.195 | 0.681–2.098 | 0.534 | 0.745 | 0.407–1.365 | 0.341 | ||||||
| PLT, ≥100×10^9/L | 0.959 | 0.383–2.397 | 0.928 | 0.533 | 0.226–1.255 | 0.15 | ||||||
| PT, >13s | 0.94 | 0.552–1.599 | 0.819 | 0.938 | 0.513–1.715 | 0.835 | ||||||
| Child-Pugh, B | 0.957 | 0.345–2.657 | 0.932 | 2.89 | 1.282–6.512 | 0.01 | ||||||
| FIB-4, >3.25 | 1.017 | 0.606–1.705 | 0.95 | 1.003 | 0.56–1.796 | 0.993 | ||||||
| PA-TACE, yes | 0.649 | 0.385–1.092 | 0.103 | 0.537 | 0.313–0.921 | 0.024 | 0.683 | 0.385–1.212 | 0.193 | 0.291 | 0.147–0.577 | <0.001 |
Notes: The bold text means P<0.2 in univariable Cox regression analysis and P<0.05 in multivariable Cox regression analysis.
Abbreviations: AFP, alpha-fetoprotein; ALP, alkaline phosphatase; FIB-4, Fibrosis-4 index; GGT, gamma-glutamyltransferase; HBV, hepatitis B virus; IQR, interquartile range; MaVI, macrovascular invasion; MVI, microvascular invasion; PA-TACE, postoperative adjuvant transarterial chemoembolization; PALB, prealbumin; PLT, platelet; PSM, propensity score matching; PT, prothrombin time; TBIL, total bilirubin.
Table 4.
Univariate and Multivariate Analysis of Recurrence and Overall Survival for HCC with BDTT After PSM
| Parameters | Recurrence | Overall Survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| HR | CI95% | P | HR | CI95% | P | HR | CI95% | P | HR | CI95% | P | |
| Age, >50 years | 1.159 | 0.609–2.206 | 0.654 | 1.724 | 0.774–3.841 | 0.183 | ||||||
| Sex, male | 0.935 | 0.361–2.423 | 0.89 | 5.032 | 0.681–37.18 | 0.113 | ||||||
| HBV, yes | 0.995 | 0.414–2.391 | 0.991 | 1.667 | 0.629–4.419 | 0.304 | ||||||
| Cirrhosis, yes | 0.691 | 0.364–1.31 | 0.257 | 0.751 | 0.353–1.6 | 0.458 | ||||||
| Tumor Number, multiple | 1.026 | 0.516–2.039 | 0.942 | 1.473 | 0.621–3.496 | 0.379 | ||||||
| Tumor Size, >5cm | 2.787 | 0.85–9.136 | 0.091 | 1.311 | 0.394–4.364 | 0.659 | ||||||
| Satellite Nodules, yes | 1.492 | 0.777–2.867 | 0.229 | 1.757 | 0.802–3.849 | 0.159 | 2.976 | 1.124–7.884 | 0.028 | |||
| Differentiation, III+IV | 1.471 | 0.611–3.541 | 0.39 | 2.062 | 0.619–6.867 | 0.238 | ||||||
| Capsule, yes | 1.773 | 0.688–4.569 | 0.236 | 2.672 | 0.632–11.302 | 0.182 | ||||||
| MVI, yes | 1.067 | 0.55–2.068 | 0.848 | 0.644 | 0.302–1.371 | 0.253 | ||||||
| MaVI, yes | 1.165 | 0.508–2.675 | 0.718 | 2.542 | 1.061–6.088 | 0.036 | 2.917 | 1.138–7.48 | 0.026 | |||
| BDTT type, II+III | 1.427 | 0.751–2.712 | 0.278 | 1.407 | 0.658–3.01 | 0.379 | ||||||
| AFP, >400ug/L | 1.442 | 0.754–2.758 | 0.269 | 2.099 | 0.955–4.613 | 0.065 | 2.688 | 1.13–6.394 | 0.025 | |||
| ALP, >150U/L | 0.981 | 0.519–1.857 | 0.954 | 1.219 | 0.57–2.608 | 0.61 | ||||||
| GGT, >150U/L | 1.972 | 0.757–5.138 | 0.165 | 2.908 | 1.071–7.9 | 0.036 | 1.948 | 0.584–6.494 | 0.278 | |||
| TBIL, >34.2umol/L | 1.218 | 0.61–2.434 | 0.576 | 1.328 | 0.577–3.058 | 0.505 | ||||||
| PALB, >170mg/L | 1.686 | 0.818–3.475 | 0.157 | 1.009 | 0.44–2.313 | 0.983 | ||||||
| PLT, ≥100×10^9/L | 1.417 | 0.433–4.639 | 0.565 | 0.825 | 0.248–2.744 | 0.753 | ||||||
| PT, >13s | 0.977 | 0.299–3.193 | 0.969 | 1.971 | 0.583–6.657 | 0.275 | ||||||
| Child-Pugh, B | 0.831 | 0.438–1.578 | 0.572 | 0.652 | 0.302–1.406 | 0.276 | ||||||
| FIB-4, >3.25 | 1.085 | 0.566–2.08 | 0.806 | 0.678 | 0.304–1.513 | 0.343 | ||||||
| PA-TACE, yes | 0.398 | 0.201–0.79 | 0.008 | 0.319 | 0.156–0.655 | 0.002 | 0.356 | 0.162–0.785 | 0.01 | 0.177 | 0.068–0.46 | <0.001 |
Notes: The bold text means P<0.2 in univariable Cox regression analysis and P<0.05 in multivariable Cox regression analysis.
Abbreviations: AFP, alpha-fetoprotein; ALP, alkaline phosphatase; FIB-4, Fibrosis-4 index; GGT, gamma-glutamyltransferase; HBV, hepatitis B virus; IQR, interquartile range; MaVI, macrovascular invasion; MVI, microvascular invasion; PA-TACE, postoperative adjuvant transarterial chemoembolization; PALB, prealbumin; PLT, platelet; PSM, propensity score matching; PT, prothrombin time; TBIL, total bilirubin.
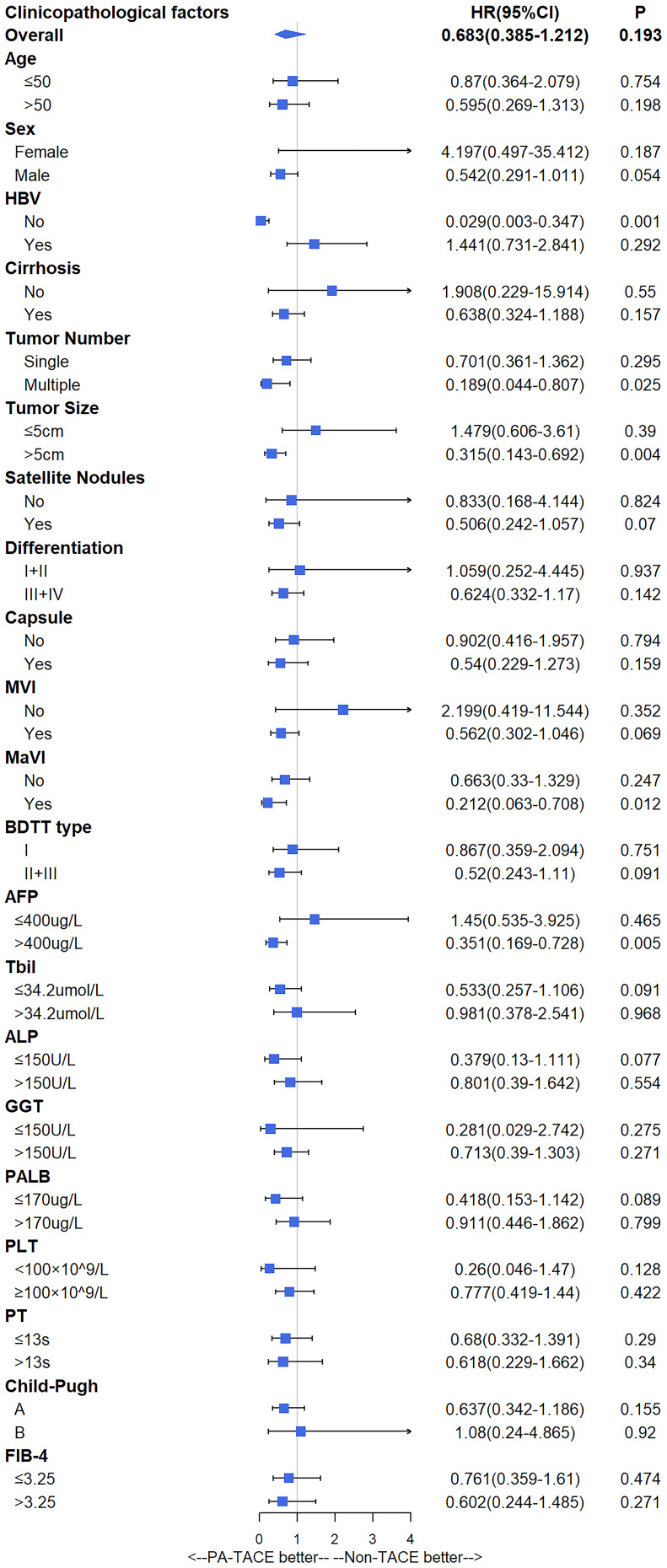
For OS, multivariate analysis showed that multiple tumors (HR=3.409, 95% CI=1.609–7.223, P=0.001), macrovascular invasion (HR=4.947, 95% CI=2.321–10.542, P=<0.001), AFP > 400 ng/mL (HR=2.376, 95% CI=1.289–4.382, P=0.006), and PA-TACE (HR=0.266, 95% CI=0.133–0.529, P<0.001) were independent risk factors before PSM (Table 3), whereas satellite nodules (HR=2.976, 95% CI=1.124–7.884, P=0.028), macrovascular invasion (HR=2.917, 95% CI=1.138–7.48, P=0.026), AFP > 400 ng/mL (HR=2.688, 95% CI=1.13–6.394, P=0.025), and PA-TACE (HR=0.177, 95% CI=0.068–0.46, P<0.001) were independent risk factors after PSM (Table 4).
Subgroup Analysis
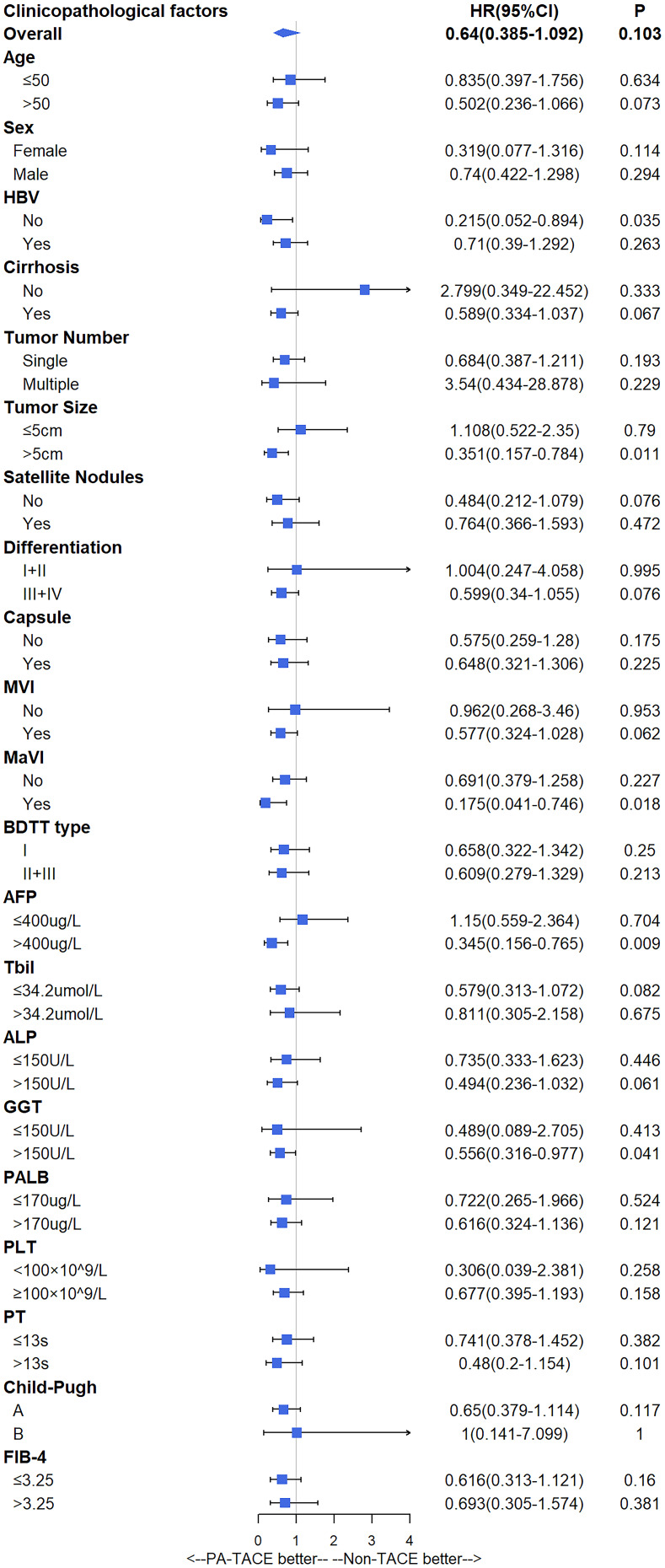
Subgroup analysis showed that patients who were not infected with HBV (HR=0.312, 95% CI=0.103–0.948, P=0.04), had tumor size > 5 cm (HR=0.351, 95% CI=0.157–0.784, P=0.011), macrovascular invasion (HR=0.175, 95% CI=0.041–0.746, P=0.018), AFP > 400 ng/mL (HR=0.345, 95% CI=0.156–0.765, P=0.009), or GGT > 150 U/L (HR=0.556, 95% CI=0.316–0.977, P=0.041) benefited from PA-TACE on recurrence significantly (Figure 2), whereas patients who were not infected with HBV (HR=0.029, 95% CI=0.003–0.347, P=0.001), had multiple tumors (HR=0.189, 95% CI=0.044–0.807, P=0.025), tumor size > 5 cm (HR=0.315, 95% CI=0.143–0.692, P=0.004), macrovascular invasion (HR=0.212, 95% CI=0.063–0.708, P=0.012), or AFP > 400 ng/mL (HR=0.351, 95% CI=0.169–0.728, P=0.005) benefited from PA-TACE on OS significantly (Figure 3).
Figure 2.
Subgroup analysis of recurrence.
Figure 3.
Subgroup analysis of overall survival.
Discussion
Generally, tumor recurrence is classified into early and late recurrence according to a cutoff interval of 2 years. More than 70% of tumor recurrences occur within 2 years of surgical resection.20 One of the main reasons for the high early recurrence rate is the presence of invisible intrahepatic metastases before surgery, which are difficult to remove completely through surgical resection. For this reason, many radical surgeries do not achieve “radical cure;” hence, postoperative adjuvant therapy has great clinical significance in these patients.
PA-TACE is a widely used anti-recurrence therapy in the clinic setting. In terms of local treatment, the concentration of chemotherapeutic drugs in the liver is 100–400-fold higher than that in the whole body; this is because hepatic artery perfusion results in considerably higher concentrations than those achieved via oral administration or intravenous injection. The accumulation of drugs in HCC lesions due to TACE resulted in 5–10-times higher drug concentrations in tumor areas than in normal liver tissue.21 There have been many previous studies on PA-TACE. Xie et al21 suggested that PA-TACE significantly improves the prognosis of low-risk patients. Gao’s study22 showed that PA-TACE was beneficial for patients with high-risk factors. Tong’s research23 indicated that single, postoperative, adjuvant TACE was beneficial for selected patients, such as patients with stage I with tumors sized <5 cm (low-risk) or those with high preoperative serum alpha-fetoprotein levels or positivity for alpha-fetoprotein on pathological analysis (high-risk). In contrast, Jiang et al24 reported that postoperative adjuvant TACE does not improve OS or reduce recurrence in HCC patients. However, whether patients with HCC with macroscopic BDTT can benefit from PA-TACE after R0 resection remains unknown. To our knowledge, this was the first study to investigate the clinical value of PA-TACE for HCC accompanied by macroscopic BDTT.
Our study showed that there were significant differences in recurrence rates and OS between the non-TACE and PA-TACE groups within 6 months after resection, and after PSM, these differences were observed for a duration of 12 months, although the differences faded gradually thereafter. Multivariate analyses confirmed that PA-TACE was an independent prognostic factor for recurrence and OS. Hence, we believe that PA-TACE could confer short-term survival benefits in HCC patients with macroscopic BDTT. This phenomenon was also observed in a previous study.25,26 The following may be the reasons for this phenomenon: (1) the different cycles of PA-TACE had diverse clinical benefits, and (2) the long-term survival after recurrence was affected by the treatment after recurrence also.
In this study, a high GGT level was another independent risk factor for recurrence. The relationship between high GGT levels and early recurrence has been reported, and this relationship may be based on the following mechanisms: (1) high GGT levels may induce DNA instability, leading to tumor formation; and (2) GGT may be associated with the degree of malignancy of HCC (in terms of vascular invasion, tumor metastasis, or poor tumor differentiation).27 Most patients in our cohort had elevated GGT levels, poor differentiation, and a high microvascular invasion incidence, consistent with the results of previous study.27 However, the correlation between GGT levels and BDTT needs to be explored further.
The subgroup analysis in our study showed that patients could significantly benefit from PA-TACE in prolonging the time to recurrence if they had no HBV infection, cirrhosis, a tumor size >5 cm, poor differentiation, macrovascular invasion, AFP levels > 400 ng/mL, or GGT levels >150 U/L. Patients without an HBV infection, multiple tumors, a tumor size >5 cm, macrovascular invasion, or AFP levels >400 ng/mL benefited from PA-TACE in terms of OS. HBV infection has been commonly regarded as a low-risk factor, while cirrhosis, tumor size >5 cm, poor differentiation, macrovascular invasion, high AFP levels, and high GGT levels have been regarded as high-risk factors. In the PA-TACE group of this study, both the time to recurrence and OS were significantly prolonged in patients with no HBV infection compared with those in patients with HBV infection. The reason for this difference is that TACE might induce HBV re-activation,28 which increases the risk of HCC recurrence and counteracts the partial anti-recurrence effect of PA-TACE. Consequently, there was no obvious survival benefit in patients with HBV infection. Regarding high-risk factors, different studies29–32 have reported that patients with a tumor size >5 cm, multiple tumors, high AFP levels, high GGT levels, or macrovascular invasion could benefit from PA-TACE, consistent with our results. Based on the abovementioned information, we thought it might be inappropriate to judge whether patients could benefit from PA-TACE by classifying patients into high- and low-risk groups.
It is difficult to conduct a randomized controlled trial due to the low incidence of BDTT. This retrospective study had several limitations. First, it was difficult to avoid selection bias, although propensity-score matching was conducted. Second, due to the lack of imaging data for some patients, it was impossible to judge whether the BDTT was separated from the primary lesion; therefore, we were unable to distinguish between type II and type III BDTT. Third, the lack of data on PA-TACE cycles and antiviral therapy precluded further subgroup analysis.
Conclusion
In summary, PA-TACE could improve the short-term prognosis of patients with HCC with macroscopic BDTT after R0 resection. PA-TACE should be recommended for selected patients with no HBV infection, multiple tumors, tumor size >5 cm, macrovascular invasion, AFP levels >400 ng/mL, or GGT levels >150 U/L.
Funding Statement
This study was sponsored by Key Clinical Specialty Discipline Construction Program of Fuzhou, Fujian, P.R.C (201,912,002).
Abbreviations
AFP, alpha-fetoprotein; ALP, alkaline phosphatase; BDTT, bile duct tumor thrombus; cHCC-ICC, combined hepatocellular carcinoma-intrahepatic cholangiocarcinoma; FIB-4, Fibrosis-4 index, GGT, gamma-glutamyltransferase; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; IQR, interquartile range; MaVI, macrovascular invasion; MVI, microvascular invasion; OS, overall survival; PA-TACE, postoperative adjuvant transarterial chemoembolization; PALB, prealbumin; PLT, platelet; PSM, propensity-score matching; PT, prothrombin time; TBIL, total bilirubin.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Zhang B, Zhang B, Zhang Z, et al. 42,573 cases of hepatectomy in China: a multicenter retrospective investigation. Sci China Life Sci. 2018;61(6):660–670. doi: 10.1007/s11427-017-9259-9 [DOI] [PubMed] [Google Scholar]
- 2.Meng KW, Dong M, Zhang WG, et al. Clinical characteristics and surgical prognosis of hepatocellular carcinoma with bile duct invasion. Gastroenterol Res Pract. 2014;2014:604971. doi: 10.1155/2014/604971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiomi M, Kamiya J, Nagino M, et al. Hepatocellular carcinoma with biliary tumor thrombi: aggressive operative approach after appropriate preoperative management. Surgery. 2001;129(6):692–698. doi: 10.1067/msy.2001.113889 [DOI] [PubMed] [Google Scholar]
- 4.Yang X, Qiu Z, Ran R, et al. Prognostic importance of bile duct invasion in surgical resection with curative intent for hepatocellular carcinoma using PSM analysis. Oncol Lett. 2018;16(3):3593–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang DD, Wu LQ, Wang ZS. Prognosis of hepatocellular carcinoma with bile duct tumor thrombus after R0 resection: a matched study. Hepatobiliary Pancreat Dis Int. 2016;15(6):626–632. doi: 10.1016/S1499-3872(16)60143-1 [DOI] [PubMed] [Google Scholar]
- 6.Qiao W, Yu F, Wu L, et al. Surgical outcomes of hepatocellular carcinoma with biliary tumor thrombus: a systematic review. BMC Gastroenterol. 2016;16:11. doi: 10.1186/s12876-016-0427-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang YB, Zhong JH, Luo XL, et al. Clinicopathological characteristics and liver stem cell marker expression in hepatocellular carcinoma involving bile duct tumor thrombi. Tumour Biol. 2016;37(5):5879–5884. doi: 10.1007/s13277-015-4446-3 [DOI] [PubMed] [Google Scholar]
- 8.Orimo T, Kamiyama T, Yokoo H, et al. Hepatectomy for hepatocellular carcinoma with bile duct tumor thrombus, including cases with obstructive jaundice. Ann Surg Oncol. 2016;23(8):2627–2634. doi: 10.1245/s10434-016-5174-7 [DOI] [PubMed] [Google Scholar]
- 9.Navadgi S, Chang CC, Bartlett A, et al. Systematic review and meta-analysis of outcomes after liver resection in patients with hepatocellular carcinoma (HCC) with and without bile duct thrombus. HPB (Oxford). 2016;18(4):312–316. doi: 10.1016/j.hpb.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao W, Sui C, Liu Z, et al. Surgical outcome of hepatocellular carcinoma patients with biliary tumor thrombi. World J Surg Oncol. 2011;9:2. doi: 10.1186/1477-7819-9-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An J, Lee KS, Kim KM, et al. Clinical features and outcomes of patients with hepatocellular carcinoma complicated with bile duct invasion. Clin Mol Hepatol. 2017;23(2):160–169. doi: 10.3350/cmh.2016.0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng BG, Liang LJ, Li SQ, et al. Surgical treatment of hepatocellular carcinoma with bile duct tumor thrombi. World J Gastroenterol. 2005;11(25):3966–3969. doi: 10.3748/wjg.v11.i25.3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon DB, Hwang S, Wang HJ, et al. Surgical outcomes of hepatocellular carcinoma with bile duct tumor thrombus: a Korean multicenter study. World J Surg. 2013;37(2):443–451. doi: 10.1007/s00268-012-1845-0 [DOI] [PubMed] [Google Scholar]
- 14.Wei W, Jian PE, Li SH, et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: a randomized clinical trial of efficacy and safety. Cancer Commun (Lond). 2018;38(1):61. doi: 10.1186/s40880-018-0331-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Ren Z, Chen Y, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res. 2018;24(9):2074–2081. doi: 10.1158/1078-0432.CCR-17-2899 [DOI] [PubMed] [Google Scholar]
- 16.Zhong C, Guo RP, Li JQ, et al. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135(10):1437–1445. doi: 10.1007/s00432-009-0588-2 [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Ke Q, Deng M, et al. Adjuvant transarterial chemoembolization for patients with hepatocellular carcinoma after radical hepatectomy: a real world study. Scand J Gastroenterol. 2019;54(11):1403–1411. doi: 10.1080/00365521.2019.1684986 [DOI] [PubMed] [Google Scholar]
- 18.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7(3):462–503. doi: [DOI] [PubMed] [Google Scholar]
- 19.Satoh S, Ikai I, Honda G, et al. Clinicopathologic evaluation of hepatocellular carcinoma with bile duct thrombi. Surgery. 2000;128(5):779–783. doi: 10.1067/msy.2000.108659 [DOI] [PubMed] [Google Scholar]
- 20.Chan AW, Chan SL, Wong GL, et al. Prognostic Nutritional Index (PNI) predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann Surg Oncol. 2015;22(13):4138–4148. doi: 10.1245/s10434-015-4516-1 [DOI] [PubMed] [Google Scholar]
- 21.Xie H, Tian S, Cui L, et al. Adjuvant trans-arterial chemoembolization after hepatectomy significantly improves the prognosis of low-risk patients with R0-stage hepatocellular carcinoma. Cancer Manag Res. 2019;11:4065–4073. doi: 10.2147/CMAR.S195485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Z, Du G, Pang Y, et al. Adjuvant transarterial chemoembolization after radical resection contributed to the outcomes of hepatocellular carcinoma patients with high-risk factors. Medicine (Baltimore). 2017;96(33):e7426. doi: 10.1097/MD.0000000000007426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tong Y, Li. Z, Liang Y, et al. Postoperative adjuvant TACE for patients of hepatocellular carcinoma in AJCC stage I: friend or foe? A propensity score analysis. Oncotarget. 2017;8(16):26671–26678. doi: 10.18632/oncotarget.15793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang JH, Guo Z, Lu HF, et al. Adjuvant transarterial chemoembolization after curative resection of hepatocellular carcinoma: propensity score analysis. World J Gastroenterol. 2015;21(15):4627–4634. doi: 10.3748/wjg.v21.i15.4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuang X, Ye J, Xie Z, et al. Adjuvant transarterial chemoembolization to improve the prognosis of hepatocellular carcinoma following curative resection. Oncol Lett. 2018;16(4):4937–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng HY, Wang X, Chen D, et al. The value and limitation of transcatheter arterial chemoembolization in preventing recurrence of resected hepatocellular carcinoma. World J Gastroenterol. 2005;11(23):3644–3646. doi: 10.3748/wjg.v11.i23.3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song P, Inagaki Y, Wang Z, et al. High levels of gamma-glutamyl transferase and indocyanine green retention rate at 15 min as preoperative predictors of tumor recurrence in patients with hepatocellular carcinoma. Medicine (Baltimore). 2015;94(21):e810. doi: 10.1097/MD.0000000000000810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang SS, Liu JX, Zhu J, et al. Effects of TACE and preventive antiviral therapy on HBV reactivation and subsequent hepatitis in hepatocellular carcinoma: a meta-analysis. Jpn J Clin Oncol. 2019;49(7):646–655. doi: 10.1093/jjco/hyz046 [DOI] [PubMed] [Google Scholar]
- 29.Gu J, Zhang X, Cui R, et al. Prognostic predictors for patients with hepatocellular carcinoma receiving adjuvant transcatheter arterial chemoembolization. Eur J Gastroenterol Hepatol. 2019;31(7):836–844. doi: 10.1097/MEG.0000000000001346 [DOI] [PubMed] [Google Scholar]
- 30.Ye JZ, Chen JZ, Li ZH, et al. Efficacy of postoperative adjuvant transcatheter arterial chemoembolization in hepatocellular carcinoma patients with microvascular invasion. World J Gastroenterol. 2017;23(41):7415–7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao WC, Zhang HB, Yang N, et al. Preoperative predictors of short-term survival after hepatectomy for multinodular hepatocellular carcinoma. World J Gastroenterol. 2012;18(25):3272–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W, Ma T, Zhang J, et al. A systematic review and meta-analysis of adjuvant transarterial chemoembolization after curative resection for patients with hepatocellular carcinoma. HPB (Oxford). 2020;22(6):795–808. [DOI] [PubMed] [Google Scholar]