Abstract
Objective
To compare the effectiveness and safety of neoadjuvant chemotherapy with carboplatin/ paclitaxel followed by interval debulking surgery (NACT-IDS) to primary debulking surgery plus postoperative chemotherapy (PDS) for advanced ovarian cancer.
Methods
A comprehensive systematic review and meta-analysis were conducted by an Expert Panel of the Japan Society of Gynecologic Oncology Ovarian Cancer Committee. Multiple public search engines including PubMed/MEDLINE and the Cochrane Database, were searched in March 2019 using the entry keywords “ovarian cancer [all fields]” AND “interval debulking surgery [all fields]”, AND “neoadjuvant chemotherapy [all fields]”. Key inclusion criteria were prospective clinical trials examining platinum-based NACT for stage II-IV epithelial ovarian cancer. The primary outcome of interest was survival, and the secondary outcome was adverse events with each intervention.
Results
After screening 333 studies, four phase III randomized clinical trials were identified that met the inclusion criteria. These trials included 1692 women (847 receiving NACT-IDS and 845 receiving PDS). It was found that NACT-IDS and PDS had similar overall survival (hazard ratio [HR]: 0.97, 95% confidence interval [CI]: 0.87–1.07, P = 0.53) and progression-free survival (HR: 0.98, 95%CI: 0.90–1.08, P = 0.74). In contrast, NACT-IDS was associated with significantly lower rates of perioperative complications (odds ratio [OR] 0.27, 95%CI: 0.20–0.36, P < 0.001) and perioperative mortality (OR: 0.17, 95%CI: 0.06–0.50, P < 0.001) compared to PDS.
Conclusion
This systematic review and meta-analysis suggests that NACT-IDS with carboplatin and paclitaxel does not negatively impact the survival of women with advanced ovarian cancer compared to PDS, while perioperative complications and mortality are significantly reduced by 70–80%.
Keywords: Ovarian cancer, Neoadjuvant chemotherapy, Survival, Perioperative complication, Systematic review, Meta-analysis
Introduction
Worldwide, ovarian cancer is the 7th most common female malignancy, and more than half of the women with ovarian cancer have advanced disease at presentation [1]. The standard initial treatment for advanced ovarian cancer has been primary debulking surgery (PDS) followed by platinum-based chemotherapy [2]. The quality of surgery is an important prognostic factor for survival in women with advanced ovarian cancer, and performing maximal cytoreductive surgery to resect all macroscopic disease is the general principal for treating advanced ovarian cancer [3,4]. However, patients with advanced ovarian cancer frequently have unresectable disease or medical comorbidity that the primary surgery may not be feasible to conduct [5]. Complications after PDS may also delay the initiation of postoperative chemotherapy.
Previous randomized controlled trials (RCTs) have found no difference in the overall survival (OS) between women with advanced ovarian cancer who received neoadjuvant chemotherapy followed by interval debulking surgery (NACT-IDS) and those given only chemotherapy [6-8]. These trials did not compare NACT-IDS with PDS and did not assess carboplatin plus taxane chemotherapy that is currently considered the standard first-line therapy for ovarian cancer treatment [9,10].
Few comprehensive meta-analyses have investigated the survival, mortality, and morbidity in women with advanced ovarian cancer treated using these two different strategies. Therefore, we conducted a comprehensive meta-analysis to compare NACT-IDS with conventional PDS.
Materials and methods
A systematic review of literature and meta-analysis were performed by an Expert Panel of the Japan Society of Gynecologic Oncology Ovarian Cancer Committee. In March 2019, a literature search was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA). PubMed/ MEDLINE and the Cochrane Database were searched for relevant articles between January 2000 and December 2018 using the entry keywords “ovarian cancer [all fields],” “primary debulking surgery [all fields],” and “neoadjuvant chemotherapy [all fields]” (Supplemental Tables S1 and S2) 11]. This study period was chosen because taxane/carboplatin chemotherapy regimen was considered the standard therapy in the first-line treatment of women with advanced ovarian cancer for almost two decades.
Eligible studies compared PDS plus postoperative chemotherapy (PDS arm) with NACT followed by IDS (NACT-IDS arm) in women with stage II-IV ovarian cancer according to the International Federation of Gynecology and Obstetrics (FIGO) staging system [12]. All the histological studies of epithelial ovarian tumors, RCTs, meta-analyses, and case-control series reported in the English literature with adequate data on patient demographics, treatment, response, and follow-up were included.
The references of each selected article were reviewed, and any article that met the inclusion criteria was assessed. If multiple publications on the same clinical trial were available, the most recent publication or presentation was chosen for the analyses. Retrospective studies, systematic reviews, reports on nonepithelial histology (including borderline malignancy), and reports on chemotherapy except carboplatin plus paclitaxel were excluded.
Clinical information
The following variables were extracted from the selected studies: year of publication, age at diagnosis, performance status (PS), FIGO stage, histological subtypes, details of initial surgical treatment (operating time, estimated blood loss, performance of lymphadenectomy, and resection of other organs), details of chemotherapy (agents and number of administered cycles), perioperative and postoperative complications, residual disease after initial surgery (complete, optimal, and suboptimal surgery), and survival outcome (OS) and progression-free survival (PFS).
Surgical complications were defined as serious adverse events (SAEs) and were classified according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTC-AE) [13]. Complete surgery was defined as complete resection with no visible or palpable residual disease in the abdomen, and optimal surgery was defined as complete resection or residual disease <1 cm in diameter [14]. OS was defined as the time period between disease diagnosis and death from any cause. PFS was defined as the time period between initial treatment and tumor progression or death from any cause. Surgical mortality was defined as perioperative/postoperative death within 28 days of surgery.
Statistical analysis
The primary objective of this study was to examine whether NACT-IDS offers any advantages over conventional PDS for FIGO stage II-IV epithelial ovarian cancer. The secondary objective was to compare the mortality and complications between these two approaches.
Time-to-event data were calculated using the Parmer method [15], and the logarithm of the hazard ratio (HR) and its standard error were calculated. For dichotomous variables, the number of women in each treatment arm who experienced an event was compared to estimate the risk ratio (RR) [16]. For continuous variables, the final value and standard deviation were determined to find the difference in the mean values.
Data extraction and management
Data were entered into a reference database and extracted independently by three reviewers who were blinded for the review each other (H.M., H.T., and staff personnel from the Japan Medical Library Association). The quality of the studies was independently assessed by the reviewers (H.M. and H.T.); disagreements were resolved via discussion with a third reviewer from the Expert Panel of the Japan Society of Gynecologic Oncology Ovarian Cancer Committee.
If data were missing or methods were unclear, further information was obtained from other published literature on the same trials or by direct inquiry from the authors. For each study, we recorded the detailed methods, study population and sample size, inclusion and exclusion criteria, interventions and comparisons, perioperative complications, and survival outcome.
Assessment of the risk of bias
Using the Cochrane collaboration tool, the risk of bias was independently assessed by two authors for each study (H.M. and H.T.), including selection bias, detection bias, attrition bias, reporting bias, and other possible types of bias (Fig. 1) [17]. Because it was not possible to blind either participants or physicians to the assigned treatment, the blinding (performance bias and detection bias) was only assessed for outcomes. To investigate publication bias, we performed a funnel plot analysis [18].
Fig. 1.
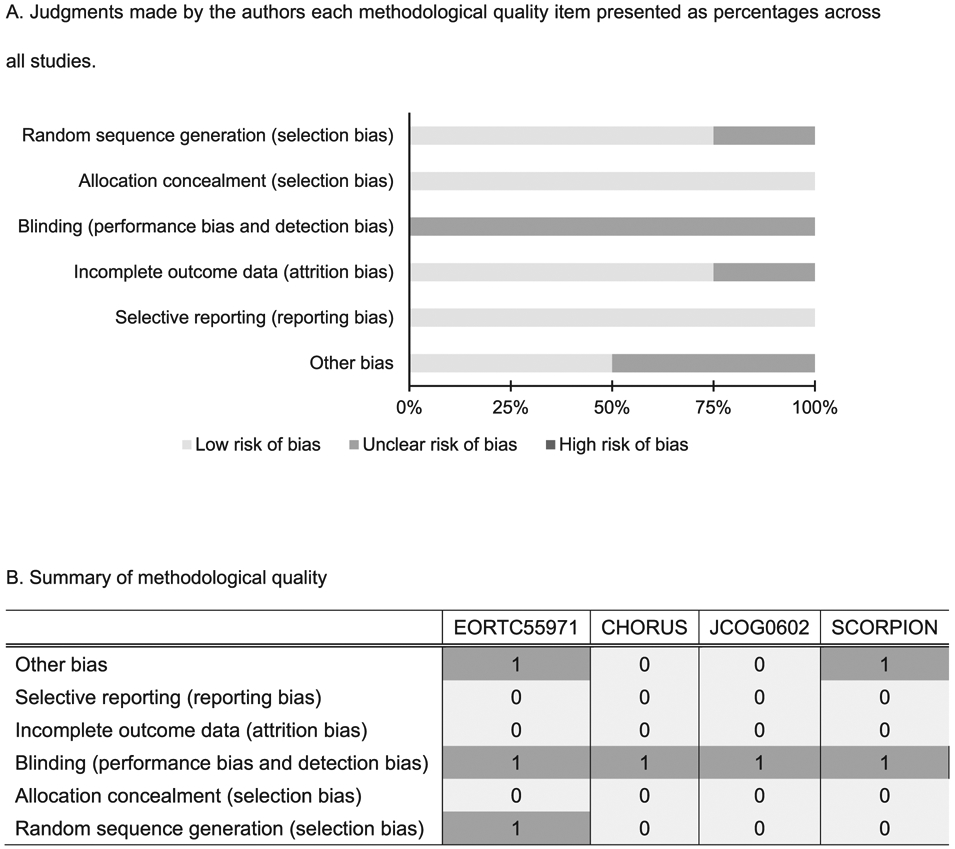
Assessment of methodological quality. A. Judgments made by the authors each methodological quality item presented as percentages across all studies.B. Summary of methodological quality. Judgments made by the authors about each methodological quality item for each study are shown. Each item was scored as follows: high risk of bias = 2, intermediate or unclear risk of bias = 1, and low risk of bias = 0.
Assessment of heterogeneity
Heterogeneity of each study was assessed by visual inspection of forest plots and by statistical evaluation using Cochran's Q test and the I2 test [19]. Synthesis of data from the studies was performed to obtain overall estimates of treatment effects. Meta-analysis was done by using random effects models with inverse variance weighting [17]. Review manager software (version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) was employed.
The level of confidence in summary data was examined by using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for studies of interventions and diagnostic test accuracy [20]. All statistical analyses were two-tailed and P-value <0.05 was considered significant.
Results
The literature search identified 333 articles published during the target period (Fig. 2). Among them, 305 articles were excluded because of being reports on ongoing trials without survival outcomes, retrospective studies, reports on non-target diseases, or non-English articles. The remaining 28 articles met the criteria for further assessment, being reports of studies that compared PDS with carboplatin/taxane-based NACT followed by IDS for advanced epithelial ovarian cancer, and full content review of these articles was performed (Supplemental Table S3) [6-8,21-40].
Fig. 2.
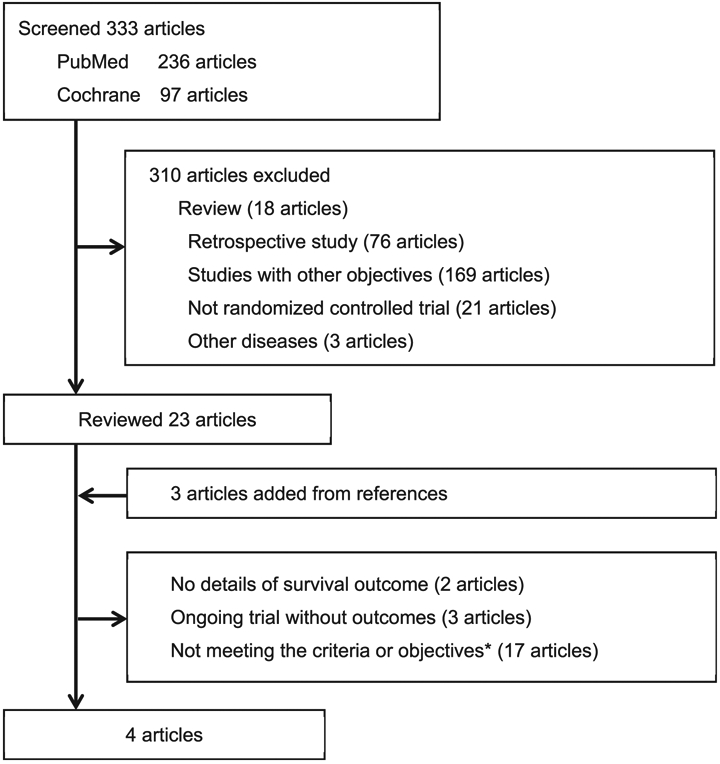
Flow diagram of study selection for systematic review.*Studies that did not compare PDS with NACT-IDS.
Finally, four RCTs were identified (EORTC 55971, CHORUS, JGOG0602, and SCORPION), which enrolled patients with FIGO stage II-IV ovarian cancer and met the inclusion criteria for this review (Fig. 2) [39,41-43]. These 4 RCTs reported data on a total of 1692 women, including 845 women who received PDS and 847 women who received NACT-IDS.
The demographic profile of patients in the four RCTs is shown in Table 1. The PDS group and the NACT group had a similar median age at diagnosis (PDS versus NACT: 59.8 versus 59.2 years). The majority of women in the NACT group had a performance status of 0–1, stage III disease, serous histology, and received 6 cycles of carboplatin plus taxane chemotherapy. There were no significant differences of these factors between the NACT group and the PDS group (performance status 0–1: 85.3% versus 85.0%; stage III disease: 75.9% versus 75.0%; serous histology: 77.3% versus 72.3%; FIGO III stage: 75.9% versus 75.0%; carboplatin plus taxane chemotherapy: 77.8% versus 69.5%; all P > 0.05). Initial tumor size is strongly associated with the likelihood of complete primary debulking and survival [44,45], but it was not significant difference between the PDS group and NACT group in this study (P < 0.79).
Table 1.
Demographic profile of patients in the four studies of PDS versus NACT-IDS.
| Study | EORTC55971 | CHORUS | JCOG0602 | SCOPION |
|---|---|---|---|---|
| Year of publication | 2010 | 2015 | 2016† | 2016† |
| Countries | Belgium, Canada, UK, Netherlands, Italy, Norway, and Spain | UK and New Zealand | Japan | Italy |
| No. of patients | 336 vs 334 | 276 vs 274 | 149 vs 152 | 84 vs 87 |
| Age (years) | 62 vs 63 | 66 vs 65 | 59 vs 60.5 | 54.8 vs 56.2 |
| PS | ||||
| 0-1 | 294 (88) vs 290 (87) | 221 (80) vs 221 (81) | 130 (87) vs 131 (86) | 51 (93) vs 50 (91) |
| 2-3 | 40 (12) vs 44 (13) | 54 (20) vs 53 (19) | 19 (13) vs 21 (14) | 4 (7) vs 5 (9) |
| Unknown | 2 (0.6) vs 0 | 1 vs 0 | 0 | 0 |
| CA125 (U/ml) | 1130 vs 1180 | Not specified | Not specified | 2653 vs. 2100 |
| Initial tumor size (cm) | ||||
| Up to 2 cm | 4(1.2) vs 10(3) | 13(5) vs 15(5) | 32(22) vs 42(28) | Not specified |
| >2-5 cm | 90(27) vs 85(25) | 59(21) vs 60(22) | 42(28) vs 51(34) | |
| >5-10 cm | 90(27) vs88(26) | 111(40) vs 110(40) | 40(27) vs 35(23) | |
| >10cm | 131(39) vs 137(41) | 86(32) vs 86(32) | 35(24) vs 24(16) | |
| Unknown | 21(6.3) vs 14(4.2) | 7(3) vs 5(2) | 0 vs 0 | |
| FIGO Stage | ||||
| II | 0 vs. 0 | 12 (5) vs. 7 (3) | 0 vs 0 | 0 vs 0 |
| III | 257 (77) vs. 253 (76) | 190 (78) vs.165 (80) | 100 (67) vs 105 (69) | 71 (85) vs 79 (91) |
| IV | 77 (23) vs. 81 (24) | 41 (17) vs. 31 (15) | 49 (33) vs 47 (31) | 13 (16) vs 8 (9) |
| Others | 2 (0.6) vs 0 | 12 vs 16 | 0 | 0 |
| Histology | ** | |||
| Serous | 220 (66) vs 194 (58) | 219 (86) vs 185 (85) | 116 (78) vs 110 (72) | 82 (98) vs 87 (100) |
| Clear cell | 6(2) vs 4 (1.2) | 4 (2) vs 13 (6) | 12(8) vs 4 (3) | 1 (1.2) vs 0 |
| Endometrioid | 11 (3) vs 5 (1.5) | 11 (4) vs 5 (2) | 6 (4) vs 4 (3) | 0 vs 0 |
| Mucinous | 8 (2) vs 11 (3) | 2 (1) vs 4 (2) | 2 (1.4) vs 2 (1.5) | 0 vs 0 |
| Others | 91 (27) vs 120 (36) | 19 (7) vs 12 (5) | 12 (8) vs 18 (13) | 1 (1.2) vs 0 |
| Surgery | ||||
| Operating time (min) | 165 vs 180 | 120 vs 120 | 341 vs 273 | 461 vs 253 |
| Blood loss (ml) | Not specified | Not specified | 3447 vs 620 | Not specified |
| Lymphadenectomy* | 26(8) vs 49(15) | 3(1) vs 1(0.5) | 29(20) vs 64(49) | 21(38) vs 7(14) |
| Abdominal organs resected | 48(16) vs 28(9) | 27(12) vs 18(8) | 51(35) vs 33(25) | 83(99) vs 33(38) |
| Complete surgery | 61(19) vs 151(51) | 39(17) vs 79(39) | 45(31) vs 83(64) | 40(48) vs 57(77) |
| Optimal surgery | 131(42) vs 238(81) | 96(41) vs147(73) | 92(63) vs107(82) | 78(93) vs 74(100) |
| Complications | ||||
| G3-4 postoperative AEs | 68 (22) vs 21 (6.5) | 74 (29) vs 31 (14) | 24 (16) vs 7 (4.6) | 39 (46) vs 7 (9.5) |
| G3-4 thromboembolism | 8 (3) vs 0 | 5 (2) vs 0 | 7 (5) vs 5 (3) | Not specified |
| G3-4 infection | 25 (8.1) vs 5(2) | 16 (6) vs 8 (3) | 1 (0.7) vs 1 (0.8) | Not specified |
| Postoperative death | 8 (2.5) vs 2(0.7) | 14 (6) vs1 (0.5) | 1 (0.7) vs 0 | 3 (3.6) vs 0 |
| Chemotherapy | ||||
| Carboplatin plus taxane | 243 (78) vs 283 (88) | 138 (61) vs 178 (70) | 148 (100) vs150 (100) | 50 (98) vs 52 (100) |
| Platinum only | 25 (8) vs 20 (6) | 89 (39) vs 75 (30) | 0 vs 0 | 1(2) vs 0 |
| Others | 21 (7) vs 19 (6) | 1(0.1) vs 1(0.1) | 0 vs 0 | 0 vs 0 |
| Cycles | Not specified | |||
| 0 | 21 (7) vs 0 | 1 (1) vs 0 | 11 (7) vs 2 (1) | |
| 1-3 | 25 (8) vs 39 (12) | 24 (11) vs 36 (17) | 6 (4) vs 17 (12) | |
| 4-5 | 11 (4) vs 7 (2) | 16 (5) vs 17 (7) | 9 (6) vs 10 (7) | |
| >6 | 253 (82) vs 276 (86) | 188 (82) vs 201 (79) | 123 (83) vs 123 (81) | |
| PFS (months) | 12 vs 12 | 11 vs 12 | 15.1 vs 16.4 | Not specified |
| OS (months) | 29 vs 30 | 23 vs 24 | 49 vs 44.3 | Not specified |
The number (%) or median is shown for PDS versus NACT-IDS.
Lymphadenectomy includes the pelvic and paraaortic lymph nodes.
For operated patients, histology was diagnosed from surgical specimens in the JCOG 0602 trial.
Both study including latest data presented at ASCO 2018.
Abbreviations: PDS, primary debulking surgery; NACT-IDS, neoadjuvant chemotherapy followed by interval debulking surgery; No., number; AE, adverse event; PFS, progression-free survival; and OS, overall survival.
Compared with the PDS group, the NACT group had a significantly shorter operating time (median 217 versus 400 min), a higher rate of pelvic and para-aortic lymphadenectomy (15.5% versus 10.0%), and a lower resection rate of other intra-abdominal organs (15.0% versus 27.0%) (all, P < 0.05).
The NACT-IDS and PDS groups showed a similar rate of discontinuing further treatment (NACT without IDS in 17.4% versus PDS without adjuvant chemotherapy in 17.3%, P = 0.97). In the NACT-IDS group, the main reason for not proceeding IDS was disease progression or death (38.0%), followed by complications of chemotherapy (16.2%). In the PDS group, the main reason for not receiving adjuvant chemotherapy was disease progression or death (50.0%), followed by postoperative complications (16.7%).
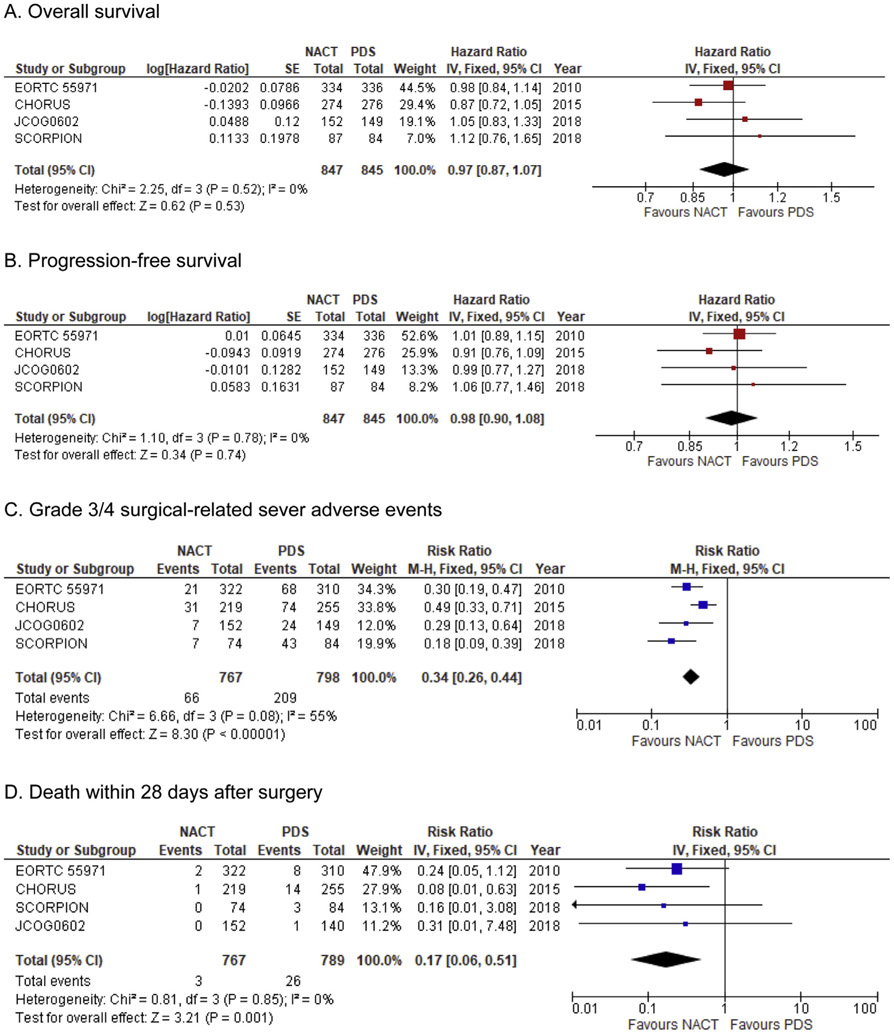
Meta-analysis of data from the four RCTs yielded the following results (Fig. 3). There was no significant difference of OS between the NACT-IDS group and the PDS group (HR: 0.97, 95% CI: 0.83 to 1.22, P = 0.53; Fig. 3A). There was also no significant difference of PFS between the two groups (HR: 0.98, 95% CI: 0.90 to 1.08, P = 0.74; Fig. 3B). When the extent of residual disease at the initial cytoreductive surgery was compared, complete resection was significantly more likely to be achieved in the NACT-IDS group compared with the PDS group (NACT-IDS versus PDS: 48.2% versus 23.2%, RR: 2.08, 95% CI: 1.80–2.39, P < 0.001). Optimal surgery was also significantly more likely to be achieved in the NACT-IDS group than the PDS group (73.8% versus 49.7%, RR: 1.48, 95%CI: 1.37–1.61, P < 0.001).
Fig. 3. Forest plots for comparison NACT-IDS versus PDS in advanced-stage ovarian cancer.
Weights were obtained from a fixed-effects model. Abbreviations: NACT, neoadjuvant chemotherapy; PDS, primary debulking surgery.
SAEs and mortality related to surgery were also examined by meta-analysis of grade 3/4 SAEs reported during the perioperative period in the four RCTs. The frequency of SAEs was significantly lower in the NACT-IDS group compared to the PDS group (26.2% versus 8.6%, RR: 0.34, 95%CI: 0.26–0.44, P < 0.001; Fig. 3C).
When specific types of perioperative and postoperative SAEs were examined, grade 3/4 venous thromboembolism and grade 3/4 infection were significantly less frequent in the NACT-IDS group compared with the PDS group (venous thromboembolism: 0.6% versus 2.8%, RR: 0.26, 95%CI: 0.11–0.63, P = 0.002; and infection: 0.6% versus 2.8%, RR: 0.31, 95%CI: 0.18–0.56, P < 0.001). Surgical mortality was also significantly less frequent in the NACT-IDS group than in the PDS group (0.4% versus 3.3%, RR: 0.17, 95% CI: 0.06–0.50, P = 0.001; Fig. 3D).
Discussion
This investigation on women with advanced ovarian cancer revealed that survival after carboplatin plus taxane-based NACT-IDS was not inferior to survival after PDS. Moreover, perioperative morbidity and mortality were 70%–80% lower among women who underwent NACT-IDS than among those who underwent PDS, and complete resection was achieved more frequently with NACT-IDS.
These findings indicate that performing carboplatin plus taxane-based NACT followed by IDS does not negatively impact the survival in women with advanced-stage ovarian cancer; however, it significantly reduces perioperative complications and mortality. Our results may have particularly important implications for women with a poor performance status, significant comorbidities, or fragility, who are ineligible for conventional PDS and could be good candidates for the NACT-IDS.
Furthermore, perioperative complications, such as infection and venous thromboembolism, were significantly less frequent with NACT-IDS than with PDS. Perioperative and postoperative complications increase the health care costs and resource utilization [46]; thus, reduced treatment costs for patients with advanced ovarian cancer could be another advantage of NACT-IDS.
In patients with advanced ovarian cancer with unresectable tumor, NACT may be a reasonable strategy to achieve tumor shrinkage as well as obtain as much resection as possible at the subsequent IDS. In particular, this meta-analysis showed a lower resection rate of other intra-abdominal organs in the NACT group than in the PDS group. Surgery was less invasive in the NACT group, and the rates of complete resection were higher. These results suggest that adopting the NACT-IDS strategy may be beneficial for institutions with fewer resources, such as low-volume hospitals. Therefore, carboplatin plus taxane-based NACT followed by IDS may be more feasible and more effective than PDS in such settings.
However, there is a concern with respect to the use of the NACT-IDS strategy. In cases where the tumor is larger at the time of initial treatment, the risk of spontaneous mutation is higher, and this increases the likelihood of chemo-resistance [46,47]. The NACT-IDS strategy may led to the development of a progressive disease during NACT, called the platinum-refractory disease. Usually, women with platinum-refractory disease have poor prognosis [45]. Therefore, most of them may not be able to undergo IDS because of disease progression [45]. In fact, our study showed that approximately 7% of those in the NACT group did not undergo IDS because of disease progression. Therefore, we need to consider both the tumor burden and the risk of refractory/resistant disease before initiating NACT.
Another factor related to chemo-resistance is tumor histology. The histological subtype of ovarian cancer is an important prognostic factor, and the response to chemotherapy varies with tumor histology. High-grade serous carcinoma is reported to have a very high response rate of 73%–81% to platinum-based chemotherapy and a low incidence of progressive disease; however, clear cell carcinoma has a low response rate of 11%–45% and a high incidence of progressive disease [47,48]. Mucinous histology can be another factor; however, it has not been well investigated because of its rarity [49]. In the present meta-analysis, most patients had chemo-sensitive serous carcinoma. Therefore, further studies should be performed to explore whether NACT-IDS is also a suitable strategy for other histological types of ovarian cancer.
The main strength of this study was that we performed a systematic review and meta-analysis to collected information. Thus, the data we obtained were more reliable than those obtained from an individual investigation [50]. We assessed all the studies published during the previous 20 years, the period during which carboplatin plus taxane chemotherapy was considered the standard first-line treatment for ovarian cancer.
This study has certain limitations. We could not obtain information about cancer genetics, patient's comorbidities, type of surgeon (gynecological oncologist or general gynecologist), the quality of care, and the hospital type. These factors have been shown to influence the survival of patients with ovarian cancer [51,52]. The use of antiangiogenic agent (i.e., bevacizumab) following platinum-based chemotherapy has an impact on perioperative morbidity and more importantly on survival, especially in patients with residual disease [53]. Moreover, the likelihood of response to platinum-based chemotherapy in high-grade serous adenocarcinoma with advanced ovarian cancer could be influenced by BRCA mutation status [54].
Furthermore, the frequency of systematic lymphadenectomy and other surgical procedures varied widely among the studies we reviewed, and various procedures were employed for cytoreductive surgery even within each trial. Therefore, there might be substantial heterogeneity in the surgical methods among the four RCTs in this systematic review (Supplemental Fig. 3).
RCTs that are currently underway, such as the Study of Upfront Surgery Versus Neoadjuvant Chemotherapy in Patients with Advanced Ovarian Cancer (SUNNY trial) being performed in China, and the Trial on Radical Upfront Surgery in Advanced Ovarian Cancer (TRUST trial) in Germany using antiangiogenic agent, may provide more detailed information about these factors [26,31].
Despite such limitations, the findings of this meta-analysis have important implications for women with advanced ovarian cancer, particularly those with unresectable disease, because NACT with carboplatin plus taxane followed by IDS appears an alternative strategy for their management. Our findings also suggest that patient selection for the NACT-IDS approach may be tailored based on tumor histology, with women who have high-grade serous tumors being candidates for the NACT-IDS strategy.
In conclusion, this meta-analysis demonstrated that women with advanced ovarian cancer had comparable survival after NACT-IDS and PDS; however, the perioperative mortality and morbidity were lower with NACT-IDS. Thus, appropriate patient selection for NACT-IDS would attribute to the improved survival in patients with advanced ovarian cancer.
Supplementary Material
Acknowledgements
This project was assisted by an Expert Panel of the Japan Society of Gynecologic Oncology, via Medical Information Network Distribution Service which received financial support from the Ministry of Health, Labor and Welfare of Japan as a consignment project.
Funding support
None.
Footnotes
Declaration of competing interest
Honorarium, Chugai, textbook editorial, Springer, and investigator meeting attendance expense, VBL therapeutics (K.M.); none for others.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejso.2019.11.520.
References
- [1].Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer biol med 2017;14:9–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Epithelial ovarian cancer/fallopian tube cancer/primary peritoneal cancer and less common histopathologies. NCCN guideline version1 2019. https://www.nccn.org/professionals/physician_gls/default.aspx. accessed June 5th 2019.
- [3].Eisenkop SM, Spirtos NM, Friedman RL, Lin WC, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol Oncol 2003;90:390–6. [DOI] [PubMed] [Google Scholar]
- [4].Winter WE 3rd, Maxwell GL, Tian C, Sundborg MJ, Rose GS, Rose PG, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol : Off J Am Soc Clini Oncol 2008;26:83–9. [DOI] [PubMed] [Google Scholar]
- [5].Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol 2007;197 676.e1–7. [DOI] [PubMed] [Google Scholar]
- [6].van der Burg ME, van Lent M, Buyse M, Kobierska A, Colombo N, Favalli G, et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N Engl J Med 1995;332:629–34. [DOI] [PubMed] [Google Scholar]
- [7].Rose PG, Nerenstone S, Brady MF, Clarke-Pearson D, Olt G, Rubin SC, et al. Secondary surgical cytoreduction for advanced ovarian carcinoma. N Engl J Med 2004;351:2489–97. [DOI] [PubMed] [Google Scholar]
- [8].Redman CW, Warwick J, Luesley DM, Varma R, Lawton FG, Blackledge GR. Intervention debulking surgery in advanced epithelial ovarian cancer. Br J Obstet Gynaecol 1994;101:142–6. [DOI] [PubMed] [Google Scholar]
- [9].McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996;334: 1–6. [DOI] [PubMed] [Google Scholar]
- [10].Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol : Off J Am Soc Clini Oncol 2003;21:3194–200. [DOI] [PubMed] [Google Scholar]
- [11].Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clini Res ed) 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Prat J Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet: Off Organ Int Fed Gynaecol Obstet 2014;124: 1–5. [DOI] [PubMed] [Google Scholar]
- [13].National Institutes of Health National Cancer Institute published. https://ctepcancer.gov; 2009. Accessed on June 5th 2019.
- [14].Hennessy BT, Coleman RL, Markman M. Ovarian cancer Lancet (London, England) 2009;374:1371–82. [DOI] [PubMed] [Google Scholar]
- [15].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17: 2815–34. [DOI] [PubMed] [Google Scholar]
- [16].Woods BS, Hawkins N, Scott DA. Network meta-analysis on the log-hazard scale, combining count and hazard ratio statistics accounting for multi-arm trials: a tutorial. BMC Med Res Methodol 2010;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cochrane handbook for systematic reviews of interventions, [chapter 8] (accessed June 5th 2019).
- [18].Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 2001;54:1046–55. [DOI] [PubMed] [Google Scholar]
- [19].Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clini Res ed) 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ (Clini Res ed) 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sebastian AT A, Chandy R, Peedicayil A, Thomas V. A randomised control trial of systematic lymphadenectomy versus removal of bulky nodes at interval debulking for advanced epithelial ovarian cancer. Int J Gynecol Cancer 2018;28:830. [Google Scholar]
- [22].van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med 2018;378:230–40. [DOI] [PubMed] [Google Scholar]
- [23].Harter P, Sehouli J, Lorusso D, Reuss A, Vergote I, Marth C, et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N Engl J Med 2019;380:822–32. [DOI] [PubMed] [Google Scholar]
- [24].Rouzier R, Gouy S, Selle F, Lambaudie E, Floquet A, Fourchotte V, et al. Efficacy and safety of bevacizumab-containing neoadjuvant therapy followed by interval debulking surgery in advanced ovarian cancer: results from the ANTHALYA trial Eur J Cancer 2017;70:133–42. Oxford, England: : 1990. [DOI] [PubMed] [Google Scholar]
- [25].Gill SE, McGree ME, Weaver AL, Cliby WA, Langstraat CL. Optimizing the treatment of ovarian cancer: neoadjuvant chemotherapy and interval debulking versus primary debulking surgery for epithelial ovarian cancers likely to have suboptimal resection. Gynecol Oncol 2017;144:266–73. [DOI] [PubMed] [Google Scholar]
- [26].S Mahner FH, Burges A, Reuss A, Kraemer B, Schmalfeldt B, Sehouli J, et al. TRUST: trial of radical upfront surgical therapy in advanced ovarian cancer (ENGOT ov33/AGO-OVAR OP7 2017;35:15. [Google Scholar]
- [27].Daniele G, Lorusso D, Scambia G, Cecere SC, Nicoletto MO, Breda E, et al. Feasibility and outcome of interval debulking surgery (IDS) after carboplatinpaclitaxel-bevacizumab (CPB): a subgroup analysis of the MITO-16A-MaNGO OV2A phase 4 trial. Gynecol Oncol 2017;144:256–9. [DOI] [PubMed] [Google Scholar]
- [28].I Jaffre FL, Houvenaeghel G, Lambaudie E, Pomel C, Vergote I, Harter P, et al. Morbidity and survival of patients treated for an advanced ovarian cancer by retroperitoneal lymphadenectomy: caraco, a French randomized trial. Int J Gynecol Cancer 2017;27:1529. [Google Scholar]
- [29].Rauh-Hain JA, Melamed A, Wright A, Gockley A, Clemmer JT, Schorge JO, et al. Overall survival following neoadjuvant chemotherapy vs primary cytoreductive surgery in women with epithelial ovarian cancer: analysis of the national cancer database. JAMA oncol 2017;3:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ferron GR G, Ray-Coquard IL, Lesoin A, Joly F, Lortholary A, Raban N, et al. The CHIVA study: a GINECO randomized double blind phase II trial of nintedanib versus placebo with the neo-adjuvant chemotherapy (NACT) strategy for patients (pts) with advanced unresectable ovarian cancer (OC). In: Report of the interval debulking surgery (IDS) safety outcome Annals of oncology Conference: 41st european society for medical oncology congress ESMO; 2016. Denmark 2016. [Google Scholar]
- [31].Group SGO. Study of Upfront surgery versus neoadjuvant chemotherapy in patients with advanced ovarian cancer (SUNNY). 2016http://clinicaltrials.gov/ct2/show/NCT02859038. accessed June 5th 2019. [Google Scholar]
- [32].Meyer LA, Cronin AM, Sun CC, Bixel K, Bookman MA, Cristea MC, et al. Use and effectiveness of neoadjuvant chemotherapy for treatment of ovarian cancer. J Clin Oncol : Off J Am Soc Clini Oncol 2016;34:3854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Petrillo M, Paris I, Vizzielli G, Amadio G, Cosentino F, Salutari V, et al. Neoadjuvant chemotherapy followed by maintenance therapy with or without bevacizumab in unresectable high-grade serous ovarian cancer: a case-control study. Ann Surg Oncol 2015;22(Suppl 3):S952–8. [DOI] [PubMed] [Google Scholar]
- [34].Greimel E, Kristensen GB, van der Burg ME, Coronado P, Rustin G, del Rio AS, et al. Quality of life of advanced ovarian cancer patients in the randomized phase III study comparing primary debulking surgery versus neo-adjuvant chemotherapy. Gynecol Oncol 2013;131:437–44. [DOI] [PubMed] [Google Scholar]
- [35].Rutten MJ, Gaarenstroom KN, Van Gorp T, van Meurs HS, Arts HJ, Bossuyt PM, et al. Laparoscopy to predict the result of primary cytoreductive surgery in advanced ovarian cancer patients (LapOvCa-trial): a multicentre randomized controlled study. BMC Canc 2012;12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chi DS, Musa F, Dao F, Zivanovic O, Sonoda Y, Leitao MM, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT). Gynecol Oncol 2012;124:10–4. [DOI] [PubMed] [Google Scholar]
- [37].Le T, Latifah H, Jolicoeur L, Weberpals J, Faught W, Hopkins L, et al. Does intraperitoneal chemotherapy benefit optimally debulked epithelial ovarian cancer patients after neoadjuvant chemotherapy? Gynecol Oncol 2011;121: 451–4. [DOI] [PubMed] [Google Scholar]
- [38].Verleye L, Ottevanger PB, Kristensen GB, Ehlen T, Johnson N, van der Burg ME, et al. Quality of pathology reports for advanced ovarian cancer: are we missing essential information? An audit of 479 pathology reports from the EORTC-GCG 55971/NCIC-CTG OV13 neoadjuvant trial Eur JCancer 2011;47: 57–64. Oxford, England: : 1990. [DOI] [PubMed] [Google Scholar]
- [39].Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet (London, England) 2015;386:249–57. [DOI] [PubMed] [Google Scholar]
- [40].Chan YMNT, Ngan HY, Wong LC. Quality of life in women treated with neoadjuvant chemotherapy for advanced ovarian cancer: a prospective longitudinal study. Gynecol Oncol 2003;88:6–16. [DOI] [PubMed] [Google Scholar]
- [41].Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943–53. [DOI] [PubMed] [Google Scholar]
- [42].Fagotti A, Ferrandina G, Vizzielli G, Fanfani F, Gallotta V, Chiantera V, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): final analysis of peri-operative outcome Eur J Cancer 2016;59:22–33. Oxford, England: : 1990. [DOI] [PubMed] [Google Scholar]
- [43].Onda T, Satoh T, Saito T, Kasamatsu T, Nakanishi T, Nakamura K, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/ IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602 Eur J Cancer 2016;64:22–31. Oxford, England: : 1990. [DOI] [PubMed] [Google Scholar]
- [44].Petrillo M, Vizzielli G, Fanfani F, Gallotta V, Cosentino F, Chiantera V, et al. Definition of a dynamic laparoscopic model for the prediction of incomplete cytoreduction in advanced epithelial ovarian cancer: proof of a concept. Gynecol Oncol 2015;139:5–9. [DOI] [PubMed] [Google Scholar]
- [45].Elzarkaa AA, Shaalan W, Elemam D, Mansour H, Melis M, Malik E, et al. Peritoneal cancer index as a predictor of survival in advanced stage serous epithelial ovarian cancer: a prospective study. J Gynecol Oncol 2018;29:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Healy MA, Mullard AJ, Campbell DA Jr, Dimick JB. Hospital and payer costs associated with surgical complications. JAMA surgery 2016;151:823–30. [DOI] [PubMed] [Google Scholar]
- [47].Pectasides D, Pectasides E, Psyrri A, Economopoulos T. Treatment issues in clear cell carcinoma of the ovary: a different entity? The Oncologist 2006;11: 1089–94. [DOI] [PubMed] [Google Scholar]
- [48].Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 2000;88:2584–9. [PubMed] [Google Scholar]
- [49].Gore M, Hackshaw A, Brady WE, Penson RT, Zaino R, McCluggage WG, et al. An international, phase III randomized trial in patients with mucinous epithelial ovarian cancer (mEOC/GOG 0241) with long-term follow-up: and experience of conducting a clinical trial in a rare gynecological tumor. Gynecol Oncol 2019;153:541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ferreira Gonzalez I, Urrutia G, Alonso-Coello P. Systematic reviews and metaanalysis: scientific rationale and interpretation. Rev Esp Cardiol 2011;64: 688–96. [DOI] [PubMed] [Google Scholar]
- [51].Nagle CM, Dixon SC, Jensen A, Kjaer SK, Modugno F, deFazio A, et al. Obesity and survival among women with ovarian cancer: results from the Ovarian Cancer Association Consortium. Br J Canc 2015;113:817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wright JD, Chen L, Hou JY, Burke WM, Tergas AI, Ananth CV, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol 2017;130:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gonzalez Martin A, Oza AM, Embleton AC, Pfisterer J, Ledermann JA, Pujade-Lauraine E, et al. Exploratory outcome analyses according to stage and/or residual disease in the ICON7 trial of carboplatin and paclitaxel with or without bevacizumab for newly diagnosed ovarian cancer. Gynecol Oncol 2019;152:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gorodnova TV, Sokolenko AP, Ivantsov AO, Iyevleva AG, Suspitsin EN, Aleksakhina SN, et al. High response rates to neoadjuvant platinum-based therapy in ovarian cancer patients carrying germ-line BRCA mutation. Cancer Lett 2015;369:363–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.