Abstract
Background
The addition of lapatinib (L) to trastuzumab (T) was previously found to be synergistic in preclinical models and in the neoadjuvant setting. Prior to the results of the ALTTO trial, this study assessed the safety and feasibility of adding L to the standard adjuvant docetaxel, carboplatin and trastuzumab (TCH) regimen in early stage HER2-positive breast cancer (HER2+ BC).
Methods
In this single-arm, 2-stage, phase II study, patients with stages I–III HER2+ BC received TCH plus L at 1000 mg daily for a total of 12 months. The primary endpoint was the safety and tolerability, including the rate of diarrhea. Secondary endpoints included adverse event (AE) profile using the NCI CTCAE v3.0 and cardiac safety.
Results
Thirty eligible patients were enrolled. Median follow-up is 5.3 years. Diarrhea was the most common AE with 50% Grade (G)1/2 and 43% G3 diarrhea. However, it was responsive to dose reduction of L (750 mg) and institution of anti-diarrheal medications. Cardiovascular AE were infrequent and no patients experienced congestive heart failure while on treatment.
Conclusion
TCHL was a tolerable regimen at a starting L dose of 750 mg PO daily when given concurrently with chemotherapy.
Keywords: Breast cancer, HER2 cardiac, Gastrointestinal, Tolerability, Lapatinib, Adjuvant
INTRODUCTION
The human epidermal growth factor receptor type 2 (HER2, ERBB2) is a trans-membrane protein overexpressed in approximately 15–25% of invasive breast cancers1,2. Patients with HER2-amplified breast cancer have an increased risk for disease relapse, progression, and mortality compared to HER2-negative breast cancer2–6. The addition of trastuzumab—a recombinant humanized monoclonal antibody against the extracellular domain of HER2—to adjuvant chemotherapy in early-stage breast cancer led to a significant improvement in disease-free survival (DFS) and overall survival (OS) as compared with adjuvant chemotherapy alone7. However, a subset of patients treated with trastuzumab and chemotherapy still experience relapse, which over the years has motivated a search for additional strategies to more effectively block HER2 signaling in an effort to further improve clinical outcomes7–12. Prior to the widespread use of neoadjuvant systemic therapy in HER2-positive breast cancer, and the modern approach of further escalating adjuvant therapy based on pathologic response13, the neoadjuvant paradigm was primarily used to provide preliminary (and faster) evidence of drug activity as assessed by pathologic response and provide rationale for larger adjuvant studies evaluating long-term benefit.
Lapatinib is an oral dual HER1 and HER2, tyrosine kinase inhibitor targeting the intracellular domains of these receptors14. The use of lapatinib in combination with capecitabine led to improved time to progression in women with metastatic HER2-positive disease that had previously progressed after treatment with trastuzumab15, and it is approved for this patient population in combination with either capecitabine or endocrine therapy16. The North Central Cancer Treatment Group (NCCTG) study 083E (N083E) reported herein is a multicenter, single-arm, phase II clinical trial designed to assess the safety and tolerability of the addition of lapatinib to the standard adjuvant regimen of docetaxel, carboplatin and trastuzumab (TCH regimen as delivered in the BCIRG-006 phase III study)17. When N083E was designed, there was extensive preclinical data supporting that the combination of lapatinib and trastuzumab was synergistic18,19, and there was rationale to explore such combination in the treatment of early-stage HER2-positive breast cancer. However, an increased incidence of adverse events (AEs) had been reported when both drugs were combined concurrently with chemotherapy20. As such, N083E was designed to generate safety data (including rates of diarrhea and cardiac safety) that would be utilized to inform the incorporation of this regimen as part of Design 2 of the phase III ALTTO (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) clinical trial21. This manuscript provides long-term follow-up data of this combination (TCHL) as pursued in the N803E study, and serves to put these results in the context of the previously reported ALTTO trial22.
MATERIALS and METHODS
Patient eligibility
Eligible patients had centrally-confirmed HER2-positive, surgically resected, invasive breast cancer. HER2 status was determined by an immunohistochemistry (IHC) score of 3+ (by Dako HercepTest®, FDA approved guideline) or by the presence of gene amplification as determined by fluorescence in situ hybridization (FISH) defined by a ratio of greater than or equal to 2.2, as per the 2007 American Society of Clinical Oncology (ASCO) and College of American of Pathologists (CAP) guidelines23. Pathology was centrally reviewed at Mayo Clinic, Rochester, MN. Baseline left ventricular ejection fraction (LVEF) greater than or equal to 50% measured by multiple-gated acquisition (MUGA) scan or echocardiogram (Echo) was mandatory. Other selected eligibility requirements included age of 18 or older, Eastern Cooperative Oncology Group (ECOG) performance status (PS) less than or equal to one, and adequate hematologic, hepatic, and renal function. Patients with history of prior mediastinal irradiation (except internal mammary node irradiation for breast cancer), prior myocardial infarction, active angina pectoris, history of congestive heart failure, LVEF less than 50%, uncontrolled hypertension, or other clinically significant cardiac disease were ineligible. Patients with gastrointestinal disorders that might interfere with the absorption of lapatinib were also excluded. Institutional Review Board (IRB) approval was required and informed consent was obtained from all patients.
Study design and treatment
N083E was a two-stage phase II study designed to determine the safety and tolerability of docetaxel plus carboplatin in combination with trastuzumab and lapatinib (TCHL), including the rate of gastrointestinal (diarrhea) and cardiac safety. The treatment schedule consisted of six cycles of docetaxel (T: 75 mg/m2 day 1) and carboplatin (C: AUC of 6 day 1) every 3 weeks, plus trastuzumab (H: 4 mg/kg loading dose on week 1 followed by 2 mg/kg weekly for 17 weeks, then at 6 mg/kg every 3 weeks for 12 additional cycles), and lapatinib (L: 1,000 mg PO daily for 52 weeks). The study allowed reducing the dose of lapatinib from 1,000 to 750 mg/day depending on the incidence and severity of the AEs, and specifically for diarrhea, changes in LVEF, abnormal liver function tests and acneiform rash attributed to this drug. Growth factor support was given following cycles 1 through 6 as part of standard of care. Selection of filgrastim or peg-filgrastim was at the investigator’s discretion. Common inhibitors or inducers of CYP3A4 were not allowed within 7 and 14 days respectively prior to the administration of the first cycle of lapatinib or during the treatment period. Patients received radiation and adjuvant endocrine therapy according to standard treatment guidelines based on prior surgical approach, stage, and tumor characteristics.
Toxicity evaluation
The AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Evaluation of LVEF by Echo or MUGA was performed at baseline, at cycle 6 day ≥15; at cycle 12, at cycle 18, every 6 months during year 2, and then once yearly from year 3 until year 10.
Treatment dose modifications
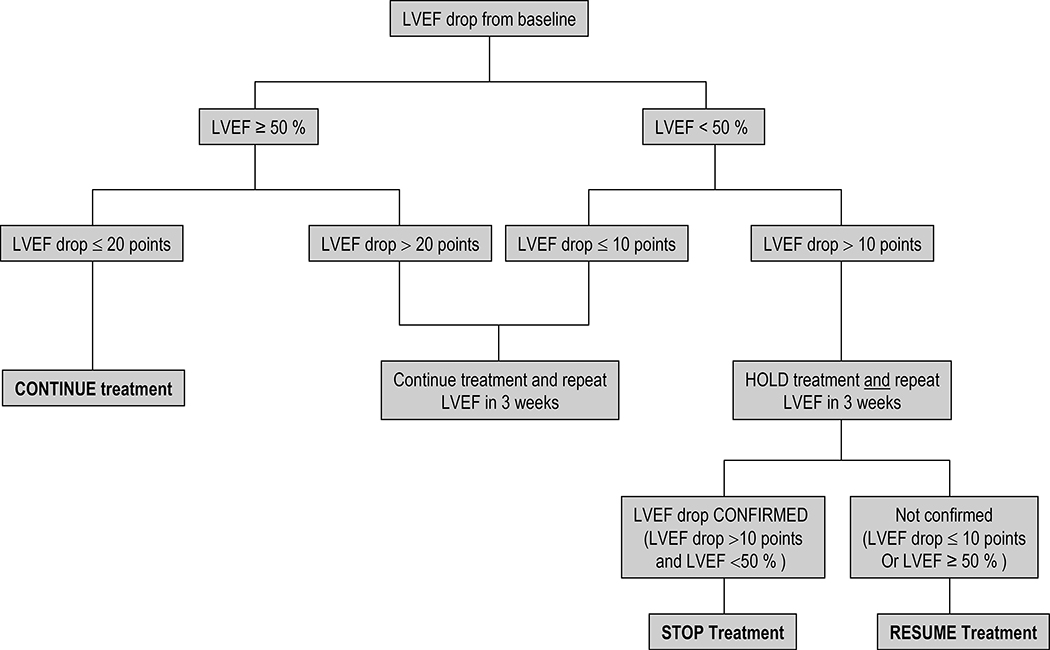
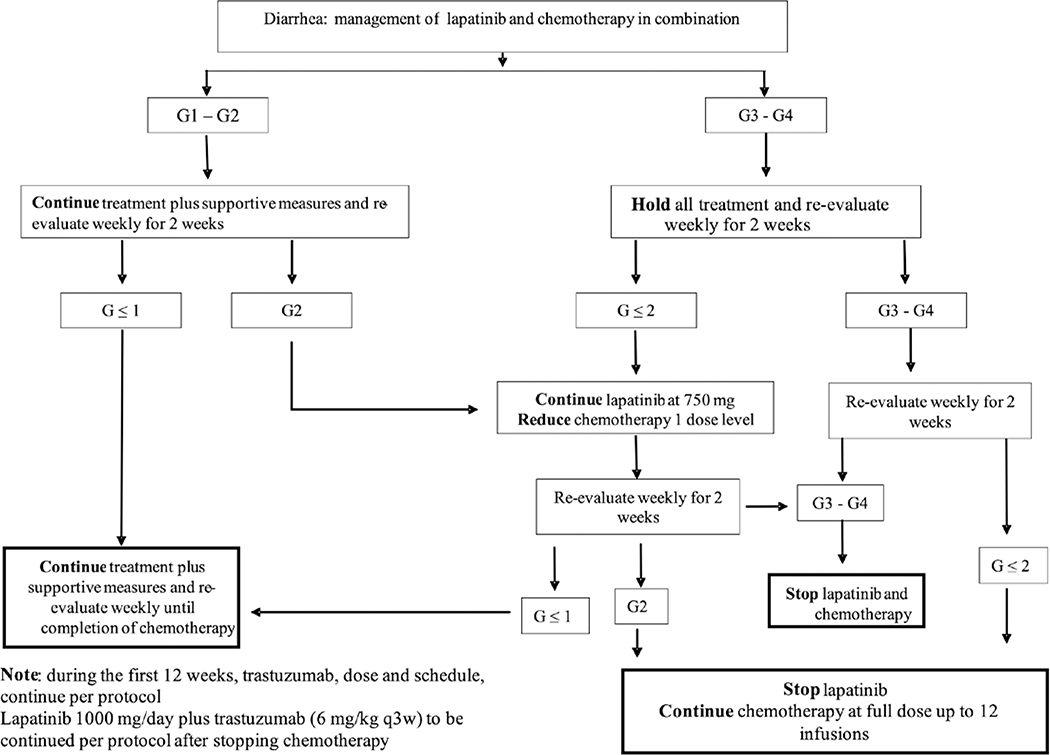
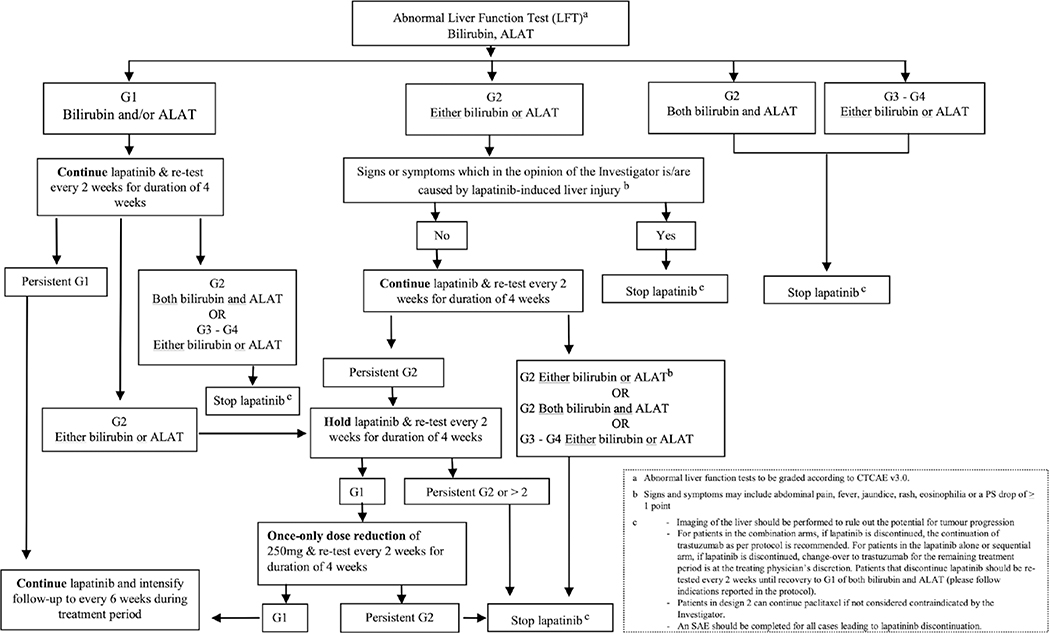
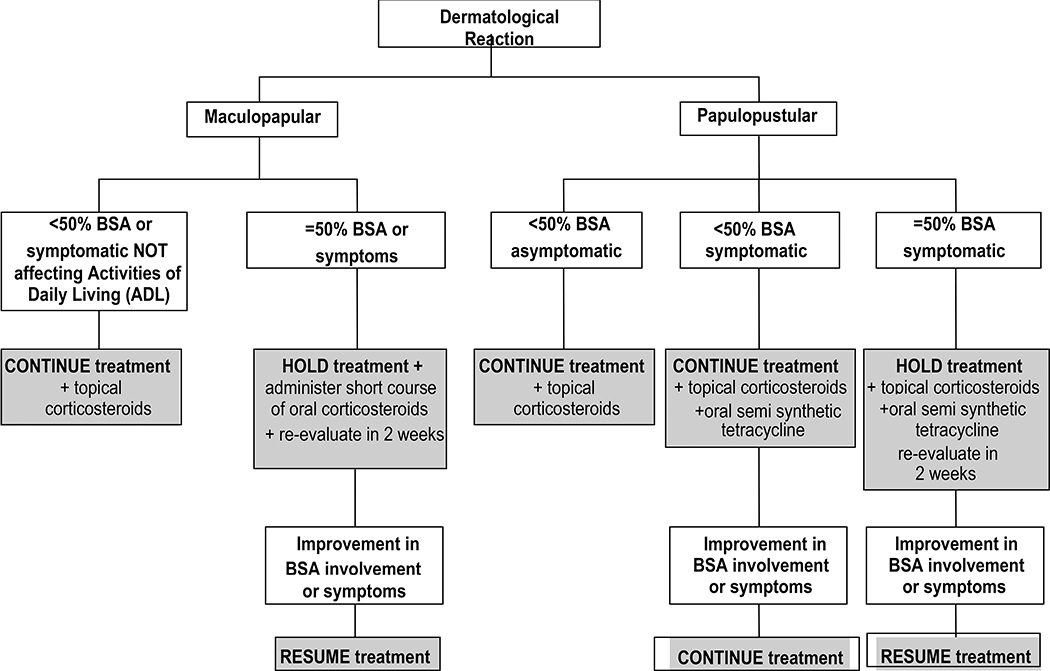
Treatment dose modification algorithms were designed for the management of cardiac toxicity, diarrhea, abnormal liver function tests and dermatological reactions (Figs. 1, 2, 3 and 4).
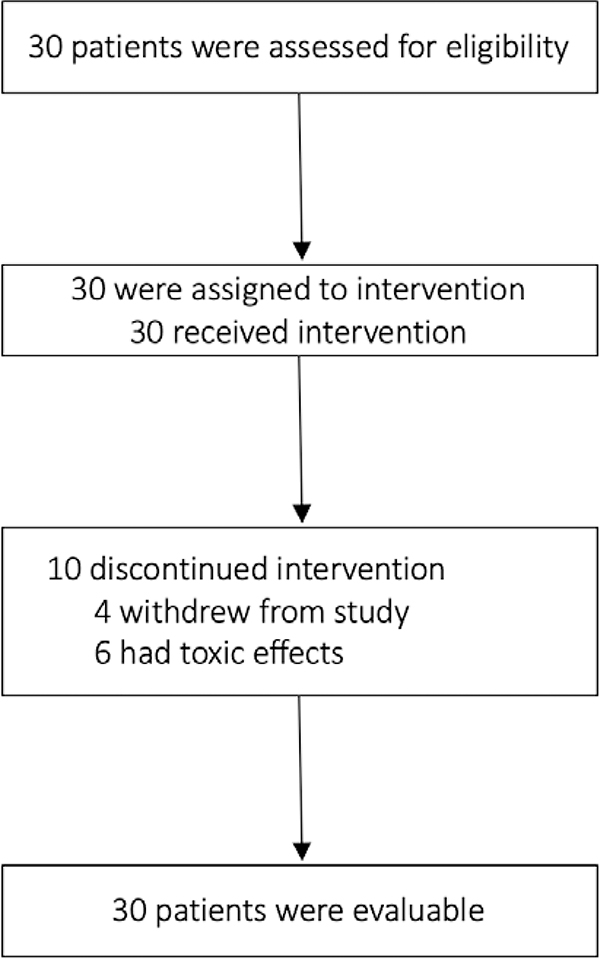
Figure 1.
CONSORT diagram
Figure 2.
Algorithm for the management of cardiac toxicity in N083E
Figure 3.
Algorithm for the management of diarrhea in N083E
Figure 4.
Algorithm for the management of abnormal liver function tests in N083E
One dose reduction in lapatinib (from 1,000 to 750 mg/day was also permitted for other Grade (G) 3/4 lapatinib-related AE. Treatment could be delayed for up to 3 weeks to allow for resolution of the AE. If treatment had to be delayed more than 3 weeks, or in the case of G3/4 confirmed interstitial pneumonitis, active study treatment with lapatinib was permanently discontinued. One- and two-level dose reductions were allowed for adverse events associated with docetaxel and carboplatin (docetaxel reduction to 60 and 45 mg/m2 and carboplatin reduction to AUC of 5 and 4 for levels −1 and −2, respectively).
Statistical considerations
The primary endpoint was the safety and tolerability of docetaxel plus carboplatin in combination with trastuzumab and lapatinib (TCHL), including the proportion of patients experiencing G3 or 4 diarrhea. While this study is descriptive in nature, an overall G3 or 4 diarrhea rate of ≤20% was defined as acceptable and a rate of ≥40% as unacceptable. The first-stage decision rule was based on this premise, and the final overall conclusions were to be predicated on this hypothesis. A minimum of 20 and a maximum of 30 evaluable patients were to be accrued onto this phase II study for evaluation of safety, unless accrual was terminated early due to diarrhea or other undue severe AEs. Ten patients were to be enrolled onto the first stage of the study. After 10 patients were enrolled, the study was to be suspended to accrual, and enhanced monitoring was to take place to ensure the safety and tolerability of the TCHL regimen. If 4 or more patients experienced G3 or 4 diarrhea during the first cycle of treatment, the safety data would be discussed with the NCI Cancer Therapy Evaluation Program (CTEP) and the sponsor GlaxoSmithKline Inc (GSK), and the study dose of lapatinib was to be reduced to 750 mg PO daily. The study would then reopen and enroll 20 additional patients using the modified regimen with the lower dose of lapatinib agreed upon by CTEP/GSK/NCCTG. Safety in those 20 patients would continue to be monitored via a pre-specified Adverse Event Stopping Rule based on the G4 or higher non-hematologic adverse event rate observed on the previous clinical trial RC0639 (phase II adjuvant study of doxorubicin/cyclophosphamide x 4 cycles followed by weekly paclitaxel/trastuzumab and daily lapatinib x 12 weeks, then 9 more months of trastuzumab plus lapatinib, in early stage HER2-positive breast cancer) 24. LVEF was to be assessed at each time point. Additionally, the proportion of patients in various categories of changes in LVEF was to be computed (such as the proportion of patients who experience ≥10% drop in LVEF between two time points). Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. Data locked May 16, 2019.
RESULTS
Patient baseline characteristics
From March 2009 to November 2009, 30 patients were enrolled. Study enrollment and treatment discontinuation are detailed in Figure X. Median follow-up at the time of this report was 5.3 years (range 1.6–9.5). Baseline characteristics of the 30 evaluable patients are presented in Table 1. Median patient age was 54.5 years (range 29–70). Two thirds (67%) of patients were post-menopausal and the same number of patients were hormone receptor-positive. Sixty percent of patients had tumors T2 or larger in size and 43% had node positive disease (10% of node-positive patients had 4 or more positive lymph nodes). Most patients (77%) underwent mastectomy and almost half planned to receive adjuvant radiation therapy.
Table 1.
Patient Characteristics (N=30)
| Characteristic | N (%) |
|---|---|
| Age (median) | 54.5 (29-70) |
| Age Group: | |
| <40 | 4 (13) |
| 40-49 | 7 (23) |
| 50-59 | 9 (30) |
| ≥ 60 | 10 (16) |
| Race: | |
| White | 29 (97) |
| Other | 1 (3) |
| Menopausal Status: | |
| Pre-menopausal | 10 (33) |
| Post-menopausal | 20 (67) |
| ER/PR Status: | |
| ER or PR Positive | 20 (67) |
| ER and PR Negative | 10 (33) |
| Previous Breast Surgery: | |
| Breast Conserving | 7 (23) |
| Mastectomy | 23 (77) |
| Nodal Status: | |
| Node Positive (1-3+ nodes) | 9 (30) |
| Node Positive (4-9+ nodes) | 1 (3) |
| Node Positive (10+ nodes) | 3 (10) |
| Node Negative | 17 (57) |
| Nottingham Tumor Grade: | |
| Well /Intermediate | 7 (23) |
| Poor | 23 (77) |
| Histology: | |
| InvasiveDuctal | 26 (87) |
| InvasiveLobular | 4 (13) |
| Size (cm) | |
| <1 | 1 (3) |
| 1-2 | 11 (37) |
| 2-5 | 13 (43) |
| > 5 | 5 (17) |
| Side of Tumor | |
| Left | 15 (50) |
| Right | 15 (50) |
| Performance Score | |
| 0 | 21 (70) |
| 1 | 9 (30) |
| Smoking Status | |
| Never | 20 (67) |
| Current | 4 (13) |
| Former | 6 (20) |
| History of Diseases: | |
| Diabetes | 2 (7) |
| Hypertension | 9 (30) |
| Other | 9 (30) |
| None | 20 (67) |
| Any Prior Cancer | |
| Yes | 1 (3) |
| No | 29 (97) |
| Planned Hormone Therapy | |
| Tamoxifen | 6 (20) |
| Aromatase Inhibitor | 10 (33) |
| None | 14 (47) |
| Planned Radiation Therapy | |
| Yes | 14 (47) |
| No | 16 (53) |
Treatment and Adverse Event Profile
A median of 18 treatment cycles (range, 1–18 cycles; total, 396 cycles) were given. Seven patients (23%) completed the study at the 1000 mg dose level of lapatinib without a dose modification. Dose omissions/reductions of lapatinib occurred in 23 patients (77%). The most common reason for dose modifications was diarrhea (47%), but also included dermatologic toxicity (10%), elevated liver enzymes (7%), hypersensitivity (3%), oral mucositis/stomatitis (3%), need for surgery (3%), and neutropenia (3%). Fourteen patients (47%) with dose omissions/reductions of lapatinib completed all cycles of the study. Nine (30%) of the remaining patients with a dose modification of L went off study treatment (6 due to adverse events and 3 due to patient refusal to continue on the trial). Among the 23 patients with L dose modifications, nine (30%) also had dose omissions/reductions of docetaxel, 4 (13%) of carboplatin and 9 (30%) of trastuzumab.
All thirty patients were evaluable for AEs. Twenty-four (80%) patients experienced one or more G3 or higher AEs. G4 AEs included thrombocytopenia (2 pts), neutropenia (6 pts) and colitis (1 pt). G3 AEs occurring in 5% or more of patients include diarrhea (43%), nausea (10%), neutropenia (17%), hypersensitivity (10%), hypokalemia (10%), leukopenia (7%), rash (7%), and rash acneiform (7%). The anti-diarrheal agent loperamide was recommended to be started at the first onset of diarrhea. In the first stage, only 1 of 10 patients (10%) developed G3 diarrhea during the first cycle and the starting dose of L was maintained at 1000 mg PO daily. However, for the entire study population, there was 53% G1/2 diarrhea (16/30 pts) and 43% (13/30 pts) G3 diarrhea. Eleven patients experienced G3 diarrhea during cycle 1 but four of those 11 patients had resolution of symptoms with dose level reduction of L to 750 mg PO daily; 2 had resolution of diarrhea on study with discontinuation of L; 4 went off study treatment (3 due to AEs, 1 due to patient refusal); 1 had resolution of diarrhea without a dose level reduction in L. Two other patients experienced G3 diarrhea; one at cycle 2 and one at cycle12. In both of these patients, diarrhea resolved after a dose level reduction in L to 750 mg PO daily. The protocol guidance for management of AEs are shown in Figures 2 through 5
Figure 5.
Algorithm for the management of dermatological reactions in N083E
Cardiac Safety
None of the patients in this study experienced symptomatic congestive heart failure or cardiac death while on active treatment. Four patients experienced G1 or 2 left ventricular failure (defined as asymptomatic resting LVEF of 50 to 60% and 40–50% respectively). Two of the patients experienced transient G1 LVEF, one patient had persistent G1 LVEF through Year 2 and she had a dose reduction of lapatinib for the remaining cycles, and one patient had persistent G2 LVEF through Year 3; this patient discontinued lapatinib after cycle 1 due to elevated liver enzymes but continued all other treatment interventions without dose reductions. Twenty-six patients have follow-up cardiac data. The mean LVEF for those 26 pts at baseline was 62.6% and at 4, 8, 12, and 18 months were 61.4%, 60.8%, 62.5%, and 64.2, respectively. The rate of change in LVEF percentage over time was −0.02%/month (p=0.85).
Disease-free survival and overall survival
At this time, with a median follow-up of 5.3 years (range 1.6–9.5), there have been 6 DFS events and 3 deaths. The estimated 5-year DFS was 84.3% (95% CI: 71.5–99.8%) and no deaths occurred prior to the 5 year time point.
DISCUSSION
Despite the significant benefit obtained in the last decade from the addition of trastuzumab to chemotherapy for the adjuvant treatment of HER2 positive breast cancer, trastuzumab resistance remains a clinically important problem7–10. Preclinical and clinical data have suggested that more complete HER2 signaling blockade with dual HER2-directed therapy can lead to improved outcomes in the metastatic and neoadjuvant settings. However, the use of dual HER2-blockade in the adjuvant setting has resulted only in very modest disease-free survival improvements25,26.
The first dual HER2-blockade efforts successfully translated to the clinic came from the phase III CLEOPATRA trial, where the addition of pertuzumab to trastuzumab and docetaxel as first-line treatment for metastatic HER2-positive breast cancer led to significantly improved DFS and OS compared to docetaxel and trastuzumab alone (DFS 18.5 vs. 12.4 months; hazard ratio [HR] 0.62, 95% confidence interval [CI], 0.51 to 0.75; p<0.001; and OS: 56.6 vs. 40.8 months; HR 0.68, 95% CI 49.3 to not reached; p<0.001)27. This dramatic improvement in the metastatic setting motivated exploring similar strategies in the early-stage setting. In the randomized phase 2 NeoSPHERE trial, a similar regimen of docetaxel, trastuzumab and pertuzumab administered in the neoadjuvant setting, led to an improvement in the pathologic complete response (pCR, defined as ypT0/is ypN0) rates in comparison with docetaxel and trastuzumab alone (pCR 39.3% vs 21.5%, respectively, p=0.0063). These results led to the U. S. Food and Drug Administration granting accelerated approval for the use of pertuzumab in the neoadjuvant setting in 201325,. Unfortunately, despite the magnitude of the pCR benefit seen in the neoadjuvant setting, the subsequent APHINITY trial only found a very small benefit in long-term outcomes with the addition of pertuzumab to trastuzumab and chemotherapy in the adjuvant setting. In APHINITY, an improvement in the 3-year invasive-disease-free survival (IDFS) of a mere 0.9% was seen with the addition a full year of pertuzumab (3y IDFS 94.1% vs 93.2%, HR 0.81; 95% CI 0.66 to 1.00; p=0.045).
A similar strategy of dual HER2-blockade—adding oral lapatinib to trastuzumab—was pursued in the neoadjuvant CALGB 40601 and NeoALTTO trials. CALGB 40601 randomized patients to paclitaxel and trastuzumab (TH), paclitaxel, trastuzumab, and lapatinib (THL), or trastuzumab and lapatinib (TL)28. Oral lapatinib was administered at a daily dose of 750 mg. Because of excessive toxicities and lower efficacy, the TL arm was terminated early and the primary endpoint centered on the TH and THL arms. The breast pCR rates were 51% for THL, 40% for TH, and 32% for TL. Although numerically higher, the difference in pCR observed with THL vs TH was not statistically significant (p=0.11). The number of grade 3 toxicities was higher for patients receiving lapatinib. Exploratory analysis of the correlative studies showed that patients with the “HER2-enriched” molecular subtype had the highest pCR rate (75%) compared with other subtypes within the HER2+ cohort, such as Luminal A (35%), Luminal B (29%), or basal-like (36%).
Subsequently, in the NeoALTTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) phase III study, women with operable HER2-positive breast cancer >2 cm were randomized to one of three regimens for 6 weeks: lapatinib (1500 mg daily) vs. trastuzumab vs. lapatinib (1000 mg daily) plus trastuzumab27. Paclitaxel was introduced in all groups at week 6, and patients continued on neoadjuvant therapy for an additional 6 weeks. The study showed a significant improvement in the rate of pCR when using lapatinib and trastuzumab as a combination versus either drug alone (51.3% with the combination, vs 29.5% with trastuzumab alone and 24.7% with lapatinib alone, p=0.0001). In the lapatinib-containing arms, over 20% of patients developed G3 diarrhea, compared to 2% only for patients on trastuzumab alone. Diarrhea was also noted to increase over time and the rate of G3 diarrhea went from 11% to 22% by the end of the neoadjuvant phase of this study. The rate of G3 hepatic abnormalities and G3 skin disorders were 9.9% and 6.6% respectively for the patients receiving both lapatinib and trastuzumab.
The NeoALTTO study was a prelude to the large ALTTO phase III adjuvant clinical trial that enrolled 8381 patients with early stage HER2-positive breast cancer in 50 countries across six continents. Patients were enrolled according to one of two design schemas (Design 1 and Design 2), and were randomized to one of four anti-HER2 treatment regimens within each design schema (trastuzumab alone x 52 weeks vs. lapatinib alone for 52 weeks vs. sequential trastuzumab for 12 weeks followed by lapatinib for 34 weeks vs. concurrent trastuzumab plus lapatinib x 52 weeks). In Design 1, the intention was to complete all (neo)adjuvant chemotherapy prior to administering the anti-HER2 therapy. In Design 2, the intention was to administer a taxane (paclitaxel or docetaxel) concurrently with the anti-HER2 therapy after any anthracycline-based (neo) adjuvant chemotherapy. Ultimately, the incorporation of lapatinib to the adjuvant therapy of early-stage HER2-positive breast cancer in the ALTTO study did not lead to significant improvement sin DFS compared to trastuzumab as the sole HER2-blocking strategy. Furthermore, patients treated with lapatinib experinced more diarrhea, cutaneous rash, and hepatic toxicity. As such, the use of lapatinib has remained confined to the metastatic setting.
As noted above, all regimens in the ALTTO trial were anthracycline-based. However, there was a growing interest in the oncologic community to have the option of using non-anthracycline-based regimens in the early-stage setting. In 2005, Slamon et al presented the first interim results of the BCIRG 006 phase III adjuvant clinical trial (n = 3222 early stage HER2+ breast cancer patients), demonstrating that the combination of docetaxel, carboplatin and trastuzumab (TCH) led to similar outcomes than anthracycline-containing regimens, with fewer cardiac events and no secondary leukemias29. However, no safety data existed on the combination of TCH with lapatinib at the time. The N083E study reported herein was designed to assess the safety and tolerability of the addition of lapatinib to the standard TCH regimen in anticipation that these data would be utilized to make a decision to incorporate this regimen as part of Design 2 of the ALTTO study.
As initially expected and based on our previous experience from the RC0639 clinical trial exploring cardiac safety of paclitaxel plus trastuzumab and lapatinib24, diarrhea was the most common non-hematologic AE observed in the N083E study. Although the rate of G3 diarrhea (43%) crossed the pre-specified “unacceptable” rate of 40%, the early implementation of an algorithm for the clinical management of diarrhea and the dose reduction of lapatinib to 750 mg daily allowed for relatively good control of this adverse event. Additionally, no significant cardiac toxicity was observed in this small study with the concurrent use of chemotherapy and dual HER2 blockade. Similarly, no significant or uncontrollable hepatic or skin toxicity were noted in the patients who participated in this adjuvant trial. The clinical management algorithms for the management of diarrhea, cardiac toxicity, abnormal liver function tests and dermatological reactions, outlined in Figures 1 to 4, proved to be very helpful for handling these potential AEs of the combination of chemotherapy, lapatinib and trastuzumab. Although this study is very small, very few events have been observed with a median follow-up of 5.3 years
CONCLUSION
Diarrhea is a common toxicity of lapatinib plus chemotherapy and trastuzumab, but is responsive to dose reductions and delays. Based on the experience gained on the studies RC0639 and N083E, we concluded that the appropriate dose of lapatinib concurrently with trastuzumab plus taxane-based chemotherapy is 750 mg daily, and the dose can be safely increased to 1000 mg daily in combination with trastuzumab without chemotherapy. The ALTTO treatment Design 2 was subsequently amended to allow TCHL to be a treatment option. While ALTTO did not support the addition of lapatinib to management of early-stage HER2-positive breast cancer, the insights on toxicity management learned on N083E may be relevant for select metastatic HER2-positive breast cancer patients utilizing combination regimens including lapatinib. Furthermore the excellent long-term outcomes of patients who participated in this small study add to the historical perspective on the development of modern-day regimens for the treatment of early-stage HER2-positive breast cancer.
Supplementary Material
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10 CA035103, UG1 CA189808, UG1 CA189812, U10 CA035415, and U10 CA063849. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST STATEMENT
Dr. Leon Ferre reports travel support from Immunomedics, not relevant to the presented work. Dr. A. Perez reports institutional research support from Genentech, AstraZeneca, Immunomedics, Nektar and Macrogenics, not relevant to the presented work. Dr. Moreno-Aspitia reports institutional research funding from GlaxoSmithKline and Genentech, not relevant to the presented work. All remaining authors have no conflicts of interest to declare.
Footnotes
DATA AVAILABILITY STATEMENT
De-identified patient data may be requested from Alliance for Clinical Trials in Oncology via concepts@alliancenctn.org if data is not publicly available. A formal review process includes verifying the availability of data, conducting a review of any existing agreements that may have implications for the project, and ensuring that any transfer is in compliance with the IRB. The investigator will be required to sign a data release form prior to transfer.
REFERENCES
- 1.Coussens L, Yang-Feng TL, Liao YC, et al. : Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science 230:1132–9, 1985 [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, et al. : Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–82, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Wright C, Nicholson S, Angus B, et al. : Relationship between c-erbB-2 protein product expression and response to endocrine therapy in advanced breast cancer. Br J Cancer 65:118–21, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benz CC, Scott GK, Sarup JC, et al. : Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat 24:85–95, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Winstanley J, Cooke T, Murray GD, et al. : The long term prognostic significance of c-erbB-2 in primary breast cancer. Br J Cancer 63:447–50, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross JS, Fletcher JA: The HER-2/neu Oncogene in Breast Cancer: Prognostic Factor, Predictive Factor, and Target for Therapy. Oncologist 3:237–252, 1998 [PubMed] [Google Scholar]
- 7.Perez EA, Romond EH, Suman VJ, et al. : Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol 29:3366–73, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romond EH, Perez EA, Bryant J, et al. : Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673–84, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. : Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–72, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Slamon D, Eiermann W, Robert N, et al. : Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365:1273–83, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldhirsch A, Piccart-Gebhart M, Procter M, et al. : HERA TRIAL: 2 years versus 1 year of trastuzumab after adjuvant chemotherapy in women with HER2-positive early breast cancer at 8 years of median follow up Cancer Res 72:Abstract nr S5–2, 2012 [Google Scholar]
- 12.Gianni L, Dafni U, Gelber RD, et al. : Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol 12:236–44, 2011 [DOI] [PubMed] [Google Scholar]
- 13.von Minckwitz G, Huang C-S, Mano MS, et al. : Trastuzumab emtansine for residual invasive HER2-positive breast cancer. New England Journal of Medicine 380:617–628, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Xia W, Mullin RJ, Keith BR, et al. : Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 21:6255–63, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Geyer CE, Forster J, Lindquist D, et al. : Lapatinib plus capecitabine for HER2-positive advanced breast cancer. New England Journal of Medicine 355:2733–2743, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Hansen KD, Irizarry RA, Wu Z: Removing technical variability in RNA-seq data using conditional quantile normalization. Biostatistics 13:204–16, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slamon D, Eiermann W, Robert N, et al. : Adjuvant trastuzumab in HER2-positive breast cancer. New England Journal of Medicine 365:1273–1283, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storniolo AM, Pegram MD, Overmoyer B, et al. : Phase I dose escalation and pharmacokinetic study of lapatinib in combination with trastuzumab in patients with advanced ErbB2-positive breast cancer. J Clin Oncol 26:3317–23, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Blackwell KL, Burstein HJ, Storniolo AM, et al. : Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 28:1124–30, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Dang C, Lin N, Moy B, et al. : Dose-dense doxorubicin and cyclophosphamide followed by weekly paclitaxel with trastuzumab and lapatinib in HER2/neu-overexpressed/amplified breast cancer is not feasible because of excessive diarrhea. J Clin Oncol 28:2982–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah SP, Roth A, Goya R, et al. : The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 486:395–9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccart-Gebhart M, Holmes E, Baselga J, et al. : Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2–positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. Journal of Clinical Oncology 34:1034, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolff AC, Hammond ME, Schwartz JN, et al. : American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Archives of pathology & laboratory medicine 131:18–43, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Moreno-Aspitia A, Dueck AC, Ghanem-Canete I, et al. : RC0639: phase II study of paclitaxel, trastuzumab, and lapatinib as adjuvant therapy for early stage HER2-positive breast cancer. Breast Cancer Res Treat 138:427–35, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Von Minckwitz G, Procter M, De Azambuja E, et al. : Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. New England Journal of Medicine 377:122–131, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin M, Holmes FA, Ejlertsen B, et al. : Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. The lancet oncology 18:1688–1700, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Baselga J, Bradbury I, Eidtmann H, et al. : Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379:633–40, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carey LA, Berry DA, Ollila D, et al. : Clinical and translational results of CALGB 40601: A neoadjuvant phase III trial of weekly paclitaxel and trastuzumab with or without lapatinib for HER2-positive breast cancer. J Clin Oncol 31:500, 2013 [Google Scholar]
- 29.Slamon D, Eiermann W, Robert N, et al. : Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC → T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC → TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2 positive early breast cancer patients: BCIRG 006 study. Breast Cancer Res & Treat 94:S5, 2005 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.