Abstract
Background
Infection with the novel severe acute respiratory syndrome coronavirus 2 has been associated with a hypercoagulable state. Emerging data from China and Europe have consistently shown an increased incidence of venous thromboembolism (VTE). We aimed to identify the VTE incidence and early predictors of VTE at our high-volume tertiary care center.
Methods
We performed a retrospective cohort study of 147 patients who had been admitted to Temple University Hospital with coronavirus disease 2019 (COVID-19) from April 1, 2020 to April 27, 2020. We first identified the VTE (pulmonary embolism [PE] and deep vein thrombosis [DVT]) incidence in our cohort. The VTE and no-VTE groups were compared by univariable analysis for demographics, comorbidities, laboratory data, and treatment outcomes. Subsequently, multivariable logistic regression analysis was performed to identify the early predictors of VTE.
Results
The 147 patients (20.9% of all admissions) admitted to a designated COVID-19 unit at Temple University Hospital with a high clinical suspicion of acute VTE had undergone testing for VTE using computed tomography pulmonary angiography and/or extremity venous duplex ultrasonography. The overall incidence of VTE was 17% (25 of 147). Of the 25 patients, 16 had had acute PE, 14 had had acute DVT, and 5 had had both PE and DVT. The need for invasive mechanical ventilation (adjusted odds ratio, 3.19; 95% confidence interval, 1.07-9.55) and the admission D-dimer level ≥1500 ng/mL (adjusted odds ratio, 3.55; 95% confidence interval, 1.29-9.78) were independent markers associated with VTE. The all-cause mortality in the VTE group was greater than that in the non-VTE group (48% vs 22%; P = .007).
Conclusions
Our study represents one of the earliest reported from the United States on the incidence rate of VTE in patients with COVID-19. Patients with a high clinical suspicion and the identified risk factors (invasive mechanical ventilation, admission D-dimer level ≥1500 ng/mL) should be considered for early VTE testing. We did not screen all patients admitted for VTE; therefore, the true incidence of VTE could have been underestimated. Our findings require confirmation in future prospective studies.
Keywords: COVID-19 coagulopathy, COVID-19 VTE, Hypercoagulable state in COVID-19
Article Highlights.
-
•
Type of Research: Single-center, retrospective cohort study
-
•
Key Findings: We found venous thromboembolism (VTE) incidence rates of 3.5% in patients admitted to a coronavirus disease 2019 unit and 17% in a population tested because of clinical suspicion. We identified an admission D-dimer level of ≥1500 ng/mL and the need for invasive mechanical ventilation as independent predictors of VTE occurrence.
-
•
Take Home Message: Our study represents one of the earliest reported from the United States on the incidence rate of VTE in patients with coronavirus disease 2019. Patients with a high clinical suspicion and identified risk factors (ie, invasive mechanical ventilation, D-dimer admission level of ≥1500 ng/mL) should be considered for early VTE testing. Our findings require confirmation in future prospective studies.
Since first described in Wuhan, Hubei Province, China, in December 2019, the novel severe acute respiratory syndrome coronavirus 2 has spread worldwide and was declared a pandemic by the World Health Organization on March 11, 2020.1 As of August 14, 2020, >21 million people had been infected worldwide, with >5.2 million cases and 167,000 deaths in the United States alone.2 Coronavirus disease 2019 (COVID-19), the disease caused by severe acute respiratory syndrome coronavirus 2, appears to be associated with a hypercoagulable state that results in an increased incidence of thromboembolic complications.3 The emerging data from Europe and China have shown a high incidence of venous thromboembolism (VTE) in patients with COVID-19.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 However, data that have identified risk factors for the prediction of VTE in COVID-19 are limited. We aimed to identify the VTE incidence and the risk factors for VTE occurrence in a high-volume, tertiary referral center.
Methods
We performed a retrospectively cohort study of all patients who had been admitted to the Temple University Hospital COVID-19 unit from April 1, 2020 to April 27, 2020 and had undergone VTE diagnostic testing because of a high index of clinical suspicion. Patients were included in the present analysis if they had tested positive for COVID-19 using nasopharyngeal swab reverse transcription polymerase chain reaction or had had findings (eg, multifocal ground glass opacities, crazy paving pattern, patchy consolidations, radial and curvilinear bands, halo sign, reversed halo sign) on computed tomography of the chest and clinical symptoms indicative of a high likelihood of COVID-19 and had received specific COVID-19 therapies. The institutional review board approved the present study and waived the requirement for patient informed consent in accordance with the local standards (protocol no. 27,012). All the patients had received VTE prophylaxis at admission (enoxaparin 40 mg daily, heparin 5000 U every 8 hours, or sequential compression devices if anticoagulant prophylaxis was contraindicated). Screening was not limited, irrespective of the preadmission anticoagulation status, nor for other reasons such as limited code status or to preserve personal protective equipment. Screening for deep vein thrombosis (DVT) was limited to proximal DVT only to limit exposure to the vascular technicians. Using the results of VTE testing, we divided our cohort into a VTE and no-VTE group. A follow-up analysis was performed to assess the outcomes of those with continued admission at the end of the initial study period.
Data are presented as counts and percentages for categorical variables and the median and range or interquartile range for continuous variables. We performed univariable analysis to compare the demographic parameters, inflammatory biomarkers, laboratory test values, treatments, and clinical outcomes between the VTE and no-VTE groups (Table I ). Group comparisons were performed using the t test or nonparametric test (Wilcoxon rank sum test) for continuous variables and the Fisher exact test or χ 2 test for categorical variables. Odds ratios (ORs) and their 95% confidence intervals were computed from the univariable logistic regression models for VTE occurrence. Multivariable logistic regression analysis was performed to identify risk factors for VTE occurrence in our cohort using a stepwise regression method. The multivariate model was constructed by including only variables with P value <.05 on univariate analysis. Adjusted ORs and 95% confidence intervals from the final multivariable logistic regression model were reported, reflecting the OR of a VTE incident occurring for a specific risk factor after adjustment for other independent predictors. P values <.05 were considered statistically significant. SAS, version 9.4 (SAS Institute Inc, Cary, NC), was used for all statistical analyses.
Table I.
Baseline demographic, laboratory, and treatment data
| Variable | VTE |
P Value | OR (95% CI) | |
|---|---|---|---|---|
| Yes (n = 25) | No (n = 122) | |||
| Demographic | ||||
| Age ≥65 years | 14 (56) | 47 (38) | .106 | 2.03 (0.85-4.84) |
| Male sex | 15 (60) | 57 (46) | .226 | 1.71 (0.712-4.10) |
| BMI ≥30 kg/m2 | 15 (60) | 77 (63) | .770 | 0.87 (0.36-2.11) |
| Race | .652 | 0.81 (0.33-1.97) | ||
| Black | 15 (60) | 79 (64) | ||
| Hispanic | 5 (20) | 24 (20) | ||
| Comorbidities | ||||
| History of VTE | 2 (8) | 9 (7) | .914 | 1.09 (0.22-5.38) |
| Cancer | 3 (12) | 9 (7) | .442 | 1.71 (0.42-6.83) |
| CAD | 5 (20) | 18 (14) | .511 | 1.44 (0.48-4.34) |
| CHF | 1 (4) | 11 (9) | .404 | 0.42 (0.05-3.41) |
| Stroke | 4 (16) | 18 (14) | .874 | 1.10 (0.33-3.58) |
| Diabetes | 7 (28) | 51 (41) | .198 | 0.54 (0.21-1.39) |
| COPD | 2 (8) | 14 (11) | .611 | 0.67 (0.14-3.15) |
| CKD | 5 (20) | 16 (13) | .370 | 1.65 (0.54-5.03) |
| Admission laboratory test results | ||||
| D-dimer ≥1500 ng/mL (normal, <500 ng/mL) | 18 (72) | 54 (44) | .011 | 3.20 (1.26-8.30) |
| Fibrinogen >385 or <200 U/L (normal, 200-385 U/L) | 14 (56) | 94 (77) | .073 | 0.99 (0.99-1.00) |
| LDH >241 U/L (normal, 87-241 U/L) | 20 (80) | 87 (71) | .374 | 1.00 (0.99-1.00) |
| Ferritin >388 ng/mL (normal, 8-388 ng/mL) | 17 (68) | 73 (60) | .445 | 1.00 (0.99-1.00) |
| Platelet count <150,000 (normal, 150-450 K/mm3) | 10 (40) | 24 (20) | .036 | 2.72 (1.08-6.80) |
| Treatment and outcome | ||||
| ICU admission | 19 (76) | 65 (53) | .036 | 2.77 (1.03-7.43) |
| Mechanical ventilation | 14 (56) | 32(26) | .003 | 2.82 (1.47-8.68) |
| Cytokine treatment | 11 (44) | 53 (43) | .959 | 1.02 (0.45-2.43) |
| Discharge | ||||
| Death | 12 (48) | 27 (22) | .007 | 3.24 (1.32-7.93) |
| Home | 8 (32) | 64 (52) | .062 | 2.34 (0.94-5.83) |
BMI, Body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; LDH, lactate dehydrogenase; OR, odds ratio; VTE, venous thromboembolism.
Data presented as number (%), unless otherwise noted.
Results
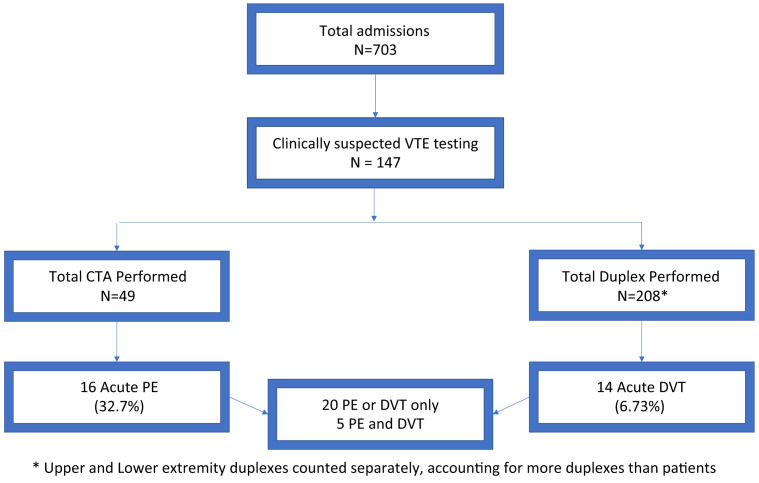
A total of 703 patients had been admitted to the Temple University Hospital COVID-19 unit from April 1, 2020 to April 27, 2020. Of the 703 patients, 147 (20.9%) had undergone diagnostic tests for acute VTE because of high clinical suspicion using extremity venous duplex ultrasonography or pulmonary computed tomography angiography, or both (Fig ). Patients with chronic DVT were excluded from our analysis. Chronic DVT shown on venous duplex ultrasonography was defined as a vein that was incompressible, narrow, and irregular and that showed echogenic thrombus attached to the venous walls with the development of collaterals.
Fig.
Flowchart showing the inclusion and exclusion of the patients. CTA, Computed tomography angiography; DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
VTE incidence
Overall, the acute VTE incidence was 3.5%. The incidence of acute VTE in the patients who had undergone VTE testing was 17% (25 of 147). Of the 25 patients, 16 had acute PE (11 with PE only and 5 with concurrent acute DVT). Fourteen patients had acute DVT (nine with DVT only and five with concurrent PE). Of these 14 patients, 9 had lower extremity DVT, 2 had upper extremity DVT, and 3 had both upper and lower extremity DVT. One case of upper extremity DVT was associated with a peripherally inserted central catheter and one was on the side of a previously ligated arteriovenous fistula. All cases of DVT were located in the proximal upper and lower extremities in our cohort.
Thromboprophylaxis
Of the 25 patients, 11 had been diagnosed with VTE within 48 hours of admission. Of the remaining 14 patients with VTE diagnosed >48 hours after admission, 3 patients had received thromboprophylaxis secondary to a recent history of bleeding, 6 patients had received standard thromboprophylaxis before the VTE diagnosis, and 5 patients had required an increase in their thromboprophylaxis dosing because of increasing D-dimer levels and individualized recommendations from the inpatient hematology consultation team. Four of these five patients had received intermediate-dose thromboprophylaxis (enoxaparin 0.5 mg/kg every 12 hours) and the dosage was increased to full therapeutic anticoagulation with heparin infusion for one patient before the VTE diagnosis secondary to a high clinical suspicion and a D-dimer level >20,000 ng/mL. All the patients had received therapeutic dose anticoagulation after the diagnosis of VTE. No inferior vena cava filters were placed
VTE risk factors
We found that an admission D-dimer level of ≥1500 ng/mL, admission platelet count <150,000, intensive care unit (ICU) admission, and the need for mechanical ventilation were significantly different statistically between the VTE and no-VTE groups on univariable analysis (Table I). Subsequently, multivariate logistic regression analysis revealed a D-dimer level of ≥1500 ng/mL and the need for invasive mechanical ventilation were independent predictors of VTE occurrence (Table II ). All other comorbidities, laboratory test results, and cytokine storm treatment were not significantly different between the two groups (Table I). We did not find a statistically significant difference in the D-dimer trends in the patients evaluated for VTE >48 hours after admission who had had positive or negative findings for VTE. An upward trend was noted in most of the patients in both the positive and the negative groups (85.7% vs 69.2%; P = .21).
Table II.
Multivariate logistical regression model for venous thromboembolism prediction
| Variable | OR | SE | Z value | P value | 95% CI |
|---|---|---|---|---|---|
| ICU admission | 1.26 | 0.77 | 0.38 | .707 | 0.37-4.23 |
| Admission platelet count <150,000 | 2.28 | 1.17 | 1.61 | .108 | 0.83-6.27 |
| Admission D-dimer ≥1500 ng/mL | 3.57 | 1.84 | 2.47 | .013 | 1.30-9.83 |
| Admission fibrinogen >385 or <200 U/L | 0.43 | 0.21 | −1.68 | .093 | 0.16-1.15 |
| Invasive mechanical ventilation | 3.39 | 1.92 | 2.16 | .031 | 1.12-10.30 |
CI, Confidence interval; ICU, intensive care unit; OR, odds ratio; SE, standard error.
Outcomes
Patients with VTE had had a greater incidence of ICU admission (76% vs 51%; P = .036), invasive mechanical ventilation (56% vs 26%; P = .003), and all-cause mortality (48% vs 22%; P = .007) and a lower rate of home discharge (32% vs 52%; P = .062) compared with the no-VTE group (Table I).
Discussion
The VTE incidence rate was 3.5% for the patients admitted to our COVID-19 unit and 17% in a population tested because of clinical suspicion. We did not identify race or obesity as risk factors in our analysis for VTE incidence. A trend was found toward significance for patient age >65 years (P = .106). However, ICU admission and the need for invasive mechanical ventilation were highly significant variables associated with VTE in our COVID-19 population (Table I). The VTE group had had greater all-cause mortality compared with the no-VTE group (48% vs 22%; P = .007). Nearly one half of our VTE-positive cohort (11 of 25) had been diagnosed within 48 hours of admission. We did not find any influence of cytokine storm treatment on VTE occurrence in our cohort. We identified an admission D-dimer level of ≥1500 ng/mL (three times the upper limit of normal) and a need for invasive mechanical ventilation as independent predictors of VTE occurrence.
Our finding of an increased risk of adverse outcomes, including the need for ICU admission and invasive mechanical ventilation and increased all-cause mortality, in the VTE cohort is supported by reported studies from other centers.4 , 6 Nearly one half of our VTE cohort (11 of 25) had been diagnosed within 48 hours of admission. We believe that the early VTE presentation in patients with COVID-19 might represent late presentation with severe COVID-19. However, the reported data have remained too limited to generalize the concept of empiric therapeutic anticoagulation for all patients with COVID-19.4 , 6 , 9 , 17 High-risk patients who cannot be tested for VTE should be considered for empiric therapeutic anticoagulation on a case by case basis.
Our incidence rates for VTE were lower than those previously reported from Europe (France, the Netherlands, Italy, and the UK) and China, with rates of 17% to 100%4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 (Table III ). However, data from U.S. centers have remained sparse. Two recent studies from the United States that focused primarily on critically ill patients reported thrombotic event rates of 22.7% and 7.7%.18 , 19 Several factors could have contributed to our lower VTE rates. All patients admitted to our institution had received VTE prophylaxis. With the recognition of an increased risk of VTE in patients with COVID-19.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 VTE prophylaxis was continuously intensified during the study period, which has also been reported by other centers.6 Additionally, our overall approach has been to use noninvasive ventilator support (high-flow oxygen therapy and bilevel positive airway pressure) and avoid the immobilization that occurs with invasive mechanical ventilation. Invasive mechanical ventilation has been identified as a risk factor for ≤69% of patients with VTE.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 The rates of invasive mechanical ventilation, high-flow oxygen nasal therapy, and bilevel positive airway pressure usage were ∼8.5%, ∼24.5%, and ∼3.3% at the end of our study period. Our rates of invasive mechanical ventilation were lower than that in other VTE studies but broadly in line with larger cohorts in China.20, 21, 22 Our lower use of invasive mechanical ventilation might have contributed to our lower reported rates of VTE. We did not screen all admitted patients for VTE; therefore, it is possible that the true incidence of VTE was underestimated.
Table III.
Current data on venous thromboembolism in patients with coronavirus disease 2019
| Investigator | ICU vs floor | Anticoagulation | VTE (total, PE, DVT) | MV rate | Mortality |
|---|---|---|---|---|---|
| United States | |||||
| Zier et al,18 (n = 66) | ICU only | NR | VTE, 22.7% | 100% | 16.7% |
| Goyal et al,19 (n = 393) | ICU and floor (details NR) | NR | VTE total, 3.3%; ICU, 7.7%; non-ICU, 1.1% | 30.8% | 10.2% |
| The Netherlands | |||||
| Middeldorp et al,4 (n = 198) | ICU, 37%; floor, 63% | Prophylactic, 84%; therapeutic (started before admission), 9.6% | VTE, 17%; PE ± DVT, 5.6%; proximal LE DVT, 6.6%; distal LE DVT, 4.0%; UE DVT, 0.5% | 67% | 14% |
| Klok et al,5 (initial; n = 184) | ICU only | Prophylactic, 90.8%; therapeutic (started before admission), 9.2% | VTE, 31% (PE, 80.6%; DVT, 9.6%; arterial thrombosis, 9.6%) | NR | 13% |
| Klok et al,6 (extended analysis; n = 184) | ICU only | Prophylactic, 90.8%; therapeutic (started before admission), 9.2% | VTE, 57% (PE, 86%; DVT, 4%; arterial thrombi, 9.3%) | NR | 22% |
| France | |||||
| Leonard-Lorant et al,7 (n = 106) | ICU, 75%; floor 25% | Prophylactic, 78%; therapeutic, 6% | PE, 30%; DVT, NR | NR | NR |
| Grillet et al,8 (n = 100) | ICU, 39%; floor 61% | NR | PE, 23%; DVT, NR | 34%; 65% in PE group | NR |
| Llijtos et al,9 (n = 26) | ICU only | Prophylactic, 31%; therapeutic (at discretion of treating physician), 69% | DVT in prophylactic group, 100%; DVT in therapeutic group, 56%; PE in prophylactic group, 0%; PE in therapeutic group, 33% | 100% | 12% |
| Poissey et al,10 (n = 107) | ICU only | Prophylactic, 90.9%; therapeutic (started before admission), 9.1% | PE, 20.6%; DVT, 4.7% | 77.3% | 14% |
| Helms et al,11 (n = 150) | ICU only | Prophylactic, 70%; therapeutic (started before admission, NR), 30% | VTE, 42.6%; PE, 16.7%; RRT circuit clot, 18.6%; DVT, NR; hemorrhagic complications, 2.7% | 100% | 8.7% |
| Italy | |||||
| Lodigiani et al,12 (n = 388) | ICU, 16%; floor, 84% | ICU: prophylactic, 96.8%; therapeutic (started before admission), 3.2%; floor: prophylactic, 68.3%; therapeutic (started before admission), 6.7% | VTE, 7.7%; PE, 2.8% | NR | 26% |
| Marone et al,13 (n = 30) | NR | NR | VTE, 53.3% | NR | NR |
| United Kingdom | |||||
| Thomas et al,14 (n = 63) | ICU only | Prophylactic, 98.4%; therapeutic (started before admission), 1.6% | VTE, 27%; PE, 7.9% | 83% | 16% |
| China | |||||
| Cui et al,15 (n = 81) | ICU only | NR | DVT, 25%; PE, NR | NR | 10% |
| Xu et al,16 (n = 138) | ICU, 10.9%; floor, 90.1% | ICU: prophylactic, 100%; floor: prophylactic, 21.5% | DVT, 2.9% | 75% of patients with DVT | 25% of patients with DVT |
DVT, Deep vein thrombosis; ICU, intensive care unit; LE, lower extremity; MV, mechanical ventilation; NR, not reported; PE, pulmonary embolism; RRT, renal replacement therapy; UE, upper extremity; VTE, venous thromboembolism.
To the best of our knowledge, no previous studies have evaluated the role of cytokine storm treatment in VTE incidence. Cytokine storm treatment at our institution consisted of high-dose steroids (≥125 mg of methylprednisolone) and other advanced therapies such as sarilumab, gimsilumab, tocilizumab, anakinra, intravenous immunoglobulin, and etoposide. However, this advanced treatment was not predictive of VTE incidence in our cohort. Inflammatory markers such as D-dimer values have been investigated as VTE predictors in previous studies.7 , 11 , 12 , 15 We assessed the D-dimer values at admission, VTE diagnosis, VTE peak, and discharge as potential predictors of VTE. We demonstrated that a D-dimer level of ≥1500 ng/mL at admission was a predictor of a VTE event. The traditional cutoff of 500 ng/mL or age-adjusted values do not appear to be helpful because most of our cohort had had abnormal D-dimer values at admission.23 Previous studies have examined cutoff values of 2660 ng/mL and 3000 ng/mL and reported a sensitivity and specificity ranging from 76.9% to 100% and 67% to 94.9%, respectively.7 , 15 We focused on the admission D-dimer values, because the substantial fluctuations in D-dimer values during the course of COVID-19 has made the D-dimer levels difficult to use as a predictor of VTE.
Study limitations
Our study had several limitations. We performed a single-center, retrospective study; thus, generalizability could be limited. More than one half of the screened population were still hospitalized at the data analysis. Our institutional VTE prophylaxis guidelines were revised during the pandemic, and the use of more aggressive VTE prophylaxis might have affected our reported rate of VTE occurrence. We had not screened all admitted patients for VTE unless clinically indicated; therefore, the true incidence in our population could have been underestimated. The use of multivariate analysis on a small sample size with a relatively lower event rate was another limitation.
Conclusions
Our study represents one of the earliest reported from the United States on the incidence rate of VTE in patients with COVID-19. Our overall incidence rate of 3.5% in our patient cohort with COVID-19 and 17% in a selectively screened population were lower than those reported by previous studies, potentially owing to the aggressive treatment of cytokine storm, aggressive VTE prophylaxis, and greater use of noninvasive ventilation. We identified an elevated D-dimer level ≥1500 ng/mL at admission and the need for invasive mechanical ventilation as potential risk factors for VTE occurrence. These risk factors could allow for risk stratification of high-risk patients and trigger earlier VTE screening. Further prospective study are needed.
Author contributions
Conception and design: PR, OO, LO, OS, RW, CM, PD, NA, MB, JP, RB, VL, RC, RG, CD, KM, AR, GC, GJC, EC
Analysis and interpretation: PR, OO, LO, DY, OS, RW, CM, PD, NA, MB, AL, XL, EC
Data collection: PR, OO, OS, RW, CM, AS, AL, EC
Writing the article: PR, OO, LO, OS, CM, PD, NA, EC
Critical revision of the article: PR, OO, LO, DY, OS, RW, CM, PD, NA, AS, MB, AL, JP, RB, VL, RC, RG, CD, KM, XL, AR, GC, GJC, EC
Final approval of the article: PR, OO, LO, DY, OS, RW, CM, PD, NA, AS, MB, AL, JP, RB, VL, RC, RG, CD, KM, XL, AR, GC, GJC, EC
Statistical analysis: LO, DY, XL, EC
Obtained funding: Not applicable
Overall responsibility: PR
PR and EC contributed equally to this article and share co-senior authorship.
Appendix.
Additional material for this article may be found online at www.jvsvenous.org.
Contributor Information
Temple University COVID-19 Research Group:
Aaron Mishkin, Abbas Abba, Abhijit S. Pathak, Abhinav Rastogi, Adam Diamond, Aditi Satti, Adria Simon, Ahmed Soliman, Alan Braveman, Albert J. Mamary, Aloknath Pandya, Amy Goldberg, Amy Kambo, Andrew Gangemi, Anjali Vaidya, Ann Davison, Anuj Basil, Beata Kosmider, Charles T. Bakhos, Bill Cornwell, Brianna Sanguily, Brittany Corso, Carla Grabianowski, Carly Sedlock, Catherine Myers, Catherine Myers, Charles Bakhos, Chenna Kesava Reddy Mandapati, Cherie Erkmen, Chethan Gangireddy, Chih-ru Lin, Christopher T. Burks, Claire Raab, Deborah Crabbe, Crystal Chen, Daniel Edmundowicz, Daniel Sacher, Daniel Salerno, Daniele Simon, David Ambrose, David Ciccolella, Debra Gillman, Dolores Fehrle, Dominic Morano, Donnalynn Bassler, Edmund Cronin, Eduardo Dominguez, Ekam Randhawa, Ekamjeet Randhawa, Eman Hamad, Eneida Male, Erin Narewski, Francis Cordova, Frederic Jaffe, Frederich Kueppers, Fusun Dikengil, Jonathan Galli, Andrew Gangemi, Jamie Garfield, Gayle Jones, Gennaro Calendo, Gerard Criner, Gilbert D'Alonzo, Ginny Marmolejos, Matthew Gordon, Gregory Millio, Rohit Gupta, Fernandez Gustavo, Hannah Simborio, Harwood Scott, Heidi Shore-Brown, Hernan Alvarado, Ho-Man Yeung, Ibraheem Yousef, Ifeoma Oriaku, Iris Jung-won Lee, Isaac Whitman, James Brown, Jamie L. Garfield, Janpreet Mokha, Jason Gallagher, Jeffrey Stewart, Jenna Murray, Jessica Tang, Jeyssa Gonzalez, Jichuan Wu, Jiji Thomas, Jim Murrett, Joanna Beros, John M. Travaline, Jolly Varghese, Jordan Senchak, Joseph Lambert, Joseph Ramzy, Joshua Cooper, Jun Song, Junad Chowdhury, Kaitlin Kennedy, Karim Bahmed, Karim Loukmane, Karthik Shenoy, Kathleen Brennan, Keith Johnson, Kevin Carney, Kraftin Schreyer, Kristin Criner, Maruti Kumaran, Lauren Miller, Laurie Jameson, Laurie Johnson, Laurie Kilpatrick, Lii-Yoong Criner, Lily Zhang, Lindsay K. McGann, Llera A. Samuels, Marc Diamon, Margaret Kerper, Maria Vega Sanchez, Mariola Marcinkienwicz, Maritza Pedlar, Mark Aksoy, Mark Weir, Marla R. Wolfson, Marla Wolfson, Robert Marron, Martin Keane, Massa Zantah, Mathew Zheng, Matthew Delfiner, Matthew Gordon, Maulin Patel, Megan Healy, Melinda Darnell, Melinda Darnell, Melissa Navaro, Meredith A. Brisco-Bacik, Michael Bromberg, Michael Gannon, Michael Jacobs, Mira Mandal, Nanzhou Gou, Erin Narewski, Nathaniel Marchetti, Nathaniel Xander, Navjot Kaur, Neil Nadpara, Nicole Desai, Nicole Mills, Norihisa Shigemura, Ohoud Rehbini, Oisin O'Corragain, Omar Sheriff, Oneida Arosarena, Osheen Abramian, Paige Stanley, Parag Desai, Parth Rali, Patrick Mulhall, Pravin Patil, Priju Varghe, Puja Dubal, Puja Patel, Rachael Blair, Rajagopalan Rengan, Rami Alashram, Randol Hooper, Rebecca A. Armbruster, Regina Sheriden, Robert Marron, Roberto Caricchio, Rogers Thomas, Rohit Gupta, Rohit Soans, Roman Petrov, Roman Prosniak, Romulo Fajardo, Ruchi Bhutani, Ryan Townsend, Sabrina Islam, Samantha Pettigrew, Samantha Wallace, Sameep Sehgal, Samuel Krachman, Santosh Dhungana, Sarah Hoang, Sean Duffy, Seema Rani, Shapiro William, Sheila Weaver, Shelu Benny, Sheril George, Shuang Sun, Shubhra Srivastava-Malhotra, Stephanie Brictson, Stephanie Spivack, Stephanie Tittaferrante, Stephanie Yerkes, Stephen Priest, Steve Codella, Steven G. Kelsen, Steven Houser, Steven Verga, Sudhir Bolla, Sudhir Kotnala, Sunil Karhadkar, Sylvia Johnson, Tahseen Shariff, Tammy Jacobs, Thomas Hooper, Tom Rogers, Tony S. Reed, Tse-Shuen Ku, Uma Sajjan, Victor Kim, Whitney Cabey, Wissam Chatila, Wuyan Li, Zach Dorey-Stein, Zachariah Dorey-Stein, and Zachary D. Repanshek
Appendix (online only).
The list of personnel for the Temple University COVID-19 Research Group follows: Aaron Mishkin, Infectious Disease; Abbas Abbas, Thoracic Medicine and Surgery; Abhijit S. Pathak, Surgery; Abhinav Rastogi, Administration; Adam Diamond, Pharmacy; Aditi Satti, Thoracic Medicine and Surgery; Adria Simon, Emergency Medicine; Ahmed Soliman, Thoracic Medicine and Surgery; Alan Braveman, Thoracic Medicine and Surgery; Albert J. Mamary, Thoracic Medicine and Surgery; Aloknath Pandya, Thoracic Medicine and Surgery; Amy Goldberg, Surgery; Amy Kambo, Thoracic Medicine and Surgery; Andrew Gangemi, Thoracic Medicine and Surgery; Anjali Vaidya, Cardiology; Ann Davison, Thoracic Medicine and Surgery; Anuj Basil, Cardiology; Beata Kosmider, Thoracic Medicine and Surgery, Charles T. Bakhos, Thoracic Medicine and Surgery; Bill Cornwell, Thoracic Medicine and Surgery; Brianna Sanguily, Thoracic Medicine and Surgery; Brittany Corso, Internal Medicine; Carla Grabianowski, Thoracic Medicine and Surgery; Carly Sedlock, Infectious Disease; Catherine Myers, Thoracic Medicine and Surgery; Catherine Myers, Thoracic Medicine and Surgery; Charles Bakhos, Thoracic Medicine and Surgery; Chenna Kesava Reddy Mandapati, Thoracic Medicine and Surgery; Cherie Erkmen, Thoracic Medicine and Surgery; Chethan Gangireddy, Cardiology; Chih-ru Lin, Thoracic Medicine and Surgery; Christopher T. Burks, Laboratory Administration; Claire Raab, Internal Medicine; Deborah Crabbe, Cardiology; Crystal Chen, Internal Medicine; Daniel Edmundowicz, Cardiology; Daniel Sacher, Thoracic Medicine and Surgery; Daniel Salerno, Thoracic Medicine and Surgery; Daniele Simon, Emergency Medicine; David Ambrose, Thoracic Medicine and Surgery; David Ciccolella, Thoracic Medicine and Surgery; Debra Gillman, Thoracic Medicine and Surgery; Dolores Fehrle, Thoracic Medicine and Surgery; Dominic Morano, Thoracic Medicine and Surgery; Donnalynn Bassler, Thoracic Medicine and Surgery; Edmund Cronin, Cardiology; Eduardo Dominguez, Thoracic Medicine and Surgery; Ekam Randhawa, Thoracic Medicine and Surgery; Ekamjeet Randhawa, Thoracic Medicine and Surgery; Eman Hamad, Cardiology; Eneida Male, Thoracic Medicine and Surgery; Erin Narewski, Thoracic Medicine and Surgery; Francis Cordova, Thoracic Medicine and Surgery; Frederic Jaffe, Thoracic Medicine and Surgery; Frederich Kueppers, Thoracic Medicine and Surgery; Fusun Dikengil, Thoracic Medicine and Surgery; Jonathan Galli, Thoracic Medicine and Surgery; Andrew Gangemi, Thoracic Medicine and Surgery; Jamie Garfield, Thoracic Medicine and Surgery; Gayle Jones, Thoracic Medicine and Surgery; Gennaro Calendo, Thoracic Medicine and Surgery; Gerard Criner, Thoracic Medicine and Surgery; Gilbert D'Alonzo, Thoracic Medicine and Surgery; Ginny Marmolejos, Thoracic Medicine and Surgery; Matthew Gordon, Thoracic Medicine and Surgery; Gregory Millio, Internal Medicine; Rohit Gupta, Thoracic Medicine and Surgery; Fernandez Gustavo, Thoracic Medicine and Surgery; Hannah Simborio, Thoracic Medicine and Surgery; Harwood Scott, Thoracic Medicine and Surgery; Heidi Shore-Brown, Thoracic Medicine and Surgery; Hernan Alvarado, Respiratory Care; Ho-Man Yeung, Internal Medicine; Ibraheem Yousef, Thoracic Medicine and Surgery; Ifeoma Oriaku, Thoracic Medicine and Surgery; Iris Jung-won Lee, Nephrology; Isaac Whitman, Cardiology; James Brown, Thoracic Medicine and Surgery; Jamie L. Garfield, Thoracic Medicine and Surgery; Janpreet Mokha, Thoracic Medicine and Surgery; Jason Gallagher, School of Pharmacy; Jeffrey Stewart, Thoracic Medicine and Surgery; Jenna Murray, Thoracic Medicine and Surgery; Jessica Tang, Thoracic Medicine and Surgery; Jeyssa Gonzalez, Thoracic Medicine and Surgery; Jichuan Wu, Thoracic Medicine and Surgery; Jiji Thomas, Thoracic Medicine and Surgery; Jim Murrett, Ultrasound Fellow; Joanna Beros, Thoracic Medicine and Surgery; John M. Travaline, Thoracic Medicine and Surgery; Jolly Varghese, Thoracic Medicine and Surgery; Jordan Senchak, Internal Medicine; Joseph Lambert, Thoracic Medicine and Surgery; Joseph Ramzy, Thoracic Medicine and Surgery; Joshua Cooper, Cardiology; Jun Song, Medical Student; Junad Chowdhury, Thoracic Medicine and Surgery; Kaitlin Kennedy, Thoracic Medicine and Surgery; Karim Bahmed, Thoracic Medicine and Surgery; Karim Loukmane, Thoracic Medicine and Surgery; Karthik Shenoy, Thoracic Medicine and Surgery; Kathleen Brennan, Thoracic Medicine and Surgery; Keith Johnson, Thoracic Medicine and Surgery; Kevin Carney, Thoracic Medicine and Surgery; Kraftin Schreyer, Emergency Medicine; Kristin Criner, Endocrinology; Maruti Kumaran, Radiology; Lauren Miller, Thoracic Medicine and Surgery; Laurie Jameson, Thoracic Medicine and Surgery; Laurie Johnson, Thoracic Medicine and Surgery; Laurie Kilpatrick, Thoracic Medicine and Surgery; Lii-Yoong Criner, Thoracic Medicine and Surgery; Lily Zhang, Thoracic Medicine and Surgery; Lindsay K. McGann, Hospitalist; Llera A. Samuels, Thoracic Medicine and Surgery; Marc Diamond, Thoracic Medicine and Surgery; Margaret Kerper, Thoracic Medicine and Surgery; Maria Vega Sanchez, Thoracic Medicine and Surgery; Mariola Marcinkienwicz, Thoracic Medicine and Surgery; Maritza Pedlar, Thoracic Medicine and Surgery; Mark Aksoy, Thoracic Medicine and Surgery; Mark Weir, Thoracic Medicine and Surgery; Marla R. Wolfson, Thoracic Medicine and Surgery; Marla Wolfson, Thoracic Medicine and Surgery; Robert Marron, Thoracic Medicine and Surgery; Martin Keane, Cardiology; Massa Zantah, Thoracic Medicine and Surgery; Mathew Zheng, Thoracic Medicine and Surgery; Matthew Delfiner, Internal Medicine; Matthew Gordon, Thoracic Medicine and Surgery; Maulin Patel, Thoracic Medicine and Surgery; Megan Healy, Emergency Medicine; Melinda Darnell, Thoracic Medicine and Surgery; Melinda Darnell, Thoracic Medicine and Surgery; Melissa Navaro, Thoracic Medicine and Surgery; Meredith A. Brisco-Bacik, Cardiology; Michael Bromberg, Hematology; Michael Gannon, Cardiology; Michael Jacobs, Thoracic Medicine and Surgery; Mira Mandal, Thoracic Medicine and Surgery; Nanzhou Gou, Thoracic Medicine and Surgery; Erin Narewski, Thoracic Medicine and Surgery; Nathaniel Marchetti, Thoracic Medicine and Surgery; Nathaniel Xander, Thoracic Medicine and Surgery; Navjot Kaur, Thoracic Medicine and Surgery; Neil Nadpara, Internal Medicine; Nicole Desai, Internal Medicine; Nicole Mills, Thoracic Medicine and Surgery; Norihisa Shigemura, Surgery; Ohoud Rehbini, Thoracic Medicine and Surgery; Oisin O'Corragain, Thoracic Medicine and Surgery; Omar Sheriff, Thoracic Medicine and Surgery; Oneida Arosarena, Otolaryngology; Osheen Abramian, Thoracic Medicine and Surgery; Paige Stanley, Thoracic Medicine and Surgery; Parag Desai, Thoracic Medicine and Surgery; Parth Rali, Thoracic Medicine and Surgery; Patrick Mulhall, Pulmonary; Pravin Patil, Cardiology; Priju Varghese, Internal Medicine; Puja Dubal, Thoracic Medicine and Surgery; Puja Patel, Thoracic Medicine and Surgery; Rachael Blair, Thoracic Medicine and Surgery; Rajagopalan Rengan, Thoracic Medicine and Surgery; Rami Alashram, Thoracic Medicine and Surgery; Randol Hooper, Thoracic Medicine and Surgery; Rebecca A. Armbruster, Chief Medical Officer; Regina Sheriden, Thoracic Medicine and Surgery; Robert Marron, Thoracic Medicine and Surgery; Roberto Caricchio, Rheumatology; Rogers Thomas, Thoracic Medicine and Surgery; Rohit Gupta, Thoracic Medicine and Surgery; Rohit Soans, Surgery; Roman Petrov, Thoracic Medicine and Surgery; Roman Prosniak, Thoracic Medicine and Surgery; Romulo Fajardo, Surgery; Ruchi Bhutani, Thoracic Medicine and Surgery; Ryan Townsend, Thoracic Medicine and Surgery; Sabrina Islam, Cardiology; Samantha Pettigrew, Internal Medicine; Samantha Wallace, Thoracic Medicine and Surgery; Sameep Sehgal, Thoracic Medicine and Surgery; Samuel Krachman, Thoracic Medicine and Surgery; Santosh Dhungana, Thoracic Medicine and Surgery; Sarah Hoang, Thoracic Medicine and Surgery; Sean Duffy, Thoracic Medicine and Surgery; Seema Rani, Thoracic Medicine and Surgery; Shapiro William, Thoracic Medicine and Surgery; Sheila Weaver, Thoracic Medicine and Surgery; Shelu Benny, Thoracic Medicine and Surgery; Sheril George, Thoracic Medicine and Surgery; Shuang Sun, Thoracic Medicine and Surgery; Shubhra Srivastava-Malhotra, Thoracic Medicine and Surgery; Stephanie Brictson, Thoracic Medicine and Surgery; Stephanie Spivack, Infectious Disease; Stephanie Tittaferrante, Internal Medicine; Stephanie Yerkes, Thoracic Medicine and Surgery; Stephen Priest, Internal Medicine; Steve Codella, Thoracic Medicine and Surgery; Steven G. Kelsen, Thoracic Medicine and Surgery; Steven Houser, Research; Steven Verga, Thoracic Medicine and Surgery; Sudhir Bolla, Thoracic Medicine and Surgery; Sudhir Kotnala, Thoracic Medicine and Surgery; Sunil Karhadkar, Surgery; Sylvia Johnson, Thoracic Medicine and Surgery; Tahseen Shariff, Thoracic Medicine and Surgery; Tammy Jacobs, Thoracic Medicine and Surgery; Thomas Hooper, Thoracic Medicine and Surgery; Tom Rogers, Thoracic Medicine and Surgery; Tony S. Reed, Chief Medical Officer; Tse-Shuen Ku, Thoracic Medicine and Surgery; Uma Sajjan, Thoracic Medicine and Surgery; Victor Kim, Thoracic Medicine and Surgery; Whitney Cabey, Emergency Medicine; Wissam Chatila, Thoracic Medicine and Surgery; Wuyan Li, Thoracic Medicine and Surgery; Zach Dorey-Stein, Thoracic Medicine and Surgery; Zachariah Dorey-Stein, Thoracic Medicine and Surgery; and Zachary D. Repanshek, Emergency Medicine.
References
- 1.WHO Director-General’s opening remarks at the media briefing on COVID-19—11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Available at:
- 2.Johns Hopkins Coronavirus Resource Center COVID-19 map. https://coronavirus.jhu.edu/map.html Available at:
- 3.Han H., Yang L., Liu R., Liu F., Wu K.-L., Li J., et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 4.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Muller M.C.A., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. https://www.preprints.org/manuscript/202004.0345/v1 Available at: [DOI] [PMC free article] [PubMed]
- 5.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klok F.A., Mjha K., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonard-Lorant I., Delabranche X., Severac F., Helms J., Pauzet C., Collange O., et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020;296:E189–E191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020;296:E186–E188. doi: 10.1148/radiol.2020201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llitjos J.-F., Leclerc M., Chochois C., Monsallier J.-M., Ramakers M., Auvray M., et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poissy J., Goutay J., Caplan M., Parmentier E., Duburcq T., Lassalle F., et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 11.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marone E.M., Rinaldi L.F. Upsurge of deep venous thrombosis in patients affected by COVID-19: preliminary data and possible explanations. J Vasc Surg Venous Lymphat Disord. 2020;8:694–695. doi: 10.1016/j.jvsv.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas W., Varley J., Johnston A., Symington E., Robinson M., Sheares K., et al. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb Res. 2020;191:76–77. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu P., Zhou Q., Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99:1205–1208. doi: 10.1007/s00277-020-04019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paranjpe I., Fuster V., Lala A., Russak A., Glicksberg B.S., Levin M.A., et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;99:1205–1208. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziehr D.R., Alladina J., Petri C.R., Maley J.H., Moskowitz A., Medoff B.D., et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201:1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Righini M., Es J.V., Exter P.L.D., Roy P.-M., Verschuren F., Ghuysen A., et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311:1117–1124. doi: 10.1001/jama.2014.2135. [DOI] [PubMed] [Google Scholar]