Abstract
Objective:
To examine rapidly emerging ventilator technologies during coronavirus disease 2019 and highlight the role of CRISIS, a novel 3D printed solution.
Data Sources:
Published articles, literature, and government guidelines that describe and review emergency use ventilator technologies.
Study Selection:
Literature was chosen from peer-reviewed journals and articles were limited to recent publications.
Data Extraction:
All information regarding ventilator technology was extracted from primary sources.
Data Synthesis:
Analysis of technology and relevance to coronavirus disease 2019 physiology was collectively synthesized by all authors.
Conclusions:
The coronavirus disease 2019 pandemic has placed massive stress on global supply chains for ventilators due to the critical damage the virus causes to lung function. There is an urgent need to increase supply, as hospitals become inundated with patients requiring intensive respiratory support. Coalitions across the United States have formed in order to create new devices that can be manufactured quickly, with minimal resources, and provide consistent and safe respiratory support. Due to threats to public health and the vulnerability of the U.S. population, the Food and Drug Administration released Emergency Use Authorizations for new or repurposed devices, shortening the approval timeline from years to weeks. The list of authorized devices varies widely in complexity, from automated bagging techniques to repurposed sleep apnea machines. Three-dimensional printed ventilators, such as “CRISIS,” propose a potential solution to increase the available number of vents for the United States and abroad, one that is dynamic and able to absorb the massive influx of hospitalized patients for the foreseeable future.
Keywords: acute respiratory distress syndrome, coronavirus disease 2019, intensive care unit; mechanical ventilation, severe acute respiratory syndrome coronavirus 2; three-dimensional printing
On March 11, 2020, the World Health Organization declared coronavirus disease 2019 (COVID-19), an upper respiratory infection caused by severe acute respiratory syndrome coronavirus 2 , a pandemic. This came months after the virus had begun its spread across the globe (1). Citizens watched as their country’s respective leaders scrambled to assess the threat to public health and create plans of action to protect them against a virus with little understanding of its epidemiology or pathologic effects on the human body. Soon, numbers across the globe exploded. The United States had the highest numbers of total confirmed cases and deaths, reaching a landmark 100,000 lives lost on May 27, 2020 (2). Varying accessibility to testing and tracing across the country has extended the period of uncertainty regarding statistics, community spread, and prevention strategies.
Acute respiratory failure is a key manifestation of COVID-19. The Center for Disease Control and Prevention reported that as high as 19% of patients require hospitalization and 6% require admission to the ICU (3)—most requiring support with mechanical ventilation. Mechanical ventilators are devices that take over the task of breathing for a patient, using an endotracheal tube system that can control the composition, pressure, volume, and rate of the air that enters the lungs. Infection with this virus exacerbates preexisting conditions, including chronic obstructive pulmonary disease, increasing the respiratory effort of lungs already laden with disease. Patients with histories of cardiovascular disease become infected and suffer from myocardial infarctions or strokes, likely caused by a COVID-induced hypercoagulable state (4). Add in the number of patients that at baseline flood hospitals for scheduled procedures and traumatic injuries, and the need for ventilators becomes staggering.
Overwhelmed medical centers in New York received a limited number of emergency ventilators from the federal stockpile, many of which arrived damaged, with missing parts, and unusable due to a lack of maintenance (5). Healthcare workers have been forced to reassign non-ICU ventilators from surgical suites, repurpose noninvasive respiratory support devices to support intubated ICU patients, and, in some areas, have attempted to support more than one patient on a single ventilator in order to care for the overwhelming numbers of patients requiring invasive ventilation (6). Medical professionals in New York and Italy faced ethical dilemmas when deciding how to allocate a limited number of ventilators to huge numbers of patients requiring respiratory support, sometimes redirecting patients to other care centers (7, 8). The COVID-19 pandemic has uncovered health systems that are ill-prepared to accommodate the increasing surge of patients that have rapidly outpaced the availability of mechanical ventilators and the clinicians who manage them. This sheds light on the need to increase the number of ventilators available to hospitals in preparation of what not only COVID-19 could bring but what future pandemics, natural disasters, and health emergencies could require.
Some estimates place United States need near 1 million ventilator units (7). An estimate of available ventilators before pandemic efforts ramped up is shockingly low, with only 62,000 full-featured machines and an additional 98,000 that can provide only basic functionality (9) in a country of 330 million people. Including the federal stockpile, there are optimally 173,000 available ventilators (9). This gap of hundreds of thousands of units is placing incredible strain on supply chains, with device manufacturing frequently taking place overseas. In response, the U.S. Food and Drug Administration (FDA) created an Emergency Use Authorization (EUA) to allow both new and repurposed ventilators (10) specifically to treat COVID-19.
Almost immediately, coalitions across the country came together to attack the nation’s leading problem: supply shortages. Liken to the efforts during World War II, engineers, medical professionals, high-school teachers, and even kids were building, printing, and donating medical supplies assembled in labs and on kitchen counters. Groups from Massachusetts Institute of Technology (MIT), National Aeronautics and Space Administration (NASA), General Motors (GM), Nike, and hospitals such as Rice, Duke, and Oregon Health and Science University (OHSU) are designing emergency ventilators—many in order to share as open source and rapidly produce while still adherent to FDA Guidelines.
Simultaneously, clinicians are waging a battle on the front lines in America’s ICUs. Acute respiratory distress syndrome (ARDS)—a syndrome defined by acute onset hypoxemic respiratory failure—is characterized by an invasion of inflammatory cells and cytokines, increasing the permeability of pulmonary vasculature and resulting in exudative fluid buildup within alveoli. Vascular damage caused by COVID-19 is bringing renewed focus to the contribution of the endothelium to ARDS. The injured lung is susceptible to ventilator-induced lung injury—a combination of injuries caused by excessive distention (strain) and pressures (stress) from high tidal volumes in traditional ARDS invasive ventilation strategies (11). Repeated inflation and deflation of alveoli increase stress, dead space, and hypoxemia (11). Data describing the treatment and outcomes of nearly 1600 patients in Lombardy, Italy, report a median positive end-expiratory pressure (PEEP) of 14 cm H2O to keep patients well-ventilated (12).
COVID-19, stroke, and trauma patients all have unique ventilation requirements. Inadequate or inconsistent rates and pressures from an emergency ventilator would serve little benefit. The optimal way to ventilate patients mechanically varies widely depending on the underlying cause of respiratory failure, which brings to light the question of how many settings rapidly produced ventilators must have, while meeting every FDA safety standard. Emergency ventilators must maintain as much functionality as possible, while minimizing cost and manufacturing time, a huge hurdle even for teams of experienced engineers and clinicians.
The FDA determined an urgent need to fast-track the approval process for ventilator usage outside of their initially cleared capacities, in addition to modifications to existing systems that would tailor them to the unique needs of COVID-19 patients. The federal organization outlined several Criteria for Issuance of Authorization. The FDA had evidence that when temporarily authorized devices were used, their benefits outweighed the risks. They determined that no adequate alternative existed to close the gap between the number of ventilators available to the American public and the estimated number needed in the coming months (10).
A secondary “Guidance for Industry and FDA Staff” was released, detailing the changes that would and would not be allowed to previously approved devices (13). Authorized changes include modifying FDA cleared indications to meet COVID requirements. For example, anesthesia machines could be repurposed for mechanical ventilation in a situation where conventional full-feature devices are unavailable (13).
The FDA also believes that changes to power supply, materials, new filtration, software changes, and extending product shelf life would also be plausible without creating undue risk (13). The document outlines additional product requirements, in addition to all of the safety requirements ventilators would be expected to meet under normal circumstances, including International Electrotechnical Commission and International Organization of Standardization (ISO) testing certification (14). These include new labels, instructions, and a clear delineation of claims that are and are not FDA-authorized. Finally, the FDA publishes a running list of all of the devices temporarily authorized under this EUA, listing manufacturer information, product name, device description, intended use, and date of authorization (15).
RESULTS
One of the earliest ventilator projects emerged from the University of Vermont. The Vermontilator’s (16) primary goals were creating the cheapest device to manufacture with long functioning lifespan, in an attempt to meet the needs of a patient requiring mechanical ventilation for several weeks. The project’s second goal was to optimize the airway pressure release ventilation mode (17). This method involves a prolonged high-pressure phase, ideally to maximize alveolar recruitment and prevent atelectasis, with a short low-pressure phase (18).
MIT engineers created an open-source project, based on a manual Ambu bag-valve-mask that could be shared and reconstructed worldwide (19). However, MIT will not request FDA authorization for the device, even under the EUA. The manufacturer responsible for reproducing, modifying, and improving MIT’s E-Vent that then plans to bring the device to bedside is required to go through the approval process on its own (20). The team started with a manual resuscitator with goals of automating it for longer term ventilation. The design includes mechanical fingers to hold and squeeze an Ambu bag with variable strengths and rates. To date, controls include respiratory rate (RR), tidal volume, and inspiratory (I): expiratory (E) ratio, with outlines for alarms and safety systems (17). MIT titles this project as an emergency ventilator toolbox, serving as a home for rapid prototyping ideas, materials, and electrical design (19).
New laboratory’s emergency ventilator response created Spiro Wave (20), defined as an automated resuscitator, as a spin off from the MIT design. It comes with basic pressure sensors and alarm systems, providing clinicians with control over tidal volume, RR, I:E, airway limit pressure, and a peep up to 25 cm H2O (13, 16). This system holds an Ambu bag between the mechanical fingers and squeezes at regular intervals. Manufacturing costs are estimated to be between $2,500 and $5,000 per unit (20), and the device has been authorized for use under the FDA EUA (15). The University of Minnesota’s project, the Coventor, is a comparable Ambu bag device, one that does not require an oxygen supply and can be stamped out of sheet metal or injection molded (21).
On the other side of the spectrum, GM partnered with Ventec to modify its existing ventilator, oxygen, suction, cough, nebulizer (22) to the “V+Pro,” without the cough, suction, or nebulizer functionality (17). Despite this reduction in utility, the V+Pro still covers a wide range of settings at the clinician’s control. The U.S. Department of Health and Human Services placed a 30,000 unit order with Ventec, with 600 units shipping in the month of May and the entire order expected to be completed by August 2020 (23). This contract costs the U.S. government $489.4 million.
NASA’s Jet Propulsion Laboratory has joined the race as well with its ventilator, intervention, technology accessible locally device (24), also authorized under the EUA (15). This device targets patients who do not need a full-feature ventilator, which NASA estimates to be the majority of those requiring respiratory support (24). They recently accepted applications for a licensing agreement for further development, testing, manufacturing, and planned commercialization (24). This operates on a “volume-targeted, pressure-limited, time-limited mode” (17), providing controls over tidal volume, I:E, PEEP, and RR.
One of the most unconventional methods proposed to meet ventilator needs for COVID-19 is ventilator splitting, which is the practice of ventilating more than one patient with a single mechanical ventilator and employing various strategies to output two different sets of tidal volumes, flows, and pressures (25). This introduces additional challenges of matching oxygen delivery to different physiologic properties with the same machine settings. Vent splitting is generally not recommended and only seen as a last resort, but proof-of-concept studies have demonstrated this method as a possible strategy during the most severe surges (25).
In total, the FDA has 65 ventilators and 11 ventilator tubing connectors, and four ventilator accessories covered under its EUA as of June 13, 2020 (15). Table 1 compares all of the discussed ventilators, covering origin, features, and authorization status.
Table 1.
A Comparison of Ventilators Used During the Coronavirus 2019 Pandemic
| Device Name | Origin of Device | Manufacture Setting | Comparable Ventilator Modes | Open Source | Key Features | Food and Drug Administration Authorization |
|---|---|---|---|---|---|---|
| Vermontilator (16) | University of Vermont, Burlington, VA | Industrial | APRV | No | Uses APRV | EUA pending |
| MIT E-Vent (19) | MIT, Cambridge, MA | Industrial | ACa | Yes | Uses bag-valve ventilator bags and readily available supplies | None |
| Spiro Wave (20) | New Laboratory, Brooklyn, NY | Industrial | AC | Yes | Uses bag-valve ventilator bags and readily available supplies | EUA authorized |
| V+Pro (23) | General Motors, Detroit, MI/Ventec, Bothell, WA | Industrial | AC, synchronized intermittent mandatory ventilationb, pressure control, pressure regulated volume control, bilevel, and spontaneous | No | More ventilator modes and has cough/suction assistance and nebulizer functions | 510(k) approvedc |
| Ventilator, Intervention, Technology Accessible Locally (24) | National Aeronautics and Space Administration Jet Propulsion Laboratory, Pasadena, CA | Industrial | AC | No | Not made for resterilization | EUA authorized |
| CRISIS ventilator | Oregon Health and Science University, Portland, OR | Industrial/Commercial | Pressure control, spontaneous | Yes | Can be built with commercial 3D printer and off-the-shelf supplies. Does not require power supply | EUA pending |
| Go2Vent (30) | Vortran Medical, Sacramento, CA | Industrial | Pressure control, spontaneous | No | Disposable, MRI compatible, and spontaneous breathing support | 510(k) approved |
| Coventor (21) | University of Minnesota, Minneapolis, MN | Industrial/Commercial | AC | Yes | Uses bag-valve ventilator bags and readily available supplies | EUA authorized |
AC = assist control, APRV = airway pressure release ventilation, EUA = Emergency Use Authorization, MIT = Massachusetts Institute of Technology.
aAC ventilation mode delivers the same tidal volume for every inspiration initiated by patient or machine.
bSynchronized intermittent mandatory ventilation delivers a mandatory set volume and number of breaths and allows spontaneous breathing.
c501(k): an accelerated U.S. Food and Drug Administration approval process for medical devices.
DISCUSSION
The challenges with the previously proposed solutions are either enormous costs per unit, long lists of manufacturing parts, or transportation costs to move the vents to the healthcare centers that need them most. Supply-chain shortages mean fewer available parts and less funding to meet demand. Some areas lack the extensive manufacturing and transportation system available in the United States, exacerbating challenges with sharing ventilators. A new avenue of exploration includes 3D printing medical supplies to meet shortages across the United States and on a global scale. This idea uses parts readily available around the world, while small, lightweight, and cost-effective.
The 3D printing technology is finding an increasing niche in medicine. It has benefits in medical implants and prosthetics. Patients may have lower recovery times and higher success rates from surgery if the implants have been custom-designed (26). Smaller production runs also are more cost-efficient if printed on demand and on site (26). A unique benefit to this strategy is the ability to share open-source ideas across the globe. The applications in medicine are extensive, covering printed tissue grafts and organs, implants, prosthetics, anatomical models, and drug-delivery devices (26) and, more recently, emergency ventilators.
At the same time, 3D printed ventilators come with their own challenges. There exists large variability between the printing machines, and in medical devices, fractions of a millimeter can render a product unusable. The most common type of printing includes fused deposition modeling in which a nozzle extrudes polymer in three dimensions. This extrusion process leaves large porosities within the devices, posing challenges for sterility and longevity when exposed to moisture.
Selective laser sintering (SLS) is a different method that is able to get around extrusion challenges. Instead, a laser traces the design layer by layer on a powder bed, slowly growing the modeled part at a resolution between 50 and 100 μm. This method, however, is highly subject to print orientation on the bed, as excess scaffolding or misalignment can lead to variability in terms of resolution and print times. In addition, devices can be prone to warping or contain weak zones within the print structure (27). Any products that are already prone to inconsistencies can result in a high failure rate when subject to large cyclic loading, like in the case of physiologic breathing. Regulation then becomes extensive, requiring detailed manufacturing specifications. SLS printing has been shown to release measurable, albeit acceptable, amounts of toxins including organic compounds and formaldehyde (28). It is generally advisable to manufacture these devices on industrial-scale printers. This would allow for submillimeter resolution with higher tolerance and filtering of potentially toxic particulate and fumes. Despite the urgent nature of COVID-19, adequate ventilation and safety training to those completing the printing process onsite are especially necessary to ensure biomedical staff safety. A staff that is trained in these safety protocols and is also capable of completing device leak and fit testing is certainly a significant limitation for implementation in smaller nonacademic or rural medical centers.
Because interest in the clinical applications of 3D printing has grown in recent years, rigorous safety standards and ISO specifications unique to the new technology are required by the FDA (29). Mandatory safety testing ensures that patients using the device are not prone to higher rates of infection or hypersensitivity from synthetic material. These standards require adequate sterilization processes and biocompatibility, and limiting manufacturing material residue (29). Devices that are able to be customized must also come with validation protocols to ensure safety after implementing design changes (29). Three-dimensional printed technologies have extensive requirements of safety testing due to the inherent risk of nonindustrial manufacturing, which could potentially delay the authorization of this solution in a sudden crisis situation. Nonetheless, every device must undergo rigorous testing to meet quality standards and will not receive authorization until risk has been minimized. It is also worth noting that currently many of these devices are still undergoing active investigation. During unprecedented emergencies, medical professionals are forced to consider solutions with only emerging data available on the efficacy or safety of many 3D printed devices, drugs, vaccines, and personal protective equipment.
A device developed at OHSU proposes a novel solution to the ventilator shortage created by the COVID-19 pandemic. The CRISIS device, displayed in Figure 1, is an improved design based on Go2Vent by Vortran Medical (30). Its manufacturing costs $15 per device and is produced with off-the-shelf materials and a standard 3D printer in 3 hours. The material list includes clinical grade and biocompatible nylon powder, a spring, and a stamped silicone membrane. An adjustable pressure valve allows clinicians to set tidal volume, peak inspiratory pressure, and PEEP. An additional dial also provides control over the RR. Three-dimensional printed ventilators can be rapidly produced close to hospital sites caring for patients with COVID-19 and are very small and lightweight giving them idea for shipping on demand to areas most in need. Solutions such as CRISIS are capable of addressing a large spectrum of disease, from those requiring basic mechanical ventilatory support to patients with ARDS. CRISIS operates on either O2 tanks or standard hospital 50-psi O2 wall supply, without needing a power supply or batteries. This opens the door to multiuse, mobile, and pocketsize ventilators, increasing applications in global health settings.
Figure 1.
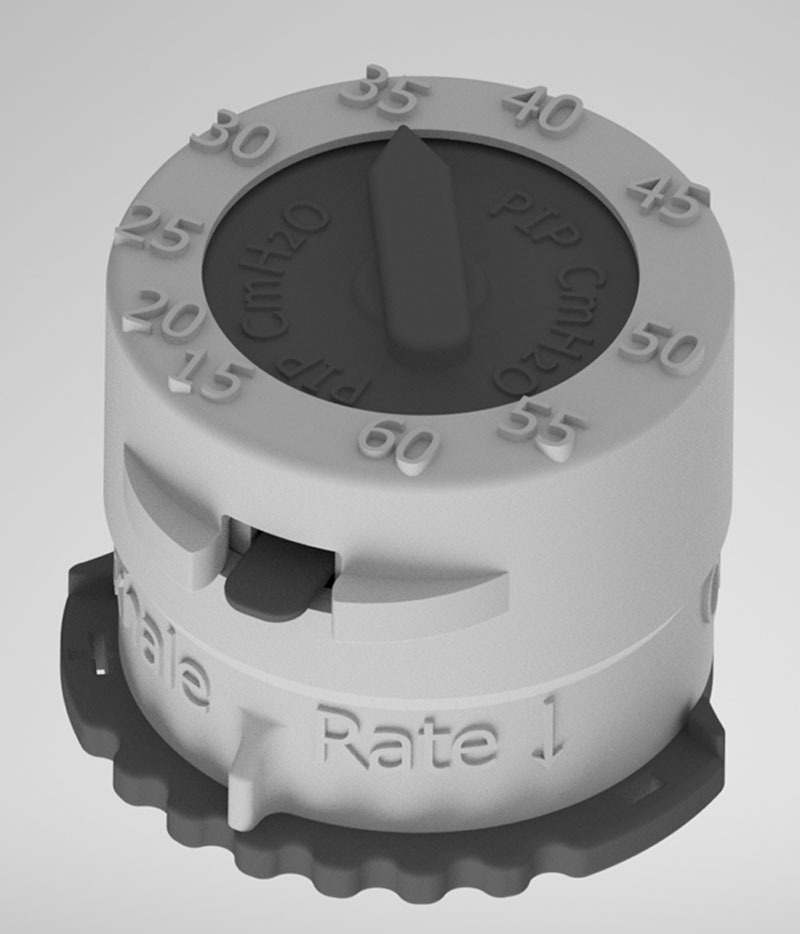
This 3D mock-up of the CRISIS ventilator displays a small ventilator device with two dials. The pressure is set by the arrow on the top of the device, whereas the rate control is set on the bottom. The device is autoclavable and capable of meeting all applicable Food and Drug Administration guidelines.
This device is still in the process of testing, and only preliminary results with the Michigan Instruments Test Lung are available (31). CRISIS has been sent to partnered medical institutions for rigorous testing in accordance with ISO 13485 and has a plan in progress for FDA EUA authorization.
CONCLUSIONS
In an ideal scenario, every patient requiring mechanical ventilation would have access to the best and most refined medical devices approved by the FDA. However, history has shown that during international crisis, whether a natural disaster or an infectious pandemic like COVID-19, experts must scramble together solutions for less than ideal situations. In this case, a global pandemic has ramped up the need for resources far beyond what the healthcare system was prepared to meet. However, teams have rapidly formed, ideas have been put together, and solutions are being created every day, which are helping reduce the burden on any one individual. No one device will solve the problem on its own, especially not with the time and resource constraints caused by disasters. However, many approaches, including 3D printing, might make bridging the gap and meeting the need, which is much easier.
Footnotes
Mr. Menzel, Mr. Fontaine, Mr. Child, Dr. Nonas, and Dr. Chi were directly involved with the development of the open-source CRISIS ventilator. The remaining authors have disclosed that they do not have any potential conflicts of interests.
REFERENCES
- 1.Center for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19) Situation Summary. Available at: https://www.cdc.gov/coronavirus. Accessed May 28, 2020
- 2.Dong E, Du H, Gardner L. An Interactive Web-Based Dashboard to Track COVID-19 in Real Time. doi: 10.1016/S1473-3099(20)30120-1. Available at: https://coronavirus.jhu.edu/map.html. Accessed May 28, 2020. [DOI] [PMC free article] [PubMed]
- 3.Center for Disease Control and Prevention. Interim Clinical Guidance for Management of Patients With Confirmed Coronavirus Disease (COVID-19). Available at: https://stacks.cdc.gov/view/cdc/89980. Accessed June 26, 2020
- 4.Lodigiani C, Iapichino G, Carenzo L, et al. ; Humanitas COVID-19 Task Force Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020; 191:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanger DE, Kanno-Youngs Z, Kulish N. . 2020
- 6.Rosenthal BM, Pinkowski J, Goldstein J. ‘The Other Option Is Death’: New York Starts Sharing of Ventilators. The New York Times. 2020 Available at: https://www.nytimes.com/2020/03/26/health/coronavirus-ventilator-sharing.html. Accessed June 26, 2020.
- 7.Ranney ML, Griffeth V, Jha AK. Critical supply shortages - the need for ventilators and personal protective equipment during the COVID-19 pandemic. N Engl J Med. 2020; 382:e41. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein J, Rothfeld M, Weiser B. 2020
- 9.Johns Hopkins Bloomberg School of Public Health Center for Health Security. Ventilator Stockpiling and Availability in the US. Available at: https://www.centerforhealthsecurity.org/resources/COVID-19/COVID-19-fact-sheets/200214-VentilatorAvailability-factsheet.pdf. Accessed June 26, 2020
- 10.Kobokovich A: Emergency Use Authorization for Ventilators. U.S. Food and Drug Administration. Available at: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/ventilators-and-ventilator-accessories-euas
- 11.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013; 369:2126–2136 [DOI] [PubMed] [Google Scholar]
- 12.Grasselli G, Zangrillo A, Zanella A, et al. ; COVID-19 Lombardy ICU Network COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020; 323:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration. Enforcement Policy for Ventilators and Accessories and Other Respiratory Devices During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency – Guidance for Industry and Food and Drug Administration Staff. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-ventilators-and-accessories-and-other-respiratory-devices-during-coronavirus. Accessed June 26, 2020
- 14.U.S. Food and Drug Administration: Emergency Use Authorization for Ventilators Appendix A – Criteria for Safety, Performance, and Labeling. Available at: https://www.fda.gov/media/136437/download. Accessed June 26, 2020
- 15.U.S. Food and Drug Administration: Emergency Use Authorization for Ventilators Appendix B – Authorized Ventilators, Ventilator Tubing Connectors, and Ventilator Accessories. Available at: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/ventilators-and-ventilator-accessories-euas#appendixb. Accessed June 26, 2020
- 16.Lamdin C. UVM Scientists, Engineers Team Up to Create ‘Vermontilator. Available at: https://www.sevendaysvt.com/. Accessed June 14, 2020.
- 17.Somers J. The Engineers Taking on the Ventilator Shortage. Available at: https://www.newyorker.com/magazine/2020/05/18/the-engineers-taking-on-the-ventilator-shortage. 2020. Accessed June 26, 2020.
- 18.Daoud EG. Airway pressure release ventilation. Ann Thorac Med. 2007; 2:176–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MIT Emergency Ventilator. MIT Emergency Ventilator Project. Available at: https://e-vent.mit.edu/. Accessed June 26, 2020
- 20.Emergency Ventilator Response. Spiro Wave, Rockefeller Philanthropy Advisors: Frequently Asked Questions. Available at: https://ventilatorresponse.com/faq.html. Accessed June 26, 2020
- 21.University of Minnesota. A Ventilator System Built for Rapid Deployment. Available at: https://med.umn.edu/covid19Ventilator. Accessed June 26, 2020
- 22.Ventec Life Systems. VOSCN: Meet the First and Only Multi-Function Ventilator. Available at: https://www.venteclife.com/. Accessed June 26, 2020
- 23.GM Corporate Newsroom: First General Motors-Ventec Critical Care V+Pro Ventilators Ready for Delivery. Available at: https://media.gm.com/media/us/en/gm/news.detail.html/content/Pages/news/me/en/2020/gm/04-16-First-General-Motors-Ventec-Critical-Care-V-Pro-Ventilators-Ready-for-Delivery.html. Accessed June 26, 2020.
- 24.California Institute of Technology: VITAL – the COVID-19 Ventilator Device. Available at: https://medeng.jpl.nasa.gov/covid-19/ventilator/. Accessed June 26, 2020.
- 25.Clarke AL, Stephens AF, Liao S, et al. Coping with COVID-19: Ventilator splitting with differential driving pressures using standard hospital equipment. Anaesthesia. 2020; 75:872–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ventola CL. Medical applications for 3D printing: Current and projected uses. P T. 2014; 39:704–711 [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison RJ, Kashlan KN, Flanangan CL, et al. Regulatory considerations in the design and manufacturing of implantable 3D-printed medical devices. Clin Transl Sci. 2015; 8:594–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hariri A, Hafidz M, Fauadi F, et al. Emission of selected environmental exposure from selective laser sintering (SLS) polyamide nylon (PA12) 3D printing process. JSHE. 2019; 1:1–6 [Google Scholar]
- 29.U.S. Food and Drug Administration: Technical Considerations for Additive Manufactured Medical Devices. Available at: https://www.fda.gov/media/97633/download.
- 30.Vortran Medical. Go2Vent. Available at: https://www.vortran.com/go2vent. Accessed June 26, 2020
- 31.Chi A, Menzel W, Fontaine E, et al. OHSU 3D printed CRISIS ventilator. J Surg Surgical Res. 2020; 6:51–55 [Google Scholar]


