Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, delirium, glial fibrillary acidic protein, neurofilament-light, severe acute respiratory syndrome coronavirus-2
Abstract
Objectives:
To provide an objective characterization of acute neurologic injury in critically ill patients with coronavirus disease 2019.
Design:
Prospective observational study. Demographics, comorbidities, and daily clinical physiologic and laboratory data were collected. Plasma levels of neurofilament-light chain, total tau, ubiquitin carboxy-terminal hydrolase L1, and glial fibrillary acidic protein were measured. The primary neurologic outcome was delirium defined by the Intensive Care Delirium Screening Checklist (scale 1–8). Associations among plasma biomarkers, respiratory failure, and inflammation were analyzed.
Setting:
Multicenter study in ICUs.
Patients:
Critically ill patients with respiratory failure, with coronavirus disease 2019, or without (ICU control).
Measurements and Main Results:
A total of 27 patients with coronavirus disease 2019 and 19 ICU controls were enrolled. Compared with ICU controls with pneumonia of other etiology, patients with coronavirus disease 2019 had significantly higher glial fibrillary acidic protein (272 pg/mL [150–555 pg/mL] vs 118 pg/mL [78.5–168 pg/mL]; p = 0.0009). In coronavirus disease 2019 patients, glial fibrillary acidic protein (rho = 0.5115, p = 0.0064), ubiquitin carboxy-terminal hydrolase L1 (rho = 0.4056, p = 0.0358), and neurofilament-light chain (rho = 0.6223, p = 0.0005) positively correlated with Intensive Care Delirium Screening Checklist score and were increased in patients with delirium (Intensive Care Delirium Screening Checklist ≥ 4) in the coronavirus disease 2019 group but not in ICU controls. There were no associations between the measures of respiratory function or cytokines with glial fibrillary acidic protein, total tau, ubiquitin carboxy-terminal hydrolase L1, or neurofilament-light chain levels in patients with coronavirus disease 2019.
Conclusions:
Plasma glial fibrillary acidic protein is two-fold higher in critically ill patients with coronavirus disease 2019 compared with ICU controls. Higher levels of glial fibrillary acidic protein, ubiquitin carboxy-terminal hydrolase L1, and neurofilament-light chain associate with delirium in patients with coronavirus disease 2019. Elevated plasma glial fibrillary acidic protein, ubiquitin carboxy-terminal hydrolase L1, and neurofilament-light chain are independent of respiratory function and peripheral cytokines.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), with greater than 20.1 million patients infected worldwide and greater than 737,000 deaths as of August 11, 2020 (1). Most critically ill patients with COVID-19 experience respiratory failure and many experience multiple organ complications, including neurologic manifestations (2, 3). General neurologic symptoms reported in COVID-19 include headache, dizziness, myalgia, and anosmia (3, 4), with additional reports of acute ischemic stroke, encephalopathy, multifocal necrotizing cerebral hemorrhages, and delirium in hospitalized patients (3–7). Current evidence is limited to descriptive studies with symptomology and neuroimaging findings and lacks comparisons against appropriate non-COVID-19 controls or insights into the underlying neurologic pathophysiology.
There are no widely used objective clinical measurements to quantify potential neurologic dysfunction in COVID-19. Delirium is a clinical expression of underlying CNS dysfunction and may stem from hypoxia, ischemia, hemorrhage, toxic or metabolic imbalance, or other diffuse neuronal dysfunction (8). Delirium is associated with adverse outcomes and mortality (9). The Intensive Care Delirium Screening Checklist (ICDSC) is a practical, validated yet subjective metric to quantify ICU-related delirium in critically ill patients. Coupling ICDSC scores with objective quantitative measures of plasma neurologic biomarkers provide an opportunity to relate biological CNS dysfunction to clinical observations in COVID-19 patients.
We measured four neurologic biomarkers in plasma on ICU admission of critically ill patients with and without COVID-19. Blood represents an accessible matrix that allows for repeated and reliable testing. Glial fibrillary acidic protein (GFAP) is an astrocyte marker; total tau (t-tau) and neurofilament-light chain (NF-L) are axonal markers, and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) is a neuronal marker. These markers are proposed as surrogate markers of neuronal (NF-L, t-tau, and UCH-L1) and neurovascular unit (GFAP) damage in conditions including, but not limited to, acute neurologic trauma and chronic neurodegenerative conditions such as Alzheimer disease (10, 11).
Our aims were: 1) to determine the differences in neurologic biomarkers between the critically ill patients with COVID-19 and ICU controls, 2) to determine the association between the neurologic biomarkers and the severity of ICU delirium in COVID-19, and 3) to determine the associations between the neurologic biomarkers and markers of COVID-19 pulmonary severity.
MATERIALS AND METHODS
We conducted a prospective multicenter observational study to investigate plasma biomarkers in critically ill patients with COVID-19 following the University of British Columbia Clinical Research Ethics Board approval (H20-00971) and trial registration on clinicaltrials.gov (NCT04363008). Research was conducted under the principles of the Helsinki declaration and reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (12) guidelines.
Patients and Controls
Description of patient management and study sites is provided in Supplemental Text, http://links.lww.com/CCX/A358. Critically ill patients consecutively admitted to the ICUs of both study sites were enrolled. COVID-19 diagnosis was based on clinical presentation compatible with pneumonia and a positive nasal or tracheal real-time reverse transcription polymerase chain reaction (RT-PCR) SARS-CoV-2 test. Consecutive non-COVID-19 critically ill control patients were identified and enrolled who were admitted to the ICU with a primary diagnosis of respiratory failure from pneumonia (community [68%] or aspiration [21%]), inhalation lung injury (5%), or urosepsis (5%) and who had two consecutive negative SARS-CoV-2 RT-PCR tracheal tests. ICU control patients were excluded if they had a primary or concomitant diagnosis of acute traumatic brain injury, CNS infection, ischemic stroke, subarachnoid hemorrhage, intracerebral hemorrhage, or hypoxic ischemic brain injury following resuscitation from cardiac arrest. Volunteer blood donors without respiratory or neurologic disease between 40 and 60 years served as healthy controls.
Data Collection and Neurologic Biomarkers
Clinical data pertaining to patient age, sex, comorbidities, premorbid medications, and dates of symptoms, hospital admission, ICU admission, mechanical ventilation, ICU, as well as hospital discharge were recorded. Premorbid neurologic characteristics pertaining to premorbid dementia and neurodegenerative diagnosis were collected. Physiologic data (median hourly per day) from the ICU admission were collected for heart rate (beats/min), mean arterial pressure (mm Hg), core body temperature (°C), propofol dose (µg/kg/min), norepinephrine dose (µg/min), end-tidal carbon dioxide tension (mm Hg), minute ventilation, Fio2 (%), positive end-expiratory pressure (cm H2O), peripheral oxygen saturation (%), serum sodium concentration (mEq/L), and hemoglobin concentration (g/dL). Daily median arterial blood gas data were also collected for pH, arterial carbon dioxide tension (Paco2, mm Hg), arterial oxygen tension (Pao2, mm Hg), and bicarbonate concentration (mEq/L). Median daily measurements of PaO2:FiO2 ratio were recorded. Daily delirium screening scores were assessed by bedside nurses as a part of routine care using the ICDSC (scale 1–8), with a threshold of greater than or equal to 4 indicating delirium (13). We collected laboratory investigations from the medical charts that were completed as part of routine care including C-reactive protein (CRP), d-dimer, complete blood count, serum ferritin, liver enzymes, and renal function (creatinine).
Daily arterial blood samples were obtained from each COVID-19 patient from days 1–10, 14, and 21 following ICU admission. For ICU controls, arterial blood samples and data collection for all variables were collected on day 1 of ICU admission. Blood biomarkers were quantified using the single-molecule array enzyme linked immunoassay (Simoa) HD-1 platform from Quanterix (Billerica, MA) following the manufacturer’s protocol. Plasma GFAP, t-tau, UCH-L1, and NF-L were measured using the Neurology-4-plex B advantage assay (103345). Plasma samples were isolated from five healthy community controls (n = 2 males [40%]; median age 54 interquartile range [IQR], 51–55) collected prior to the SARS-CoV-2 pandemic to serve as an internal biomarker calibrator as their levels align with published normative data (14, 15). Different investigators collected ICDSC scores and biomarker measurements, and each was blinded to clinical data and group assignment. Serum interleukin (IL)-6 was quantified using the Simoa HD-1 platform and Cytokine-3-plex A kit (101795).
Outcomes
Primary, secondary, and tertiary outcomes were selected a priori. The primary outcome was the difference between the acute neurologic biomarkers in patients with COVID-19 and ICU controls. Secondary outcomes were the difference between the acute neurologic biomarkers in patients with and without a diagnosis of delirium, and the relationship between the acute neurologic biomarkers and maximum ICDSC score. Tertiary outcomes were the relationships of acute neurologic biomarkers to the markers of COVID-19 respiratory disease severity including a diagnosis of acute respiratory distress syndrome (ARDS) and the Pao2:Fio2 ratio. Post hoc analysis was done to observe biomarker trajectories over time in a subgroup of subjects.
Statistical Analyses
Descriptive statistics including median and IQRs and frequency were used to describe continuous and categorical variables, respectively. Groupwise differences were determined using a Mann-Whitney test for continuous variables or Fisher exact test for categorical variables. We constructed multivariable linear regression models adjusting for age and sex to explore further the relationship between the COVID-19 status and plasma biomarkers. Normality residuals were assessed with p-p plots, q-q plots, and Shapiro-Wilk tests. Associations between the plasma biomarkers measured from the day of ICU admission and maximum ICDSC scores during the first week of ICU stay were assessed using Spearman rank correlation test. Differences between the plasma biomarker levels measured on days 1 and 7 of ICU stay were tested for using a Wilcoxon signed ranks test. All statistical tests were two-sided, and a p value of less than 0.05 was considered significant. All statistical analyses were completed using Graphpad Prism (Version 6.07; San Diego, CA) and STATA (Stata Statistical Software: Release 15; StataCorp LLC, College Station, TX).
RESULTS
Demographics
Of 46 patients enrolled between March 30, 2020 and May 5, 2020, 27 were in the COVID-19 group and 19 in the ICU control group. Demographics, neurologic symptoms, comorbidities, and exposures for patients with COVID-19 and ICU controls are shown in Table 1, and laboratory findings are shown in Supplemental Table 1 (Supplemental Digital Content, http://links.lww.com/CCX/A359). There were no significant differences between the patients with COVID-19 and ICU controls for age, sex, or frequency of dementia ascertained by medical record. Of the seven controls from whom surgical history data were available, 2 (29%) had operative interventions prior to ICU admission, whereas 0 patients with COVID-19 underwent operative procedures.
Table 1.
Demographics and Neurologic Biomarker Results From Both ICU Control and Patients With Coronavirus Disease 2019
| Variables | Reporting n Control; COVID | ICU Controls (n = 19) | COVID (n = 27) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (IQR) | 19; 2 7 | 64 (33–75) | 70 (54–76) | 0.15 |
| Males, n (%) | 19; 27 | 9 (47) | 18 (67) | 0.23 |
| Dementia, n (%) | 19; 27 | 2 (11) | 5 (19) | 0.68 |
| Neurologic clinical symptoms | ||||
| Headache, n (%) | 17; 27 | 2 (18) | 7 (26) | 0.45 |
| Delirium, n (%) | 19; 27 | 4 (21) | 11 (41) | 0.21 |
| Max Intensive Care Delirium Screening Checklist score, median (IQR) | 19; 27 | 0 (0–3) | 2 (0–6) | 0.075 |
| Glasgow Coma Scale, median (IQR) | 15; 25 | 7 (3–15) | 13 (3–15) | 0.52 |
| Comorbidities | ||||
| Hypertension, n (%) | 15; 26 | 8 (53) | 15 (58) | > 0.99 |
| Diabetes, n (%) | 14; 26 | 3 (21) | 8 (31) | 0.72 |
| Obesity, n (%) | 14; 26 | 3 (21) | 2 (80) | 0.32 |
| Dyslipidemia, n (%) | 17; 22 | 3 (18) | 10 (45) | 0.093 |
| Chronic kidney disease, n (%) | 17; 26 | 2 (11) | 4 (15) | > 0.99 |
| Coronary artery disease, n (%) | 7; 21 | 1 (14) | 5 (24) | > 0.99 |
| Chronic obstructive pulmonary disease, n (%) | 15; 26 | 2 (13) | 1 (4) | 0.54 |
| Exposures | ||||
| Previous surgery, n (%) | 7; 27 | 2(29) | 0 (0) | 0.037 |
| Sedation, n (%) | 19; 27 | 17 (89) | 15 (55) | 0.022 |
| Sedation duration, d, median (IQR) | 19; 27 | 2 (1–3) | 2 (0–5) | 0.62 |
| Intubation, n (%) | 19; 27 | 13 (68) | 14 (51) | 0.36 |
| Renal replacement therapy, n (%) | 16; 27 | 0 (0) | 3 (11) | 0.28 |
| Propofol, n (%) | 16; 27 | 11 (69) | 16 (59) | 0.75 |
| Opioids, n (%) | 16; 27 | 2 (13) | 10 (37) | 0.16 |
| Midazolam, n (%) | 16; 27 | 1 (6) | 2 (7) | > 0.99 |
| Neurologic biomarkers | ||||
| Glial fibrillary acidic protein, pg/mL, median (IQR) | 19; 27 | 118 (78.5–168) | 272 (150–555) | 0.0009 |
| Total tau, pg/mL, median (IQR) | 19; 27 | 3.54 (2.21–5.05) | 1.80 (1.13–2.83) | 0.0012 |
| Ubiquitin carboxy-terminal hydrolase L1, pg/mL, median (IQR) | 19; 27 | 20.8 (15.5–32.9) | 25.9 (14.5–41.5) | 0.24 |
| Neurofilament-light chain, pg/mL, median (IQR) | 19; 27 | 32.9 (11.4–42.1) | 36.7 (17.1–84.2) | 0.19 |
COVID = coronavirus disease, IQR = interquartile range.
Delirium, Intensive Care Delirium Screening Checklist scores, and exposures are recorded from first week of ICU stay. Plasma biomarker levels are measured from the first day of ICU admission or study enrolment. p values determined by Mann-Whitney U test for results are reported as median (IQR) and p values determined by Fisher exact test for results are reported as n (%).
Acute Biomarker Levels
Upon ICU admission, median plasma levels of GFAP, UCH-L1, and NF-L were higher in both the COVID-19 and ICU control groups than healthy controls, whereas t-tau was higher only in ICU controls (Fig. 1). Within critically ill patients, plasma GFAP was significantly higher (272 pg/mL [150–555 pg/mL] vs 118 pg/mL [78.5–168 pg/mL]; p = 0.0009) and t-tau was significantly lower (1.80 pg/mL [1.13–2.83 pg/mL] vs 3.54 pg/mL [2.21–5.05 pg/mL]; p = 0.0012) in patients with COVID-19 versus ICU controls, with no difference in plasma UCH-L1 or NF-L (Fig. 1). These significant relationships persisted after adjusting for age and sex (Supplemental Table 2, Supplemental Digital Content, http://links.lww.com/CCX/A360). There was a significant positive correlation between age and GFAP (rho = 0.633, p = 0.0004) and NF-L (rho = 0.678, p = 0.0001) in the COVID-19 group, and for GFAP (rho = 0.719, p = 0.0005) and t-tau (rho = 0.690, p = 0.0011) in the ICU control group, with no effect of sex (Supplemental Table 3, Supplemental Digital Content, http://links.lww.com/CCX/A361; and Supplemental Table 4, Supplemental Digital Content, http://links.lww.com/CCX/A362). When groups were disaggregated based on prior dementia diagnosis, NF-L was significantly higher (84.2 [57.1–269] vs 30.9 [16.0–72.6]; p = 0.023) in patients with COVID-19 and dementia than those without dementia. In the ICU control group, there was a significant increase in GFAP (409 [359–458] vs 113 [73.2–143.2]; p = 0.012) and t-tau (6.51 [5.86–7.15] vs 2.80 [2.17–4.90]; p = 0.047) in patients with dementia versus without (Supplemental Table 5, Supplemental Digital Content, http://links.lww.com/CCX/A363).
Figure 1.
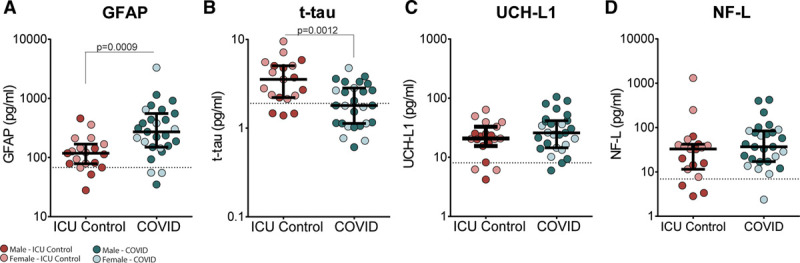
Concentrations of (A) glial fibrillary acidic protein (GFAP), (B) total tau (t-tau), (C) ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), and (D) UCH-L1 in patients with coronavirus disease (COVID) 2019 (n = 27) and ICU controls (n = 19). Concentrations are measured in plasma samples at initial ICU admission or the earliest available sample. Median with interquartile ranges is shown. Significant difference determined by Mann-Whitney U test with all significant p values is displayed. Sex disaggregation is displayed by symbol shading (light: female; dark: male); no sex differences were observed for either group for all analytes. Horizontal dotted line is median plasma levels in healthy controls (n = 5). NF-L = neurofilament-light chain.
Delirium
Correlations were determined between maximum the ICDSC scores from the first week of ICU stay and biomarker levels measured from the first day of ICU admission. There were significant positive correlations with GFAP (rho = 0.512, p = 0.0064), UCH-L1 (rho = 0.406, p = 0.036), and NF-L (rho = 0.622, p = 0.0005) in the COVID-19 group. In the ICU control group, a significant negative correlation between ICDSC and UCH-L1 was observed (rho = –0.634, p = 0.0035) (Fig. 2A–D). COVID-19 and ICU control groups were then dichotomized by delirium diagnosis using an ICDSC score of greater than or equal to 4 as the diagnostic cutoff (Fig. 2E–H). Although there were no significant associations of delirium and neurologic biomarkers in ICU controls, we observed significantly higher plasma concentrations of GFAP (438 [223–761] vs 200 [106–363], p = 0.023), UCH-L1 (40.1 [25.9–66.4] vs 16.7 [11.0–32.2]; p = 0.0079) and NF-L (77.5 [28.6–219] vs 27.7 [12.4–53.8]; p = 0.0079) in COVID-19 patients with an ICDSC score of greater than 4 compared with less than 4. Demographics and comorbidities were further investigated in these delirium groups (Supplemental Table 6, Supplemental Digital Content, http://links.lww.com/CCX/A364). In the COVID-19 group, there was a greater prevalence of dementia (p = 0.0087) and diabetes (p = 0.038) in patients with an ICDSC score of greater than 4.
Figure 2.

Delirium correlations and diagnosis dichotomization for patients with coronavirus disease (COVID) 2019 and ICU controls. Correlations among (A) glial fibrillary acidic protein (GFAP), (B) total tau (t-tau), (C) ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), and (D) neurofilament-light chain (NF-L) concentrations at ICU admission and subjects’ maximum Intensive Care Delirium Screening Checklist (ICDSC) score from the first week of ICU stay. Results of Spearman rank correlation test are displayed. E and F, Groups dichotomized by maximum ICDSC score with a cutoff of 4. Median with interquartile ranges is shown. Significant difference is determined by Mann-Whitney U test with all significant p values displayed.
Delirium scores and biomarker results were tested for their relationship with sedation time. A significantly higher proportion of ICU controls received sedation during the course of ICU stay, but no significant difference in the length of sedation between the groups was observed (Table 1). An association with delirium and sedation time exists in the COVID-19 group (rho = 0.588, p = 0.0012) but not in ICU controls (rho = 0.120, p = 0.62). Within the COVID-19 group, there was no correlation among t-tau, GFAP, and NF-L levels and sedation time (t-tau rho = 0.126, p = 0.53; GFAP rho = 0.101, p = 0.62; NF-L rho = 0.150, p = 0.45), but an association between UCH-L1 and sedation time was observed (UCH-L1 rho = 0.503, p = 0.0075). In ICU controls, there were no significant correlations between fluid biomarkers and sedation time (t-tau rho = –0.185, p = 0.45; GFAP rho = 0.00893, p = 0.97; NF-L rho = –0.155, p = 0.53; UCH-L1 rho = 0.178, p = 0.46).
Pulmonary and Inflammatory Pathophysiology
Next, we sought to determine the relationship between the neurologic biomarkers and severity of COVID-19 pulmonary pathophysiology. There were no significant correlations between initial Pao2/Fio2 and GFAP, t-tau, UCH-L1, or NF-L, nor were there are differences among COVID-19 patients diagnosed with ARDS versus those without (Supplemental Fig. 1, Supplemental Digital Content, http://links.lww.com/CCX/A366). As COVID-19 severity has been linked to cytokine storm, we further assessed whether any neurologic biomarker associated with proinflammatory markers. The only significant relationship was a negative correlation with CRP with GFAP (rho = –0.4831, p = 0.0124), t-tau (rho = –0.4023, p = 0.0416), and UCH-L1 (rho = –0.4831, p = 0.0124) (Supplemental Table 7, Supplemental Digital Content, http://links.lww.com/CCX/A365).
Biomarker Trajectories
In the COVID-19 group, 10 patients had an ICU stay that lasted at least 1 week and had plasma samples collected on both days 1 and 7. In this subgroup of patients, we preformed post hoc analysis to determine whether any neurologic biomarker levels changed throughout ICU stay. We observed a significant increase in NF-L (30.9 pg/mL [17.0–85.6 pg/mL] vs 52.7 pg/mL [28.5–267 pg/mL]; p = 0.0098) between days 1 and 7, whereas no changes were observed for GFAP, t-tau, or UCH-L1 (Fig. 3).
Figure 3.
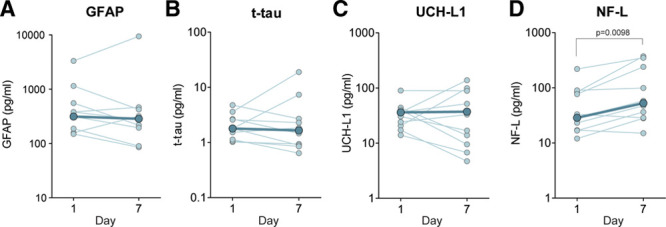
Plasma glial fibrillary acidic protein (GFAP), total tau (t-tau), ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), and neurofilament-light chain (NF-L) concentrations in participants over the first week of ICU stay measured from all patients with coronavirus disease 2019 who had day 1 and day 7 ICU specimens (n = 10). A–D, Individual subject trajectories are displayed in light blue, with group medians for each time point overlaid in dark blue. Significant differences determined by Wilcoxon signed ranked test are shown.
DISCUSSION
In this hypothesis generating study, we evaluated plasma biomarkers of neurologic damage in critically ill patients with COVID-19 relative to a critically ill ICU control group without COVID-19. Our primary finding is that, compared with ICU controls, GFAP is acutely increased in ICU patients with COVID-19. Second, we found significant associations between GFAP, UCH-L1, and NF-L and delirium. Finally, we made the notable observation that there are no relationships between the neurologic biomarkers and the measures of pulmonary severity of illness or peripheral cytokines in patients with COVID-19. As astrocytes form an integral part of the neurovascular unit and GFAP associates with blood brain barrier (BBB) disruption (16), elevated plasma GFAP is consistent with reports of vasculopathy and cerebral hemorrhage in COVID-19 (4–6) and may have potential utility in identifying patients who should be closely monitored for neurologic complications such as hemorrhage and stroke. Increased plasma GFAP could also reflect astrocytic activation unrelated to compromise the neurovascular unit. T-tau levels were elevated above healthy controls only in ICU controls but not in patients with COVID-19. This could be due to the exposure to general anesthesia or surgery that can elevate t-tau levels (17).
There is a high incidence of delirium in patients with COVID-19 (41%) during the first week of ICU stay. NF-L and GFAP are all significantly increased in COVID-19 patients with delirium, and each significantly and positively correlates with ICDSC score, a pattern not observed in ICU controls. This correlation is also observed with UCH-L1; however, it is important to note that as this biomarker also correlates with sedation time, interventions may influence UCH-L1 levels. Although we cannot ascertain causality between delirium and markers of neurologic or cerebrovascular damage, the observation that delirium and elevated neurologic biomarkers occur in critically ill patients with COVID-19 has potential physiologic relevance. Although social isolation with resultant anxiety and depression in ICU units has been proposed as a major component of delirium experienced by patients with COVID-19 (8), our data suggest that there are potential signs of acute neurologic injury reflected by elevated blood biomarker levels in patients with higher ICDSC scores.
This neurologic dysfunction may result from direct neuronal involvement by SARS-CoV-2, which has been suggested to have potential to exhibit tropism for the CNS (18–20). Vasculopathy and loss of BBB integrity may also allow viral CNS entry from the circulation via the cerebrovascular endothelium (20). As the SARS-CoV-2 viral receptor, angiotensin-converting enzyme 2, is expressed in neurons (18), CNS invasion via the olfactory nerve with subsequent infection of the hypothalamus and brain stem (21) is possible, and SARS-CoV-2 has been detected in the brain of some patients with COVID-19 (22). Thus, COVID-19-associated delirium should be further investigated through specific neurologic exams, electroencephalography, or neuroimaging to detect direct CNS injury. Our findings and the established relationship between the risk of future cognitive decline and critical illness in older adults (9, 23) further support the necessity for follow-up neurologic and neuropsychiatric evaluations.
Indirect effects are also proposed for neurologic manifestations of COVID-19 including an exaggerated host-immune response and inadequate cerebral oxygen delivery consequent to cardiorespiratory failure (24). In our study, there were no associations between the neurologic biomarkers and indices of severity for COVID-19 associated respiratory failure, namely, Pao2/Fio2 and ARDS diagnosis. This remarkable finding supports the notion that risk of neurologic injury may not be completely dependent on the severity of COVID-19 respiratory failure. Furthermore, there was no positive correlation between the neurologic biomarkers and proinflammatory cytokines, such as serum IL-6, which has been proposed as a major indicator of cytokine storm syndrome in patients with COVID-19 (25). These observations suggest that respiratory failure, inflammation, and neurologic damage may have distinct pathophysiological courses in critically ill patients.
The availability of a subcohort of patients with COVID-19 who had additional sampling on day 7 enabled analysis of the temporal relationship of these neurologic biomarkers. Although there was no difference in the NF-L levels between the COVID-19 patients and ICU controls acutely, plasma NF-L increased in patients with COVID-19 between days 1 and 7. Although few subjects remained in the ICU beyond 1 week, this finding is consistent with previous reports on the slow accumulation of NF-L in blood over days, weeks, and months following acute neurotrauma (11, 26). As NF-L is emerging as a highly sensitive but nonspecific marker of axonal injury in several neurologic conditions (27), it will be important in future studies to determine whether NF-L levels return to normal in the weeks and months after ICU discharge and recovery from COVID-19, and whether acutely elevated NF-L levels may predict future cognitive or neuropsychologic impairment in patients with COVID-19.
Although our study was not designed to distinguish among the mechanisms by which SARS-CoV-2 may affect the CNS, elevated GFAP but not t-tau raises the hypothesis that vasculopathy may play a more prominent role than direct neuronal damage in critically ill patients with COVID-19. The fact that delirium associates with neurologic biomarkers, yet there is no association with neurologic damage and measures of inflammation and respiratory distress, suggests that SARS-CoV-2 infection could potentially lead to multiple pathophysiological changes in the critical care setting. Future studies will be necessary to test further these hypotheses and determine if neurologic symptoms may also be independent of respiratory symptoms in patients with milder forms of COVID-19.
Our study has several limitations. Despite being a multicenter study, our sample size is small, limiting our ability to draw firm conclusions. Therefore, the observations reported here will require validation in a larger cohort. Notably, Kanberg et al (28) recently reported elevated plasma GFAP and NF-L levels in n = 27 COVID-19 patients with moderate to severe disease. Although we observed no age effects in our dataset, we do recognize that GFAP and NF-L vary by age and neurologic factors such as delirium and neurodegeneration. Thus, age will be an important covariate in future studies. As serial blood samples from ICU controls were not available, we cannot determine whether the observed increase in NF-L over time is specific to COVID-19. Finally, as the Quanterix Simoa platform is classified as research use only, further analytical and clinical validation is required to develop these blood biomarkers for diagnostic and prognostic contexts of use for the neurologic manifestations of COVID-19.
CONCLUSIONS
Neurologic blood biomarkers are elevated in critically ill patients and might correlate with delirium in COVID-19 patients.
ACKNOWLEDGMENTS
We thank the multidisciplinary ICU staff and colleagues who supported this study and the patient participants.
Supplementary Material
Footnotes
Ms. Cooper, Dr. Stukas, and Dr. Hoiland are co-first authors.
Drs. Sekhon and Wellington are co-senior authors.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by the Vancouver General Hospital Foundation. Dr. Sekhon is supported by the Vancouver Coastal Health Research Institute Clinician Scientist Award.
The authors have disclosed that they do not have any potential conflicts of interest.
Trial Registration: Clinicaltrials.gov (NCT04363008)
REFERENCES
- 1.World Health Organization. Coronavirus Disease (COVID-19) Situation Report – 204. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200811-covid-19-sitrep-204.pdf?sfvrsn=1f4383dd_2. Accessed August 11, 2020
- 2.Renu K, Prasanna PL, Valsala Gopalakrishnan A. Coronaviruses pathogenesis, comorbidities and multi-organ damage - A review. Life Sci. 2020; 255:117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19: A systematic review. J Neurol Sci. 2020; 413:116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020; 77:683–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vonck K, Garrez I, De Herdt V, et al. Neurological manifestations and neuro-invasive mechanisms of the severe acute respiratory syndrome coronavirus type 2. Eur J Neurol. 2020; 27:1578–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avula A, Nalleballe K, Narula N, et al. COVID-19 presenting as stroke. Brain Behav Immun. 2020; 87:115–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kandemirli SG, Dogan L, Sarikaya ZT, et al. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020; 297:E232–E235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotfis K, Williams Roberson S, Wilson JE, et al. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. 2020; 24:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girard TD, Thompson JL, Pandharipande PP, et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: A prospective cohort study. Lancet Respir Med. 2018; 6:213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrenberg AJ, Khatun A, Coomans E, et al. Relevance of biomarkers across different neurodegenerative diseases. Alzheimers Res Ther. 2020; 12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zetterberg H, Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat Rev Neurol. 2016; 12:563–574 [DOI] [PubMed] [Google Scholar]
- 12.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007; 370:1453–1457 [DOI] [PubMed] [Google Scholar]
- 13.Bergeron N, Dubois MJ, Dumont M, et al. Intensive care delirium screening checklist: Evaluation of a new screening tool. Intensive Care Med. 2001; 27:859–864 [DOI] [PubMed] [Google Scholar]
- 14.Disanto G, Adiutori R, Dobson R, et al. ; International Clinically Isolated Syndrome Study Group International Clinically Isolated Syndrome Study Group. Serum neurofilament light chain levels are increased in patients with a clinically isolated syndrome. J Neurol Neurosurg Psychiatry. 2016; 87:126–129 [DOI] [PubMed] [Google Scholar]
- 15.Mattsson N, Zetterberg H, Janelidze S, et al. ; ADNI Investigators ADNI Investigators. Plasma tau in Alzheimer disease. Neurology. 2016; 87:1827–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Z, Wang KK. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015; 38:364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiMeglio M, Furey W, Hajj J, et al. Observational study of long-term persistent elevation of neurodegeneration markers after cardiac surgery. Sci Rep. 2019; 9:7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z, Kang H, Li S, et al. Understanding the neurotropic characteristics of SARS-CoV-2: From neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. doi: 10.1007/s00415-020-09929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natoli S, Oliveira V, Calabresi P, et al. Does SARS-Cov-2 invade the brain? Translational lessons from animal models. Eur J Neurol. 2020; 27:1764–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baig AM, Khaleeq A, Ali U, et al. Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020; 11:995–998 [DOI] [PubMed] [Google Scholar]
- 21.Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: Underestimated opportunistic pathogens of the central nervous system? Viruses. 2020; 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020; 383:590–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brummel NE, Boehm LM, Girard TD, et al. Subsyndromal delirium and institutionalization among patients with critical illness. Am J Crit Care. 2017; 26:447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baig AM. Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci Ther. 2020; 26:499–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: A meta-analysis. J Med Virol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zetterberg H, Blennow K. Fluid markers of traumatic brain injury. Mol Cell Neurosci. 2015; 66:99–102 [DOI] [PubMed] [Google Scholar]
- 27.Gaetani L, Blennow K, Calabresi P, et al. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019; 90:870–881 [DOI] [PubMed] [Google Scholar]
- 28.Kanberg N, Ashton NJ, Andersson L-M, et al. Neurochemical evidence of astrocytic and neuronal injury commonly found in COVID-19. Neurology. 2020; 95:e1754–e1759 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


