Abstract
Background:
To evaluate the clinical value of circulating tumor cell (CTC) detection in peripheral blood for the diagnosis and prognosis of hepatocellular carcinoma (HCC).
Methods:
Public databases were searched, and a meta-analysis was performed to determine the specificity, sensitivity, negative- likelihood ratio (NLR) and positive-likelihood ratio (PLR), and diagnostic odds ratio (dOR) of CTC detection for the diagnosis of HCC. Hazard ratios (HRs) and 95% confidence intervals (CIs) were analyzed for the association of CTC detection with overall survival (OS) and HCC recurrence. The Meta-DiSc 1.4 and Review Manager 5.2 software programs were used for statistical analysis.
Results:
Meta-analysis of 20 studies including 1191 patients showed that the specificity, sensitivity, NLR, PLR, and dOR of CTC testing for HCC diagnosis were 0.60 (95% CI = 0.57–0.63), 0.95 (95%CI = 0.93–0.96), 0.36 (95%CI = 0.28–0.48), 11.64 (95%CI = 5.85–23.14), and 38.94 (95%CI = 18.33–82.75), respectively. Meta-analysis of 18 studies including 1466 patients indicated that the OS of CTC-positive HCC patients was less than that of CTC-negative patients (HR = 2.31; 95% CI = 1.55–3.42; P < .01). Meta-analysis of 5 studies including 339 patients revealed that the presence of CTCs in peripheral blood significantly increased the risk of HCC recurrence (HR = 3.03, 95% CI = 1.89–4.86; P < .01).
Conclusion:
CTCs in peripheral blood may be a useful marker for HCC diagnosis. In addition, the prognosis of CTC-positive HCC patients was significantly worse than that of CTC-negative HCC patients. Therefore, further studies are warranted to confirm the clinical potential of CTC detection in peripheral blood in patients with primary HCC.
Keywords: circulating tumor cells, CTCs, diagnosis, hepatocellular carcinoma, meta-analysis, prognosis
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide, and thus, constitutes a major global health burden.[1,2,3] HCC is also the leading cause of death in patients with cirrhosis.[4,5,6] Survival is relatively short among patients with end-stage liver cancer if untreated.[7,8] With advances in precision medicine in recent years, the international consensus is that different treatment options are appropriate for different HCC patients, and use of the most appropriate treatments increases patient survival. Unfortunately, due to a lack of effective markers for the detection of distant metastases and tumor recurrence, the optimal treatment time is missed for many patients, and the 3- and 5-year survival rates of HCC patients still need to be improved. Therefore, the identification of effective markers for tumor metastasis and recurrence is essential.
Circulating tumor cells (CTCs) are tumor cells that enter the circulation via invading peripheral blood vessels; CTCs are also known as dispersing tumor cells.[9] Progress in tumor biology has revealed that various components derived from tumor cells that appear in the circulation can be used as tumor-related markers. CTCs that enter the blood, lymph, and bone marrow have a high metastatic potential and can form new metastases in various organs, including the lungs, bone marrow, and brain. In recent years, the rapid development of molecular detection technology has made possible the separation and detection of CTCs in peripheral blood samples, allowing for analyses that provide tumor biological information to clinicians, including metastatic potential and resistance. Given their therapeutic potential, CTCs may be used as novel tumor markers to evaluate solid tumor progression. Recently, many studies have reported that CTC-positive patients show a poor prognosis, including decreased recurrence-free survival (RFS) and overall survival (OS), compared with their CTC-free counterparts. However, whether the prognosis in CTC-positive HCC patients can be better predicted compared with that in CTC-negative HCC patients remains controversial.[10,11,12,13,14]
The objective of the present meta-analysis was to evaluate the diagnostic accuracy of CTCs as a single test for the early detection of HCC and prediction of prognosis in HCC patients.
2. Material and methods
2.1. Database searches
We accessed several public databases, including the Cochrane Library Springer, PubMed, Elsevier Science Direct, Medline, and Google scholar, searching for all relevant articles published before May 2018. The following key words were used: “circulating tumor cells” or “hepatocellular carcinoma” and “prognosis” or “liver cancer” or “HCC” and “diagnosis” or “diagnostic” and “study” or “trial” or “research.” All searches were conducted among English language studies.
2.2. Inclusion and exclusion criteria for studies
The included studies provided detailed data to evaluate the relationship between positive detection of CTCs in peripheral blood and HCC patient OS and RFS. The methods used for CTC testing in the HCC patients were recorded, and the sample size and age range were not limited. We excluded research involving CTCs that was described only as a test within a review or a report as well as duplicated studies.
2.3. Evaluation of data quality and extraction of data
Identified studies were assessed by 3 investigators for quality using the Newcastle-Ottawa Scale (NOS) criteria,[15] including the definition and selection of the experimental and control groups, the comparability of the 2 groups, and the exposure factors. A NOS score of ≥7 points was considered high quality. We developed and extracted data after training our investigators. The data items included the details of the study (e.g., author, study year, participant area, and research design) and relevant characteristics of the participants. Two investigators verified the extracted data, and any discrepancies were resolved by discussion among the group.
2.4. Meta-analysis method
We used the recommended summary of the receiver operating characteristic (SROC) curve to represent the performance of the diagnostic test in this study.[16] The SROC curve included multiple points, and the cut-off points were determined by selecting the maximum point, which is the sum of the sensitivity and the specificity.[17] The area under the curve (AUC) and exponential Q∗ were evaluated as potential useful summaries of the curve. Based on the exact analysis of CTC detection, the upper limit was derived and the lower limit of the Q∗ was defined by the sensitivity equal to the feature point, where Q∗ is not equal to heterogeneity.[16] The asymmetry of the funnel was assessed using a logarithmic scale of size and Egger linear regression[18] in order to assess publication bias.
The Jadad score was used to evaluate the quality of each study for this meta-analysis. It was also noted whether a study mainly demonstrated a random or fixed effects model. For each publication, we used the RR and 95% CI. In addition, the Mantel-Haenszel method was used in the fixed effects model,[19] and the DerSimonian and Laid method was used in the stochastic effects model to obtain the combined estimates. In addition, we used Cochran's Q to further evaluate heterogeneity within each publication and among the rest of the included studies.[20] Moreover, we used I 2 = 100% × (Q-df)/Q to further quantify the substantial effects of heterogeneity.[21] Heterogeneity of the study was indicated by Q variables (P < .10) or I 2 statistics (I 2 > 50%), and a random effects model was used for the meta-analysis.
Statistical analysis was carried out using the Meta-DiSc (v1.4) and Review Manager v5.2 software programs. All P values were two-tailed, and a P value < .05 was considered statistically significant.
3. Results
3.1. Characteristics of included studies
The database searches yielded 608 papers, and a flow chart of the literature screening process is shown in Figure 1. After removing irrelevant or repetitive papers, a total of 76 studies were found. Upon reading of the abstracts, 23 studies were excluded (11 as commentary articles, 5 that did not perform CTC testing, and 7 that did not report HCC diagnosis). The remaining 53 articles were reviewed in full, and 21 were removed (12 that did not apply CTC testing and 9 for which the full text was not available).Overall, we included 32 studies[10,11,12,13,14,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] published between 1997 and 2016.
Figure 1.
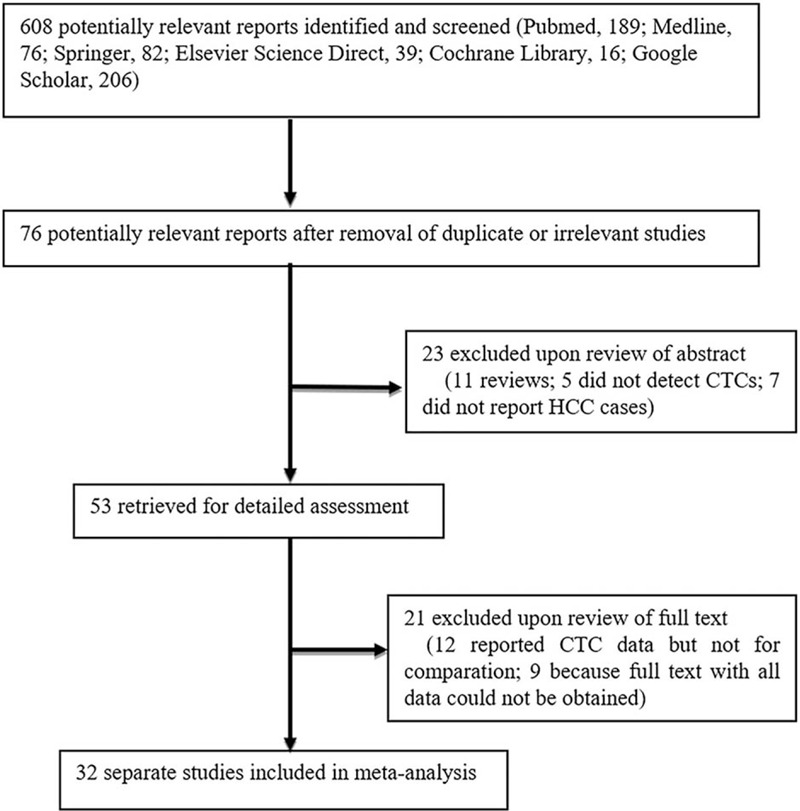
Flow diagram of study selection for the meta-analysis.
3.2. Meta-analysis of clinical value of CTC detection for the diagnosis of HCC
3.2.1. Analysis of diagnostic threshold
Exploring the threshold effect and other sources of heterogeneity is the first step in the meta-analysis of diagnostic tests. A total of 20 studies[23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] reported data evaluating the use of CTC testing in the diagnosis of HCC and were included in this meta-analysis. These studies included a total of 1191 cases. No threshold effect was observed based on the sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and other indicators for the combined data. Using Meta-DiSc software, the Spearman correlation coefficient for the study was determined to be −0.198 with a P value of .416, thus suggesting that there was no threshold effect.
3.2.2. Overall effects of CTC testing for HCC diagnosis
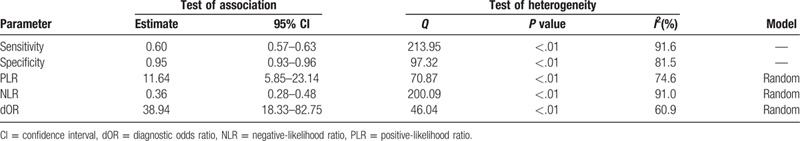
The overall meta-analysis of CTC testing for HCC diagnosis is summarized in Table 1. For the combined data from the 20 studies, we calculated the following rates: false positive (FP); true positive (TP); true negative (TN); and false negative (FN). The overall estimates of the meta-analysis showed that CTC testing may be useful for detecting HCC among patients. The specificity, sensitivity, NLR, PLR, and diagnostic odds ratio (dOR) were 0.60 (95% CI = 0.57–0.63), 0.95 (95%CI = 0.93–0.96), 0.36 (95%CI = 0.28–0.48), 11.64 (95%CI = 5.85–23.14), and 38.94 (95%CI = 18.33–82.75), respectively. The AUC and Q∗ index values were 0.91 and 0.84, respectively.
Table 1.
HCC diagnostic indices based on CTC detection.
3.3. Meta-analysis of the clinical value of CTC detection for the prognosis of HCC
A total of 18 studies[10,11,12,13,14,22,23,28,33,36,37,41,43,44,45,46,47,48] reported data for evaluating the use of CTC testing in predicting the prognosis of HCC and were included in this meta-analysis. These studies included a total of 1466 cases, of which 795 were CTC-positive and 671 were CTC-negative. The characteristics of these studies are shown in Table 2. We extracted the hazard ratio (HR) and standard error for RFS and/or OS from each study. The HR was measured by comparing CTC-positive and -negative patients. If the HR was >1, the prognosis of the CTC-positive group was poorer than that of the CTC-negative group. Data for RFS were reported in 5 studies, including 339 patients (180 CTC-positive and 159 CTC-negative cases). The summary analysis showed that the presence of CTCs in peripheral blood significantly increased the risk of recurrence in HCC patients (HR = 3.03, 95% CI = 1.89–4.86; P < .01; Fig. 2), and the heterogeneity in the studies was moderate (P = .04, I 2 = 60%).Moreover, the OS among CTC-positive primary HCC patients in the 18 studies was less than that among CTC-negative patients (HR = 2.31, 95% CI = 1.55–3.42; P < .01). We found that tumor size was associated with CTC positivity (risk ratio [RR] = 1.39, 95% CI = 1.15–1.66; P < .01 [fixed effects]), and the heterogeneity was moderate (I 2 = 41%, P = .12).The serum alpha fetoprotein (AFP) level also was associated with CTC positivity (RR = 2.32, 95% CI = 1.32–4.09; P < .01 [random effects]), and heterogeneity was high (I 2 = 86.0%, P < .01) (Fig. 3).
Table 2.
Characteristics of studies included in the meta-analysis of CTC detection for prediction of HCC prognosis.
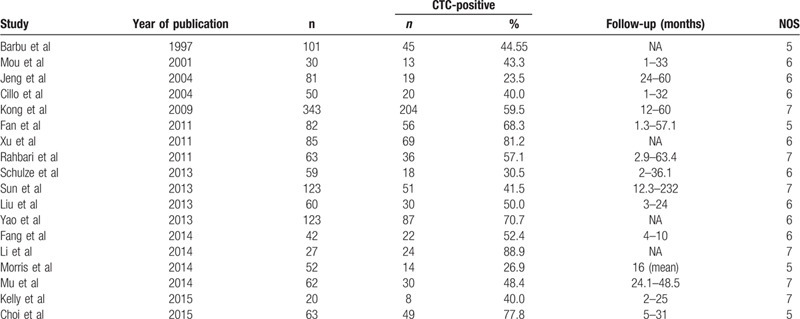
Figure 2.
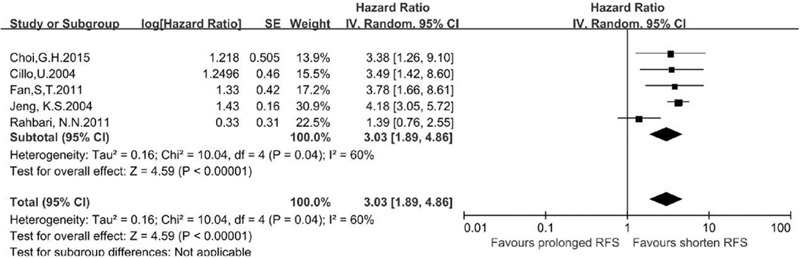
Meta-analysis of a difference in RFS between CTC-positive and CTC-negative HCC patients based on a HR.
Figure 3.
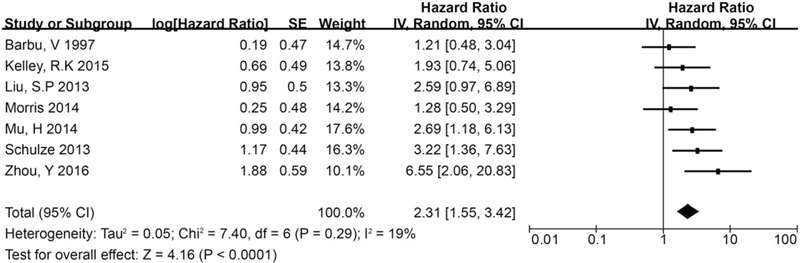
Summary estimates of HR for OS in CTC-positive HCC patients.
3.4. Publication bias
Revman 5.2 was used to construct a funnel plot for analyzing whether publication bias existed in the included literature. The analysis showed that the funnel plots for the CTC-positive and CTC-negative groups were symmetric, and there was no apparent publication bias in the included studies (Fig. 4A and B).
Figure 4.
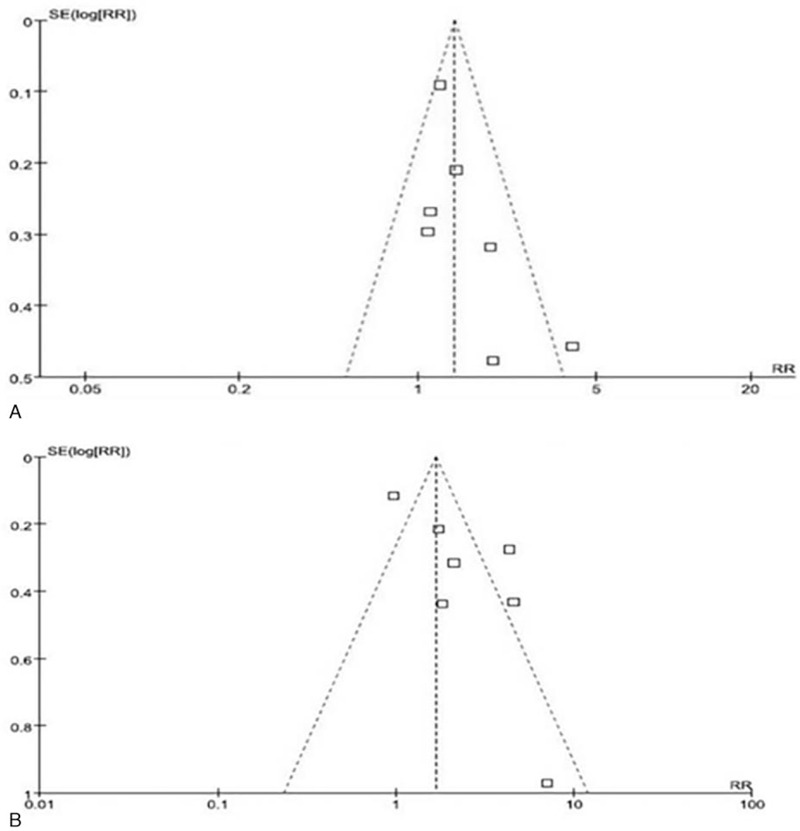
Funnel plots for the association of (A) tumor size and (B) serum AFP concentration with CTC-positivity in HCC patients.
4. Discussion
CTCs are a type of cancer cell or cancer cell cluster that enter the circulation by invading the vascular endothelium. Most individual tumor cells are either cleared by the immune system or die via anoikis, but a small number of cells or cell clusters can evade the immune system, migrate, adhere, and aggregate into tiny cancerous ridges to form metastases at distant locations. The significance of CTC testing in the diagnosis of HCC has been discussed in many studies, but the data from the individual studies were insufficient to draw a firm conclusion. Therefore, the present meta-analysis of relevant studies with a focus on CTC testing for HCC diagnosis was performed. Meta-analysis of data from 20 studies indicated the diagnostic value of CTC testing for HCC diagnosis based on specificity, sensitivity, NLR, PLR, and dOR values of 0.60 (95% CI = 0.57–0.63), 0.95 (95%CI = 0.93–0.96), 0.36 (95%CI = 0.28–0.48), 11.64 (95%CI = 5.85–23.14), and 38.94 (95%CI = 18.33–82.75), respectively.
Moreover, meta-analysis of data from 18 studies indicated the prognostic value of CTC testing in HCC patients based on the associations of CTC detection with OS and recurrence. These findings provide theoretical support for the clinical application of peripheral blood CTCs for the prediction, and therefore prevention, of post-operative recurrence and metastasis in HCC patients. Studies have shown that the presence of tumor cells in the circulation is an important risk factor for post-operative recurrence and metastasis in cancer patients,[49,50] with 1 study reporting that CTC detection in peripheral blood can be used as an early warning factor for post-operative recurrence and metastasis of liver cancer as well as in the evaluation criteria for the efficacy of post-operative chemotherapy.[47] Consistently, our results showed that the probabilities of recurrence and metastasis were greater in CTC-positive patients than in CTC-negative patients. Therefore, the detection of a CTCs within peripheral blood samples before or during chemotherapy has important clinical value for predicting the prognosis of cancer patients and can also guide decision-making regarding clinical effective treatments. By facilitating the planning of individualized treatment programs, CTC detection can improve patient survival and avoid the expenditure of medical resources for treatments less likely to be effective. Additional research is warranted to confirm these findings.
In the current meta-analysis, we used the dOR, AUC, and other indicators to evaluate the diagnostic value of CTC detection in peripheral blood. A dOR value >1 reflects a correlation between the results of the diagnostic test and HCC, with increasing values indicating better diagnostic value. If the dOR value is <1, a test may suffer from the increased occurrence of FP results. The results of our data analysis showed that the dOR value for CTC detection was 38.94, which indicated that CTCs might be a suitable diagnostic marker for HCC. At the same time, we performed SROC analysis to summarize the overall test performance and AUC assessment of the overall diagnostic efficiency. In general, AUC values >0.9 indicate excellent diagnostic value, AUC values between 0.93 and 0.96 indicate very good diagnostic value, AUC values between 0.75 and 0.92 indicate good diagnostic value, and AUC values <0.75 indicate only some clinical significance. Combined with the above criteria, our analysis indicated that CTCs have an excellent diagnostic value (AUC = 0.91), and that CTC detection has a high sensitivity and specificity for the diagnosis of HCC.
The present study does have several limitations. First, although we conducted a pooled analysis of data from relevant studies published in the literature before May 2018, we cannot rule out the potential effects of cohort studies that were not included, and we cannot draw a strong conclusion based on the number of cases included in the study. Analyses of a larger sample size are needed to verify the conclusion. Second, the included study groups were from relatively independent studies with differences in the protocols used for CTC detection, patient follow-up and clinical treatment. Thus, there was inevitably an analysis bias, which affected the credibility of the results.
Author contributions
Kai Cui designed the study and drafted the manuscript; Yang Ou acquired the data; Yangyang Shen performed the data analysis and interpretation; Sheng Li revised the manuscript; Ziqiang Sun gave advice on the statistical analysis. All authors read and approved the final manuscript.
Footnotes
Abbreviations: AFP = alpha fetoprotein, AUC = area under the curve, CI = confidence interval, CTCs = circulating tumor cells, dOR = diagnostic odds ratio, FN = false negative, FP = false positive, HCC = hepatocellular carcinoma, HR = hazard ratio, NLR = negative- likelihood ratio, NOS = Newcastle-Ottawa Scale, OS = overall survival, PLR = positive-likelihood ratio, RFS = recurrence-free survival, SROC = summary of the receiver operating characteristic, TN = true negative, TP = true positive.
How to cite this article: Cui K, Ou Y, Shen Y, Li S, Sun Z. Clinical value of circulating tumor cells for the diagnosis and prognosis of hepatocellular carcinoma (HCC): a systematic review and meta-analysis. Medicine. 2020;99:40(e22242).
This work was supported by the National Key Research and Development Program (2016YFC010600) and the Science and Technology Subject of Shandong Health Care Technology Association (SDBJKT20180138).
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Our study did not require an ethical board approval because it did not contain human or animal trials.
The authors of this work have nothing to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Yang S, Liu L, Luo Y, et al. Lower alpha fetoprotein and higher risk of hepatocellular carcinoma, study from the type 2 diabetes mellitus patients. Diabetes Res Clin Pract 2018;143:239–44. [DOI] [PubMed] [Google Scholar]
- [2].Wang ZF, Hu R, Pang JM, et al. Serum long noncoding RNA LRB1 as a potential biomarker for predicting the diagnosis and prognosis of human hepatocellular carcinoma. Oncol Lett 2018;16:1593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Uthamalingam P, Das A, Behra A, et al. Diagnostic value of glypican3, heat shock protein 70 and glutamine synthetase in hepatocellular carcinoma arising in cirrhotic and non-cirrhotic livers. J Clin Exp Hepatol 2018;8:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jia Y, French B, Tillman B, et al. Different roles of FAT10, FOXO1, and ADRA2A in hepatocellular carcinoma tumorigenesis in patients with alcoholic steatohepatitis (ASH) vs non-alcoholic steatohepatitis (NASH). Exp Mol Pathol 2018;105:144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cheung TT, Han HS, She WH, et al. The Asia Pacific consensus statement on laparoscopic liver resection for hepatocellular carcinoma: a report from the 7th Asia-Pacific primary liver cancer expert meeting held in Hong Kong. Liver Cancer 2018;7:28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].El-Wahab NM, Rashed HG, El-Sherif WT, et al. Glypican-3 and melanoma antigen genes 1 and 3 as tumor markers for hepatocellular carcinoma. Egypt J Immunol 2017;24:187–200. [PubMed] [Google Scholar]
- [7].Zhu X, Kong Q, Xie L, et al. The single-nucleotide polymorphisms in CHD5 affect the prognosis of patients with hepatocellular carcinoma. Oncotarget 2018;9:13222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kalra N, Kang M, Duseja AK, et al. Comparison of radiofrequency ablation alone & in combination with percutaneous ethanol injection for management of hepatocellular carcinoma. Indian J Med Res 2017;146: Suppl: S30–s37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011;331:1559–64. [DOI] [PubMed] [Google Scholar]
- [10].Choi GH, Kim GI, Yoo JE, et al. Increased expression of circulating cancer stem cell markers during the perioperative period predicts early recurrence after curative resection of hepatocellular carcinoma. Ann Surg Oncol 2015;22: Suppl 3: S1444–52. [DOI] [PubMed] [Google Scholar]
- [11].Fang ZT, Zhang W, Wang GZ, et al. Circulating tumor cells in the central and peripheral venous compartment - assessing hematogenous dissemination after transarterial chemoembolization of hepatocellular carcinoma. Onco Targets Ther 2014;7:1311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yao M, Yao DF, Bian YZ, et al. Values of circulating GPC-3 mRNA and alpha-fetoprotein in detecting patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2013;12:171–9. [DOI] [PubMed] [Google Scholar]
- [13].Rahbari NN, Reissfelder C, Muhlbayer M, et al. Correlation of circulating angiogenic factors with circulating tumor cells and disease recurrence in patients undergoing curative resection for colorectal liver metastases. Ann Surg Oncol 2011;18:2182–91. [DOI] [PubMed] [Google Scholar]
- [14].Kong SY, Park JW, Kim JO, et al. Alpha-fetoprotein and human telomerase reverse transcriptase mRNA levels in peripheral blood of patients with hepatocellular carcinoma. J Cancer Res Clin Oncol 2009;135:1091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [16].Walter SD. Properties of the summary receiver operating characteristic (SROC) curve for diagnostic test data. Stat Med 2002;21:1237–56. [DOI] [PubMed] [Google Scholar]
- [17].Altman DG, Bland JM. Diagnostic tests 3: receiver operating characteristic plots. BMJ 1994;309:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [20].Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–6. [DOI] [PubMed] [Google Scholar]
- [21].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cillo U, Navaglia F, Vitale A, et al. Clinical significance of alpha-fetoprotein mRNA in blood of patients with hepatocellular carcinoma. Clin Chim Acta 2004;347:129–38. [DOI] [PubMed] [Google Scholar]
- [23].Bahnassy AA, Zekri AR, El-Bastawisy A, et al. Circulating tumor and cancer stem cells in hepatitis C virus-associated liver disease. World J Gastroenterol 2014;20:18240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cheng SW, Tsai HW, Lin YJ, et al. Lin28B is an oncofetal circulating cancer stem cell-like marker associated with recurrence of hepatocellular carcinoma. PLoS One 2013;8:e80053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Choi HS, Lee HM, Kim WT, et al. Detection of mycoplasma infection in circulating tumor cells in patients with hepatocellular carcinoma. Biochem Biophys Res Commun 2014;446:620–5. [DOI] [PubMed] [Google Scholar]
- [26].Dent BM, Ogle LF, O’Donnell RL, et al. High-resolution imaging for the detection and characterisation of circulating tumour cells from patients with oesophageal, hepatocellular, thyroid and ovarian cancers. Int J Cancer 2016;138:206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Guo W, Yang XR, Sun YF, et al. Clinical significance of EpCAM mRNA-positive circulating tumor cells in hepatocellular carcinoma by an optimized negative enrichment and qRT-PCR-based platform. Clin Cancer Res 2014;20:4794–805. [DOI] [PubMed] [Google Scholar]
- [28].Kelley RK, Magbanua MJ, Butler TM, et al. Circulating tumor cells in hepatocellular carcinoma: a pilot study of detection, enumeration, and next-generation sequencing in cases and controls. BMC Cancer 2015;15:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li J, Chen L, Zhang X, et al. Detection of circulating tumor cells in hepatocellular carcinoma using antibodies against asialoglycoprotein receptor, carbamoyl phosphate synthetase 1 and pan-cytokeratin. PLoS One 2014;9:e96185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li YM, Xu SC, Li J, et al. Epithelial-mesenchymal transition markers expressed in circulating tumor cells in hepatocellular carcinoma patients with different stages of disease. Cell Death Dis 2013;4:e831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu HY, Qian HH, Zhang XF, et al. Improved method increases sensitivity for circulating hepatocellular carcinoma cells. World J Gastroenterol 2015;21:2918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu YK, Hu BS, Li ZL, et al. An improved strategy to detect the epithelial-mesenchymal transition process in circulating tumor cells in hepatocellular carcinoma patients. Hepatol Int 2016;10:640–6. [DOI] [PubMed] [Google Scholar]
- [33].Mu H, Lin KX, Zhao H, et al. Identification of biomarkers for hepatocellular carcinoma by semiquantitative immunocytochemistry. World J Gastroenterol 2014;20:5826–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ogle LF, Orr JG, Willoughby CE, et al. Imagestream detection and characterisation of circulating tumour cells - A liquid biopsy for hepatocellular carcinoma? J Hepatol 2016;65:305–13. [DOI] [PubMed] [Google Scholar]
- [35].Sabile A, Louha M, Bonte E, et al. Efficiency of Ber-EP4 antibody for isolating circulating epithelial tumor cells before RT-PCR detection. Am J Clin Pathol 1999;112:171–8. [DOI] [PubMed] [Google Scholar]
- [36].Schulze K, Gasch C, Staufer K, et al. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int J Cancer 2013;133:2165–71. [DOI] [PubMed] [Google Scholar]
- [37].Sun YF, Xu Y, Yang XR, et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology 2013;57:1458–68. [DOI] [PubMed] [Google Scholar]
- [38].Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol 2000;156:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Waguri N, Suda T, Nomoto M, et al. Sensitive and specific detection of circulating cancer cells in patients with hepatocellular carcinoma; detection of human telomerase reverse transcriptase messenger RNA after immunomagnetic separation. Clin Cancer Res 2003;9:3004–11. [PubMed] [Google Scholar]
- [40].Wu S, Liu S, Liu Z, et al. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS One 2015;10:e0123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Xu W, Cao L, Chen L, et al. Isolation of circulating tumor cells in patients with hepatocellular carcinoma using a novel cell separation strategy. Clin Cancer Res 2011;17:3783–93. [DOI] [PubMed] [Google Scholar]
- [42].Yao F, Guo JM, Xu CF, et al. Detecting AFP mRNA in peripheral blood of the patients with hepatocellular carcinoma, liver cirrhosis and hepatitis. Clin Chim Acta 2005;361:119–27. [DOI] [PubMed] [Google Scholar]
- [43].Barbu V, Bonnand AM, Hillaire S, et al. Circulating albumin messenger RNA in hepatocellular carcinoma: results of a multicenter prospective study. Hepatology 1997;26:1171–5. [DOI] [PubMed] [Google Scholar]
- [44].Fan ST, Yang ZF, Ho DW, et al. Prediction of posthepatectomy recurrence of hepatocellular carcinoma by circulating cancer stem cells: a prospective study. Ann Surg 2011;254:569–76. [DOI] [PubMed] [Google Scholar]
- [45].Jeng KS, Sheen IS, Tsai YC. Does the presence of circulating hepatocellular carcinoma cells indicate a risk of recurrence after resection? Am J Gastroenterol 2004;99:1503–9. [DOI] [PubMed] [Google Scholar]
- [46].Liu S, Li N, Yu X, et al. Expression of intercellular adhesion molecule 1 by hepatocellular carcinoma stem cells and circulating tumor cells. Gastroenterology 2013;144:1031–41.e1010. [DOI] [PubMed] [Google Scholar]
- [47].Morris KL, Tugwood JD, Khoja L, et al. Circulating biomarkers in hepatocellular carcinoma. Cancer Chemother Pharmacol 2014;74:323–32. [DOI] [PubMed] [Google Scholar]
- [48].Mou DC, Cai SL, Peng JR, et al. Evaluation of MAGE-1 and MAGE-3 as tumour-specific markers to detect blood dissemination of hepatocellular carcinoma cells. Br J Cancer 2002;86:110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213–21. [DOI] [PubMed] [Google Scholar]
- [50].Katoh S, Goi T, Naruse T, et al. Cancer stem cell marker in circulating tumor cells: expression of CD44 variant exon 9 is strongly correlated to treatment refractoriness, recurrence and prognosis of human colorectal cancer. Anticancer Res 2015;35:239–44. [PubMed] [Google Scholar]


