Abstract
Background
Prominent clinical symptoms of COVID-19 include CNS manifestations. However, it is unclear whether severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19, gains access to the CNS and whether it causes neuropathological changes. We investigated the brain tissue of patients who died from COVID-19 for glial responses, inflammatory changes, and the presence of SARS-CoV-2 in the CNS.
Methods
In this post-mortem case series, we investigated the neuropathological features in the brains of patients who died between March 13 and April 24, 2020, in Hamburg, Germany. Inclusion criteria comprised a positive test for SARS-CoV-2 by quantitative RT-PCR (qRT-PCR) and availability of adequate samples. We did a neuropathological workup including histological staining and immunohistochemical staining for activated astrocytes, activated microglia, and cytotoxic T lymphocytes in the olfactory bulb, basal ganglia, brainstem, and cerebellum. Additionally, we investigated the presence and localisation of SARS-CoV-2 by qRT-PCR and by immunohistochemistry in selected patients and brain regions.
Findings
43 patients were included in our study. Patients died in hospitals, nursing homes, or at home, and were aged between 51 years and 94 years (median 76 years [IQR 70–86]). We detected fresh territorial ischaemic lesions in six (14%) patients. 37 (86%) patients had astrogliosis in all assessed regions. Activation of microglia and infiltration by cytotoxic T lymphocytes was most pronounced in the brainstem and cerebellum, and meningeal cytotoxic T lymphocyte infiltration was seen in 34 (79%) patients. SARS-CoV-2 could be detected in the brains of 21 (53%) of 40 examined patients, with SARS-CoV-2 viral proteins found in cranial nerves originating from the lower brainstem and in isolated cells of the brainstem. The presence of SARS-CoV-2 in the CNS was not associated with the severity of neuropathological changes.
Interpretation
In general, neuropathological changes in patients with COVID-19 seem to be mild, with pronounced neuroinflammatory changes in the brainstem being the most common finding. There was no evidence for CNS damage directly caused by SARS-CoV-2. The generalisability of these findings needs to be validated in future studies as the number of cases and availability of clinical data were low and no age-matched and sex-matched controls were included.
Funding
German Research Foundation, Federal State of Hamburg, EU (eRARE), German Center for Infection Research (DZIF).
Introduction
COVID-19, the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has evolved into a global pandemic since the first recorded cases in December, 2019. Although SARS-CoV-2 primarily targets the respiratory tract,1 other organ systems such as the renal and cardiovascular systems are also affected.2, 3 Additionally, neurological symptoms are common in COVID-19 and include anosmia and ageusia, non-specific symptoms such as dizziness and headache, and severe conditions such as ischaemic stroke, haemorrhagic encephalopathy, and posterior reversible encephalopathy syndrome with epileptic seizures.4, 5, 6, 7 Furthermore, clinical data and laboratory investigations suggest that encephalitis,8, 9, 10 meningitis,9, 10 polyneuritis cranialis, and Guillain-Barré and Miller Fisher syndromes11, 12, 13 might also be associated with COVID-19.
Why SARS-CoV-2 infection leads to neurological symptoms, and whether and how the virus gains access to the CNS are not well understood. The two main competing hypotheses are based on neurotropism and direct invasion of SARS-CoV-2 into the CNS, and indirect mechanisms mediated by the cytokine storm induced by systemic SARS-CoV-2 infection.
In-depth neuropathological assessment can elucidate if and how SARS-CoV-2 gains access to or damages the brain.14 However, only a few reports of the neuropathological findings of patients with COVID-19 have been published. Two case reports showed no gross CNS abnormalities at autopsy,15 and two case series documented no signs of encephalitis or CNS vasculitis.16, 17 Additionally, loss of white matter and axonal injury were described in one case report,18 while massive intracranial haemorrhage and pan-encephalitis were described in a case series,19 and one case series reported only the detection of SARS-CoV-2 in the brain.20
Research in context.
Evidence before this study
We searched PubMed for studies focusing on the neuropathology of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, published in German or English, until Aug 2, 2020. Search terms included “COVID-19” OR “SARS-CoV-2” AND “neuropathology” OR “neurodegeneration” OR “encephalitis” OR “central nervous system” OR “brain”. Studies examining the neuropathology of COVID-19 in animal models were also considered. The literature gave a heterogeneous picture regarding the neuropathological presentation of COVID-19. Two studies described no signs of encephalitis or nervous system vasculitis, and mainly reactive changes unrelated to SARS-CoV-2 infection, whereas other studies show pan-encephalitis, cerebral haemorrhage, and areas of necrosis with loss of white matter and axonal injury attributed to SARS-CoV-2 infection. All studies were subject to sampling bias and small patient numbers, and most examined brains originated from patients who died in hospital under intensive care unit treatment, which can itself lead to neuropathological alterations independently of SARS-CoV-2 infection.
Added value of this study
To our knowledge this study represents the world's largest case series of brain autopsies from patients with COVID-19 to date. We included 43 patients who died under intensive care unit treatment, in regular hospital wards, in nursing homes, or in their own homes, ranging in age from 51 years to 94 years. Fresh ischaemic lesions were found in the brains of six patients, and almost all patients showed astrocytic reactions in all assessed brain regions. Neuroimmune activation was observed in all examined brains, with prominent involvement of the brainstem and neuroimmune reaction, in line with involvement of the adaptive and innate immune systems. The presence of SARS-CoV-2 did not seem to be associated with the severity of neuroimmune activation. Neuroimmune activation was also observed in patients who died from COVID-19 at home or in nursing homes.
Implications of all the available evidence
The emerging evidence, including the current study, shows that neuropathological alterations in the brains of patients who die from COVID-19 are relatively mild, although the virus is able to gain access to the brain. The neuropathological alterations are most likely to be immune-mediated, and there does not seem to be fulminant virus-induced encephalitis nor direct evidence for SARS-CoV-2-caused CNS damage. Further studies are needed to define how SARS-CoV-2 gains access to the brain, to define the neuroimmune activation, and to describe the distribution of SARS-CoV-2 in the brain.
The current study aimed to investigate the neuropathological features of COVID-19, including glial response, inflammatory changes, and the presence and distribution of SARS-CoV-2 in the brain of patients who died from COVID-19.
Methods
Study design and participants
Consecutive patients who had died following a diagnosis of SARS-CoV-2 infection were autopsied at the Institute of Legal Medicine, University Medical Center of Hamburg-Eppendorf (Hamburg, Germany) between March 13 and April 24, 2020, upon order issued by the Hamburg public health authorities in accordance with section 25(4) of the German Infection Protection Act. Organisation of autopsies and adequate collection of samples were logistically challenging as this time period coincided with the peak incidence of COVID-19 in Hamburg. Inclusion criteria for this study were a confirmed diagnosis of SARS-CoV-2 infection, with SARS-CoV-2 RNA detected by quantitative RT-PCR (qRT-PCR) analysis of pharyngeal swabs, and the availability of sufficient high-quality brain tissue samples. Clinical presentation and neuroradiological findings did not form part of the inclusion criteria.
The study was approved by the local ethics committee of the Hamburg Chamber of Physicians (approval number PV7311) and the study is in line with the Declaration of Helsinki.
Procedures
We assessed patients' clinical data, including pre-existing medical conditions, medical course before death, and ante-mortem diagnostic findings. Where logistically feasible, before fixation, specimens were taken from 23 brains for cryopreservation to allow investigation of the presence of SARS-CoV-2 in non-fixed tissue. All brains were fixed in buffered 4% formaldehyde, examined macroscopically, and underwent routine neuropathological workup.
Single-cell gene expression analysis
Human brain single-cell transcriptome data taken from Darmanis and colleagues' study21 were processed and analysed with use of the methods described by Marouf and colleagues.22 In brief, cell-type clusters were annotated using known marker genes as reported previously.21 Mean cell type-specific RNA levels of angiotensin-converting enzyme 2 (ACE2), cathepsin L (CTSL), transmembrane serine protease 2 (TMPRSS2), transmembrane serine protease 4 (TMPRSS4), neuropilin 1 (NRP1), and two pore segment channel 2 (TPCN2) were normalised per gene (cell type-specific expression divided by the sum of gene expression across cell types), and were subsequently plotted as a heatmap.
Histological and immunohistochemical evaluations
Formalin-fixed paraffin-embedded tissue (FFPE) samples from the olfactory bulb, superior frontal gyrus, basal ganglia including the putamen, upper and lower medulla oblongata, and cerebellar hemisphere were processed and stained with haematoxylin and eosin using standard laboratory procedures. Immunohistochemical staining was also done with a Ventana Benchmark XT Autostainer (Ventana, Tucson, AZ, USA), in accordance with the manufacturer's recommendations, using antibodies against human glial fibrillary acidic protein (GFAP; clone 6F2; Dako, Glostrup, Denmark; dilution 1:200), HLA-DR (mouse anti-HLA-DP, DQ, DR antibody, clone CR3/43; Dako; 1:200), transmembrane protein 119 (TMEM119; catalogue number ab185333; Abcam, Cambridge, UK; 1:250), ionized calcium-binding adaptor molecule 1 (IBA1; clone EPR16588; Abcam, Cambridge; 1:1000), CD68 (clone PG-M1; Dako; 1:200), and CD8 (clone SP239; Spring Bioscience, Pleasanton, USA; 1:100). Double-immunolabelling for IBA1 and CD8 was done sequentially with Permanent Red (Monosan Permanent AP-Red Kit; Monosan, Uden, Netherlands) as chromogen.
Slides were examined by experienced neuropathologists (JM, CH, and MG). At least two neuropathologists, masked to patients' clinical findings, assessed each slide, and disagreements were resolved by consensus. Slides were screened at low magnification and areas with the most pronounced changes (ie, strongest staining) were used for quantification, and were electronically scanned at high magnification (×40) as high-resolution images (1900 × 1200 pixels) with a NanoZoomer 2.0-HT (Hamamatsu Photonics, Hamamatsu, Japan).
The degree of astrogliosis and microgliosis was classified as none, slight, moderate, or severe, using a three-tiered semi-quantitative approach (appendix p 3), based on GFAP as an astrocyte marker and HLA-DR as a marker of activated microglia. CD68 was used to judge phagocytic activity, and IBA1 as an additional marker for microglia activity.
For semi-quantitative assessment of cytotoxic T lymphocyte infiltration, cells with positive CD8 staining were counted per high-power field (HPF) of 0·5 mm2. Infiltration was categorised as none, mild (one to nine cells per HPF), moderate (ten to 49 cells per HPF), or severe (≥50 cells per HPF; appendix p 3).
qRT-PCR analysis of SARS-CoV-2
qRT-PCR was used to quantify SARS-CoV-2 presence in specimens with enough high-quality material available. RNA was isolated from frozen or paraffin-embedded tissue samples. Frozen tissue was ground with a Precellys 24 tissue homogeniser (Bertin, Rockville, USA) using 2 mL tubes pre-filled with ceramic beads (Precellys Lysing Kit; Bertin) and 1 mL RNAse-free and DNAse-free PCR-grade water. 200 μL of the tissue homogenate was transferred to a MagNA Pure 96 instrument (Roche, Mannheim, Germany), and automated nucleic acid extraction was done according to the manufacturer's recommendation with whole process control (RNA Process Control Kit; Roche), with a final elution volume of 100 μL. Slides of paraffin-embedded tissue were deparaffinised and RNA was extracted with the Maxwell 16 LEV RNA FFPE Purification Kit and a Maxwell RNA extraction system (Promega, Fitchburg, USA).
PCR and virus quantification were done as previously described.20 In brief, an assay targeting the E gene of SARS-CoV-223 was used for the amplification and detection of SARS-CoV-2 RNA. A cycle threshold value for the target was determined with use of the second derivative maximum method.24 For quantification, standard in-vitro transcribed RNA of the E gene of SARS-CoV-2 was used (catalogue number 001K-03884; European Virus Archive, Charité, Berlin, Germany). The linear range of the assay is between 1 × 103 and 1 × 109 copies per mL. To normalise for input, quantitative β-globin PCR was done with a commercial TaqMan primer kit (catalogue number Hs00758889_s1; Thermo Fisher Scientific, Waltham, MA, USA), and the amount of DNA was normalised with use of a KAPA Human Genomic DNA Quantification and QC DNA Standard (catalogue number 07960638001; KAPA Biosystems, Cape Town, South Africa).
Immunohistochemical detection of SARS-CoV-2 spike protein and nucleoprotein
Antibodies for detecting SARS-CoV-2 in FFPE tissues were first validated on SARS-CoV-2-infected (Hamburg isolate) and non-infected Vero cells that were processed to FFPE blocks (appendix p 2). In specimens with sufficient high-quality tissue, we tested for the presence of the virus with immunohistochemistry using antibodies against viral nucleocapsid protein (catalogue numbers 40143-R001 [dilution 1:5000] and 40143-T62 [dilution 1:1000]; Sino Biological, Eschborn, Germany) and spike protein (clone 1A9, catalogue number GTX632604; GeneTex, Irvine, USA; dilution 1:300).25 Immunohistochemical staining was done with a Ventana Benchmark XT Autostainer. All slides were examined by two experienced morphologists (SK and MG), and any disagreements were resolved by consensus.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
From the 110 patients with diagnosed or suspected SARS-CoV-2 infection who were autopsied between March 13 and April 24, 2020, 43 (39%) with a positive qRT-PCR test for SARS-CoV-2 and adequate samples available were included in this study. The autopsy findings, excluding any neuropathological analysis, of 37 (86%) of these patients were previously reported in a separate report of the first 80 consecutive individuals who died of SARS-CoV-2 infection in Hamburg, Germany.26 The remaining six (14%) cases have not been previously reported. 40 (93%) patients had adequate samples for the detection of SARS-CoV-2 by immunohistochemistry, and 27 patients (63%) had samples available for the detection of SARS-CoV-2 by qRT-PCR. Data on SARS-CoV-2 RNA in the brain tissue from 22 of these cases have been reported previously.20
The median age of the 43 patients was 76 years (IQR 70–86; range 51–94), 16 (37%) patients were women and 27 (63%) were men. 40 (93%) had relevant pre-existing chronic medical conditions (mainly cardiorespiratory problems), and 13 (30%) had pre-existing neurological diseases, such as neurodegenerative disease or epilepsy (table ). 11 patients (26%) died outside of a hospital (five at home and six in a nursing facility) and 32 (74%) died in a hospital. 12 (28%) patients who died in hospital were treated in intensive care units (ICUs). Cause of death was mainly attributed to the respiratory system, with viral pneumonia as the underlying condition in most cases (table).
Table.
Summary of cases and brain autopsy findings
| Sex | Age, years | Place of death | Post-mortem interval, days | Cause of death | Comorbidities | Brain weight, g | Brain oedema | Brain atrophy | Arteriosclerosis | Macroscopic findings | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Female | 87 | Nursing home | 0 | Pneumonia | COPD, dementia, IHD, renal insufficiency | 1215 | None | Mild | Moderate | None |
| Case 2 | Female | 85 | Hospital ward | 0 | Pneumonia | Atrial fibrillation, cardiac insufficiency, IHD, myelofibrosis, renal insufficiency | 1240 | None | Mild | Moderate | Fresh infarction in territory of PCA |
| Case 3 | Male | 88 | Hospital ward | 5 | Pneumonia | Emphysema, IHD, renal insufficiency | 1490 | Moderate | None | Moderate | Fresh infarction in territory of MCA |
| Case 4 | Male | 75 | ICU | 4 | Pulmonary arterial embolism, pneumonia | Atrial fibrillation, emphysema, hypertension, renal insufficiency | 1475 | Mild | None | Moderate | Fresh infarction in territory of PCA |
| Case 5 | Female | 86 | Nursing home | 0 | Pneumonia | COPD, dementia, IHD | 1250 | None | Mild | Severe | Fresh infarction in territory of PCA |
| Case 6 | Male | 90 | Nursing home | 2 | Pneumonia | Atrial fibrillation, dementia, diabetes, history of stroke | 1015 | None | Moderate | Severe | Old infarctions in territory of PCA |
| Case 7 | Male | 90 | Hospital ward | 3 | Emphysema with respiratory decompensation | Cardiac insufficiency, COPD | 1440 | None | Mild | Moderate | None |
| Case 8 | Male | 77 | Hospital ward | 2 | Pneumonia | Aortic aneurysm, atrial flutter, cardiac hypertrophy, emphysema, renal insufficiency | 1590 | Moderate | None | Moderate | None |
| Case 9 | Male | 76 | ICU | 3 | Pulmonary arterial embolism, respiratory tract infection | Cardiac insufficiency, COPD | 1460 | Mild | None | Moderate | None |
| Case 10 | Male | 76 | ICU | 3 | Sepsis, aortic valve endocarditis, pneumonia | AML, cardiomyopathy, thyroid cancer | 1270 | None | Mild | Mild | None |
| Case 11 | Male | 70 | Hospital ward | 1 | Pneumonia (aspiration) | Cardiac insufficiency, COPD, IHD, Parkinson's disease | 1430 | Mild | None | Severe | None |
| Case 12 | Male | 93 | Hospital ward | 3 | Pneumonia | Diabetes, hypertension | 1400 | Mild | None | Moderate | None |
| Case 13 | Male | 66 | Emergency room | 2 | Pneumonia | Diabetes, IHD | 1450 | Mild | None | Severe | None |
| Case 14 | Female | 54 | Hospital ward | 1 | Pneumonia | Trisomy 21, epilepsy | 950 | None | Severe | Mild | Grey matter heterotopia |
| Case 15 | Male | 82 | Hospital ward | 1 | Pneumonia | Diabetes, IHD, Parkinson's disease | 1170 | None | Mild | Moderate | Old infarctions in territory of PCA |
| Case 16 | Male | 86 | Nursing home | 2 | Sepsis, pneumonia | Emphysema, epilepsy, hypoxic brain damage, IHD, renal insufficiency | 1210 | None | Mild | Moderate | None |
| Case 17 | Female | 87 | Home | 1 | Pneumonia | Cardiac insufficiency, COPD | 1180 | None | Mild | Severe | None |
| Case 18 | Female | 70 | ICU | 3 | Pneumonia | Cardiac insufficiency | 1150 | None | Mild | Moderate | None |
| Case 19 | Female | 75 | ICU | 4 | Pneumonia | Cardiac arrythmia, IHD | 1210 | None | Mild | Severe | None |
| Case 20 | Male | 93 | Hospital ward | 2 | Pneumonia | Atrial fibrillation, cardiac insufficiency, diabetes, IHD, obstructive sleep apnoea syndrome | 1000 | None | Moderate | Moderate | Old cerebellar infarction |
| Case 21 | Female | 82 | Hospital ward | 4 | Purulent bronchitis | COPD, history of pulmonary embolism, renal insufficiency | 1080 | None | Moderate | Moderate | None |
| Case 22 | Male | 63 | ICU | 1 | Pulmonary arterial embolism, pneumonia | Cardiac insufficiency | 1435 | Mild | None | Mild | Fresh infarction in territory of ACA |
| Case 23 | Male | 84 | Hospital ward | 5 | Pneumonia, septic encephalopathy | Diabetes, history of stroke, hypertension, IHD, ulcerative colitis | 1350 | Mild | None | Severe | None |
| Case 24 | Male | 71 | ICU | 2 | Pulmonary arterial embolism, pneumonia | Cardiac insufficiency, diabetes, lung granuloma | 1665 | Moderate | None | Mild | None |
| Case 25 | Male | 75 | Nursing home | 3 | Sudden cardiac death | Parkinson's disease | 1110 | Mild | None | Moderate | None |
| Case 26 | Male | 52 | Home | 1 | Pulmonary arterial embolism, pneumonia | Cardiac insufficiency | 1520 | Moderate | None | Severe | None |
| Case 27 | Male | 85 | ICU | 2 | Pneumonia | COPD, aortic valve replacement, hypertension, IHD | 1400 | Mild | None | Moderate | None |
| Case 28 | Female | 75 | Home | 2 | Pulmonary arterial embolism | Hypertension, IHD | 1095 | None | Moderate | Moderate | None |
| Case 29 | Male | 59 | Hospital ward | 12 | Pneumonia | Cardiomyopathy | 1575 | Moderate | None | Mild | None |
| Case 30 | Male | 85 | Hospital ward | 15 | Pneumonia | Atrial fibrillation, COPD, hypothyroidism, lung cancer, renal insufficiency | 1540 | Moderate | None | Moderate | Cerebellar metastasis of non-small cell lung cancer |
| Case 31 | Female | 76 | Hospital ward | 2 | Pneumonia | Breast cancer, hypertension | 1180 | None | Mild | Moderate | None |
| Case 32 | Male | 73 | Home | 9 | Sudden cardiac death | Cardiomyopathy, emphysema, IHD | 1430 | Mild | None | Severe | None |
| Case 33 | Male | 70 | ICU | 9 | Pneumonia | Dementia, IHD, hypertension | 1370 | None | None | Moderate | None |
| Case 34 | Female | 90 | Nursing home | 3 | Pneumonia | Cardiomyopathy, dementia, emphysema, renal insufficiency | 1090 | None | Severe | Moderate | None |
| Case 35 | Female | 94 | Hospital ward | 2 | Sepsis | Atrial fibrillation, cardiac insufficiency, dementia, history of stroke, IHD, renal insufficiency | 1220 | Mild | Mild | Moderate | Old infarction in territory of PCA |
| Case 36 | Female | 87 | Hospital ward | 3 | Sepsis, pneumonia | Colon cancer, emphysema, paranoid schizophrenia | 1310 | None | None | Mild | None |
| Case 37 | Female | 54 | ICU | 1 | Pneumonia | Mild cardiomyopathy | 1470 | Mild | None | Mild | None |
| Case 38 | Female | 79 | Hospital ward | 5 | Pneumonia | COPD, myelodysplastic syndrome, IHD | 1290 | None | Mild | Mild | None |
| Case 39 | Male | 51 | Home | 8 | Pneumonia | Liver cirrhosis | 1255 | Mild | Mild | Mild | None |
| Case 40 | Male | 85 | Hospital ward | 3 | Pneumonia | Atrial fibrillation, cardiac insufficiency, dysphagia, emphysema, hypertension, IHD | 1290 | Mild | None | Moderate | None |
| Case 41 | Male | 56 | Hospital ward | 3 | Pneumonia | Cardiac insufficiency, COPD, diabetes, IHD, renal insufficiency | 1230 | Mild | None | Mild | Old infarctions in territory of PCA and lenticulostriate arteries |
| Case 42 | Male | 76 | ICU | 3 | Aortic valve endocarditis, pneumonia | AML, cardiomyopathy, thyroid cancer | 1270 | Mild | None | Mild | None |
| Case 43 | Female | 59 | ICU | 1 | Pneumonia | Multiple myeloma | 1220 | Mild | None | Mild | Fresh infarction in territory of MCA |
COPD=chronic obstructive pulmonary disease. IHD=ischaemic heart disease. PCA=posterior cerebral artery. MCA=middle cerebral artery. ICU=intensive care unit. AML=acute myeloid leukaemia. ACA=anterior cerebral artery.
The mean post-mortem interval was 3·3 days (SD 3·1) after two patients with extremely long post-mortem intervals of 12 days and 14 days were excluded as outliers (table). The mean weight of the unfixed brains was 1302 g (SD 171; median 1270 g [1195–1438]; range 950–1665; table) with 23 patients (53%) showing signs of mild to moderate brain oedema, commonly seen as unspecific agonal changes. Arteriosclerosis of the basal vessels was mild in 12 (28%) patients, moderate in 22 (51%), and severe in nine (21%; table). 13 brains (30%) showed gross macroscopic abnormalities (fresh territorial ischaemic lesions in six patients, older territorial ischaemic lesions in five patients, grey matter heterotopia in one patient with trisomy 21, and cerebellar metastasis of a non-small cell lung carcinoma in one patient; table).
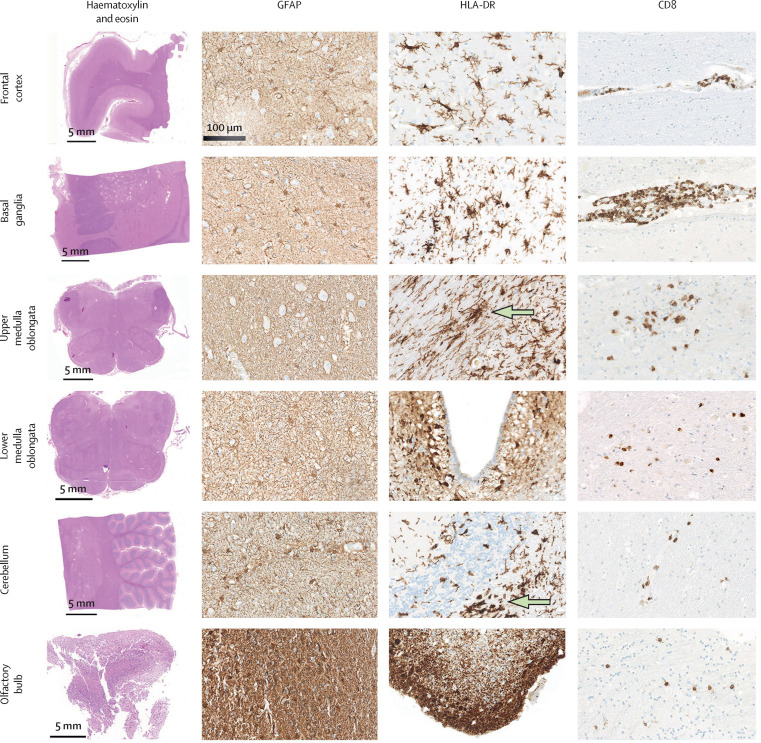
There was no evidence of cerebral bleeding or small-vessel thromboses. We found rare instances (two cases) of neuronophagy, and no acute necrotising lesions. Six (14%) patients had fresh ischaemic infarctions: three in the territory of the posterior cerebral artery, two in the territory of the anterior cerebral artery, and one in the territory of the middle cerebral artery, which were most likely due to thromboembolic events. A highly variable degree of astrogliosis was seen in all patients, with 37 patients (86%) showing astrogliosis in all assessed regions. Diffuse activation of microglia, with occasional microglial nodules, was pronounced in the brainstem and cerebellum. Additionally, we found distinct positive staining for HLA-DR in subpial and subependymal regions, a pattern not commonly observed in classic encephalitis. Parenchymal and perivascular microglia expressed the lysosomal marker CD68 while retaining the microglia core marker TMEM119 on their surfaces (Figure 1, Figure 2 ; appendix p 5).
Figure 1.
Common neuropathological findings in the brains of patients who died from COVID-19
An overview of each brain region with haematoxylin and eosin staining is shown in the first column. Immunohistochemical staining for the astrocytic marker GFAP showed variable degrees of reactive astrogliosis. Immunohistochemical staining for the microglia marker HLA-DR showed reactive activation of the microglia with occasional microglial nodules in the medulla oblongata and cerebellum (green arrows). Staining for the cytotoxic T lymphocyte marker CD8 (brown) revealed perivascular and parenchymal infiltration with CD8-positive cells. GFAP=glial fibrillary acidic protein.
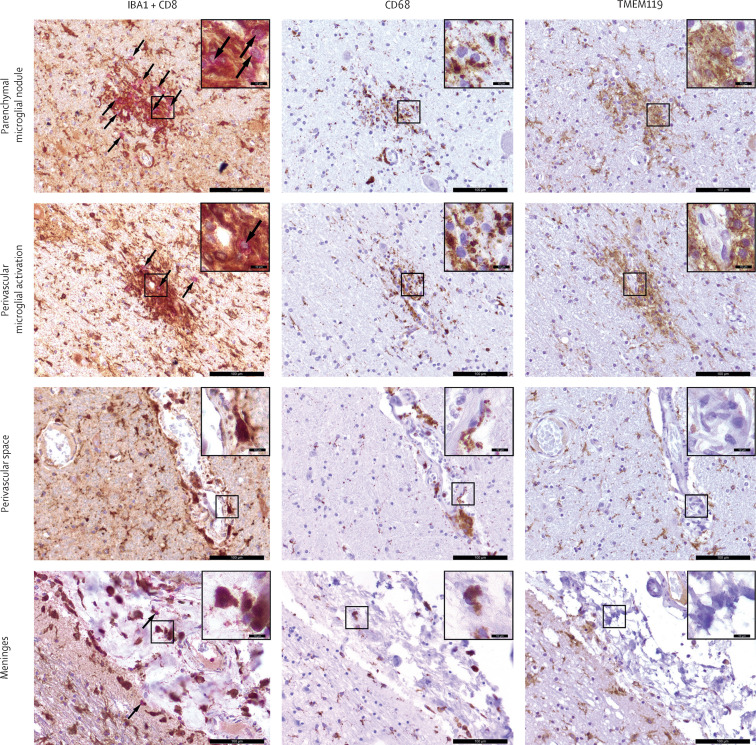
Figure 2.
Concomitant activation of the adaptive and innate immune systems in the brain of one patient (case 2) who died from COVID-19
Representative images of double-chromogenic immunohistochemical labelling for IBA1 (brown) and CD8 (pink), as well as immunohistochemical staining for CD68 (brown), and TMEM119 (brown) at different CNS interfaces in the upper medulla oblongata. Counterstaining was done with haematoxylin (blue). Scale bars represent 100 μm (10 μm in the inset images). Arrows indicate CD8-positive T cells.
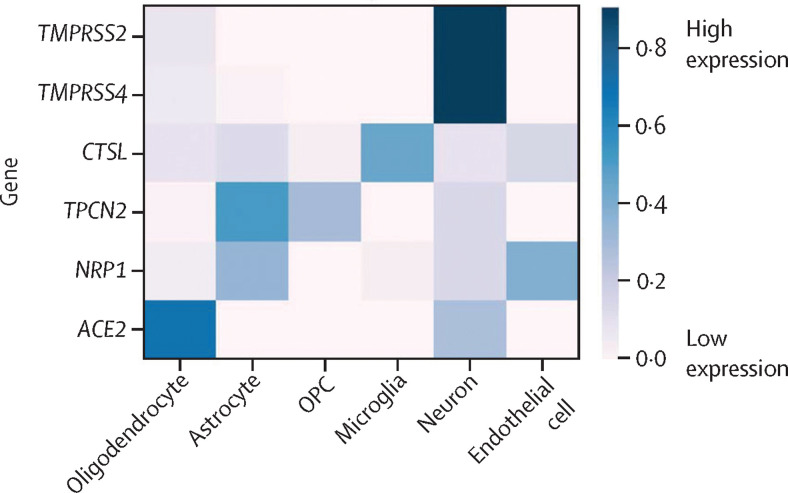
To assess which cell types in the CNS might be prone to SARS-CoV-2 infection, we did an in-silico analysis of publicly available datasets. The analysis focused on cell-specific expression within the cerebral cortex of genes that have been shown to contribute to viral entry into the cell27 and viral persistence,25 including ACE2, TMPRSS2, TPCN2, TMPRSS4, NRP1, and CTSL. Our analysis showed that these genes are expressed in neurons, glial cells, and endothelial cells, suggesting their possible capacity to support SARS-CoV-2 infection. Expression of ACE2 was highest in oligodendrocytes, while TMPRSS2 and TMPRSS4 were highest in neurons, CTSL was highest in microglia, and TPCN2 was highest in astrocytes (figure 3 ).
Figure 3.
In-silico analysis of the distribution of genes relevant to severe acute respiratory syndrome coronavirus 2 in the CNS
Human temporal lobe cell type-specific expression of TMPRSS2, TMPRSS4, CTSL, TPCN2, NRP1, and ACE2. The heatmap shows the per-gene normalised mean expression across cell types (expression sums to 1 across the cell types). OPC=oligodendrocyte precursor cell.
Cytotoxic T lymphocytes could be seen in small amounts (up to 49 cells per HPF) in the frontal cortex and basal ganglia, but their presence was more pronounced in the brainstem, where they were mostly concentrated in perivascular regions but were also observed in the parenchyma as clusters in close vicinity to IBA-1-positive microglia (Figure 1, Figure 4 ). Infiltration of the meningeal compartments by cytotoxic T lymphocytes was seen in 34 (79%) patients (moderate in six patients and mild in 28 patients; table, figure 4), with an enrichment of IBA-1-postive, CD68-positive, and TMEM119-negative perivascular and meningeal macrophages (figure 2). The olfactory bulb showed a high degree of astrogliosis and microgliosis, but only minor infiltration by cytotoxic T lymphocytes (Figure 1, Figure 4).
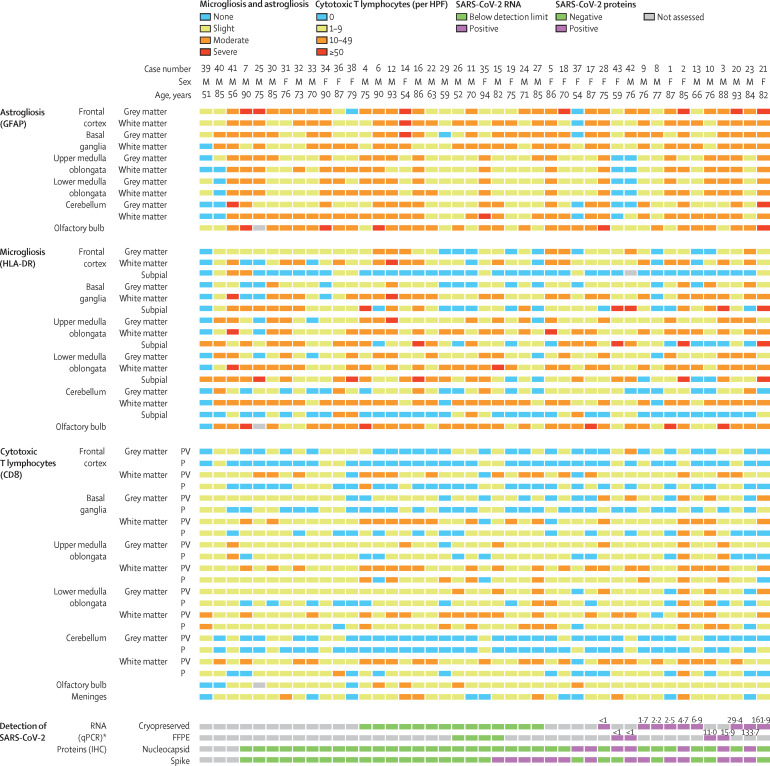
Figure 4.
Neuropathological findings and SARS-CoV-2 viral loads in studied patients (n=43)
Cases are arranged from left to right on the basis of the presence and quantity of SARS-CoV-2 in the brain. F=female. FFPE=formalin-fixed paraffin-embedded. HPF=high-power field. IHC=immunohistochemistry. M=male. P=parenchymal. PV=perivascular. qPCR=quantitative PCR. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. *Values shown for positive cases represent number of copies of SARS-CoV-2 RNA (× 103/mL); detection was done in the frontal lobe in cryopreserved specimens and in the upper medulla oblongata in FFPE specimens.
SARS-CoV-2 RNA was detected by qRT-PCR in cryopreserved frontal lobe tissue from nine (39%) of 23 patients with available samples, and in FFPE medulla oblongata tissue from four (50%) of eight patients with available samples. In total, SARS-CoV-2 was found in the brain tissues of 13 (48%) of 27 patients who had at least one available sample (four patients had both types of sample available; figure 4). A median 4700 copies of SARS-CoV-2 RNA were detected (IQR 1350–29 400; range <1000 to 1·62 × 105) among these 13 cases.
Samples from 40 (93%) patients underwent immunohistochemical staining for SARS-CoV-2 spike and nucleocapsid proteins. SARS-CoV-2-positive structures (cells and nerve fibres) were found scattered throughout the brain tissue. In eight (61%) of the 13 cases for which SARS-CoV-2 was detected in the brain by qRT-PCR, at least one SARS-CoV-2 protein could be detected (both spike and nucleocapsid in four cases, spike alone in three cases, and nucleocapsid alone in one case). Notably, in eight patients who were untested or tested negative on qRT-PCR analysis of SARS-CoV-2 RNA in the brain tissues, viral proteins were detectable by immunohistochemistry in the medulla oblongata (spike and nucleocapsid in one case, nucleocapsid alone in one case, and spike alone in six cases; figure 4). In the 16 (40%) cases positive for SARS-CoV-2 proteins on immunohistochemistry, spike protein was detected much more frequently (14 [88%] cases) than nucleocapsid protein (seven [44%] cases). By immunohistochemistry, SARS-CoV-2 could be mapped to isolated cells within the medulla oblongata and in the cranial nerves (either the glossopharyngeal or vagal nerves) originating from the brainstem (figure 5 , appendix p 4). Overall, SARS-CoV-2 RNA or proteins were detected in the brain tissues of 21 (53%) of the 40 investigated patients, with eight (20%) patients having both SARS-CoV-2 RNA and protein detected (figure 4).
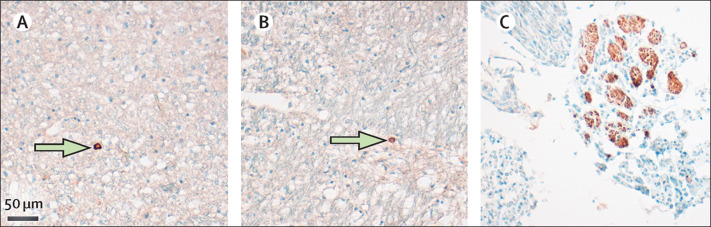
Figure 5.
Distribution of SARS-CoV-2 within the CNS
Representative images of viral protein-positive cells (green arrows) in the medulla oblongata detected by anti-nucleocapsid protein antibody (A) or anti-spike protein antibody (B). (C) SARS-CoV-2 nucleoprotein (brown staining) could also be detected in subsets of cranial nerves originating from the lower brainstem. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Cases 2, 16, 21, and 41 showed more brainstem inflammation (in terms of infiltration by cytotoxic CD8-positive T cells or activation of microglia) than all others (figure 4). Among these four cases, viral proteins were detected in the brain of one patient (case 2) and viral RNA in the brains of two patients (cases 2 and 21), and none died under ICU treatment.
Discussion
To our knowledge, this is the most comprehensive report of neuropathological findings of patients who died from COVID-19. In this post-mortem case series, we observed substantial yet highly variable degrees of astrogliosis in all assessed regions. Astrocytes are key regulators of homoeostasis, responding to stimuli through upregulation of GFAP and astroglial hypertrophy.28 Because astrogliosis occurs in a variety of pre-existing medical conditions, and because critical illness also contributes to astrogliosis, a causal connection to SARS-CoV-2 cannot be drawn at present.
Activation of microglia and infiltration of cytotoxic T lymphocytes were mostly confined to the brainstem and cerebellum, with little involvement of the frontal lobe, in line with clinical findings pointing to an involvement of the brainstem.5 The staining pattern of activated microglia with occasional microglial nodules is reminiscent of mild viral and autoimmune encephalitides.29 We observed cytotoxic T lymphocytes in close vicinity to IBA-1-positive microglia, suggesting that these glial cells activate lymphocytes and potentially induce T-cell stimulation.30 Microglia strongly expressed the lysosomal marker CD68, indicating their increased phagocytic activity. Notably, highly activated phagocyting microglia retained the microglial core marker TMEM119 on their surfaces. Anosmia has been linked to COVID-19,5 and might be related to the pronounced astrogliosis and microgliosis in the olfactory bulb observed in this study.
Clinically, nuchal rigidity has been identified in patients with COVID-19 as a possible sign of SARS-CoV-2-associated meningitis.10 We found mostly mild meningeal infiltrates consisting of cytotoxic T lymphocytes, most likely indicative of non-specific meningeal reaction rather than viral meningitis.
CNS involvement with destructive lesions has been documented in cases of SARS-CoV infection.31, 32 Additionally, influenza A virus infects the CNS in some patients with a severe disease course. In these patients, acute subarachnoid haemorrhage and acute necrotising encephalopathy with multifocal brain lesions have been observed,29 and such findings have also been reported in one patient with COVID-19.4 Pan-encephalitis and intracranial haemorrhage19 in addition to myelin loss has been described in patients with COVID-19.18 In our cohort, we did not find evidence of myelin loss, cerebral bleeding, or acute necrotising lesions, congruent with previous findings.16 Whether the absence of these pathologies was due to the small number of patients in our study or because they are not caused by SARS-CoV-2 remains an open question. Intracranial haemorrhagic lesions can also occur as a result of necessary treatments in ICUs, such as extracorporeal membrane oxygenation.33 Some patients with COVID-19 present with neurological symptoms indicative of cerebral ischaemia, and, in patients younger than 50 years of age, large-vessel stroke has been proposed to constitute a presenting feature.7 We observed signs of fresh territorial ischaemic lesions in six (16%) patients, of whom four were older than the average age of our sample and two were younger (63 years and 59 years). Thus, patients with stroke were not disproportionately younger among our cases. Morphologically, the ischaemic lesions followed a vascular pattern and presumably were of thromboembolic origin. These data are in line with previously published data showing thromboembolic events in a proportion of patients with COVID-19.2
SARS-CoV-2 was detected by qRT-PCR or immunostaining in the brains of 21 (53%) of all tested patients. Furthermore, immunohistochemical analysis revealed viral proteins in the cranial nerves (either glossopharyngeal or vagal) originating from the lower medulla oblongata and in single cells within the medulla oblongata. Although qRT-PCR might lack sensitivity, and immunostaining for viral proteins is prone to artifacts, our findings are consistent with those of previous studies of SARS-CoV, in which viral proteins could be seen in single cells in the brains of some patients who died following infection with the virus.31 Thus, effects of SARS-CoV-2 on the brainstem could be correlates of, or contribute to, unusually rapidly deteriorating respiratory function, as has been observed in some patients with COVID-19 given non-invasive ventilation.34
The presence of SARS-CoV-2 RNA and proteins in the brains of patients with COVID-19 in this study is in line with the hypothesis that SARS-CoV-2 can infiltrate the CNS.14 However, the presence of SARS-CoV-2 was not associated with the severity of neuropathological changes. Thus, CNS damage and neurological symptoms might be due to additional factors such as cytokine storm, neuroimmune stimulation, and systemic SARS-CoV-2 infection, rather than by direct CNS damage caused by the virus. We saw a surprisingly uniform presentation of neuropathological findings (ie, activation of microglia, infiltration with CD8-positive T cells) in our patients, irrespective of the clinical severity of COVID-19 in each case. Notably, the neuropathological presentation in patients who died in a domestic setting or in a nursing home did not differ from that in patients who died in hospital wards or ICUs.
The main limitation of our study is its descriptive nature and the absence of age-matched and sex-matched controls. Our study cohort was assembled during the peak of the SARS-CoV-2 pandemic and, for logistic reasons, simultaneous collection of case controls was not feasible, and it was not possible to use historical controls because of differences in sampling protocols. Thus, the proposed mechanisms of viral entry, viral replication, and putative pathophysiological principles underlying tissue damage must be interpreted in this context. Furthermore, we assessed only a small number of post-mortem specimens, and the selected regions might not be fully representative of the whole brain. Sample preservation could have influenced the analyses. Additionally, due to the heterogeneity of the places of death of studied patients, no systematically validated clinical data (eg, systematically documented neurological information) were available, and establishing conclusive clinicopathological correlations was not possible.
In summary, our results show that SARS-CoV-2 RNA and proteins can be detected in the CNS. The brain shows mild neuropathological changes with pronounced neuroinflammation in the brainstem being the most common finding. However, the presence of SARS-CoV-2 in the CNS was not associated with the severity of neuropathological changes.16, 17 Careful neuropathological interpretation will be essential to disentangle which changes are attributable to SARS-CoV-2. All such changes must be mapped against neuropathological changes caused by pre-existing medical conditions often present in patients with COVID-19, as well as neuropathological changes caused by invasive treatments that are used in severe cases of COVID-19.35
Data sharing
Data collected for the study and data from sample analyses will be made available upon reasonable request to the corresponding author.
Acknowledgments
Acknowledgments
We thank Ulrike Rumpf, Claudia Oye Attah, and Kristin Hartmann (Institute of Neuropathology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany) for technical help. MG was supported by the German Research Foundation (SFB877). ML, MDa, LA, and MG were supported by Hamburg state research funding (Landesforschungsförderung; “mechanisms of cell-communication during infection”). DSM and SB were supported by eRARE Maxomod and the German Research Foundation (SFB1286).
Contributors
JM, CH, and MG designed the study. MG and JM wrote the manuscript. JM, ML, JPS, ASS, CE, LA, MDa, AH, AF, SP, SB, HM, KP, SK, MA, MP, MS, and MG performed experiments and collected or analysed the data. ML, LA, MDa, SK, and MA analysed the presence and distribution of the virus. JM, CH, SK, MP, MS, and MG performed morphological analyses. DSM and SB performed in single-cell gene expression analysis. All authors discussed the results. JM, SK, MDo, MP, MS, and MG created the figures. MG and CG reviewed and discussed clinicopathological interpretations. All authors read, edited, and approved the manuscript.
Declaration of interests
MDa reports grants from the German Center for Infection Research during the conduct of the study and grants from the German Research Foundation outside the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Zou L, Ruan F, Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wichmann D, Sperhake JP, Lutgehetmann M. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varga Z, Flammer AJ, Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;296 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao L, Jin H, Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helms J, Kremer S, Merdji H. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oxley TJ, Mocco J, Majidi S. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YH, Jiang D, Huang JT. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav Immun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernard-Valnet R, Pizzarotti B, Anichini A. Two patients with acute meningo-encephalitis concomitant to SARS-CoV-2 infection. Eur J Neurol. 2020;27:e43–e44. doi: 10.1111/ene.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moriguchi T, Harii N, Goto J. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez-Ortiz C, Mendez A, Rodrigo-Rey S. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–e605. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toscano G, Palmerini F, Ravaglia S. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Felice FG, Tovar-Moll F, Moll J, Munoz DP, Ferreira ST. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the central nervous system. Trends Neurosci. 2020;43:355–357. doi: 10.1016/j.tins.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon IH, Normandin E, Bhattacharyya S. Neuropathological features of COVID-19. N Engl J Med. 2020 doi: 10.1056/nejmc2019373. published online June 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaller T, Hirschbuhl K, Burkhardt K. Postmortem examination of patients with COVID-19. JAMA. 2020;323 doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Weyhern CH, Kaufmann I, Neff F, Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395:e109. doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puelles VG, Lutgehetmann M, Lindenmeyer MT. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darmanis S, Sloan SA, Zhang Y. A survey of human brain transcriptome diversity at the single cell level. Proc Natl Acad Sci USA. 2015;112:7285–7290. doi: 10.1073/pnas.1507125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marouf M, Machart P, Bansal V. Realistic in silico generation and augmentation of single-cell RNA-seq data using generative adversarial networks. Nat Commun. 2020;11:166. doi: 10.1038/s41467-019-14018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfefferle S, Reucher S, Norz D, Lutgehetmann M. Evaluation of a quantitative RT-PCR assay for the detection of the emerging coronavirus SARS-CoV-2 using a high throughput system. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.9.2000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tichopad A, Dilger M, Schwarz G, Pfaffl MW. Standardized determination of real-time PCR efficiency from a single reaction set-up. Nucleic Acids Res. 2003;31:e122. doi: 10.1093/nar/gng122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockx B, Kuiken T, Herfst S. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368:1012–1015. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edler C, Schroder AS, Aepfelbacher M. Dying with SARS-CoV-2 infection—an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann M, Kleine-Weber H, Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271. doi: 10.1016/j.cell.2020.02.052. 80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verkhratsky A, Zorec R, Parpura V. Stratification of astrocytes in healthy and diseased brain. Brain Pathol. 2017;27:629–644. doi: 10.1111/bpa.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludlow M, Kortekaas J, Herden C. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol. 2016;131:159–184. doi: 10.1007/s00401-015-1511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tröscher AR, Wimmer I, Quemada-Garrido L. Microglial nodules provide the environment for pathogenic T cells in human encephalitis. Acta Neuropathol. 2019;137:619–635. doi: 10.1007/s00401-019-01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu J, Gong E, Zhang B. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desforges M, Le Coupanec A, Stodola JK, Meessen-Pinard M, Talbot PJ. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. 2014;194:145–158. doi: 10.1016/j.virusres.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Guennec L, Cholet C, Huang F. Ischemic and hemorrhagic brain injury during venoarterial-extracorporeal membrane oxygenation. Ann Intensive Care. 2018;8:129. doi: 10.1186/s13613-018-0475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glatzel M. Neuropathology of COVID-19: where are the neuropathologists? Brain Pathol. 2020;30:729. doi: 10.1111/bpa.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected for the study and data from sample analyses will be made available upon reasonable request to the corresponding author.