Abstract
Trial design:
The current study is a meta-analysis designed to assess the effect of adding magnesium to a combination of intrathecal bupivacaine and fentanyl.
Methods:
The protocol was registered in PROSPERO with the number CRD42020177618. PubMed, Cochrane library, Web of Science, and Google Scholar were searched for randomized controlled trials investigating the effect of adding magnesium to a combination of intrathecal bupivacaine and fentanyl. The continuous data were presented as Ratio of means (RoM). Risk ratio (RR) along with 95% confidence interval (CI) was utilized to assess the dichotomous data.
Results:
Ten trials were involved in the present study with 720 adult patients. Compared with control, intrathecal magnesium prolonged time to the first analgesic requirement by an estimate of 1.23 (RoM: 1.23; 95%CI: 1.13–1.33; P < .00001), prolonged adequate sensory block duration for surgery by an estimate of 1.16 (RoM: 1.16; 95%CI: 1.05–1.27; P = .003), delayed time to maximum sensory level by an estimate of 1.38 (RoM: 1.38; 95%CI: 1.07–1.78; P = .01) and reduced the incidence of shivering following spinal anesthesia (risk ratio: 0.38; 95%CI: 0.18 to 0.81, P = .01) without influence on time to full motor recovery or incidences of hypotention, bradycardia, nausea, and vomiting or pruritis.
Conclusion:
Intrathecal magnesium, when added to a combination of intrathecal bupivacaine and fentany, prolongs the analgesic duration of spinal anesthesia without increased incidences of side effects.
Keywords: bupivacaine, fentanyl, magnesium sulphate, meta-analysis, spinal anesthesia
1. Introduction
Effective treatment of perioperative pain is important because it can blunt stress reaction, and then lead to a decreased perioperative morbidity.[1] Research continues on techniques and medicines that could provide optimal operative conditions and postoperative pain relief. Various medicines such as opiates, benzodiazepines, the N-methyl D-aspartate (NMDA) receptor antagonists, α2 agonists etc, have been used clinically as adjuvants in spinal anesthesia.
The use of small dose of opioid combined with nonopioid drug as adjuvant to local anesthetic in spinal anesthesia is becoming increasingly popular for perioperative pain management. Surgical stimuli can activate NMDA receptors, which are involved in central sensitization.[2,3] Magnesium, a kind of NMDA receptor antagonist, can block NMDA channels in a voltage-dependent way, and the addition of magnesium can reduce NMDA-induced currents.[4] Therefore, magnesium has antinociceptive effect and has application in spinal anesthesia.
There are an increasing number of papers suggesting that intrathecal magnesium added to bupivacaine-fentanyl spinal anesthesia can improve the anesthetic effect. However, the relative data are inconsistent. Therefore, this meta-analysis is conducted to investigate the effect of adding magnesium to a combination of intrathecal bupivacaine and fentanyl.
2. Methods
2.1. Search strategy
Neither ethical approval nor informed consent was necessary, since it was a systematic review and meta-analysis. The present study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations.[5] The protocol was registered in PROSPERO with the number CRD42020177618. Randomized controlled trials (RCTs) investigating the effect of adding magnesium to a combination of intrathecal bupivacaine and fentanyl were selected and reviewed.
2.2. Study selection
The literature search was performed by two reviewers in PubMed, Web of Science, Cochrane library, and Google Scholar independently.
The literature search was performed by using the MESH and keywords including: “magnesium”, “fentanyl”, “anesthesia, spinal”, “injection, spinal” and “injection, subarachnoid” without language limitation. We manually searched the reference lists of related papers to find additional eligible RCTs. The latest search was done on March 20, 2020.
RCTs investigating the efficacy of adding magnesium to a combination of intrathecal bupivacaine and fentanyl were sought. The literature research was limited to human studies of subjects aged equal to or more than 18 years. Meeting papers, correspondences or editorials were excluded. If an agreement could not be reached between these 2 reviewers, the opinion of a third reviewer was obtained.
2.3. Quality and risk of bias assessment
The risk of bias and the quality of RCTs were separately evaluated using the Cochrane Collaboration Risk of Bias tool and a 5 point Jadad scale by 2 of the reviewers.[6,7] A score less than 3 was taken as low methodological quality. The third reviewer was consulted when an agreement could not be reached.
2.4. Data extraction
Data collection was performed by 2 authors. If an agreement could not be achieved, a third reviewer joined to make a decision. Extracted data included authors, publication year, surgery setting, sample size, dosages of bupivacaine, and fentanyl for spinal anesthesia, magnesium dose, as well as data on block characteristics.
2.5. Statistical analysis
Review Manager 5.3 (Cochrane Library, Oxford, England) was utilized for statistical analysis. Because of significant clinical heterogeneity of doses of bupivacaine, fentanyl and magnesium, ratio of means (RoM), standard error, and 95% confidence intervals (CIs) were calculated for continuous data to assess change from baseline for continuous data, under the assumption of equal variances in log scale and log-normal distribution.[8,9,10,11] Dichotomous data were analyzed using risk ratio (RR) and CIs. Statistical significance was considered if P value was < .05.
3. Results
3.1. Literature search
Of 165 initial papers found, 149 papers were excluded after screening. Sixteen full-text articles were found and assessed in detail, then 10 RCTs including 720 adult patients were eligible in this meta-analysis.[12,13,14,15,16,17,18,19,20,21] The detailed flowchart of the selection was presented in Figure 1.
Figure 1.
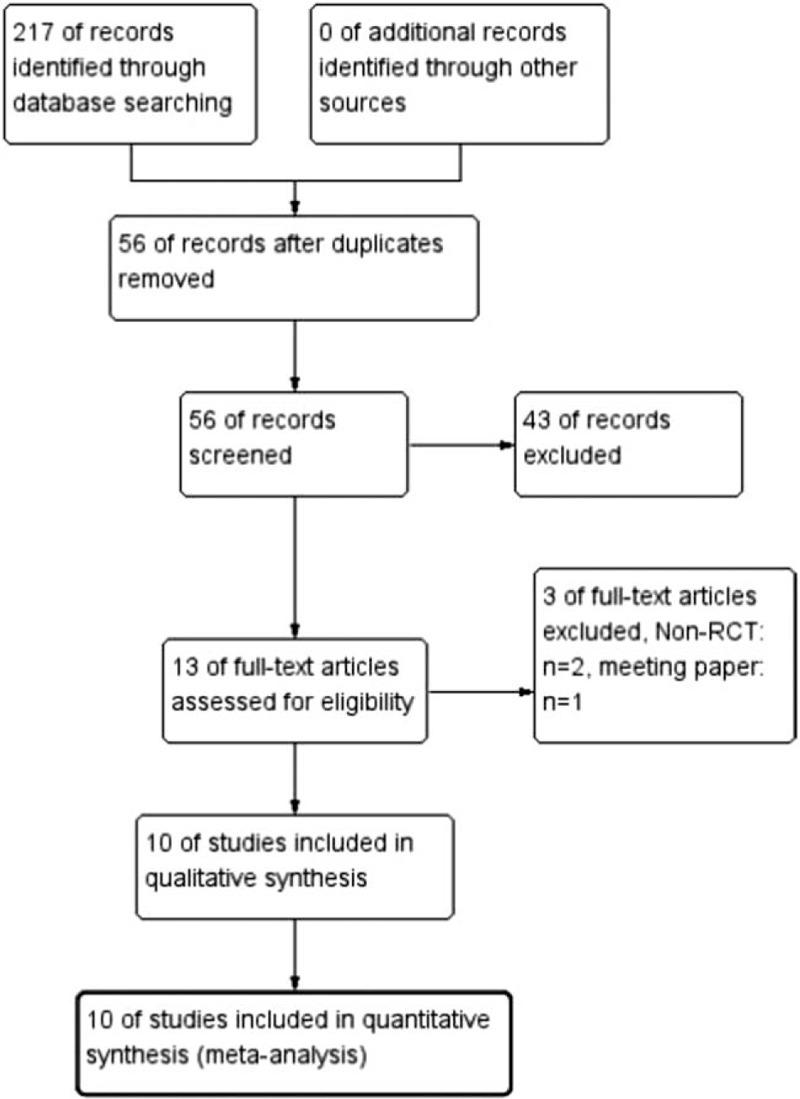
The flow chart of study selection.
3.2. Study characteristics
The details of the eligible RCTs were shown in Table 1. Intrathecal bupivacaine was used in all included trials, and the range of bupivacaine dosages used was 6 to 15 mg. The dosages of fentanyl combined with bupivacaine ranged from 10 to 25 μg. With the exception of one study[12] that used a 100 mg dose of magnesium sulphate, 50 mg magnesium was used in each of the reviewed trials.[13,14,15,16,17,18,19,20,21] The risk-of-bias plot was detailed in Figure 2.
Table 1.
Characteristics of the included randomized controlled trials.
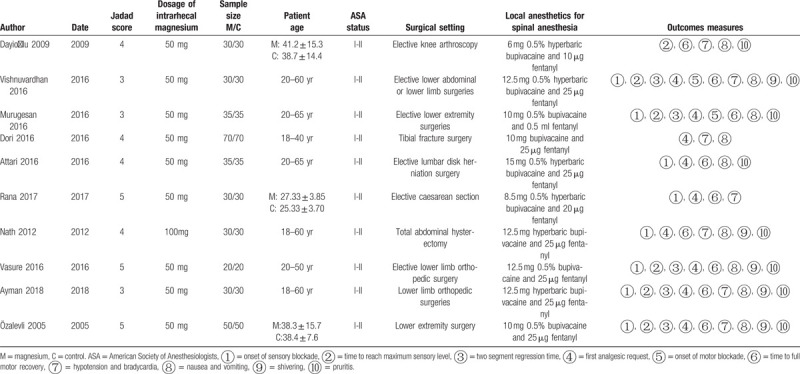
Figure 2.
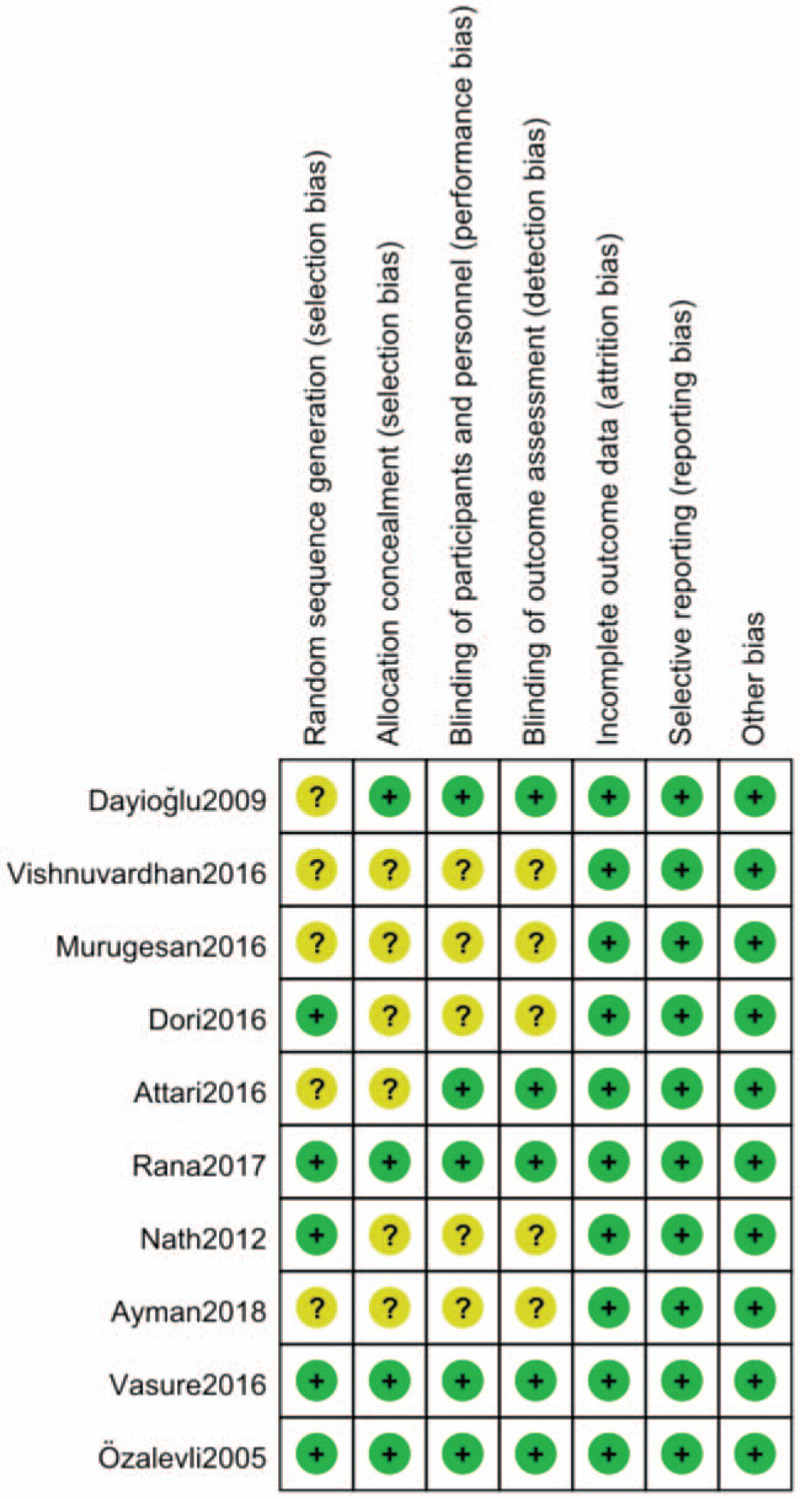
The risk of bias assessment of the included studies.
3.3. Time to the first analgesic requirement
The primary outcome was time to the first analgesic requirement which was considered as time period from intrathecal injection to the first analgesic request. Nine studies reported time to the first analgesic requirement.[12,13,14,15,16,17,18,19,20] Intrathecal magnesium prolonged time to the first analgesic requirement by an estimate of 1.23 (RoM: 1.23; 95%CI: 1.13–1.33; P < .00001; I2 = 96%) compared with control. (Fig. 3) Sensitivity analysis was conducted by removing each study individually. The reliability of the results was confirmed and no source of heterogeneity was found.
Figure 3.
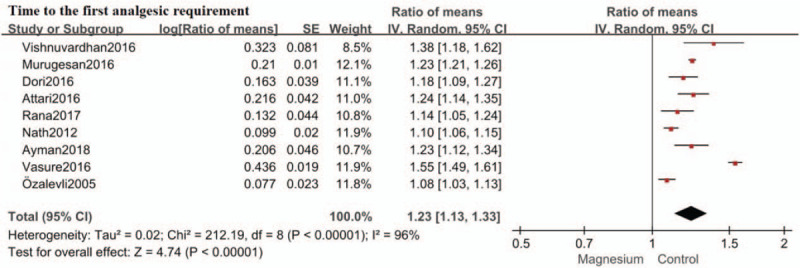
Forest plot for time to the first analgesic requirement. Confidence interval indicates confidence interval; IV = inverse variance, SE = standard error.
3.4. Time to maximum sensory level
Six studies evaluated time to maximum sensory level.[13,14,15,16,17,21] Intrathecal magnesium delayed time to maximum sensory level by an estimate of 1.38 (RoM: 1.38; 95%CI: 1.07–1.78; P = .01; I2 = 97%) compared with control (Fig. 4). Sensitivity analysis was conducted by removing each study individually. The reliability of the results was confirmed and no source of heterogeneity was found.
Figure 4.
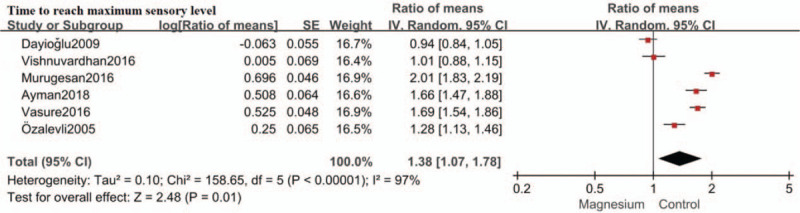
Forest plot for time to reach maximum sensory level. Confidence interval indicates confidence interval, IV = inverse variance, SE = standard error.
3.5. Adequate sensory block duration
Adequate sensory block duration for surgery was defined as two segment regression time in 5 RCTs[13,14,15,16,17] and defined as time to T10 regression in 2 RCTs.[18,19] Therefore, adequate sensory block duration for surgery was assessed in 7 trials.[13,14,15,16,17,18,19] Intrathecal magnesium prolonged adequate sensory block duration by an estimate of 1.16 (RoM: 1.16; 95%CI: 1.05–1.27; P = .003, I2 = 93%) compared with control. (Fig. 5) Sensitivity analysis was conducted by removing each study individually. The reliability of the results was confirmed and no source of heterogeneity was found.
Figure 5.
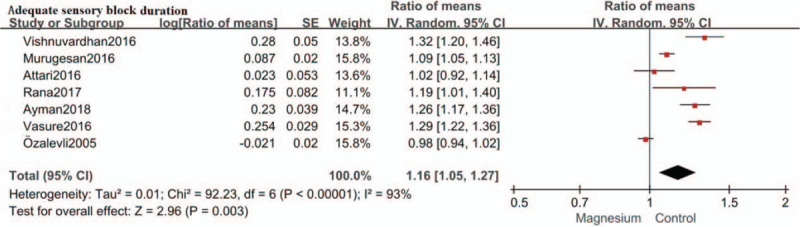
Forest plot for adequate sensory block duration. Confidence interval indicates confidence interval, IV = inverse variance, SE = standard error.
3.6. Time to full motor recovery
The effect of intrathecal magnesium on time to full motor recovery was described in 8 studies reviewed.− No significant difference was found in time to full motor recovery between the magnesium group and the control group (RoM: 1.07; 95%CI: 0.99–1.16; P = .11, I2 = 93%). (Fig. 6) Sensitivity analysis was conducted by removing each study individually. The reliability of the results was confirmed and no source of heterogeneity was found.
Figure 6.
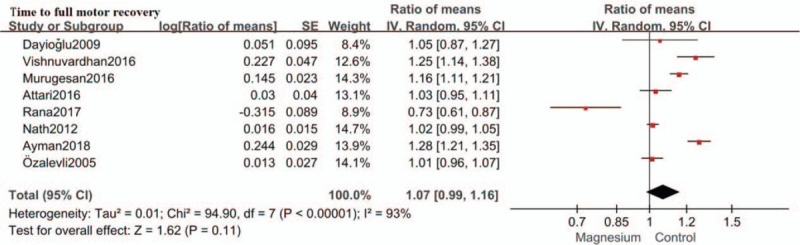
Forest plot for time to full motor recovery. Confidence interval indicates confidence interval, IV = inverse variance, SE = standard error.
3.7. The incidence of hypotention and bradycardia
Seven studies[12,13,15,17,19,20,21] reported the incidence of hypotension and 5 studies[12,13,17,20,21] reported the incidence of bradycardia. For I2 = 0%, the fixed effect model was used for meta-analysis. Our study demonstrated that intrathecal magnesium did not increase the incidence of hypotention (RR: 0.98; 95%CI: 0.70–1.37, P = .91; I2 = 0%) (Fig. 7A) and bradycardia (RR: 0.78; 95%CI: 0.44 to 1.39, P = .40; I2 = 0%; Fig. 7B), compared with control.
Figure 7.
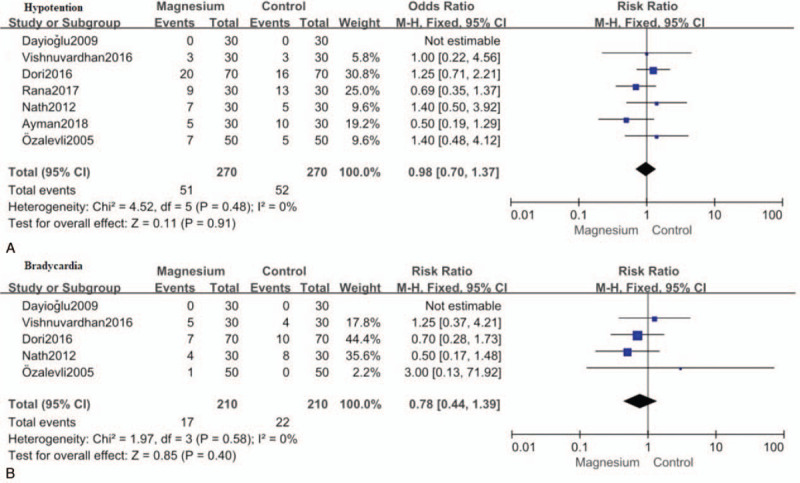
Forest plot for the incidence of hypotention (A) and bradycardia (B). CI = confidence interval, M-H = Mantel-Haenszel.
3.8. The incidence of nausea and vomiting
The incidence of nausea and vomiting was reported in all but 1 study.[19] The result showed the difference was not statistically significant in the incidence of nausea and vomiting between the magnesium group and the control group (RR: 0.90; 95%CI: 0.56 to 1.46, P = .68; I2 = 0%). (Fig. 8)
Figure 8.
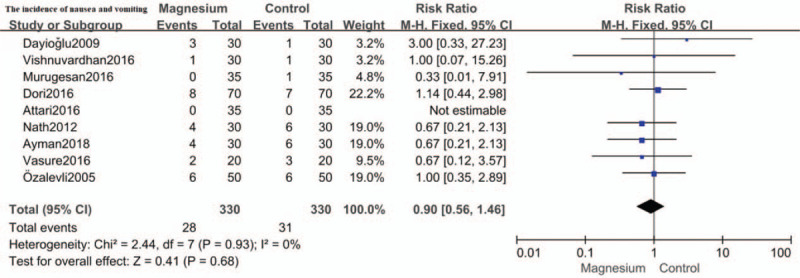
Forest plot for the incidence of nausea and vomiting. CI = confidence interval, M-H = Mantel-Haenszel.
3.9. The incidence of shivering
The incidence of shivering following spinal anesthesia was assessed in 5 trials,[12,13,15,16,17] permitting quantitative analysis. Intrathecal magnesium was associated with lower incidence of shivering following spinal anesthesia (RR: 0.38; 95%CI: 0.18–0.81, P = .01; I2 = 0%). (Fig. 9)
Figure 9.
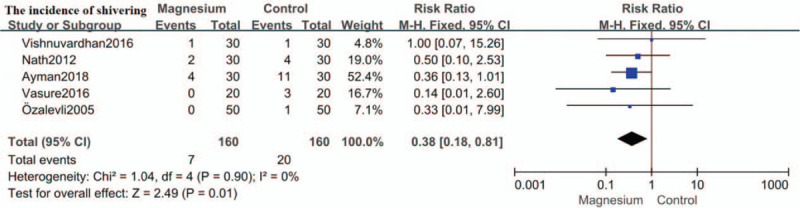
Forest plot for the incidence of shivering. CI = confidence interval, M-H = Mantel-Haenszel.
3.10. The incidence of pruritis
The incidence of pruritis was assessed in 8 trials.[12,13,14,15,16,17,18,21] Compared to placebo, no significant association of intrathecal magnesium with pruritis was found (RR: 0.89; 95%CI: 0.54 to 1.47, P = .65; I2 = 0%). (Fig. 10)
Figure 10.
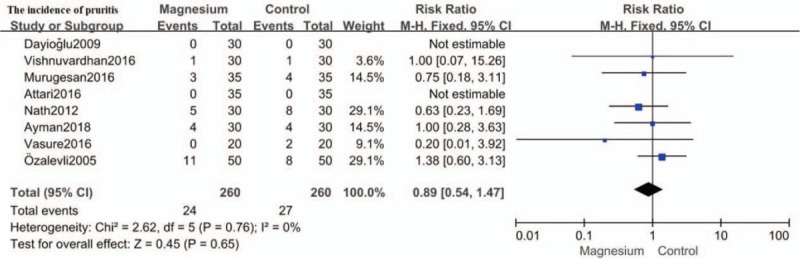
Forest plot for the incidence of pruritis. CI = confidence interval, M-H = Mantel-Haenszel.
4. Discussion
This meta-analysis demonstrates that addition of intrathecal magnesium is valuable for patients under bupivacaine-fentanyl spinal anesthesia.
The results of this meta-analysis indicate that intrathecal magnesium, when added to a combination of intrathecal bupivacaine and fentany, prolongs time to the first analgesic requirement and adequate sensory block duration for surgery, leads to a significant delay in time to maximum sensory level, and reduces the incidence of post-spinal anesthesia shivering. In addition, intrathecal magnesium sulfate does not influence time to full motor recovery or increase the incidences of hypotension, bradycardia, nausea, and vomiting or pruritis.
Magnesium sulfate is a kind of NMDA receptor antagonist. This prolongation of sensory block resulting from intrathecal magnesium is due to synergistic interaction between intrathecal local anesthetics and NMDA antagonists. Magnesium sulfate can be used as an adjuvant for intrathecal block, because it can diminish neuronal excitation caused by activation of C-fibres.[22] There are evidences suggesting that activation of the NMDA receptors is involved in both hyperalgesia after tissue injury and the development of central sensitization.[23] NMDA receptor antagonists can not only inhibit central sensitization caused by peripheral pain stimulation, but also blunt such hypersensitivity if it is formed up.[24]
The safety of intrathecal magnesium has been assessed in rat and canine studies, and no neurological deficit or histopathological change is observed after intrathecal magnesium administration.[25] In this meta-analysis, there are no serious complications associated with intrathecal magnesium reported in the included 10 RCTs.[12,13,14,15,16,17,18,19,20,21] Therefore, magnesium seems to be safe for intrathecal administration.
This meta-analysis has 2 limitations. First, statistical heterogeneity is high for some outcomes, making combination of the RCTs debatable, because of various outcome measures among the eligible RCTs. Second, we use the RoM method with assumption of equal variances and lognormal distributions. The assumption is acceptable but can not be absolutely confirmed as lacking of individual patient's data.[10]
5. Conclusion
Intrathecal magnesium, when added to a combination of intrathecal bupivacaine and fentany, prolongs the analgesic duration of spinal anesthesia, without increased incidences of side effects.
Author contributions
XXX.
Footnotes
Abbreviations: CI = confidence interval, NMDA = N-methyl D-aspartate, RCT = randomized controlled trials, RoM = ratio of means, RR = relative risk.
How to cite this article: Wang J, Wang Z, Shi B, Wang N. The effect of adding intrathecal magnesium sulphate to bupivacaine-fentanyl spinal anesthesia: a meta-analysis of randomized controlled trials. Medicine. 2020;99:40(e22524).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Sirvinskas E, Laurinaitis R. Use of magnesium sulphate in anesthesiology. Medicina 2002;38:695–8. [PubMed] [Google Scholar]
- [2].Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl D-aspartic acidreceptor activation: implications for the treatment of post-injury pain and hypersensitivity states. Pain 1991;44:293–9. [DOI] [PubMed] [Google Scholar]
- [3].Woolf CJ, Chong MS. Preemptive analgesia: treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 1993;77:362–79. [DOI] [PubMed] [Google Scholar]
- [4].Ascher P, Nowak L. Electrophysiological studies of NMDA receptors. Trends Neurosci 1987;10:284–8. [Google Scholar]
- [5].Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [6].Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration 2011; version 5.1.0. Available at: www.cochrane-handbook.org. [Google Scholar]
- [7].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [8].Friedrich JO, Adhikari NK, Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: a simulation study. BMC Med Res Methodol 2008;8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Friedrich JO, Adhikari NK, Beyene J. Ratio of means for analyzing continuous outcomes in meta-analysis performed as well as mean difference methods. J Clin Epidemiol 2011;64:556–64. [DOI] [PubMed] [Google Scholar]
- [10].Chang KV, Wu WT, Han DS, et al. Ulnar nerve cross-sectional area for the diagnosis of cubital tunnel syndrome: a meta-analysis of ultrasonographic measurements. Arch Phys Med Rehabil 2018;99:743–57. [DOI] [PubMed] [Google Scholar]
- [11].Chang KV, Wu WT, Hung CY, et al. Comparative effectiveness of suprascapular nerve block in the relief of acute post-operative shoulder pain: a systematic review and meta-analysis. Pain Physician 2016;19:445–56. [PubMed] [Google Scholar]
- [12].Nath MP, Garg R, Talukdar T, et al. To evaluate the efficacy of intrathecal magnesium sulphate for hysterectomy under subarachnoid block with bupivacaine and fentanyl: A prospective randomized double blind clinical trial. Saudi J Anaesth 2012;6:254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vishnuvardhan V, Hemalatha S, Sarika MS, et al. Effects of adding intrathecal magnesium sulphate to bupivacaine and fentanyl in lower abdominal and lower limb surgeries. IOSR J Dental Med Sci 2016;15:44–8. [Google Scholar]
- [14].Murugesan BS, Ramakrishnan CD. The effect of adding intrathecal magnesium sulphate to bupivacaine-fentanyl spinal anaesthesia. J Evolution Med Dent 2016;5:7185–91. [Google Scholar]
- [15].Ayman AM, Moustafa A, Sayed MA. Comparison between 3 different doses of magnesium sulphate as a spinal adjuvant to bupivacaine and fentanyl combination in lower limb orthopedic surgery. Med J Cairo Univ 2018;86:3253–62. [Google Scholar]
- [16].Vasure R, Ashahiya ID, Mahendra R, et al. Comparison of effect of adding intrathecal magnesium sulfate to bupivacaine alone and bupivacaine-fentanyl combination during lower limb orthopedic surgery. Int J Sci Study 2016;3:141–6. [Google Scholar]
- [17].Özalevli M, Cetin TO, Unlugenc H, et al. The effect of adding intrathecal magnesium sulphate to bupivacaine-fentanyl spinal anaesthesia. Acta Anaesthesiologica Scandinavica 2005;49:1514–9. [DOI] [PubMed] [Google Scholar]
- [18].Attari MA, Najafabadi FM, Talakoob R, et al. Comparison of the effects of 3 methods of intrathecal bupivacaine, bupivacaine-Fentanyl, and bupivacaine-fentanyl-magnesium sulfate on sensory motor blocks and postoperative pain in patients undergoing lumbar disk herniation surgery. J Neurosurg Anesthesiol 2016;28:38–43. [DOI] [PubMed] [Google Scholar]
- [19].Rana S, Singha D, Kumar S, et al. Efficacy of magnesium sulphate and/or fentanyl as adjuvants to intrathecal low-dose bupivacaine in parturients undergoing elective caesarean section. J Obstet Anaesth Crit Care 2017;7:20–5. [Google Scholar]
- [20].Dori MM, Foruzin F. The analgesic efficacy of intrathecal bupivacaine and fentanyl with added neostigmine or magnesium sulphate. Anesth Pain Med 2016;6:e9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dayioğlu H, Baykara ZN, Salbes A, et al. Effects of adding Magnesium to bupivacaine and fentanyl for spinal anesthesia in knee arthroscopy. J Anesth 2009;23:19–25. [DOI] [PubMed] [Google Scholar]
- [22].Dickenson AH. NMDA receptor antagonists: interactionwith opioids. Acta Anaesthesiol Scand 1997;41:112–5. [DOI] [PubMed] [Google Scholar]
- [23].Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia andmorphine tolerance: a current view of their possible interactions. Pain 1995;62:259–74. [DOI] [PubMed] [Google Scholar]
- [24].Ascher P, Nowak L. Electrophysiological studies of NMDAreceptors. Trends Neurosci 1987;10:284–8. [Google Scholar]
- [25].Simpson JI, Eide TR, Schiff GA. Intrathecal magnesiumsulfate protects the spinal cord from ischemic injury duringthoracic aortic cross clamping. Anesthesiology 1994;81:1493–9. [PubMed] [Google Scholar]


