Supplemental Digital Content is available in the text
Keywords: colon cancer, quality, surgery, survival, textbook outcome
Abstract
The aim of this study was to investigate the relationship between textbook outcome and survival in patients with surgically treated colon cancer. A total of 804 surgical cases were enrolled between June 1, 2010 and December 31, 2014. Textbook outcome was defined as patients who had colon cancer surgery and met the six healthcare parameters of surgery within 6 weeks, radical resection, lymph node (LN) yield ≥12, no ostomy, no adverse outcome and colonoscopy before/after surgery within 6 months. The effect of textbook outcome on 5-year disease-specific survival (DSS) was calculated using the Kaplan-Meier method. A Cox regression model was used to find significant independent variables and stratified analysis used to determine whether text-book outcome had a survival benefit. A textbook outcome was achieved in 59.5% of patients undergoing colon cancer surgery. Important obstacles to achieving textbook outcome were no stomy, no adverse outcome and LN yield ≥12. Patients with text-book outcome had statistically significant better 5-year DSS compared to those with-out (80.1% vs. 58.3%). Multivariate analyses indicated that colon cancer patients with textbook outcome had better 5-year DSS after adjusting for various confounders ([aHR], 0.44; 95% CI, 0.34–0.57). Thus, besides being an index of short-term quality of care, textbook outcomes could be used as a prognosticator of long-term outcomes, such as 5-year survival rates.
1. Introduction
Colon cancer remains one of the most commonest type of cancer that contributes to increasing cancer-related death worldwide.[1] For colon cancer, tumor resection with or without chemotherapy is the cornerstone of treatment.[2] With the advancement of these multimodal treatments, outcomes have improved in recent decades.[3] Nevertheless, significant differences in survival still exist. In the past years, quality assurance has been acknowledged as a crucial factor in the assessment of oncological surgical care and prognosis[4]; however, the influence of quality of care on colon cancer survival has not been fully investigated.
Literatures had revealed the importance of hospital accreditation and its positive impact on quality improvement.[5,6] The Quality Integration Committee of the Commission on Cancer had developed quality measures, which relied on whether the institution had reached the estimated performance rate of different indicators such as rate of 85% for at least 12 regional lymph nodes removed in resected colon cancer, or rate of 80% for needle biopsy performed to establish diagnosis of breast cancer.[7] However, most of the accreditation of cancer treatment mentioned some of these quality indicators, instead all of them.[5] Thus, when we examined the performance between hospitals, the superiority of one quality of care indicator, may be low on another. Furthermore, these quality indicators often interrelated. Therefore, using a summarized measure could give a comprehensive and overview of hospital quality of care for patients, hospitals, and government. Recently, a composite measure including all desirable outcomes called “textbook outcome” was developed from acute myocardial infarction, colon, and gastrointestinal cancer surgery.[8,9,10] Textbook outcome is defined as a patient whose healthcare team has met all the most important quality of care parameters for procedure. Analyzing from 5582 operated colon cancer patients in 82 hospitals, Kolfschoten et al,[10] reported that a textbook outcome including 6 quality indicators varied from 26% to 71%. Most patients did not achieve a textbook outcome (mean: 49%) and there was wide variation between hospitals. Thus, textbook outcome enables the production of a summary of hospital performance and provides a more comprehensive impression of the overall quality of care than less robust measures.
Previous studies have demonstrated that outcomes after colon cancer surgery vary between hospitals.[11,12,13] However, to our knowledge, the textbook outcome has not been validated in a single hospital nor has its impact on survival been studied in colon cancer. The aim of this study was to investigate the clinical relevance of textbook outcome in operated colon cancer patients. Further we also assess its impact on long term survival after surgery and establish a composite score of colon cancer surgery for future application in cancer accreditation.
2. Materials and methods
2.1. Ethical statement
This study was approved by the Ethics Committee of the Institutional Review Board of the Chi Mei Medical Center (IRB: CMFHR10707-012). Informed consent was not obtained from any participants because the IRB waived the need for individual informed consent, as this study had a non-interventional retrospective design, no human subjects or personally identifying information used, and all data were analyzed anonymously.
2.2. Patient database and selection
The data for this study were collected from the cancer registry dataset of the Chi-Mei Medical Center between January 1, 2010 and December 31, 2014. Electronic medical records and a cancer registry dataset were retrospectively reviewed. All patients were regularly monitored after diagnosis until death or last follow up. Finally, a total of 804 patients who undergo colon cancer resection with curative intent (i.e., right hemicolectomy, left hemicolectomy, or sigmoidectomy) were identified for this study. This study was limited to colon cancer because rectal cancer frequently is treated with a different sequence, such as neo-adjuvant chemo-radiotherapy. Exclusion criteria included patients with carcinoma in situ, a previous history of cancer, age <18 years, chemotherapy as the initial treatment or missing data.
2.3. Variables and end point
Our cancer registry dataset provided information on patient, tumor, and treatment characteristics: age, sex, marital status, date of diagnosis, circumferential resection margin (CRM), lymph node (LN) yield, tumor differentiation, perineural invasion (PNI), adjuvant treatment (e.g., chemotherapy and/or radiotherapy), clinical/pathologic tumor-node-metastasis stage, carcinoembryonic antigen (CEA) level, and cause of death. All staging were according to the American Joint Committee on Cancer (AJCC) staging (7th edition). The primary end point was the 5-year disease-specific survival (DSS) rate. Deaths due to cancer were recorded as events, and deaths secondary to other causes were censored.
2.4. Textbook outcome definitions
The quality parameters of care chosen for this study were modeled on those approved by the National Comprehensive Cancer Network (NCCN) guidelines for colon cancer, the quality index of our National Health Research Institute and from studies of potential quality metrics in colon cancer.[10,14,15] A textbook outcome included six separate preoperative, intra-operative and postoperative quality of care measures: surgery within 6 weeks, radical resection, LN yield ≥12, no stoma, no adverse outcome and colonoscopy before/after surgery within 6 months (Table 1). Radical tumor resection was defined as no residual tumor, including micro/macroscopic findings. Adverse outcomes were defined as any adverse outcome occurring, including readmission or reoperation within 30 days after resection. It is worth mentioning that a hospital stay of 14 days or less was not included as a quality of care measure in this study because our patients stayed longer in the hospital to complete their cancer staging and receive an operation.[10] In order to set up a suitable and comprehensive measure, the “all or none” method was used.[16] When all 6 short-term quality of care parameters were realized, a textbook outcome was achieved. If any one of the six parameters was not met, the treatment was not considered textbook outcome.
Table 1.
Textbook outcome parameters of colon cancer surgery, n = 804.

2.5. Statistical analysis
Patient, tumor, and treatment characteristics were compared between patients with and without a textbook outcome by Pearson chi-square test or Fisher exact test. False negative textbook outcomes were defined as patients with disease-specific death within 5 years. Sensitivity and negative predictive value were also estimated. The 5-year DSS rate among these 2 groups was described by the Kaplan–Meier method and the differences were compared using log-rank statistics. A multivariate Cox regression model was used to evaluate the effect of textbook outcome on 5-year DSS rates after adjusting for other confounding variables. Stratified survival analyses were also performed on different groups. All statistical analyses were performed using SPSS statistical software (version 20, SPSS Inc.; Chicago, IL). P < .05 was set as representing statistical significance. All confidence intervals (CIs) were stated at the 95% level.
3. Results
3.1. Demographic characteristics
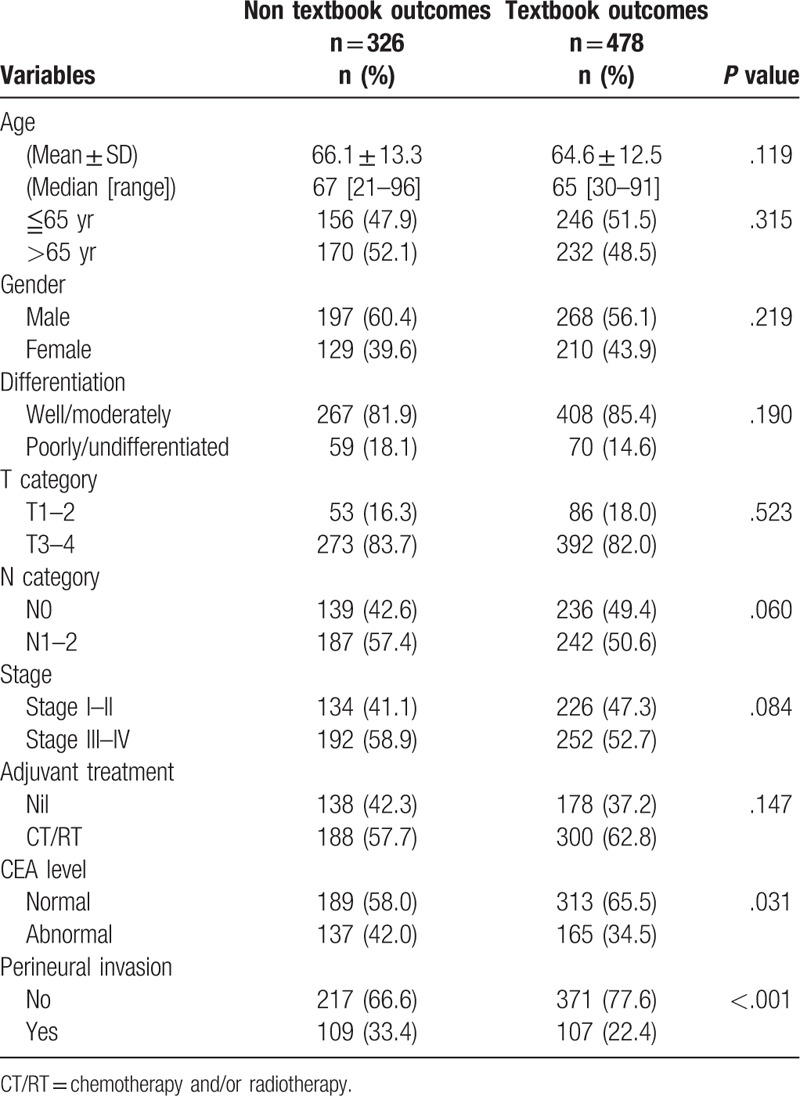
A total of 804 colon cancer patients were identified, 465 men (57.9%) and 339 women (42.1%). The mean follow-up time was 40 ± 18 months. A textbook outcome was realized in 478 patients (59.5%) and the details are presented in Fig. 1. The outcome parameters that most prevented patients from achieving a textbook outcome were LN yield >12, no stoma, and no adverse outcome (Fig. 1). Differences in demographic, clinical, and pathological characteristics according to textbook outcome are displayed in Table 2. Patients with and without a textbook outcome had no statistically significant differences in baseline characteristics, except for CEA level and PNI.
Figure 1.
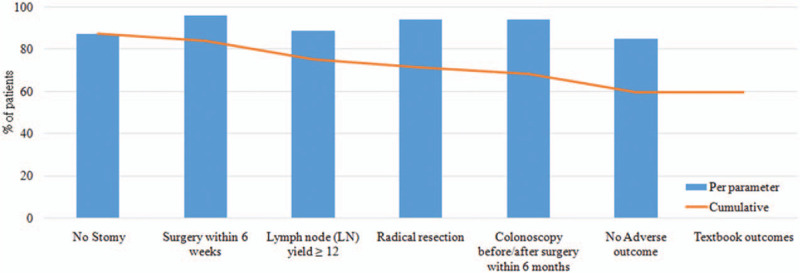
Percentage of surgically colon cancer patients (2010–2014) who fulfilled each textbook outcome parameter. A textbook outcome was achieved in 478 (59.5%) patient.
Table 2.
Demographic characteristics for surgically colon cancer patients by textbook outcomes, n = 804.
3.2. Impact of textbook outcomes
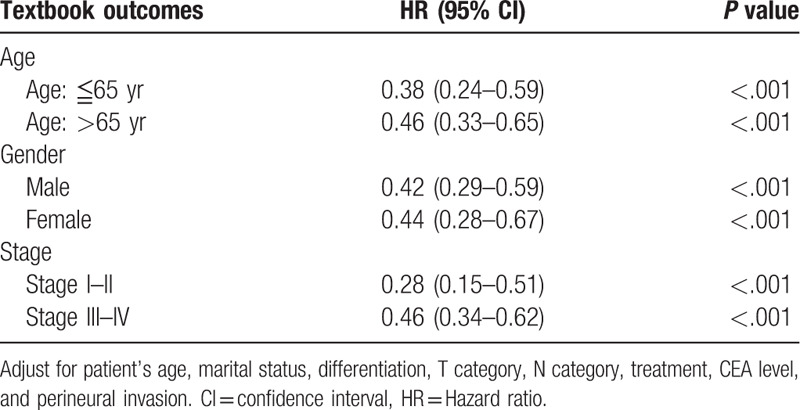
The association between textbook outcome and disease-specific death with 5 years was illustrated in supplementary Table 1. Ninety five patients (20%) with textbook outcome died within 5 years. This resulted in 59% sensitivity and 80% negative predictive value. The Kaplan–Meier survival curves were generated to compare the 5-year DSS. As presented in Fig. 2, the 5-year DSS differed significantly between subgroups of patients with and without textbook outcome. Colon cancer patients with a textbook outcome had better survival outcomes than those patients without a textbook outcome (80.1% vs 58.3%, P < .001). In accordance with univariate results, the multivariate analysis (Table 3) indicated that having a textbook outcome was still associated with longer 5-year DSS for surgically treated colon cancer patients (adjust hazard ratio [aHR], 0.44; 95% confidence interval [CI], 0.34–0.57). We then performed stratified analysis for 5-year DSS according to different demographic variables. As shown in Table 4, textbook outcome still plays a significant protective role for all age, sex, and cancer stage subgroups (all P < .001).
Figure 2.
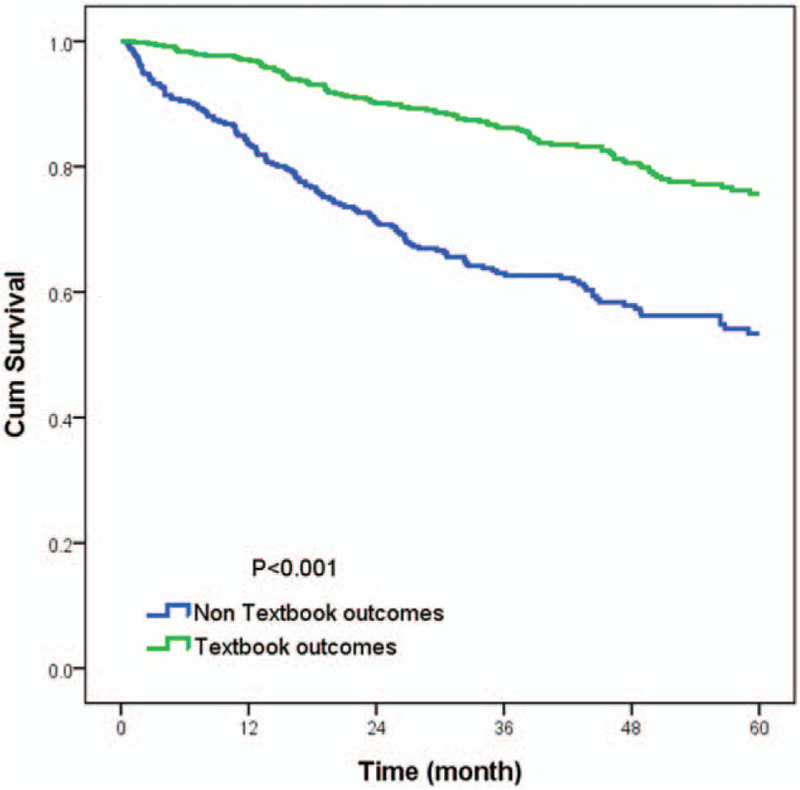
Kaplan–Meier 5-year disease specific survival curves for surgically colon cancer patients according to whether or not a textbook outcome was achieved (80.1% vs 58.3%, P < .001).
Table 3.
Univariate and multivariate analysis for the 5-year disease-specific survival, n = 804.
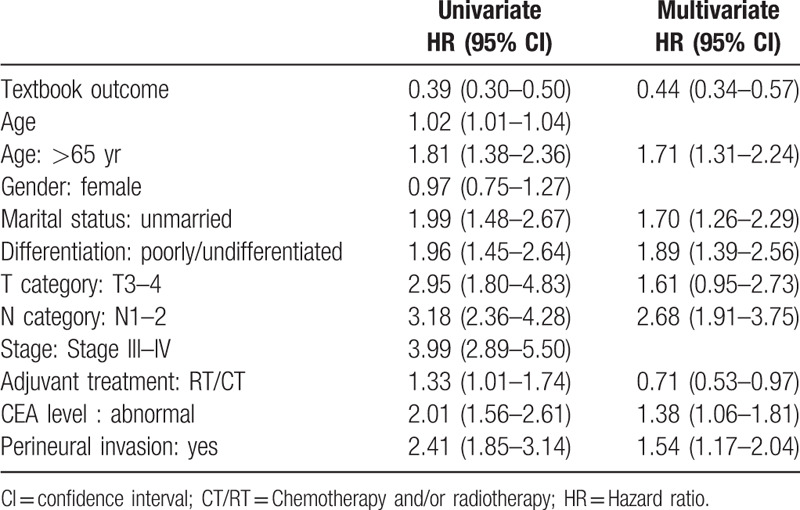
Table 4.
Stratified analysis of textbook outcome for the 5-year disease-specific survival according to age, gender, grade, and stage, n = 804.
4. Discussion
A textbook outcome was achieved in 59.5% of patients in our study. The main factors inhibiting textbook outcome were LN yield ≥12, no stoma, and no adverse outcome. Negative predictive rate of textbook outcome was 80%. Moreover, patients with a textbook outcome had better 5-year DSS than those without a textbook outcome. This means that well preparation, surgery, and postoperative care could optimize long-term survival. Therefore, the information of textbook outcome could not only be used to evaluate a summary of short-term quality of cancer care, but also to predict long-term survival in operated colon cancer patients.
This study has several strengths. To our knowledge, this is the first study to use a textbook outcome including preoperative, intraoperative, and postoperative quality parameters to predict long-term survival in colon cancer. Sensitivity (59%) and negative predictive rate (80%) of textbook outcome on long-term survival were noted. Although the sensitivity of textbook outcome was not high, the negative predictive rate was acceptable. This high negative predictive rate of textbook outcome on operated colon cancer patients could be utilized as a summary quality measure of cancer treatment. As we know, the accreditation for quality of cancer care are not simple. The comparison of disease-specific survival or overall survival between different institutions might be unfair due to selection bias and confounding effect of different stage, age, fragility of the patients and techniques, equipment, and treatment modality of the hospitals and staff.[5] However, evaluation of the textbook outcome rate of the treated patients, such as operated colon cancer patients, was feasible and simple. Second, with a longer follow-up time than previously used in similar studies,[10] our study validated a textbook outcome that is easy to generate for colon cancer patients undergoing surgery and is sufficient to estimate survival. Third, our database permitted analysis of the predisposing factors that may influence survival (e.g., CRM status, tumor grade, PNI, CEA levels, and adjuvant therapy). We were therefore able to perform an in-depth assessment of the impact of these factors on outcomes.
As reported by the Institute of Medicine, evaluating the quality of care has become increasingly more important in recent decades.[17,18] Especially for cancer patients, optimizing the quality of cancer care can reduce ineffective care and improve health care practices with proven benefit. But, quality of cancer care is complex, comprising many different aspects of the process of care, and differing also by type of cancer. For patients undergoing curative cancer surgery, the surgical process can be considered safe if no adverse outcomes (mortality and morbidity) have occurred, and effective if complete tumor removal and adequate lymphadenectomy have been achieved. These goals are represented in the composite measure textbook outcome.[10] Textbook outcome places individual outcome parameters within a broader context and approaches quality of care from a systems perspective. A better understanding of the different factors that lead to success or failure during the surgical process may potentially lead to further improvements in quality of care. A textbook outcome has also been described for patients with surgically treated esophagogastric cancer.[8,19]
Although the composite measure textbook outcome represents a comprehensive method to evaluate quality of care, most previous studies only discussed the variations of textbook outcome between different hospitals.[8,10,20] Recently, van der Kaaij et al,[19] reported that esophageal and gastric cancer patients with a textbook outcome had a better 3-year overall survival after surgery. The underlying reason for the association between textbook outcome and overall survival is textbook outcome consists of known prognostic factors, including complete staging, clear tumor resection, retrieval of at least 12 LNs, and well postoperative care.[21,22] For example, preoperative flexible sigmoidoscopy is recommended to evaluate for synchronous carcinoma or neoplastic polyps.[23] By receiving appropriate preoperative staging prior to resection, colon cancer patients had a lower risk of tumor recurrence, perioperative morbidity and mortality, leading to a better long-term survival.[24,25] A significant negative impact on survival of time factors such as longer than 6 weeks from first diagnostic test adapted in our analysis were also discussed in colon cancer.[26] Moreover, different to complete tumor resection with clear margin and sufficient lymph node retrieved which had been recognized as survival prognosticators,[22,27] intraoperative leakage and infectious complications significantly increased the serum concentration of Interleukin-6 and vascular endothelial growth factor after colorectal cancer surgery. Amplification of inflammation and angiogenesis might be the reason for the higher disease recurrence and worse survival in colon cancer.[21,28] Under the multidisciplinary team of specialists to an optimal pre-, peri-, and postoperative care path for patients undergoing colon cancer surgery, the association between textbook outcome and better overall survival would be expected. In our results, surgically treated colon patients with textbook outcome had statistically significant better 5-year DSS compared with those without (80.1% vs 58.3%) and negative predictive rate for long-term survival was up to 80%. Important obstacles to achieving textbook outcome were no stoma, no adverse outcome, and LN yield ≥12. Therefore, by combining relevant short-term outcome indicators of care into a single measure, textbook outcome meaningfully addresses all aspects of care to identify the ideal practice that leads to a favorable long term outcome.
With the advantages of this summary of hospital performance, textbook outcome can help patients to choose hospitals that provide the best quality of care and survival. For doctors, it can provide useful feedback on how often treatment is successful and drive quality improvement when the included parameters are evaluated separately.[8,10] For hospitals, because a single indicator is insufficient to reflect the overall quality of care (supplementary Table 2), using this composite measure could easy to discriminate the total quality of care and to compare long-term outcome between hospitals, especially for those new hospitals without enough follow-up time. For government, use of the measure could potentially lower treatment variations between hospitals by providing more public health grant, human resource, and education courses.[20]
This study had some limitations that should be addressed. One, our analysis could not correct for potential confounding factors adversely affecting the health of cancer patients, such as comorbidities reflecting poor general health and nutritional status. The use of instrumental variable analysis may help control for such measured and unmeasured confounding factors.[29] Second, the different textbook outcome parameters were not weighted by their unequal influence because there is no evidence to support the relative value of each. Any weights added to the textbook outcome measure would vary by hospital and would diminish its simplicity of use. We further analyzed the different combination number of indicators, ranging from 1 to 6 (supplementary Table 2). Textbook outcome defined as with 6 indicators had the highest Harrell c, sensitivity, and negative predictive rate, compared with others. Third, we only included 6 quality of care parameters in this study. Other important parameters from evidence-based medicine are potential added into current textbook outcome measure and could be discussed in the future. Finally, we focused on patients who underwent surgery as a first treatment. Patients who did not receive tumor resection with curative intent or who were treated with chemotherapy first cannot be assessed in this population.
In summary, a single indicator is not sufficient to reflect the entirely quality of care for cancers. A composite measure called textbook outcome is defined as a patient whose healthcare team has met all the most important quality of care parameters for procedure. By using this multidimensional measure, it can be used not only to drive quality improvement for healthcare teams, but also to evaluate the summary of quality of care between hospitals. Furthermore, our results demonstrated the critical importance of optimizing the quality of care on 5-year DSS in operated colon cancer patients. With a high negative predictive rate of 80%, the information from the textbook outcome is a simple, useful, and reliable tool for patients or hospital accreditation to know one hospital performance of cancer care.
Acknowledgments
The authors acknowledge the support from the following grants: Health and Welfare surcharge of tobacco products (MOHW109-TDU-B-212-134020, WanFang Hospital, Chi-Mei Medical Center, and Hualien Tzu-Chi Hospital Joing Cancer Center Grant-Focus on Colon Cancer Research); CMFHR108105 from the Chi Mei Medical center; The staff of the Cancer Center of the Chi Mei Medical Center collected the data. They were not compensated for their contribution.
Author contributions
Conceptualization: Ching-Chieh Yang, Yu-Feng Tian, Wen-Shan Liu, Chia-Lin Chou, Li-Chin Cheng, Ching-Chih Lee.
Data curation: Ching-Chieh Yang, Yu-Feng Tian, Chia-Lin Chou, Li-Chin Cheng, Shou-Sheng Chu.
Formal analysis: Ching-Chieh Yang, Ching-Chih Lee.
Investigation: Ching-Chieh Yang, Ching-Chih Lee.
Methodology: Ching-Chieh Yang, Ching-Chih Lee.
Project administration: Ching-Chieh Yang, Ching-Chih Lee.
Resources: Ching-Chieh Yang, Ching-Chih Lee.
Software: Ching-Chieh Yang, Ching-Chih Lee.
Supervision: Ching-Chih Lee.
Writing – original draft: Ching-Chieh Yang, Ching-Chih Lee.
Writing – review & editing: Ching-Chieh Yang, Ching-Chih Lee.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CEA = carcinoembryonic antigen, CI = confidence interval, CRM = circumferential resection margin, DSS = disease-specific survival, HR = hazard ratio, IOM = Institute of Medicine, LN = lymph node, NCCN = National Comprehensive Cancer Network, PNI = perineural invasion, TNM= tumor-node-metastasis.
How to cite this article: Yang CC, Tian YF, Liu WS, Chou CL, Cheng LC, Chu SS, Lee CC. The association between the composite quality measure “textbook outcome” and long term survival in operated colon cancer. Medicine. 2020;99:40(e22447).
Funding: None.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Boland GM, Chang GJ, Haynes AB, et al. Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Cancer 2013;119:1593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017;66:683–91. [DOI] [PubMed] [Google Scholar]
- [4].Mathis KL, Cima RR. Quality assurance in colon and rectal cancer surgery. Surg Oncol Clin N Am 2014;23:11–23. [DOI] [PubMed] [Google Scholar]
- [5].Orangio GR. A National Accreditation program for rectal cancer: a long and winding road. Dis Colon Rectum 2018;61:145–6. [DOI] [PubMed] [Google Scholar]
- [6].Schmaltz SP, Williams SC, Chassin MR, et al. Hospital performance trends on national quality measures and the association with Joint Commission accreditation. J Hosp Med 2011;6:454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Surgeons ACo. Cancer committee for compliance to Standards 4.4 and 4.5; 2017. Available at: https://www.facs.org/quality-programs/cancer/ncdb/qualitymeasures/development. Accessed February 13, 2019. [Google Scholar]
- [8].Busweiler LA, Schouwenburg MG, van Berge Henegouwen MI, et al. Textbook outcome as a composite measure in oesophagogastric cancer surgery. Br J Surg 2017;104:742–50. [DOI] [PubMed] [Google Scholar]
- [9].Coory M, Scott I. Analysing low-risk patient populations allows better discrimination between high-performing and low-performing hospitals: a case study using inhospital mortality from acute myocardial infarction. Qual Saf Health Care 2007;16:324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kolfschoten NE, Kievit J, Gooiker GA, et al. Focusing on desired outcomes of care after colon cancer resections; hospital variations in ’textbook outcome’. Eur J Surg Oncol 2013;39:156–63. [DOI] [PubMed] [Google Scholar]
- [11].Bilimoria KY, Stewart AK, Edge SB, et al. Lymph node examination rate, survival rate, and quality of care in colon cancer. JAMA 2008;299:896–7. author reply 897–898. [DOI] [PubMed] [Google Scholar]
- [12].Chow CJ, Al-Refaie WB, Abraham A, et al. Does patient rurality predict quality colon cancer care?: a population-based study. Dis Colon Rectum 2015;58:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Elferink MA, Wouters MW, Krijnen P, et al. Disparities in quality of care for colon cancer between hospitals in the Netherlands. Eur J Surg Oncol 2010;36: suppl: S64–73. [DOI] [PubMed] [Google Scholar]
- [14].Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 2001;93:583–96. [DOI] [PubMed] [Google Scholar]
- [15].Schofield JB, Mounter NA, Mallett R, et al. The importance of accurate pathological assessment of lymph node involvement in colorectal cancer. Colorectal Dis 2006;8:460–70. [DOI] [PubMed] [Google Scholar]
- [16].O’Brien SM, Shahian DM, DeLong ER, et al. Quality measurement in adult cardiac surgery: part 2--statistical considerations in composite measure scoring and provider rating. Ann Thorac Surg 2007;83: 4 suppl: S13–26. [DOI] [PubMed] [Google Scholar]
- [17].Hurria A, Naylor M, Cohen HJ. Improving the quality of cancer care in an aging population: recommendations from an IOM report. JAMA 2013;310:1795–6. [DOI] [PubMed] [Google Scholar]
- [18].Moulton G. IOM report on quality of cancer care highlights need for research, data expansion. Institute of Medicine. J Natl Cancer Inst 1999;91:761–2. [DOI] [PubMed] [Google Scholar]
- [19].van der Kaaij RT, de Rooij MV, van Coevorden F, et al. Using textbook outcome as a measure of quality of care in oesophagogastric cancer surgery. Br J Surg 2018;105:561–9. [DOI] [PubMed] [Google Scholar]
- [20].Salet N, Bremmer RH, Verhagen M, et al. Is Textbook Outcome a valuable composite measure for short-term outcomes of gastrointestinal treatments in the Netherlands using hospital information system data? A retrospective cohort study. BMJ Open 2018;8:e019405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alonso S, Pascual M, Salvans S, et al. Postoperative intra-abdominal infection and colorectal cancer recurrence: a prospective matched cohort study of inflammatory and angiogenic responses as mechanisms involved in this association. Eur J Surg Oncol 2015;41:208–14. [DOI] [PubMed] [Google Scholar]
- [22].Lykke J, Jess P, Roikjaer O, et al. A high lymph node yield in colon cancer is associated with age, tumour stage, tumour sub-site and priority of surgery. Results from a prospective national cohort study. Int J Colorectal Dis 2016;31:1299–305. [DOI] [PubMed] [Google Scholar]
- [23].Alberti LR, Garcia DP, Coelho DL, et al. How to improve colon cancer screening rates. World J Gastrointest Oncol 2015;7:484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Amri R, Bordeianou LG, Sylla P, et al. Impact of screening colonoscopy on outcomes in colon cancer surgery. JAMA Surg 2013;148:747–54. [DOI] [PubMed] [Google Scholar]
- [25].Leal TB, Holden T, Cavalcante L, et al. Colon cancer staging in vulnerable older adults: adherence to national guidelines and impact on survival. Ann Hematol Oncol 2014;1:1012. [PMC free article] [PubMed] [Google Scholar]
- [26].Simunovic M, Rempel E, Theriault ME, et al. Influence of delays to nonemergent colon cancer surgery on operative mortality, disease-specific survival and overall survival. Can J Surg 2009;52:E79–86. [PMC free article] [PubMed] [Google Scholar]
- [27].Rajput A, Romanus D, Weiser MR, et al. Meeting the 12 lymph node (LN) benchmark in colon cancer. J Surg Oncol 2010;102:3–9. [DOI] [PubMed] [Google Scholar]
- [28].Bohle B, Pera M, Pascual M, et al. Postoperative intra-abdominal infection increases angiogenesis and tumor recurrence after surgical excision of colon cancer in mice. Surgery 2010;147:120–6. [DOI] [PubMed] [Google Scholar]
- [29].Sainani KL. Instrumental variables: uses and limitations. PM R 2018;10:303–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


