Abstract
Purpose:
To review the literature on the efficacy and safety of Chaihu Longgu Muli decoction (CLMD) for insomnia.
Methods:
A systematic literature search was performed for five databases up to May of 2019 to identify randomized control trials involving CLMD for patients with insomnia. The experimental group was CLMD monotherapy or CLMD plus conventional treatment. Comparators were placebo, no treatment, or conventional medicine. The main comparison was CLMD against conventional drugs. The primary outcome was sleep quality (assessed using the Pittsburgh Sleep Quality Index, PSQI). The secondary outcomes were clinical effectiveness rate, total sleep time, and adverse event rate. RevMan 5.3 software was used for meta-analysis with effect estimate presented as relative risk (RR) and mean difference (MD) with 95% confidence interval (CI).
Results:
A total of 22 studies involving 2029 patients were included. All the included studies presented some risk of bias, especially risks of performance, and detection bias. The main meta-analysis showed that CLMD alone was more effective than conventional medications by reducing PSQI (MD = −2.80, 95% CI [−5.48, −0.13], P = .04), improving the clinical effectiveness rate (RR = 1.23, 95% CI [1.16, 1.31], P < .00001), and prolonging total sleep time (MD = 1.01, 95% CI [0.19, 1.83], P = .002). The adverse event rate in the CLMD group was lower than that of the control group (RR = 0.22, 95% CI [0.09, 0.51], P = .0005). CLMD also improved sleep quality better than conventional medications as an adjunct therapy (P < .05). The funnel plot was symmetrical, representing a low risk of publication bias.
Conclusion:
CLMD presented better efficacy and safety than conventional medications and had the potential to become an alternative to conventional medications for the treatment of insomnia. However, as the included studies showed significant risks of bias, these results will need to be confirmed by future double-blind randomized controlled trials.
PROSPERO registration number:
CRD42019133103.
Keywords: Chaihu Longgu Muli decoction, insomnia, Chinese herbal medicine, systematic review
1. Introduction
Insomnia is the most prevalent sleep disorder that can present independently or as a comorbidity of other medical or psychiatric disorders. It is the most prevalent sleep disorder that affects large proportions of the population on a situational, recurrent, or persistent basis.[1] It is characterized by difficulties initiating or maintaining sleep, and is associated with significant distress or daytime impairments, despite adequate sleep opportunities.[2] In one study, 25% of adults reported dissatisfaction with their sleep; 10% to 15% reported symptoms of insomnia associated with daytime consequences, and 6% to 10% met criteria for an insomnia disorder.[1] A study on the economic burden of insomnia found that the average annual per-person costs were $5010 for individuals with insomnia syndrome, $1431 for individuals presenting with symptoms, and $421 for good sleepers.[3] These findings suggest that an affordable and effective therapy for insomnia is desirable.
The treatments recommended by recent clinical guidelines are pharmacotherapy and cognitive-behavioral therapy (CBT) for insomnia.[4] Pharmacotherapies for insomnia include benzodiazepines, nonbenzodiazepine prescription drugs, and nonbenzodiazepine benzodiazepine receptor agonists.[5] Eszopiclone, zolpidem, and suvorexant may improve insomnia in the short term; however, their comparative effectiveness and long-term efficacies are unclear. Pharmacotherapies for insomnia may lead to cognitive and behavioral changes, and may be associated with serious harms.[6] CBT is largely unavailable to people with insomnia though it is considered as first-line treatment for adults with chronic insomnia disorder.[7] Due to the above shortcomings, it is necessary to explore better treatment. There have been many randomized-controlled trials (RCTs) evaluating the efficacy of complementary and alternative medicine (CAM) because of the significant health risks associated with insomnia. According to the National Health Interview Survey analysis, over 1.6 million civilian and non-institutionalized adult US citizens use CAM to treat insomnia or trouble sleeping.[8] Traditional Chinese Medicine (TCM) plays an important role in China. TCM includes Chinese herbal medicine (CHM), acupuncture, moxibustion, massage, and other non-drug therapies. In a typical CHM prescription, a complex integration of two or more single Chinese herbs are combined to achieve additive or synergistic effects.[9] A systematic review indicated that oral CHM may improve subjective sleep in people with insomnia.[10] Chaihu Longgu Muli decoction (CLMD) was first recorded in ShangHanLun (Treatise on Febrile Diseases), one of the four classical Chinese medical books. It is one of the most famous herbal prescriptions for insomnia that is widely used in China.[11] CLMD was found to be effective for insomnia in many clinical studies and experiments.[12]
Critical appraisal of evidence such as systematic reviews or meta-analysis is necessary to justify the clinical application and recommendation of CLMD. At present, there are two Chinese systematic reviews, both of which have substantial limitations.[13,14] Their outcome measures were incomplete and did not include the latest research. Therefore, we aimed to review the efficacy and safety of CLMD for insomnia systematically based on current RCTs.
2. Methods
This study was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.[15] The review protocol was registered in PROSPERO (ID: CRD42019133103, https://www.crd.york.ac.uk/prospero/). Because this study is a secondary study of past clinical trials, ethical approval is not necessary.
2.1. Eligibility criteria
2.1.1. Types of studies
All RCTs evaluating CLMD for insomnia were included, except those containing inaccurate or incomplete data. There were no restrictions on language, geography, population characteristics, or publication type. Quasi-randomized trials that used date of birth, date of admission, hospital numbers, or alternative methods of allocation were excluded. Duplicated publications reporting the same groups of participants were also excluded.
2.1.2. Types of participants
Patients diagnosed with insomnia were included, regardless of age, gender, course of disease, or ethnicity. The diagnostic criteria were based on the Chinese classification of mental disorders CCMD-2-R and the update version CCMD-3,[16] the Guideline for Clinical Trials of New Patent Chinese Medicines (GCTNPCM) criteria,[17] the Guidelines for Diagnosis and Treatment of Insomnia in Adults in China (GDTIAC),[18] the Criteria for the Diagnosis and Therapeutic Effect of Diseases and Syndromes in Traditional Chinese Medicine (CDTEDSTCM),[19] the Diagnostic and Statistical Manual of Mental Disorders (DSM-4) (1994),[20] and the update version in 2014 (DSM-5).[21] We excluded secondary insomnia caused by craniocerebral injury, kidney, adrenal gland, and goiter.
2.1.3. Types of interventions
RCTs using CLMD as a monotherapy or as adjunct therapy in the experimental group were included. There were no restrictions regarding dosage, including form, frequency, dose, and intensity. There was no limit to the duration of these treatments. The definition of modified CLMD was addition or subtraction of a few herbs on the basis of syndrome differentiation and treatment; however, the prescription must include at least 80% herbs of CLMD and the principal herbs, including Chaihu, Longgu, and Muli. Interventions in the control group were placebo, no treatment, or conventional medicine. In this research, conventional medicine refers to the drugs commonly used for insomnia, including benzodiazepines, non-benzodiazepines, and antidepressants.
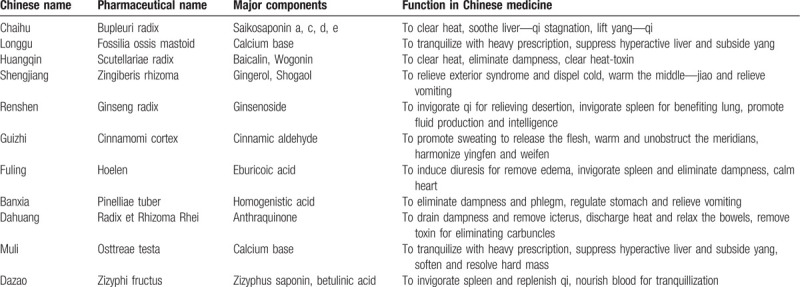
The individual herbs composing CLMD are Bupleuri radix, Fossilia ossis mastoid, Scutellariae radix, Zingiberis rhizoma, Ginseng radix, Cinnamomi cortex, Hoelen, Pinelliae tuber, Radix et Rhizoma Rhei, Osttreae testa, Zizyphi fructus, all of which are recorded in the Chinese Pharmacopoeia (Version 2010).[22] According to CHM theory, this prescription is reported to clear heat and eliminate dampness, and tranquilize with heavy prescription. The details of CLMD are summarized in Table 1.
Table 1.
Details of CLMD.
2.1.4. Types of outcome measures
The primary outcome was sleep quality measured using the Pittsburgh Sleep Quality Index (PSQI). The secondary outcome measures were clinical effectiveness rate, total sleep time, and adverse event rate.
The PSQI is a specific questionnaire formulated by Buysse[23] in 1989. It is used to evaluate sleep quality of patients with sleep disorders and mental disorders, and also suitable for the evaluation of sleep quality of general people. It is a self-rated scale with a range of respectively 0 to 21 points, for which a lower score is better. At present, the PSQI is a well-validated and commonly used instrument worldwide for sleep quality assessment.[24] The clinical effectiveness rate was based on response evaluation criteria in TCM treatment of insomnia[17] and was calculated using the following formula: (number of clinical cured patients + number of improved patients)/total number of patients ×100%. It is commonly used as an outcome in clinical trials and meta-analyses of TCM.[7,13,14] In GCTNPCM,[17] evaluation standards for clinical therapeutic effects were as follows:
-
1.
clinical cure: sleep time to restore normal sleep time or the night-time sleep duration of more than 6 h, deep sleep, full of energy after waking up;
-
2.
markedly effective: significant improvement of insomnia; sleep time increased over 3 h compared with the previous sleep time; an increase of the depth of sleep;
-
3.
effective: amelioration in symptoms; sleep time increased <3 h compared with the previous sleep time;
-
4.
ineffective: no significant improvement of insomnia, or deteriorated after treatment.
Subjective total sleep time refers to sleep duration measured using a self-reported questionnaire. The measurement of the outcomes should be reported in the studies. The authors were contacted when relevant data were missing.
2.2. Literature search
RCTs assessing the administration of CLMD for insomnia were located by searching the following databases: PubMed, EMBASE, China Biology Medicine database (CBM), the Chinese National Knowledge Infrastructure (CNKI), and Wan Fang database. The publication time was from the start of each database up to May 2019. The following search strategy was used for PubMed and was modified to suit the other databases.
PubMed search strategy:
#1 insomnia [MeSH Terms]
#2 disorders of initiating and maintaining sleep [Title/Abstract]
#3 early awakening [Title/Abstract]
#4 awakening, early [Title/Abstract]
#5 insomnia [Title/Abstract]
#6 sleeplessness [Title/Abstract]
#7 sleep [Title/Abstract]
#8 insomnias [Title/Abstract]
#9 OR #1-#8
#10 chaihu longgu muli decoction [Title/Abstract]
#11 chaihu longgu muli tang [Title/Abstract]
#12 chaihu longgu muli [Title/Abstract]
#13 chaihu [Title/Abstract]
#14 chai hu [Title/Abstract]
#15 OR #10-#14
#16 #9 AND #15
2.3. Study selection and data extraction
Two reviewers (XW and JJ) independently selected and checked the eligible studies. The search results from the different databases were imported into the document management software application Note Express 2.0. The two reviewers screened the studies for duplications, excluding irrelevant titles and abstracts, and then selecting eligible studies by reviewing full texts. Disagreements were discussed and resolved by consultation with a third investigator (HX).
Two reviewers (XW and JL) independently extracted the data from the selected studies, and then performed crosschecks. A standardized data extraction form was applied to extract data, including the authors’ names, year of publication, study size, age, and sex of the participants, interventions, disease duration, outcomes, course of interventions, and follow-up. Disagreements were resolved by discussion and consensus was reached through a third investigator (HX). The authors were contacted when relevant data were missing.
2.4. Risk of bias in individual studies
Two reviewers (XW and JJ) independently evaluated the risk of bias of the included articles using the Cochrane Handbook for Systematic Reviews of Interventions (updated September 2009). Disagreements were resolved through discussion.
2.5. Data synthesis and analysis
Data analysis was performed using Review Manager version 5.3 software recommended by the Cochrane's Collaboration. Dichotomous data were expressed as relative risks (RR), and continuous data were expressed as mean differences (MD) both with 95% confidence intervals (CIs). Statistical heterogeneity was evaluated using the Cochran chi-square test, and was quantified using I 2. A random-effect model was used to estimate the overall effect instead of a fixed-effect model because the former weighs study outcomes according to within-trial as well as between-trial variance so as to provide a more conservative result.[25] Publication bias was explored inspection of funnel plots if sufficient numbers of studies were found.
3. Results
3.1. Description of studies
We identified 220 potentially relevant articles from CNKI, CBM, Wan Fang database, PubMed, and Embase. A total of 22 studies were finally included in the meta-analyses. The details of literature screening were shown in a PRISMA 2009 flow diagram (Fig. 1).
Figure 1.
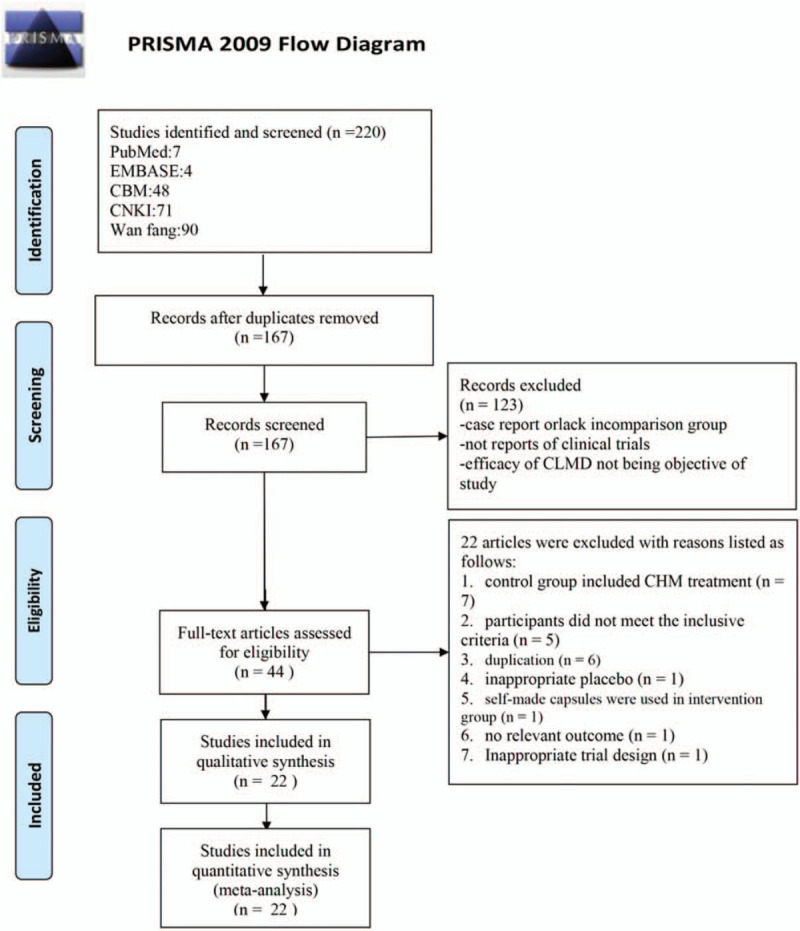
Flow diagram of the search method and selection process.
3.2. Characteristics of included studies
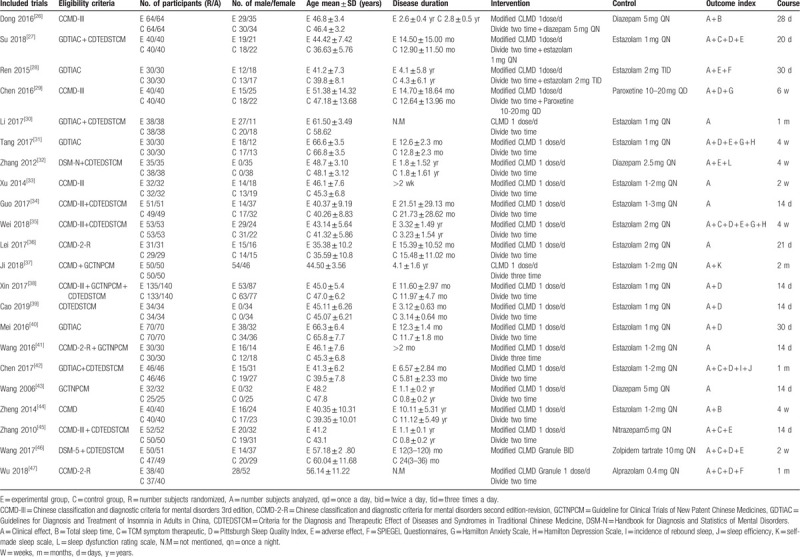
The characteristics of the 22 trials[26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] are summarized in Table 2. All 22 RCTs were published in Chinese. A total of 2029 adult participants with insomnia were included. The sample size varied from 57 to 268 and the age of participants ranged from 35.38 to 66.8 years old. The courses of treatment were between 14 days and 2 months. All trials adopted CLMD monotherapy or adjunct therapy in the experimental groups and conventional medicine in the control groups.
Table 2.
Summary of the characteristics of the included trials.
3.3. Risk of bias in included studies
The risk of bias assessment of included trials according to the criteria recommended by Cochrane Handbook for Systematic Reviews. The randomized allocation of participants was mentioned in all but one trial.[30] Eleven studies (50%) reported adequate methods of random sequence generation, including random number table,[26,29,34,35,38,39,42] computer-generated random number,[28,46] and drawing of lots.[31,45] Only one trial[46] described adequate allocation concealment.
Notably, none of the trials described blinding methods. Three trials (13.6%) reported dropout or withdrawal for detailed reasons.[38,46,47] Non-selective reporting was found in all 22 trials. All trials provided baseline data for the comparability among groups. The result of the assessment of risk of bias was presented in a graph of bias risk figure produced by RevMan 5.3 automatically (Fig. 2).
Figure 2.
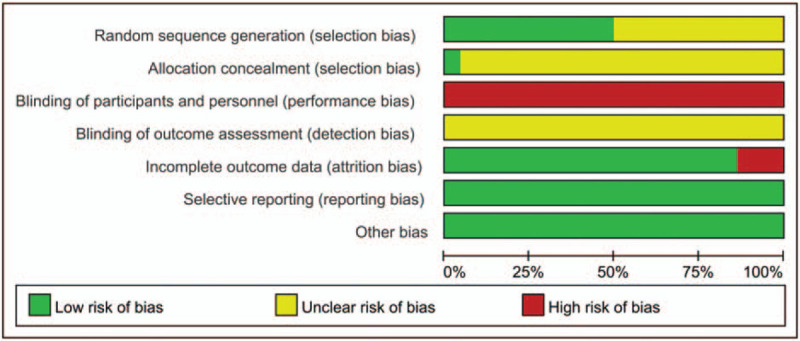
Risk of bias graph: review authors’ judgement about each risk of bias item presented as percentages across all included studies.
3.4. Primary outcome
We used the PSQI-measured sleep quality as primary outcome. Ten studies assessed PSQI scores.[27,29,31,35,38,39,40,42,46,47] The result of meta-analysis revealed that the PSQI scores of CLMD were lower than those of conventional medicine (MD = −2.80, 95% CI [−5.48, −0.13], P = .04) (Fig. 3). Also, the PSQI score of CLMD plus conventional medicine was lower than that of conventional medicine (MD = −2.55, 95% CI [−4.09, −1.01], P = .001). Compared with the control, CLMD can reduce the PSQI either used as a monotherapy or an adjunct therapy (MD = −2.72, 95% CI [−5.00, −0.44], P = .02).
Figure 3.
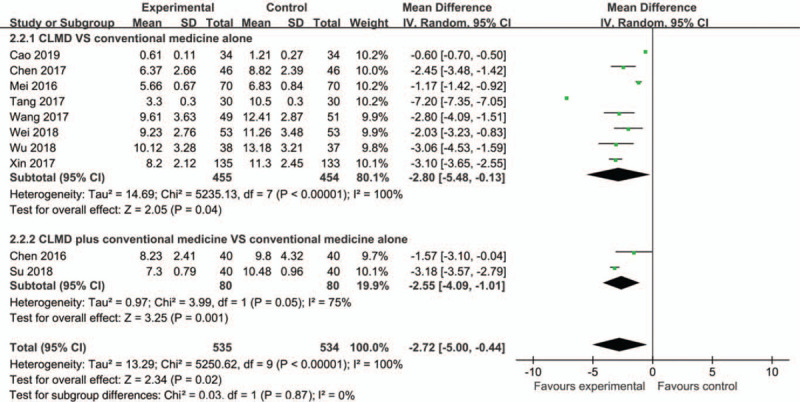
Forest plots based on the PSQI scores.
3.5. Secondary outcomes
3.5.1. Clinical effectiveness rate
All 22 studies reported clinical effectiveness rate. Eighteen trials[30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] indicated that the clinical effectiveness rate of CLMD was higher than that of conventional medicine (RR = 1.23, 95% CI [1.16, 1.31], P < .00001) (Fig. 4). Four trials[26,27,28,29] showed that CLMD plus conventional medicine was more effective than conventional medicine (RR = 1.22, 95% CI [1.20, 1.34], P = .0001). CLMD can significantly improve the clinical effectiveness whether as a monotherapy or an adjunct therapy (RR = 1.23, 95% CI [1.17, 1.29], P < .00001).
Figure 4.
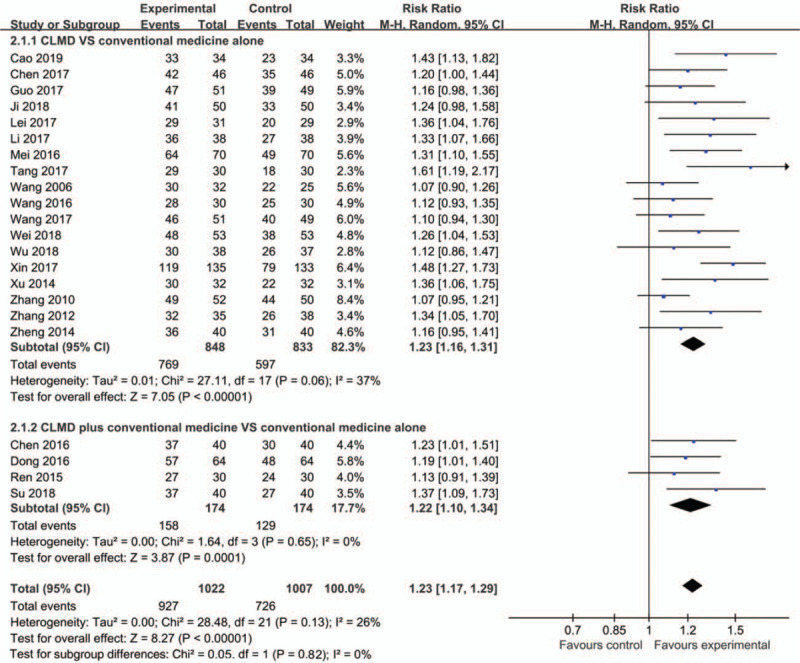
Forest plot of clinical effectiveness rate.
3.5.2. Total sleep time
In terms of total sleep time reported by patients, CLMD was better than conventional medicine in prolonging total sleep time[44] (MD = 1.01, 95% CI [0.19, 1.83], P = .002) (Fig. 5). Similarly, one study[26] indicated that CLMD plus conventional medicine was significant for prolonging total sleep time compared with conventional medicine alone (MD = 1.50, 95% CI [1.15, 1.85], P < .00001). In general, CLMD was more effective than conventional medicine whether as a monotherapy or an adjunct therapy (MD = 1.40, 95% CI [1.01, 1.79], P < .00001).[26,44]
Figure 5.
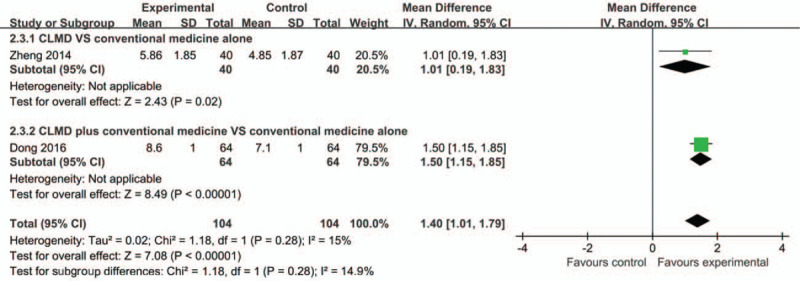
Forest plots based on the total sleep time.
3.5.3. Adverse event rate
Adverse event monitoring was only mentioned in seven studies,[27,28,31,32,35,45,46] and was not mentioned in the other 15 trials. Among these seven trials, five[31,32,35,45,46] compared CLMD with conventional medicine. The other two trials[27,28] compared CLMD plus conventional medicine with conventional medicine alone. As shown in Figure 6, the pooled data demonstrated that CLMD was safer than conventional medicine (RR = 0.22, 95% CI [0.09, 0.51], P = .0005) (Fig. 6). The other two trials[27,28] were not included in the meta-analysis because they claimed no adverse event happened. The adverse events in the experimental group were headache, dizziness, drowsiness, and blurred vision.
Figure 6.
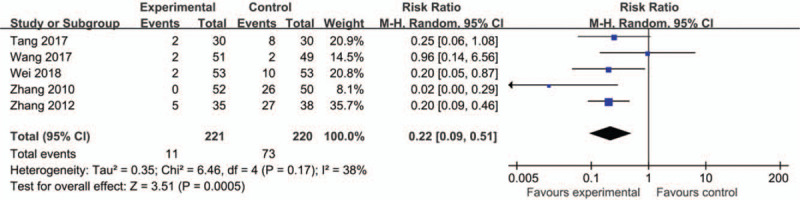
Forest plots of adverse event rate.
3.6. Assessment of publication bias
We generated an inverted funnel plot to assess the influence of publication bias (Fig. 7). The funnel plot was made according to RCTs that reported clinical effectiveness rate. As shown, it was generally symmetrical, representing a low risk of publication bias. It is important to note that there can still be publication bias owing to publishing factors of these RCTs despite the symmetrical appearance.
Figure 7.
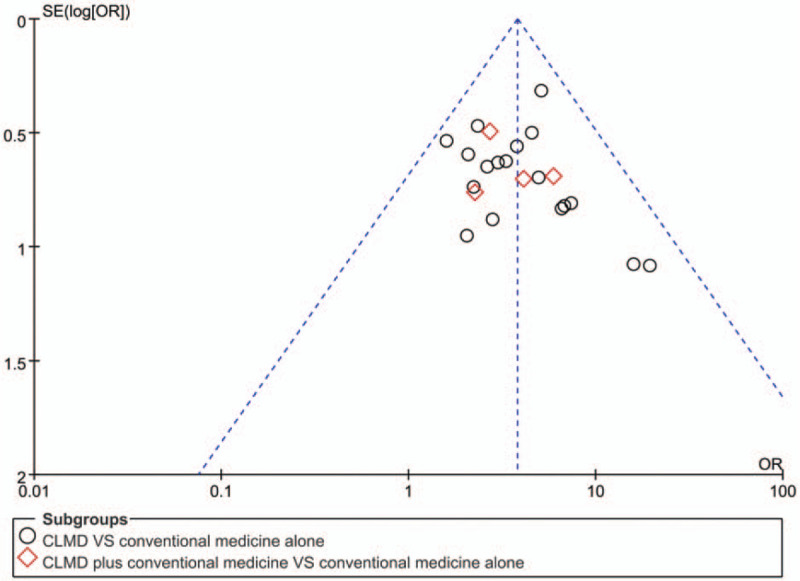
Funnel plot of the clinical effectiveness rate for assessing publication bias.
4. Discussion
4.1. Summary of evidence
This study provides up-to-date and comprehensive evidence of the relative safety and efficacy of CLMD for insomnia. All 22 eligible trials were based on Chinese participants, because CLMD is mostly practiced in China. No relevant trials assessed CLMD in countries other than China. The main meta-analysis showed that CLMD alone was more effective than conventional medications by reducing PSQI (MD = −2.80, 95% CI [−5.48, −0.13], P = .04), improving the clinical effectiveness rate (RR = 1.23, 95% CI [1.16, 1.31], P < .00001), prolonging total sleep time (MD = 1.01, 95% CI [0.19, 1.83], P = .002). This systematic review suggests that CLMD was superior to conventional drugs whether it was used as a monotherapy or as adjunct therapy. CLMD may be safer than conventional medicine for treatment of insomnia (RR = 0.22, 95% CI [0.09, 0.51], P = .0005). This is likely due to the small sample size, flawed methodologies of the included trials, or the differing follow-up durations. The funnel plot was generally symmetrical and represented low risk of publication bias.
4.2. Possible mechanisms of CLMD for insomnia
Some studies have explored the physiological mechanisms of CLMD for insomnia. CLMD treats anxiety and insomnia by regulating the hypothalamus–pituitary–adrenal axis as well as brain monoamine neurotransmitters (norepinephrine, dopamine, and 5-hydroxytryptamine).[48,49] CLMD inhibits adrenocorticotropic hormone and corticosterone, both of which may be biological mechanisms of regulating insomnia.[50] Jin et al found that CLMD may treat insomnia by inhibiting the activation of MEK/ERK pathway.[51] Generally, the main physiological mechanism of CLMD is the regulation of the hypothalamic–pituitary–adrenal axis and the levels of monoamine neurotransmitters in the brain. These mechanisms deserve further research.
4.3. Limitations
Several limitations must be acknowledged prior to drawing conclusions from this review. First, the main limitation is the high risk of the original RCTs, because it possibly affected the reliability and accuracy of the final results. Few studies provided information about allocation concealment, intention-to-treat analysis, and drop-out accounting. Half of the studies did not describe the generation of random sequences in detail. Moreover, none of the trials gave a detailed description of the blinding process. This may directly lead to performance and detection biases occasioned by patients and researchers being aware of the therapeutic interventions. In non-placebo-controlled or non-double-blind trials, the placebo effect might increase the complexity of interpreting the conclusion. Although it was difficult to conduct double-blinding due to the certain features of TCM, blinding to the outcome assessors and data analyzer could be feasible. In addition, no trials conducted pretrial estimation of sample size, suggesting the lack of statistical power to ensure appropriate estimation of the clinical efficacy for insomnia.
Second, there was high heterogeneity between studies, which may have resulted from different treatment duration, doses, additions, and subtractions of the CLMD. Furthermore, the use of composite outcome measures to evaluate overall improvement of symptoms may become a limitation of the universality of the results, including clinical cure rate, marked effectiveness, effectiveness, and ineffectiveness.
Finally, compared with conventional medicine, the CLMD evaluated in this review generally appeared to be safe and well tolerated by patients with insomnia. Nevertheless, the safety of CLMD could not be confirmed because only seven trials mentioned adverse events. We cannot rule out the possibility that investigators of these studies underrated possible adverse events. Also, no study compared CLMD to placebo. Therefore, we can only conclude about the relative safety of the formula. Therefore, specific evidence for safety of CLMD is suggested in the future.
Despite these limitations, our review represents the best available evidence concerning CLMD for insomnia.
4.4. Implications for future research and clinical practice
The literature search of the past two Chinese reviews covered up to 2018[13,14]; however, a few new studies have appeared that were not included in the previous review. The new studies are better designed and are therefore more persuasive. We believe the present meta-analysis provides more positive results than previous meta-analyses. Despite the fact that the present review has limitations, its significance for clinical application and future research should be taken seriously. The strengths of the present systematic review are the following:
-
1.
We identified a new area in treating insomnia that deserves attention. Although the present evidence remains weak for supporting the efficacy of CLMD, it is nevertheless a promising starting point for further relevant clinical research[52];
-
2.
we identified current problems that deserve improvement in further studies, including low quality of study designs and neglect of reports of adverse events.
We recommend that the Consolidated Standards of Reporting Trials (CONSORT) 2010 statement[53] should be employed as a guideline in reporting RCTs.
5. Conclusion
We reviewed the efficacy and safety of CLMD for insomnia objectively and comprehensively. The results of this review and meta-analysis support the relative safety and efficacy of CLMD for relieving insomnia. CLMD provided more benefit than conventional medications and had the potential to become an alternative to conventional medications for the treatment of insomnia. However, as the included studies showed significant risks of bias, these results will need to be confirmed by future double-blind randomized controlled trials.
Author contributions
Conceptualization: Xinyi Wang, Hao Xu.
Data curation: Xinyi Wang, Jianqing Ju, Jingen Li, Yixuan Fan.
Formal analysis: Xinyi Wang, Jianqing Ju, Jingen Li.
Methodology: Xinyi Wang, Jianqing Ju, Jingen Li.
Project administration: Hao Xu.
Supervision: Hao Xu.
Writing – original draft: Xinyi Wang, Jingen Li.
Writing – review & editing: Xinyi Wang, Jianqing Ju, Jingen Li, Yixuan Fan, Hao Xu.
Footnotes
Abbreviations: CAM = complementary and alternative medicine, CBM = China biology medicine database, CBT = cognitive-behavioral therapy, CHM = Chinese herbal medicine, CI = confidence interval, CLMD = Chaihu Longgu Muli decoction, CNKI = Chinese National Knowledge Infrastructure, CONSORT = Consolidated Standards of Reporting Trials, MD = mean differences, PRISMA = Preferred Reporting Items for Systematic reviews and Meta-Analyses, PSQI = Pittsburgh Sleep Quality Index, RCT = randomized-controlled trial, RR = relative risks, TCM = Traditional Chinese Medicine.
How to cite this article: Wang X, Ju J, Li J, Fan Y, Xu H. Chaihu Longgu Muli decoction, a Chinese herbal formula, for the treatment of insomnia: A systematic review and meta-analysis. Medicine. 2020;99:40(e22462).
The research was financially supported by the funding from Special Project for Professional Construction of National Clinical Research Center of Traditional Chinese Medicine (NO. JDZX2015263) and Capital's Funds for Health Improvement and Research (NO. 2018-1-4171).
The authors have no conflict of interests to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Morin CM, Benca R. Chronic insomnia. Lancet 2012;379:1129–41. [DOI] [PubMed] [Google Scholar]
- [2].Araújo T, Jarrin DC, Leanza Y, et al. Qualitative studies of insomnia: current state of knowledge in the field. Sleep Med Rev 2017;2:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Daley M, Morin CM, LeBlanc M, et al. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep 2009;32:55–64. [PMC free article] [PubMed] [Google Scholar]
- [4].Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2016;165:125–33. [DOI] [PubMed] [Google Scholar]
- [5].Erman MK. Therapeutic options in the treatment of insomnia. J Clin Psychiatry 2005;66:18–23. [PubMed] [Google Scholar]
- [6].Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med 2016;165:103–12. [DOI] [PubMed] [Google Scholar]
- [7].Birling Y, Jia M, Li G, et al. Zao Ren An Shen for insomnia: a systematic review with meta-analysis. Sleep Med 2020;69:41–50. [DOI] [PubMed] [Google Scholar]
- [8].Pearson NJ, Johnson LL, Nahin RL. Insomnia, trouble sleeping, and complementary and alternative medicine: analysis of the 2002 national health interview survey data. Arch Intern Med 2006;166:1775–82. [DOI] [PubMed] [Google Scholar]
- [9].Xie CL, Gu Y. Efficacy and safety of Suanzaoren decoction for primary insomnia: a systematic review of randomized controlled trials. BMC Complement Altern Med 2013;13:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ni X, Shergis JL, Guo X, et al. Updated clinical evidence of Chinese herbal medicine for insomnia: a systematic review and meta-analysis of randomized controlled trials. Sleep Med 2015;16:1462–81. [DOI] [PubMed] [Google Scholar]
- [11].Wang B, Zhang T, Qiu L, et al. Therapeutic effect of Bupleurum Falcatum plus dragon bone and oyster shell decoction on insomnia based on theory of Bupleurum Falcatum Decoction Originated from ZHANG Zhongjing. Chin Arch Tradit Chin Med 2016;34:1430–3. [Google Scholar]
- [12].Chen Q, Yang DS, Li SM, et al. Research progress in Bupleurum Falcatum plus dragon bone and oyster shell decoction. Med Recapit 2016;22:3441–4. [Google Scholar]
- [13].Rui YQ, Lv Y, Li WT, et al. Meta-analysis of modified Chaihu Longgu Muli Decoction in the treatment of insomnia. Shandong J Tradit Chin Med 2019;38: 1123-30 + 1136. [Google Scholar]
- [14].He SS, Chen XF, Wang XN. Meta-analysis of modified Chaihu Longgu Muli Decoction in the treatment of insomnia. Hunan J Tradit Chin Med 2019;35:125–8. [Google Scholar]
- [15].Moher D, Liverati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jinan Shandong Press of Science and Technology, Chinese Society of Psychiatry. CCMD-3: Chinese Classification of Metal Disorders. 2001. [Google Scholar]
- [17].Ministry of Health of the PRC. Guideline for Clinical Trials of New Patent Chinese Medicines. 11993;Beijing: Ministry of Health of the People's Republic of China, 186–187. [Google Scholar]
- [18].Primary Insomnia Group of Society of Neurology in Chinese Medical Association Guidelines for Diagnosis and Treatment of Insomnia in Adults in China. Chin J Neurol 2012;45:534–40. [Google Scholar]
- [19].State Administration of Traditional Chinese Medicine Criteria for the Diagnosis and Therapeutic Effect of Diseases and Syndromes in Traditional Chinese Medicine. 1994;Nanjing: Nanjing University Press, 19–20. [Google Scholar]
- [20].American Psychiatric Association. The Diagnostic and Statistical of Mental Disorders. 4Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- [21].American Psychiatric Society. Handbook on Diagnosis and Statistics of Mental Disorders (Desk Reference Book). 52014;Beijing: Peking University Press, (translated by Zhang Daolong). [Google Scholar]
- [22].Wang X, Zou Z, Shen Q, et al. Involvement of NMDA-AKT-mTOR signaling in rapid antidepressant-like activity of Chaihu-jia-Longgu-Muli-tang on olfactory bulbectomized mice. Front Pharmacol 2018;9:1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- [24].Tsai PS, Wang SY, Wang MY, et al. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual Life Res 2005;14:1943–52. [DOI] [PubMed] [Google Scholar]
- [25].Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care 1990;6:5–30. [DOI] [PubMed] [Google Scholar]
- [26].Dong F. An analysis of treating 128 cases of intractable insomnia with the Chaihu Guizhi decoction with the Longgu Muli decoction. Clin J Chin Med 2016;8:115–7. [Google Scholar]
- [27].Su LX, He JR, Huang SN, et al. Clinical effect of modified Chaihu Longgu Muli decoction in treatment of insomnia with liver depression transforming into fire: an analysis of 40 cases. Hunan J Tradit Chin Med 2018;34:5–7. [Google Scholar]
- [28].Ren YX. Clinical observation on treatment of chronic insomnia with modified Chaihu Longgu Muli decoction. Guangming J Chin Med 2015;30:293–5. [Google Scholar]
- [29].Chen L, Zhang YX, Chen LJ. Clinical observation of Chaihu Longgu Muli decoction combined with Paroxetine in the treatment of anxiety insomnia. J Pract Tradit Chin Med 2016;32:899–900. [Google Scholar]
- [30].Li KX, Liu MJ. The effect of Chaihu and keel oyster soup on insomnia after ischemic stroke. Electron J Clin Med Lit 2017;4:16767–8. [Google Scholar]
- [31].Tang RZ. Randomized parallel controlled study on the treatment of post stroke insomnia with Bupleurum Chinense plus Oyster Shell decoction. J Pract Tradit Chin Intern Med 2017;31:17–20. [Google Scholar]
- [32].Zhang R, Song LD. Curative effect of Chaihujialonggumulitang decoction in women with perimenopausal insomnia. Chin J Clin Med 2012;19:175–6. [Google Scholar]
- [33].Xu CF, Yang XM. Clinical observation on 32 cases of insomnia treated with Chaihu Jialonggu Oyster decoction. Pract Clin J Integr Tradit Chin Western Med 2014;14:8–9. [Google Scholar]
- [34].Guo YQ. Clinical observation on treating 51 cases of insomnia with the Chaihu Longgu Muli decoction. Clin J Chin Med 2017;9:15–7. [Google Scholar]
- [35].Wei B, Yang SY, Song SL. Clinical observation of Bupleurum and Longgu Oyster decoction in the treatment of insomnia and anxiety caused by hyperthyroidism. Intern Med 2018;13:99–101. [Google Scholar]
- [36].Lei HM. Thirty-one cases of insomnia of liver Qi stagnation treated with Bupleurum combined with Os Draconis and Concha Ostreae decoction. Henan Tradit Chin Med 2017;37:213–4. [Google Scholar]
- [37].Ji ZL, Zhang CJ. Clinical observation of Chaihu Jialonggu Oyster decoction in the treatment of insomnia due to liver depression and fire. Diet Health 2018;5:90–1. [Google Scholar]
- [38].Xin H, Zhang GZ. Clinical observation on 135 cases of insomnia treated with Chaihu Longgu Muli decoction. Hebei J Tradit Chin Med 2017;39:377–80. [Google Scholar]
- [39].Cao X. Clinical experience of Chaihu Jia longgu muli decoction in the diagnosis and treatment of insomnia after breast cancer operation. China Pract Med 2019;14:133–4. [Google Scholar]
- [40].Mei SY. Clinical study on modified Chaihu Longgu Muli decoction in the treatment of insomnia after ischemic stroke. Yiyao Qianyan 2016;6:348–9. [Google Scholar]
- [41].Wang JP. Clinical observation on treatment of insomnia with Chaihu and Longgu Muli decoction. China Health Care Nutr 2016;26:156–7. [Google Scholar]
- [42].Chen XZ. Clinical observation on 46 cases of insomnia with Qi depression, Phlegm and heat coagulation syndrome treated by modified Chaihu Longgu Muli decoction. Hunan J Tradit Chin Med 2017;33:52–4. [Google Scholar]
- [43].Wang PJ. Therapeutic effect of Chaihu Longgu Muli decoction on menopausal insomnia. Chin J Basic Med Tradit Chin Med 2006;12:369–82. [Google Scholar]
- [44].Zheng GL. Clinical observation of Chaihu Longgu Muli decoction in the treatment of insomnia of liver depression and fire. J Sichuan Tradit Chin Med 2014;32:110–1. [Google Scholar]
- [45].Zhang Y. Clinical observation on the treatment of insomnia of liver depression and fire with Chaihu Longgu Muli decoction. Beijing J Tradit Chin Med 2010;29:527–8. [Google Scholar]
- [46].Wang JL, Xing J, He LJ, et al. Observation of short term effects of modified Chaihu Jia Longgu Muli decoction in treating insomnia with syndrome of internal disturbance of phlegm-heat. China J Tradit Chin Med Pharmacy 2017;32:1548–52. [Google Scholar]
- [47].Wu HY, Deng LL, Wang WQ. Therapeutic effect of Chaihu Guizhi Longgu Muli decoction on chronic arthritis complicated with chronic insomnia. Home Med 2018;7:53–4. [Google Scholar]
- [48].Dong FQ, Zhu WE, Xie WT, et al. Effect of Chaihu plus Longgu Muli decoction on psychological stress reaction in rats. J Zhejiang Chin Med Univ 2007;31:58–60. [Google Scholar]
- [49].Wang WX, Sun FJ, Li F, et al. Effect of Longgu Muli decoction with Chaihu added on monoamine transmitters in anxiety rats. Tradit Chin Drug Res Clin Pharmacol 2008;19:340–2. [Google Scholar]
- [50].Ou BY, Li Y, Yang ZM, et al. Mechanism of Bupleurum Falcatum Plus Dragon Bone and Oyster Shell Decoction (CJLM) on insomnia. Lishizhen Med Mater Med Res 2010;21:1887–8. [Google Scholar]
- [51].Jin X, Zhang W, Zhang DX, et al. Study on the mechanism of Chaihu Longgu Muli decoction in the treatment of insomnia rats based on MEK/ERK pathway. Pharmacol Clin Chin Mater Med 2020;36:51–4. [Google Scholar]
- [52].Gagnier JJ, Boon H, Rochon P, et al. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med 2006;144:364–7. [DOI] [PubMed] [Google Scholar]
- [53].Cheng CW, Wu TX, Shang HC, et al. CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann Int Med 2010;167:W7–20. [DOI] [PubMed] [Google Scholar]


