Abstract
To evaluate the association between gene polymorphisms of MTHFR (C677T, A1298C) and MTRR (A66G), and the recurrent spontaneous abortion (RSA) risk in Asia.
Related case-control studies were collected, selected, and screened. A meta-analysis was conducted by Stata 12.0 software to assess the association between polymorphisms of target genes and RSA.
Altogether 30 studies examining the relationship between genetic polymorphism of folate metabolism and RSA risk were included, among which 20 studies were related to MTHFR C677T, 11 to MTHFR A1298C and 6 to MTRR A66G. The studies suggested that MTHFR C677T polymorphism was closely connected with RSA risk under all models (P < .05). Furthermore according to the subgroup analysis of ethnicity, the correlation between C677T polymorphism and RSA was stronger in north of China when compared with south of China and other Asian countries (P > . 05). For MTHFR A1298C, it was closely related to RSA risk in all gene models except for (AC vs AA) (P < .05). However, when it comes to MTRR A66G, there was no significant correlation between gene A66G polymorphism and RSA risk except for the additive gene model (G vs A) (P < .05).
The present evidence shows that the correlation between gene polymorphisms and RSA risk can be found in MTHFR C677T, A1298C (except for heterozygote model) and MTRR A66G (only in additive genotypes), and the detection of the correlated gene polymorphisms mentioned above is of certain guiding significance for preventing RSA and screening high-risk groups.
Keywords: methylenetetrahydrofolate reductase (MTHFR), methioninesynthase reductase (MTRR), polymorphism genetic, abortion, meta-analysis
1. Introduction
Recurrent spontaneous abortion (RSA) is defined as 2 or more times of pregnancy loss in the first 20 weeks of pregnancy which can be clinically detected.[1] It is a common complication of pregnancy, which accounts for 5% of the women in childbearing age.[2] In addition to clear etiology such as uterine anatomical defects, chromosome aberration, hormone disorders, blood system diseases[3] et al, there are still 60% causes remaining to be explored[4] which is defined as unexplained recurrent spontaneous abortion (URSA). It is believed that hyperhomocysteinemia caused by abnormal metabolism of folic acid is one of the independent risk factors for many pregnancy-related disorders,[5,6] because of its facilitation to embolization in placenta arteries,[7] which can further affect embryo's blood supply and result in villi necrosis, lapoptosis of placental trophoblast, thus leading to the lower secretion of maternal gonadotropin,[8] which finally results in the recurrent embryo loss.
Crucially, MTHFR and MTRR are key enzymes in folate/homocysteine pathway.[9] Some single nucleotide polymorphisms (SNPs) in genes involved in the folate metabolic pathway have been considered to be closely related to a low level of folate and a high level of homocysteine such as polymorphisms of MTHFR and MTRR,[10] which is also the most concerned by scholars in home and abroad. Over the last 10 years, a number of molecular epidemiological studies have been conducted worldwide to study the correlation between MTHFR or MTRR polymorphisms and RSA risks, but the results remain inconsistent.[11,12]
The inconsistency of results may be due to differences in race, regional, eating habits, as well as the fact that the sample size is too small to objectively reflect the relevance. In order to compare the different results of the researches more scientifically and objectively. Meta-analysis on this issue being widely carried out also draws conflicting conclusion.[13,14] Moreover the articles included in the latest meta-analysis by previous researchers were 8 years ago, of which the research scope only focused on 1 or 2 gene loci in the area of China.[15] Therefore, based on this situation, we carry out a meta-analysis including the genotype data from all eligible investigations in the latest years involving as extensive countries and regions in Asia as possible, as well as covering 3 gene loci(C677T and A1298C of MTHFR and A66G of MTRR) so as to provide a more accurate evaluation of the connection between folate metabolism polymorphisms and RSA susceptibility.
2. Materials and methods
2.1. Search strategy
This research was carried in adherence to the Meta-analysis of Observational Studies in Epidemiology guidelines[16] and we searched the following academic databases: China National Knowledge Infrastructure (CNKI), China Wanfang Database, PubMed, EMBASE, Cochrane library, Web of Science and Wiley for relevant studies since the establishment of the library to November of 2019. The following search words were combined according to different retrieval methods of each database: “methylenetetrahy-drofolatereductase”, “MTHF”, “C677T”, “A1298C”, “methioninesynthase reductase”, “MTRR” or “A66G”, “Unexplained recurrent spontaneous abortion” or “recurrent pregnancy loss”. In addition, we screened the bibliography of the included studies for any additional relevant study. Furthermore all magazines were retrieved from the first issue, and the relevant conference literature was tracked.
2.2. Statement
The ethical approval is not necessary, as this study is about the association between gene polymorphism of folate metabolism and RSA: a meta-analysis. It is not a clinical trial study, thus ethical approval and informed consent are not required. All the articles included in this meta-analysis have passed ethical approval and informed consent.
2.3. Inclusion and exclusion criteria
Studies that meet the following criteria will be adopted:
-
1.
The literature must be a case–control study published with good balance and comparability.
-
2.
Languages are limited to Chinese or English.
-
3.
The research should involve RSA risk and gene polymorphisms about MTHFR C677T, A1298C or MTRR A66G loci.
-
4.
Patients with RSA should have experienced 2 or more times abortion in the first trimester that have ruled out definite etiologies and the control group should have at least 1 successful pregnancy.
-
5.
Distribution each genotype and individual number in the case and control groups should be listed in the literature.
Studies with the following characteristics will be excluded:
-
1.
Those are not associated with MTHFR C677T A1298C, MTRR A66G polymorphism and URSA;
-
2.
Those are not case–control studies;
-
3.
Those that case groups did not exclude the clinical abortion factors;
-
4.
Frequency of genotype or allele in the literature are incomplete or unclear.
2.4. Data extraction and quality evaluation
The selection procedure was independently replicated by 2 reviewers (X-X.Z and Y-L.P) to avoid biasing. Firstly, the title and summary of the studies were viewed. Then they read the full text to eliminate studies that did not accord with the above-mentioned inclusion criteria. We attempted to contact the corresponding author to obtain important information that was missing. If that failed, the literature would be deleted. We used the nine - star Newcastle - Ottawa scale to evaluate the quality of the studies.[17] It included 3 aspects: study object selection, group comparability, and exposure factor measurement. In brief, a maximum of 9 points was assigned to each study: 4 for selection, 2 for comparability, and 3 for outcomes. A final score above 6 was regarded as high quality. They extracted relevant data from each article: The first authors name, years of publication, country and region, genotype frequencies in the observation group, and control group, the number of miscarriages defining RSA, Hardy-Weinberg equilibrium and Quality score of case-control study were showed in the table (Fig. 1).
Figure 1.
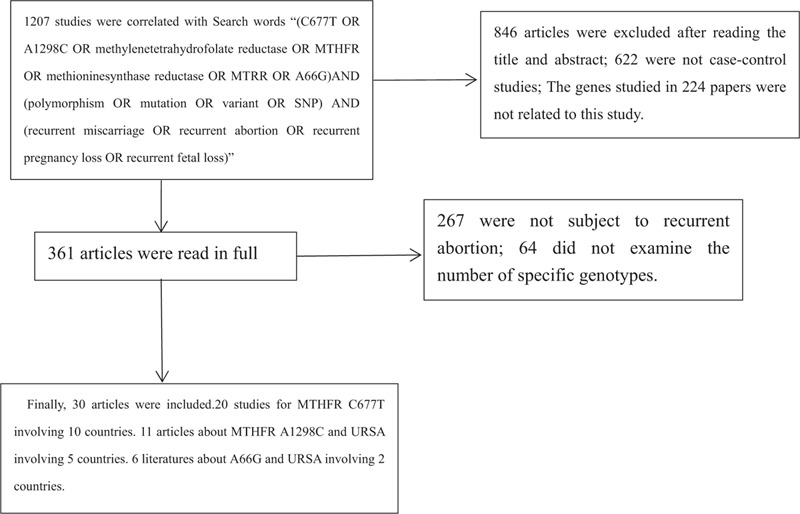
Article screening flowchart.
2.5. Statistical analysis
All the data were analyzed by Stata 12. 0 software and the flow charts were drawn below. Based on the odds ratio (OR) with a corresponding 95% confidence interval (CI), we counted the pooled odds to analyze the effect on the association. While crossing these studies, Q test and I 2 were first used to test the heterogeneity of the included literatures. When I 2 > 50%, it proved that there was heterogeneity between the studies, and the random effect model was used, and if not, the fixed effect model was applied instead. In order to search for the sources of heterogeneity, we mainly conducted subgroup analysis according to different countries and regions. In order to evaluate the stability of the combined results, a sensitivity analysis was conducted for the meta-analysis results after each removal of a case-control study. The Begg funnel plot was utilized as a criterion for evaluating publication bias.
3. Results
3.1. Characteristics of the included studies
Overall, a total of 30 out of 1207 articles were selected for the final meta-analysis.[18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] Among the included articles, 20 studies described the relationship between polymorphisms of MTHFR C677T and RSA with 2413 cases and 2923 controls involving 10 countries. Eleven articles[18,21,22,27,30,38,39,40,41,42,43] demonstrated the association between MTHFR A1298C and RSA with 1889 cases and 1947 controls involving 5 countries. Six literatures described the relationship between A66G and URSA with 918 cases and 942 controls involving 2 countries. The baseline characteristics of the participants by MTHFR C677T, A1298C, and MTRR A66G were respectively shown in Tables 1–3. All of the 30 articles were published before June 2019. In addition, 13 manuscripts were published in English, and 17 manuscripts were in Chinese, as shown in Tables 1–3.
Table 1.
Characteristics of studies on the association between C677T gene polymorphisms of MTHFR and URSA risk.
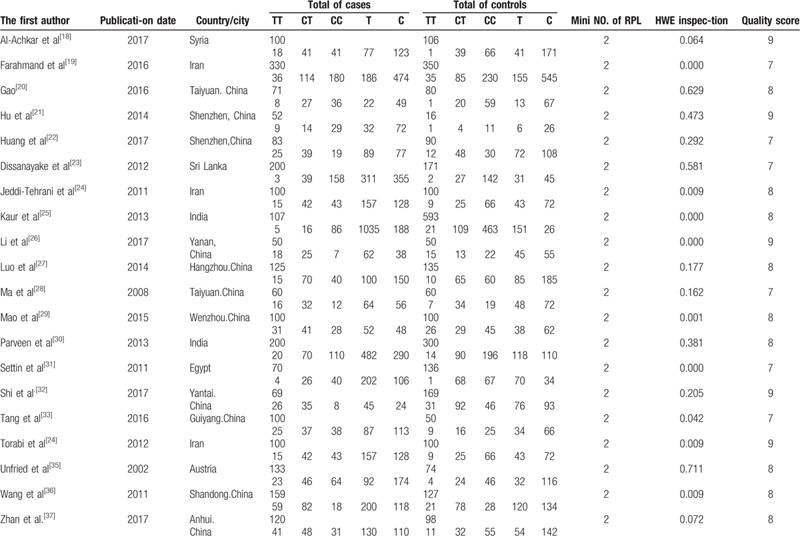
Table 3.
Characteristics of studies on the association between A66G gene polymorphisms of MTRR and URSA risk.

4. Results of the overall meta-analysis
4.1. Meta-analysis of MTHFR C677T polymorphism and URSA risk
Twenty articles were related to C677T and RSA risk. The results showed that the polymorphisms of MTHFR C677T were significantly correlated with RSA under dominant gene model (TT + CT vs CC; OR 1.781, 95% CI 1.574–2.014), recessive gene model (TT vs CC + CT; OR 2.155, 95% CI 1.798–2.583), heterozygote gene model (CT vs CC; OR 1.600, 95% CI 1.401–1.826), homozygote gene model (TT vs CC; OR 2.813, 95% CI 2.300–3.442) and additive gene model (T vs C; OR1.686, 95%CI 1.539–1.848) (Table 4).
Table 4.
Meta-analysis of MTHFR C677T polymorphism and URSA risk.

5. Sub-group analysis
5.1. Subgroup analysis of (TT vs CC) genotypes in China and other countries
As shown in Table 2, compared with other genetic models, odds ratio in homozygote gene model (TT vs CC)(OR = 2.81) was the highest, which mean that population with homozygous genotype TT were more likely to suffer from RSA. We made an in-depth analysis of the results. Since 11 of the 20 articles were from China and 9 were from other countries, we conducted subgroup analysis of (TT vs CC) genotypes according to countries. The pooled OR was 3.420 in China [95%CI = (2.579–4.536)], and 2.273 in other countries [95%CI = (1.700–3.039)]. It can be concluded that population in China contributed more to the correlation between TT genotype and RSA (Fig. 2).
Table 2.
Characteristics of studies on the association between A1298C gene polymorphisms of MTHFR and URSA risk.
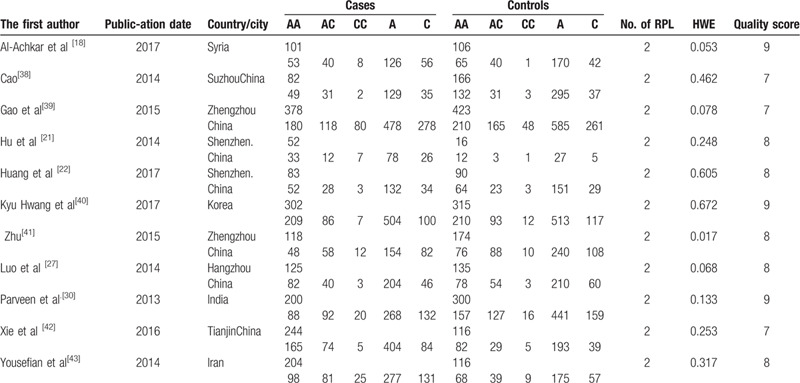
Figure 2.
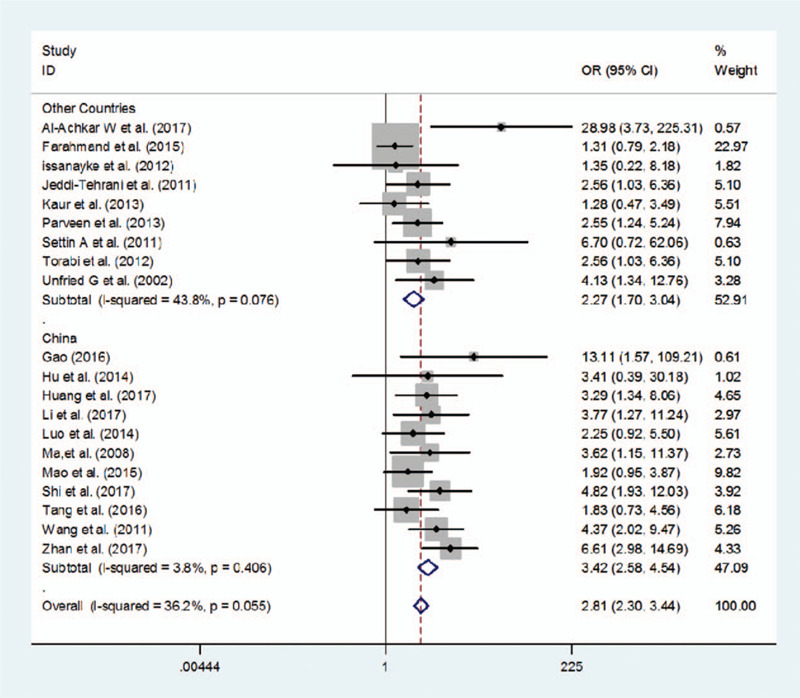
Subgroup analysis of (TT vs CC) genotypes in China and other countries.
Of the 11 literatures from China, 6 were carried out in the south and 5 were in the north. Subgroup analysis was conducted on (TT vs CC) genotype in terms of RSA susceptibility according to the population from south or north of China. The pooled OR was 4.580 [95%CI (2.896–7.244)] and 2.852 [95%CI = (1.990–4.088)] respectively in north and south of China. It was obvious that the population in the north of China contributed more to high probability of TT genotype and RSA (Fig. 3).
Figure 3.
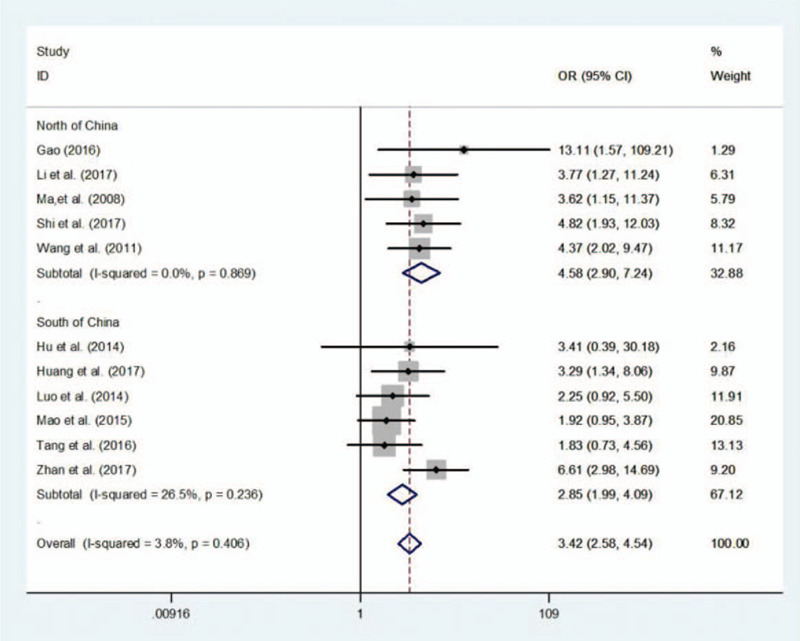
Subgroup analysis of TT vs CC genotype in north and south China.
5.2. Test for heterogeneity
The results of the meta-analysis presented in the above table showed that I 2 was <50%, indicating that the included studies had clinical significance and statistical homogeneity.
5.3. Publication bias evaluation
In the evaluation process, we found that there were various differences in subjects included in the literatures such as countries, regions, races, and miscarriage times, etc. Two groups of gene funnel plot analysis showed asymmetry indicating the possibility of publication bias (Fig. 4).
Figure 4.
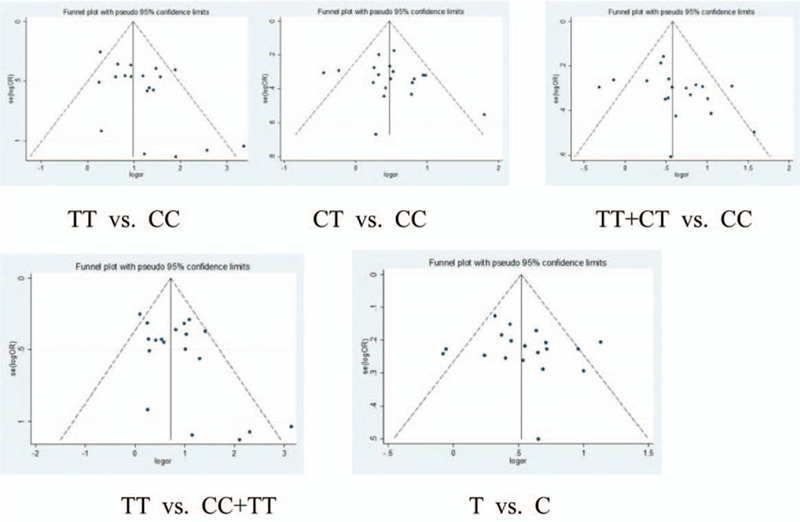
The publication bias of articles on the relationship between C677T and URSA risk was shown in the funnel figures.
5.4. Meta-analysis of MTHFR A1298C polymorphism and URSA risk
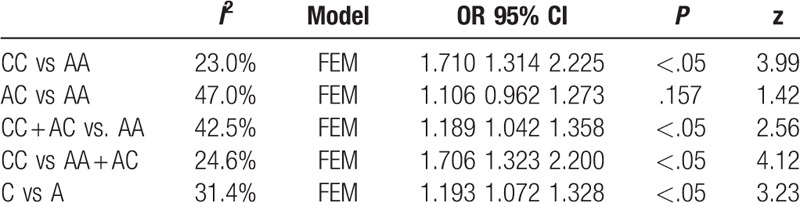
Eleven articles were associated with A1298C and the chance of RSA. The results showed that the polymorphism of MTHFR A1298C was closely related to RSA under dominant model (CC + AC vs AA; OR 1.189, 95%CI 1.042–1.358), recessive model (CC vs AA + AC; OR 1. 706, 95%CI 1.323–2.200), heterozygote model (AC vs AA; OR 1.106, 95% CI 0.962–1.273) homozygote model (CC vs AA; OR 1.710, 95%CI 1.314–2.225) and additive model (C vs A; OR 1.193, 95%CI 1.072–1.328). And there was no significant association between (AC vs AA; OR1.106, 95%CI 0.962–1.273, P = .157). The result was shown in Table 5.
Table 5.
Results of MTHFR A1298C polymorphism and URSA risk.
5.5. Test for heterogeneity
The results of the meta-analysis presented in the above table showed that I 2 was <50%, indicating that the included studies had clinical significance and statistical homogeneity.
5.6. Publication bias
In the same situation as C677T, we analyzed the publication bias of articles on the relationship between A1298C and RSA risk. The 2 groups of gene funnel plot analysis showed asymmetry, indicating the possibility of publication bias (Fig. 5).
Figure 5.
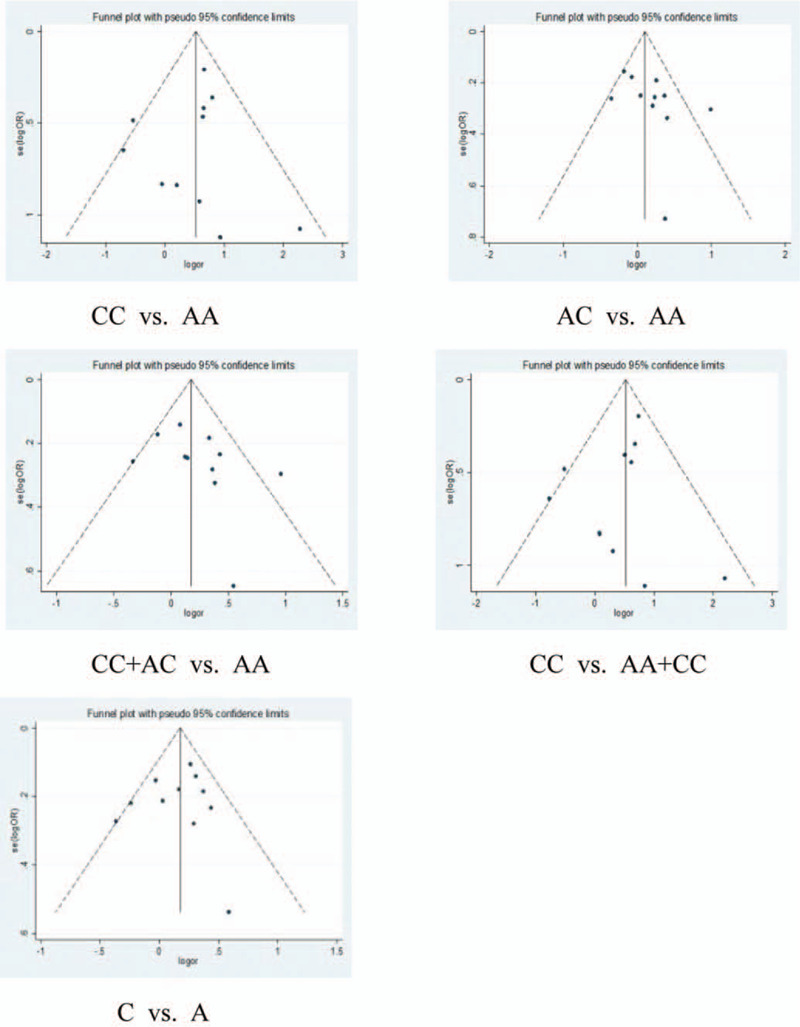
The publication bias of articles on the relationship between A1298C and URSA risk was shown in the Funnel figure.
5.7. Meta-analysis of A66G polymorphism and URSA risk
Six articles were associated with A1298C and RSA. In all genotypes of MTRR A66G polymorphism, it was the additive gene model (G vs A; OR1.204, 95%CI 1.044–1.390) rather than the dominant (GG + AG vs AA), recessive (GG vs AA + AG; OR 1.290, 95%CI 0.956–1.740), heterozygote (AG vs AA; OR1.075, 95%CI 0.874–1.322), homozygote gene model (GG vs AA; OR 1.343, 95%CI 0.966–1.867) that had association with RSA risk. The result was shown in Table 6.
Table 6.
Results of MTRR A66G polymorphism and URSA risk.
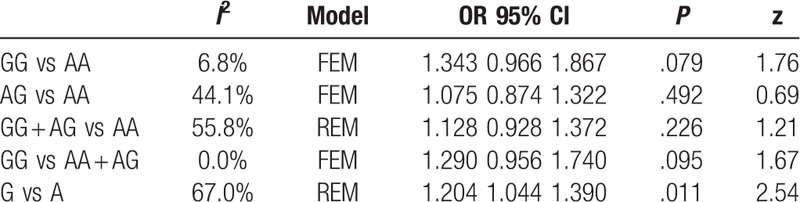
5.8. Test for heterogeneity
In the heterogeneity test for the A66G genotypes of each gene model, I 2 of (GG + AG vs AA) and (G vs A) were greater than 50%, indicating that the included studies had heterogeneity. Subgroup analysis was necessary for heterogeneous sources.
5.9. Subgroup analysis
Of the 6 articles included, 5 were from China and 1 was from Korea. Therefore, the subgroup analysis of (GG + AG vs AA) and (G vs A) were conducted according to the source of the countries. The meta-analysis of 5 Chinese literature showed that I 2 of (GG + AG vs AA) and (G vs A) groups were both lesser than 50%, indicating that different countries exerted an impact on heterogeneity. And the subgroup analysis showed that the MTRR A66G gene mutation was dramatically connected with RSA risk under the dominant group (GG + AG vs AA)[OR = 1.301, 95%CI = (1.022–1.657)] and additive group (G vs A) [OR = 1.373, 95%CI = (1.157–1.630)]. The results was shown in Table 7, Figures 6 and 7.
Table 7.
Results of MTRR A66G mutation and URSA risk in subgroup analysis.

Figure 6.
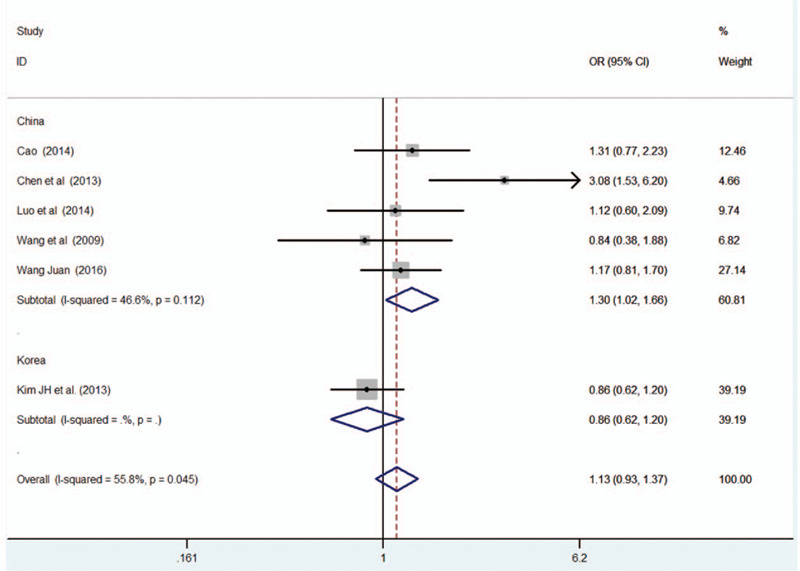
Results of MTRR A66G mutation and URSA risk in subgroup analysis.
Figure 7.
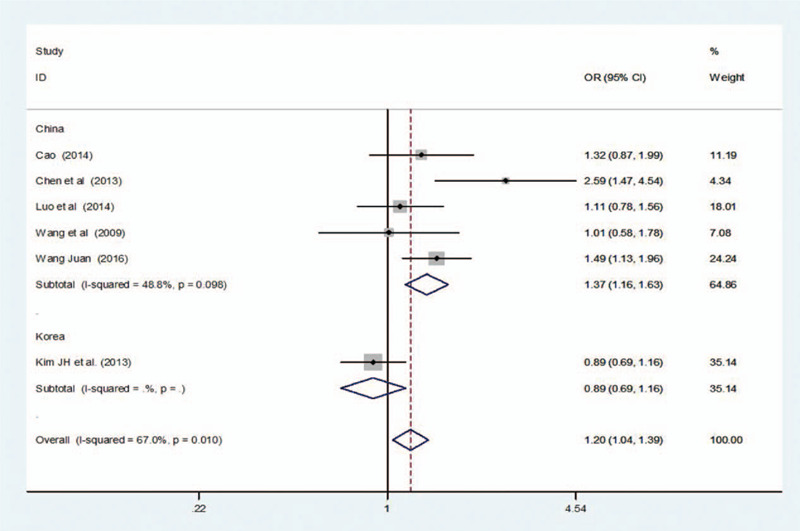
Results of MTRR A66G mutation and URSA risk in subgroup analysis.
5.10. Publication bias
We also analyzed the publication bias of articles on the relationship between A66G and URSA risk. The gene funnel plot analysis of the 2 groups showed asymmetry indicating the possibility of publication bias (Fig. 8).
Figure 8.
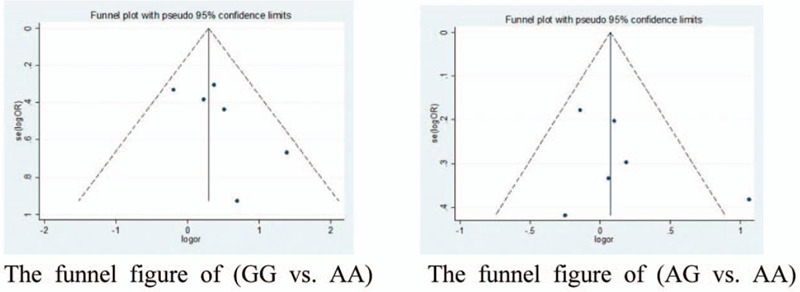
The publication bias of articles on the relationship between A1298C and URSA risk was shown in the funnel figure.
6. Discussion
At present, disorders of coagulation mechanism in the pathogenesis of RSA have attracted extensive attention in the academic world,[48] which can be classified into 2 categories: acquired and hereditary coagulation disorders, and the latter is closely related to gene polymorphisms.[49] MTHFR and MTRR are the 2 vital enzymes contributing to the folate metabolism.[50] Abnormal activities of these enzymes can lead to hyperhomocysteinemia, which is one of the important reasons for hypercoagulable state, and act as an independent risk for various arteriovenous thromboembolic diseases, including pregnancy loss.[51] Thrombus in placenta can limit blood supply to the embryo and cause villi necrosis, which is a momentous component of all RSA etiologies.[52] Homocystein(Hcy), catalyzed by methionine synthetase, accompanied with vitamin B12 to receive methyl from 5-methyl tetrahydrofolic acid so as to produce methionine. MTHFR catalyzes the formation of 5, 10 methylenetetrahydrofolate, which can be transformed to 5-methyl tetrahydrofolate.[53] Moreover, vitamin B12 needs MTRR to catalyze its methylation to maintain the activity of co-enzyme so that the catalytic reaction of methionine synthetase is normally carried out.[54] MTHFR and MTRR genes show the characteristics of polymorphism, among which the single nucleotide polymorphisms of genes C677T, A1298C in MTHFR, and A66G in MTRR are the most studied. The MTHFR gene is located in chromosome 1q36. 3. At present, there are 15 types of point mutations in human MTHFR, and the most important one is the mutation of 677 siting on the fifth exon called C677T mutation. The allele “C”is mutated to “T”, making the original encoding alanine (Ala) mutate to valine (Val), which can affect the enzymes heat sensitivity.[55] A1298C is another important mutation site located in the eighth exon. After mutation, the encoding amino acid is changed from Glu to Ala, leading to the decrease of enzyme activity.[56] MTRR can catalyze the conversion of cysteine to methionine. When the “A” lying on the 66th site of MTRR mutates to “G”, the amino acid it encodes will transform from methionine to isoleucine, resulting in the decrease of enzyme activity in MTRR.
Chen[22] published a meta-analysis in 2016, discussing the relationship between MTHFR mutation and RSA in China, and drew the conclusion that MTHFR C677T gene mutations were connected with the onset of RSA in China, while there was no significant correlation between A1298C mutation and RSA susceptibility. However, in this meta-analysis, only 5 studies discussed about A1298C, thus the result were inevitably showed limitation to some extent, and there was no detailed subgroup analysis of the different regions between the north and south. Moreover 11 out of 21 articles were published before 2010. In recent years, relevant researches have emerged endlessly. Based on this, it is necessary to carry out a new research to collect more updated achievements, involving more different provinces in China and more countries in Asia.
In our current meta-analysis, a total of 30 studies were accepted to review the relationship between MTHFR, MTRR gene polymorphism and RSA risk, among which 20 were related to the C677T polymorphism, 11 to A1298C polymorphism and 6 studies to polymorphism of A66G. The results displayed that the mutation of MTHFR C677T was associated with RSA risk in Asian population under dominant group (TT + CT vs CC; OR 1.781, 95% CI 1.574–2.014), recessive group(TT vs CC + CT; OR 2.155, 95%CI 1.798–2.583), heterozygote group(CT vs CC; OR 1.600, 95% CI 1.401–1.826), homozygote group(TT vs CC; OR 2.813, 95% CI 2.300–3.442), and additive group (T vs C; OR 1.686, 95%CI 1.539–1.848), especially in the model of (TT vs CC) (OR = 2. 81). Stratified analysis obviously reduces the heterogeneities and the results showed a higher risk of RSA in Chinese [OR = 3.420, 95%CI = (2.579–4.536)], indicating that population in China contributed more to the correlation between genotype of TT and RSA. And we further conducted subgroup analysis on (TT vs CC) and RSA risk according to the population from south or north of China. The pooled OR was respectively 4.580[95%CI (2.896–7.244)] and 2.852[95%CI = (1.990–4.088)] in north and south. The result further showed that the existence of TT genotypes was closely related to the susceptibility to RSA in northern China.
For A1298C, 11 articles were associated with A1298C and RSA risk. The results demonstrated that the polymorphism of MTHFR A1298C was dramatically connected with RSA risk under the dominant model (CC + AC vs AA; OR 1.189, 95% CI 1.042–1.358), recessive model (CC vs AA + AC; OR1. 706, 95% CI 1.323–2.200), heterozygote model(AC vs AA; OR1.106, 95%CI 0.962–1.273) homozygote model (CC vs AA; OR 1.710, 95%CI 1.314–2.225), and additive model (C vs A;OR1.193, 95%CI 1.072–1.328). And there was no significant association between (AC vs AA; OR1.106, 95% CI 0.962–1.273, P = .157) and RSA risk.
When it comes to A66G, 6 articles are associated with A1298C and RSA risk. In all genotypes of MTRR A66G polymorphism, it was the additive model (G vs A; OR 1. 204, 95%CI 1.044–1.390) that showed association with the risk of RSA, rather than the dominant (GG + AG vs AA), recessive (GG vs AA + AG; OR 1.290, 95%CI 0.956–1.740), heterozygote (AG vs AA; OR1.075, 95%CI 0.874–1.322) and homozygote gene models (GG vs AA; OR1.343, 95%CI 0.966–1.867). Of the 6 articles included, 5 were from China and 1 was from Korea. Therefore, the subgroup analysis was conducted. After the exclusion of South Korea, the meta-analysis showed that I 2 of (GG + AG vs AA) and (G vs A) gene groups were both lesser than 50%, indicating that different countries contribute to heterogeneity. And the subgroup analysis displayed that the MTRR A66G gene mutation was significantly associated with RSA risk in China under dominant group (GG + AG vs AA)[OR = 1.301, 95%CI = (1.022–1.657)], and additive group (G vs A) [OR = 1.373, 95% CI = (1.157–1.630)].
The present evidence showed that identification of MTHF C677T and A1298C mutation was of certain practical significance for preventing RSA and screening high-risk groups, especially MTHFR C677T mutation in north of China when compared with other regions and countries in Asia. This may be due to the difference in living environment and diet between north and south China. Furthermore, we found that the risk of RSA was significantly increased in women carrying the mutant TT or TC of C677T and CC of A1298C. Except for the gene model (G vs A), the genetic A66G polymorphism was not associated with the risk of RSA. Large-scale clinical studies were needed to verify the conclusions in terms of the quantity and quality of the study.
Author contributions
Xiaoxuan Zhao was responsible for designing and writing this article; Yunlu Ping was in charge of study selection and information extraction; Yang Zhao was responsible for meta-analysis; Lu Chen took charge of reviewing and revising the article; Xiaoling Feng was responsible for the supervision and submission work.
Footnotes
Abbreviations: Ala = alaninemutate, Hcy = homocysteinemia, MTHFR = methylenetetrahydrofolate reductase, MTRR = methioninesynthase reductase, RSA = recurrent spontaneous abortion, SNPs = single nucleotide polymorphisms, URSA = unexplained recurrent spontaneous abortion, Val = valine.
How to cite this article: Zhao X, Zhao Y, Ping Y, Chen L, Feng X. Association between gene polymorphism of folate metabolism and recurrent spontaneous abortion in Asia: a meta-analysis. Medicine. 2020;99:40(e21962).
This work was supported by grants from the National Natural Science Foundation of China (81973894, 81574014) and Post Doctoral Fund Project of Heilongjiang Province (LBH-Z16199).
All authors declare that they have no conflict of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009;92:1520–4. [DOI] [PubMed] [Google Scholar]
- [2].Hady El Hachem, Vincent Crepaux, Pascale May-Panloup, et al. Recurrent pregnancy loss: current perspectives. Int J Womens Health 2017;9:331–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grimstad F, Krieg S. Immunogenetic contributions to recurrent pregnancy loss. J Assist Reprod Genet 2016;33:833–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Diejomaoh MF. Recurrent spontaneous miscarriage is still a challenging diagnostic and therapeutic quagmire. Med Princ Pract. 2015;24 Suppl 1(Suppl 1):38-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bergen NE, Jaddoe VW, Timmermans S, et al. Homocysteine and folate concentrations in early pregnancy and the risk of adverse pregnancy outcomes: the Generation R Study. BJOG 2012;119:739–51. [DOI] [PubMed] [Google Scholar]
- [6].Byrne J. Periconceptional folic acid prevents miscarriage in Irish families with neural tube defects. Ir J Med Sci 2011;180:59–62. [DOI] [PubMed] [Google Scholar]
- [7].Simcox LE, Ormesher L, Tower C, et al. Thrombophilia and pregnancy complications. Int J Mol Sci 2015;16:28418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yamada T, Morikawa M, Yamada T, et al. First-trimester serum folate levels and subsequent risk of abortion and preterm birth among Japanese women with singleton pregnancies. Arch Gynecol Obstet 2013;287:9–14. [DOI] [PubMed] [Google Scholar]
- [9].Goodman CS, Coulam CB, Jeyendran RS, et al. Which thrombophilic gene mutations are risk factors for recurrent pregnancy loss? Am J Reprod Immunol 2006;56:230–6. [DOI] [PubMed] [Google Scholar]
- [10].Shahzad K, Hai A, Ahmed A, et al. A Structured-based model for the decreased activity of Ala222Val and Glu429Ala Methylenetetrahydrofolate Reductase (MTHFR) mutants. Bioinformation 2013;9:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hwang KR, Choi YM, Kim JJ, et al. Methylenetetrahydrofolate reductase polymorphisms and risk of recurrent pregnancy loss: a case-control study. J Korean Med Sci 2017;32:2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aiko M, Tamao N, Mayumi SO, et al. Methylenetetrahydrofolate reductase and an endothelial nitric oxide synthase polymorphism with recurrent pregnancy loss. AJRI 2004;52:60–6. [DOI] [PubMed] [Google Scholar]
- [13].ANair RR, Khanna A, Singh R, et al. Association of maternal and fetal MTHFR A1298C polymorphism with the risk of pregnancy loss: a study of an Indian population and a meta-analysis. Fert Ster 2013;99:1311–8. [DOI] [PubMed] [Google Scholar]
- [14].Hong HH, Hu Y, Yu XQ, et al. Associations of C677T polymorphism in methylenetetrahydrofolate reductase(MTHFR) gene with male infertility risk: A meta-analysis. Eur J Obstet Gynecol Reprod Biol 2017;212:101–9. [DOI] [PubMed] [Google Scholar]
- [15].Chen H, Yang X, Lu M. Methylenetetrahydrofolate reductase gene polymorphisms and recurrent pregnancy loss in China: a systematic review and meta-analysis. Arch Gynecol Obstetr 2016;293:283–90. [DOI] [PubMed] [Google Scholar]
- [16].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [17].Han Y, Xia Z, Guo S, et al. Laparoscopically assisted anorectal pullthrough versus posterior sagittal anorectoplasty for high and intermediate anorectal malformations: a systematic review and meta-analysis. PLoS One 2017;12:e0170421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Al-Achkar W, Wafa A, Ammar S, et al. Association of Methylenetetrahydrofolate Reductase C677T and A1298C gene polymorphisms with recurrent pregnancy loss in syrian women. Reprod Sci 2017;24:1275–9. [DOI] [PubMed] [Google Scholar]
- [19].Farahmand K, Totonchi M, Hashemi M, et al. Thrombophilic genes alterations as risk factor for recurrent pregnancy loss. J Matern Fetal Neonatal Med 2016;29:1269–73. [DOI] [PubMed] [Google Scholar]
- [20].Gao Q. Study on the correlation between the polymorphism of B vitamins, serum homocysteine and MTHFR C677T gene in patients with recurrent spontaneous abortion. Chin Maternal Child Health 2016;31:3794–7. [Google Scholar]
- [21].Hu XD, Liang PY, Diao LH, et al. The relationship between the gene mutation of methyl-tetrahydrofolate reductase gene and recurrent miscarriage of unknown cause. China Eugen Genet J 2014;22:87–9. [Google Scholar]
- [22].Huang SY, Tang GL, Liu QZ, et al. Correlation analysis of gene polymorphisms of MTHFR and unexplained recurrent abortion. Int J Reprod Health/Fam Plan 2017;36:382–4. [Google Scholar]
- [23].Dissanayake VH, Sirisena ND, Weerasekera LY, et al. Candidate gene study of genetic thrombophilic polymorphisms in pre-eclampsia and recurrent pregnancy loss in Sinhalese women. J Obstet Gynaecol Res 2012;38:1168–76. [DOI] [PubMed] [Google Scholar]
- [24].Jeddi-Tehrani M, Torabi R, Mohammadzadeh A, et al. Investigating association of three polymorphisms of coagulation factor XIII and recurrent pregnancy loss. Am J Reprod Immunol 2010;64:212–7. [DOI] [PubMed] [Google Scholar]
- [25].Kaur L, Puri M, Kaushik S, et al. Genetic thromobophilia in pregnancy: a case–control study among North Indian women. J Thromb Thrombolysis 2013;35:250–6. [DOI] [PubMed] [Google Scholar]
- [26].Li H, Huang JJ. Correlation analysis of 677 polymorphisms and recurrent abortion of methyl-tetrahydrofolate reductase gene. J Shanxi Med Sci 2017;46:855–7. [Google Scholar]
- [27].Luo L. Folic acid metabolism pathway main enzymes encoding gene polymorphism and unexplained recurrent miscarriage association studies. Zhejiang province medical association inspection medical branch, Zhejiang medical doctor association of physicians branch. Laboratory medicine academic conference proceedings in Zhejiang Province in 2014. Zhejiang province medical association inspection medical branch, zhejiang medical doctor association of physicians branch 2014;1:232–8. [Google Scholar]
- [28].Ma SF, Zheng ML. The relationship between MTHFRC677T gene polymorphism and recurrent abortion. Health Care Med Res Pract 2008;4:4–5. [Google Scholar]
- [29].Mao YL, Li XM, Xu F. Analyze folinic acid (methylene reductase gene polymorphism and the correlation of spontaneous abortion Chinese. Journal of Traditional Chinese Medicine 2015;1:2250. [Google Scholar]
- [30].Parveen F, Tuteja M, Agrawal S. Polymorphisms in MTHFR, MTHFD, and PAI-1 and recurrent miscarriage among North Indian women. Arch Gynecol Obstet 2013;288:1171–7. [DOI] [PubMed] [Google Scholar]
- [31].Settin A, Elshazli R, Salama A, et al. Methylenetetrahydrofolate reductase gene polymorphisms in Egyptian women with unexplained recurrent pregnancy loss. Genet Test Mol Biomarkers 2011;15:887. [DOI] [PubMed] [Google Scholar]
- [32].Shi CH, Yan Q, Jiang L. Study on the correlation between the polymorphismof MTHFR gene C677T site in yantai area and the cause of unexplained recurrent spontaneous abortion. China Continuing Med Sci Educ 2017;9:75–6. [Google Scholar]
- [33].Tang DL, Wu ZQ, Jin YQ, et al. Relationship between MTHFR C677 T gene polymorphism and plasma HCY and recurrent miscarriage. Chin J Eugen Genet 2016;24:15–6. [Google Scholar]
- [34].Torabi R, Zarei S, Zeraati H, et al. Combination of thrombophilic gene polymorphismsas a cause of increased the risk of recurrent pregnancy loss. J Reprod Infertil 2012;13:89–94. [PMC free article] [PubMed] [Google Scholar]
- [35].Unfried G, Griesmacher A, Weismüller W, et al. The C677T polymorphism of the methylenetetrahydrofolate reductase gene and idiopathic recurrent miscarriage. Obstet Gynecol 2002;99:614–9. [DOI] [PubMed] [Google Scholar]
- [36].Wang SM, Jia YF, Yang DT, et al. Correlation between MTHFR gene C677T polymorphism and unexplained recurrent spontaneous abortion. Chin Maternal Child Health Care 2011;26:1385–7. [Google Scholar]
- [37].Zhan QQ, He LZ. Correlation analysis of MTHFR gene and PAI-1 gene polymorphism and recurrent abortion. J Southern Anhui Med Coll 2017;36:38–41. [Google Scholar]
- [38].Cao YL. Meta-analysis and correlation analysis of single nucleotide polymorphisms of recurrent miscarriage and coagulation related genes and folate metabolism. Fudan university 2014;1:59–63. [Google Scholar]
- [39].Gao J, Wang T, Xiao H, et al. Correlation analysis of folate metabolism gene MTHFR and MTRR polymorphism and recurrent abortion in henan. Clin Med 2015;35:1–4. [Google Scholar]
- [40].Hwang KR, Choi YM, Jin JK, et al. DNA microarray analysis of gene expression in eutopic endometrium from patients with endometriosis. Adv Reprod Sci 2017;05:75–96. [Google Scholar]
- [41].Zhu L. Polymorphisms in the methylene tetrahydrofolate reductase and methionine synthase reductase genes and their correlation with unexplained recurrent spontaneous abortion susceptibility. Genetics & Molecular Research Gmr 2015;14:8500. [DOI] [PubMed] [Google Scholar]
- [42].Xie XY, Zhang Y, Xin L, et al. Relationship between folate metabolic enzyme MTHFR, MTRR gene polymorphism and unexplained recurrent abortion. Tianjin Med 2016;44:1243–6. [Google Scholar]
- [43].Yousefian E, Kardi MT, Allahveisi A. Methylenetetrahydrofolate Reductase C677T and A1298C polymorphism in iranian women with idiopathic recurrent pregnancy losses. Iran Red Crescent Med J 2014;16:e16763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen HL, Tang LY, Chen CB, et al. Study on the genetic polymorphism of thereductase and methionine synthase reductase and methionine synthase in the unknown cause. Chin J Eugen Genet 2013;21:29–30. [Google Scholar]
- [45].Kim JH, Jeon YJ, Lee BE, et al. Association of methionine synthase and thymidylate synthase genetic polymorphisms with idiopathic recurrent pregnancy loss. Fertil Steril 2013;99:1674–80. [DOI] [PubMed] [Google Scholar]
- [46].Wang SM, Shi XY, Shen R, et al. Study on the polymorphisms of folate metabolic enzymes and recurrent spontaneous abortion. Chin J Eugen Genet 2009;17:12–3. [Google Scholar]
- [47].Wang J. ANXA5, KDR, MTHFR, analysis on the correlation between MTRR gene single nucleotide polymorphism and the risk of recurrent spontaneous abortion in Chinese Han women. Chongqing Med Univ 2016;1:26–7. [Google Scholar]
- [48].Dasarathy J, Gruca LL, Bennett C, et al. Methionine metabolism in human pregnancy. Am J Clin Nutr 2010;91:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Stangler HS, Zagradišnik B, Erjavec ŠA, et al. MTHFR C677T and A1298C genotypes and haplotypes in slovenian couples with unexplained infertility problems and in embryonic tissues from spontaneous abortions. Balkan J Med Genet 2013;16:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li WX, Cheng F, Zhang AJ, et al. Folate deficiency and gene polymorphisms of MTHFR, MTR and MTRR elevate the hyperhomocysteinemia risk. Clin Lab 2017;63:523–33. [DOI] [PubMed] [Google Scholar]
- [51].Zhao JY, Qiao B, Duan WY, et al. Genetic variants reducing MTTR gene expression increase the risk of congenital heart disease in Han Chinese populations. Eur Heart J 2014;35:733–42. [DOI] [PubMed] [Google Scholar]
- [52].Chatzidimitriou M, Chatzidimitriou D, Mavridou M, et al. Thrombophilic gene polymorphisms and recurrent pregnancy loss in Greek women. Int J Lab Hematol 2017;39:590–5. [DOI] [PubMed] [Google Scholar]
- [53].Reilly R, Mcnulty H, Pentieva K, et al. MTHFR 677TT genotype and disease risk: is there a modulating role for B-vitamins? Proc Nutr Soc 2014;73:47. [DOI] [PubMed] [Google Scholar]
- [54].Gaughan DJ, Kluijtmans LA, Barbaux S, et al. Corrigendum to “The methionine synthase reductase (MTRR) A66G polymorphism is a novel genetic determinant of plasma homocysteine concentrations”. Atherosclerosis 2003;167:373–4. [DOI] [PubMed] [Google Scholar]
- [55].Ueland PM, Hustad S, Schneede J, et al. Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci 2001;22:195–201. [DOI] [PubMed] [Google Scholar]
- [56].Yang B, Fan S, Zhi X, et al. Geographical and ethnic distribution of MTHFR gene polymorphisms and their associations with diseases among Chinese population. Clin Genet 2016;92: [DOI] [PubMed] [Google Scholar]


