Abstract
This study aims to assess the survival status of patients with Primary gallbladder cancer (PGC) and analyze the prognosis factors to facilitate the exploration of the prevention and therapeutic strategies of PGC.
Data from 2433 PGC patients collected from 2010 to 2015 were extracted from the Surveillance, Epidemiology, and End Results (SEER) database. The SEER∗Stat, SPSS 23.0 and GraphPad Prism 8 were used for statistical analyses. Kaplan Meier analysis was performed for the survival curve, log-rank test analyses were used to compare the survival rate difference and Cox regression analyses were performed to determine the prognosis factors.
A total of 2433 PGC cases were reported from 2010 to 2015. The median age was 64.2 ± 10.4 years old and the percentages of the white patients were 73.7% (1794/2433). The percentage of patients who received surgery treatment was 82.1% (1998/2433). The overall median survival time of all patients was 19 months and the 5-year survival rate was 28.8%. The 5-year survival rate of PGC patients in pN2 stage dropped to 0% and the 5-year survival rate for PGC patients with distant metastasis was only 2.7%. Age, tumor size, grade, pT stage, pM stage were risk factors for prognosis, surgery or not and radiation or not were protective factors for prognosis.
Survival analysis of PGC patients based on the SEER database have provided an opportunity for understanding PGC prognosis and the basis for the exploration of viable PGC prevention and therapeutic strategies.
Keywords: primary gallbladder cancer, prognosis, survival
1. Introduction
Primary gallbladder cancer (PGC) accounts the fifth most common malignancy of the gastrointestinal tract, and is characterized by poor prognosis and low survival rates.[1] It is estimated to constitute 2.3% (219,420 new cases) of the total cancer incidence worldwide and 1.7% (165,087 new cases) of all cancer deaths in 2018.[2] PGC remains to be aggressive with an overall dismal outcome despite biomedical, technological, surgical, and chemotherapeutic advancements in the past.[3]
PGC shows differences in geographic distribution. The incidence of PGC varies by geography,[4] ethnicity and culture. Its incidence ranges from 1.5 per 100,000 in North America to 27.3 per 100,000 in South America.[5,6] Despite its decline over the past several decades, the incidence of PGC appears to be on the increase in some European countries,[7] including women in Netherlands (1.7 per 100,000 in 2008 to 2.0 per 100,000 in 2012) and Poland (3.5 per 100,000 in 2007 to 4.6 per 100,000 in 2012).[8] Also, the high rates of PGC in South America and in parts of Asia, such as Pakistan, Korea, and Japan, have been attributed to high rates of cholecystitis and salmonella infection, both of which are known risk factors for PGC.[9,10] Although PGC is less prevalent in North America, where PGC incidence is 1.4%, it is still associated with extremely poor prognosis.[5,11,12]
PGC has been largely understudied in comparison to other cancers.[6] For example, there have only been a few studies published in the last decade on epidemiology and survival of PGC. Therefore, it is necessary to assess the epidemiological features and survival status of PGC patients in order to provide a timely and comprehensive understanding of PGC and to explore its prevention and treatment policy. This study explores PGC patients in the United States in an effort to describe and analyze the survival status over the last decade.
2. Source and methods
2.1. Inclusion and exclusion criteria
We obtained data of patients clearly diagnosed with PGC between 2010 and 2015 from the Surveillance, Epidemiology, and End Result (SEER) database, the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) Code C23.9 was used as a reference for selection. The inclusion criteria included three points: the diagnostic age was between 20 and 80, the diagnostic year was from 2004 to 2009 and the tumor site was gallbladder. The exclusion criteria included 5 aspects: multisource tumor, cancer in situ, incomplete information on cancer type, differentiation degree and the AJCC 7th staging, unknown surgical method, incomplete information on follow-up and patients died in 30 days. The dataset used is publicly available, and approval by the institutional review board at Chinese Center for Disease Control and Prevention (Beijing, China) was waived.
2.2. Data extraction
Patient information was collected based on the patient's registered information at the time of the first diagnosis. The following data were extracted: patient's ID, age at diagnosis, sex, gender, marital status, race, region, tumor size, pathological type, histological grade, pTNM stage, type of surgery, radiotherapy, and follow-up (including survival time, follow-up and death). The primary endpoint was cancer-specific survival.
2.3. Statistical analysis
All statistical analyses were performed with SPSS version 23.0 software, the survival curves were drawn by GraphPad Prism 8. The survival rate of the patients was analyzed by the Kaplan–Meier method, and the comparison of survival rates of two or more groups was analyzed by log-rank test. In the process of establishing the Cox regression model, univariate analysis was carried out first to screen out the variables with statistical significance. And considering the information integrity, some variables would be included in the regression model as continuous variables. Meaningful variables from univariate analysis were integrated into the Cox regression model (backward method) for the multivariate analysis in further to obtain the independent factors influencing the prognosis of PGC patients. All tests were conducted with a two-sided probability, P < .05 was considered statistically significant.
3. Results
3.1. Demographics
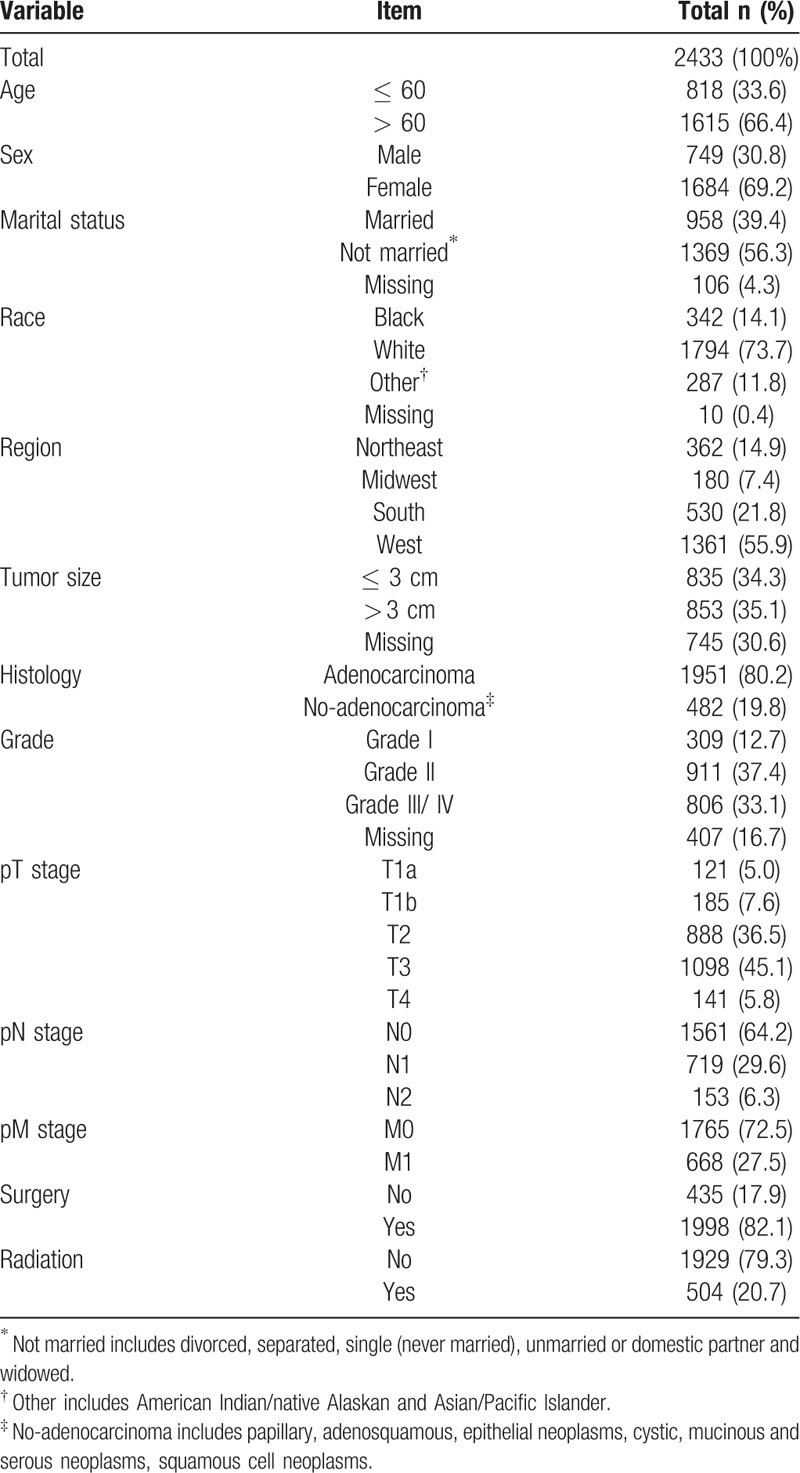
A total of 2433 PGC cases were reported from 2010 to 2015. The median age was 64.2 ± 10.4 years old, and the patients aged over 60 years accounted for 66.4% (1615/2433). The percentages of the female patients and the white patients were 69.2% (1684/2433) and 73.7% (1794/2433) respectively. The median tumor size was 3.2 cm (quartile range was 3.38 cm). The majority of patients were diagnosed as adenocarcinoma (80.2%, 1951/2433). There were 45.1% (1098/2433) patients in pT3 stage, 64.2% (1561/2433) patients in pN0 stage and 72.5% (1765/2433) patients in pM0 stage. The percentages of patients who received surgery and radiation treatment were 82.1% (1998/2433) and 20.7% (504/2433) respectively (Table 1).
Table 1.
Demographics and clinical profile of 2433 PGC patients from the SEER database (2010–2015).
3.2. PGC patients at different TNM stages had different overall survival rates
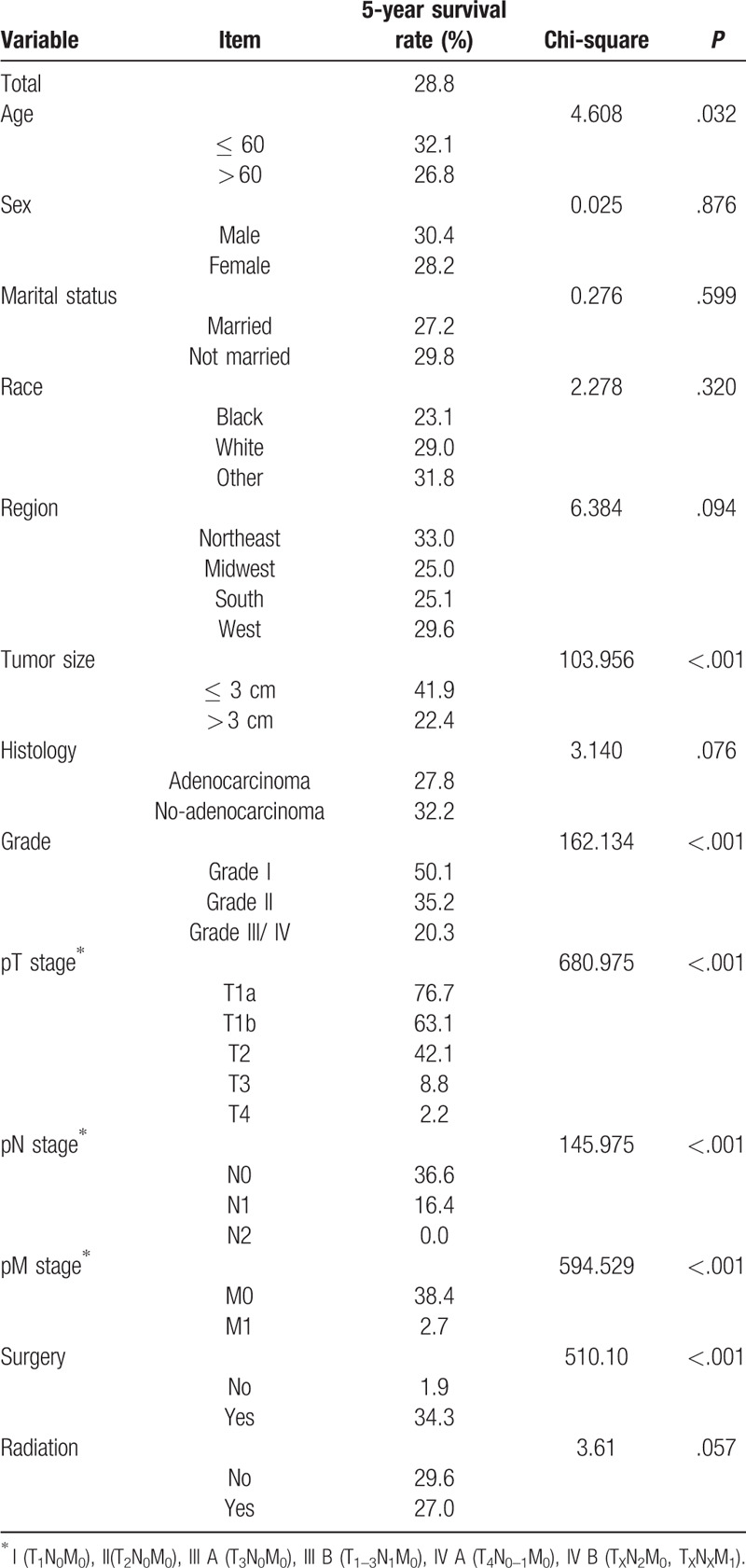
The overall median survival time of all patients was 19 months and the 5-year survival rate was 28.8%. Different degree of infiltration showed different survival rates, the Figure 1 showed that the 5-year survival rate sharply decreased on pT stage (Fig. 1). The increased number of positive lymph node meant the decreased survival rate and the 5-year survival rate of PGC patients in pN2 stage dropped to 0%. The overall survival rate differed whether or not there was distant metastasis and the 5-year survival rate for PGC patients with distant metastasis was only 2.7% (Fig. 2, Table 2).
Figure 1.
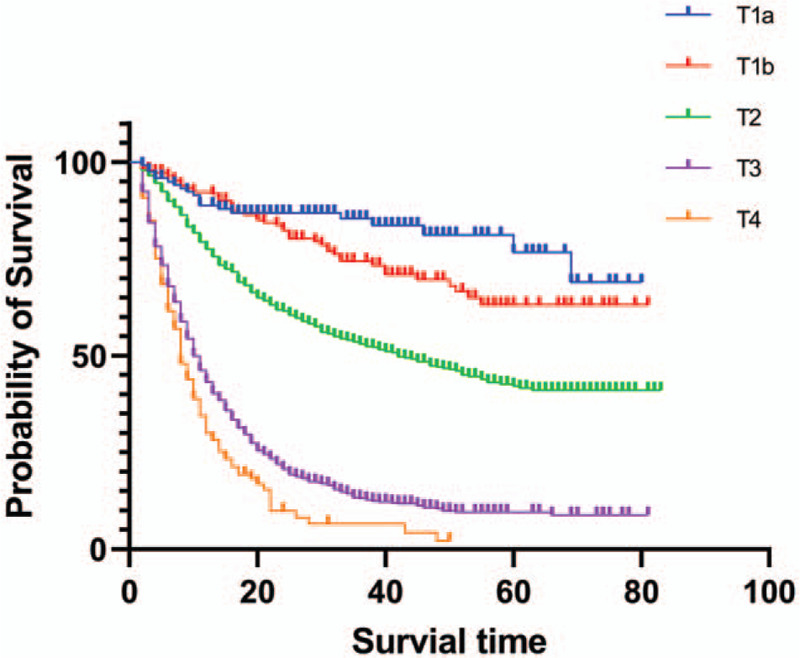
Survival of GBC patients based on pT stage. The survival rate of the patients was analyzed by the Kaplan–Meier method.
Figure 2.
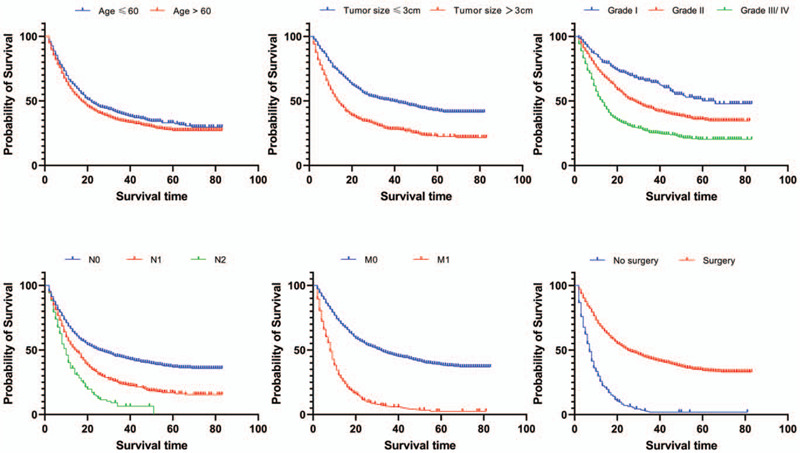
Survival of GBC patients based on age, tumor size, grade, pN stage, pM stage and surgery. Log rank test was used to evaluate the prognostic factors in PGC patients.
Table 2.
The survival of PGC patients and the results of log-rank test.
3.3. Univariate and multivariate Cox regression analysis for the prognostic factors in PGC patients
Log-rank test was used to evaluate whether the survival rates of GPC patients with different groups of categorical variables were different. The results showed that the survival rate of GPC patients in different age groups, different tumor size groups, different grades, different pT stages, different pN stages, different pM stages and whether to perform surgery were statistically different (Table 2).
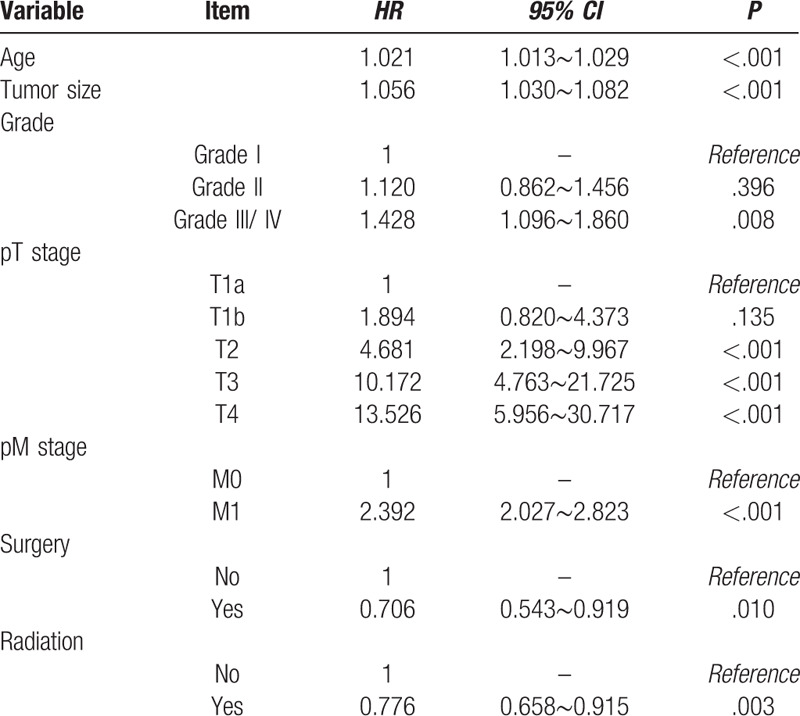
At the same time, univariate analysis of the Cox regression model was performed. In order to preserve the information of the variables as much as possible, the age and tumor size were analyzed in the form of continuous variables. The results showed that age, tumor size, grade, pT stage, pM stage, pM stage and surgery or not were statistically significant variables (Table 3). In addition, considering the P value of radiation or not was around 0.05, it was included in the Cox regression model multivariate analysis together with the above seven variables. The results of multivariate analysis revealed that age, tumor size, grade, pT stage, pM stage, surgery or not and radiation or not were independent factors influencing the prognosis of PGC patients. Age, tumor size, grade, pT stage, pM stage were risk factors for prognosis, surgery or not and radiation or not were protective factors for prognosis. Among PGC patients, each year the patient's age increases, the risk of death increases by 1.021 times (95% CI = 1.013∼1.029, P < .001). Similarly, each 1-cm increase in tumor size, the risk of death in patients increased by 1.056 times (95% CI = 1.030∼1.082, P < .001). Patients at higher grade had higher mortality risk (Grade II vs Grade I: HR = 1.120, Grade III/ IV vs Grade I: HR = 1.428); also, patients at advanced degree of infiltration had higher mortality risk (T1b vs T1a: HR = 1.894, T2 vs T1a: HR = 4.681, T3 vs T1a: HR = 10.172, T4 vs T1a: HR = 13.526). The hazard ratio of patients with presence of distant metastasis was 2.392 compared to those absence of distant metastasis (95% CI = 2.027∼2.823, P < .001). Referred to the above two protective factors, patients received surgery treatment had lower mortality risk than those not (HR = 0.706; 95% CI = 0.543∼0.919, P = .010) and patients received radiation treatment had lower mortality risk than those not (HR = 0.776; 95% CI = 0.658∼0.915, P = .003). (Table 4)
Table 3.
Relative hazards of death in PGC patients according to the Cox univariate analysis.
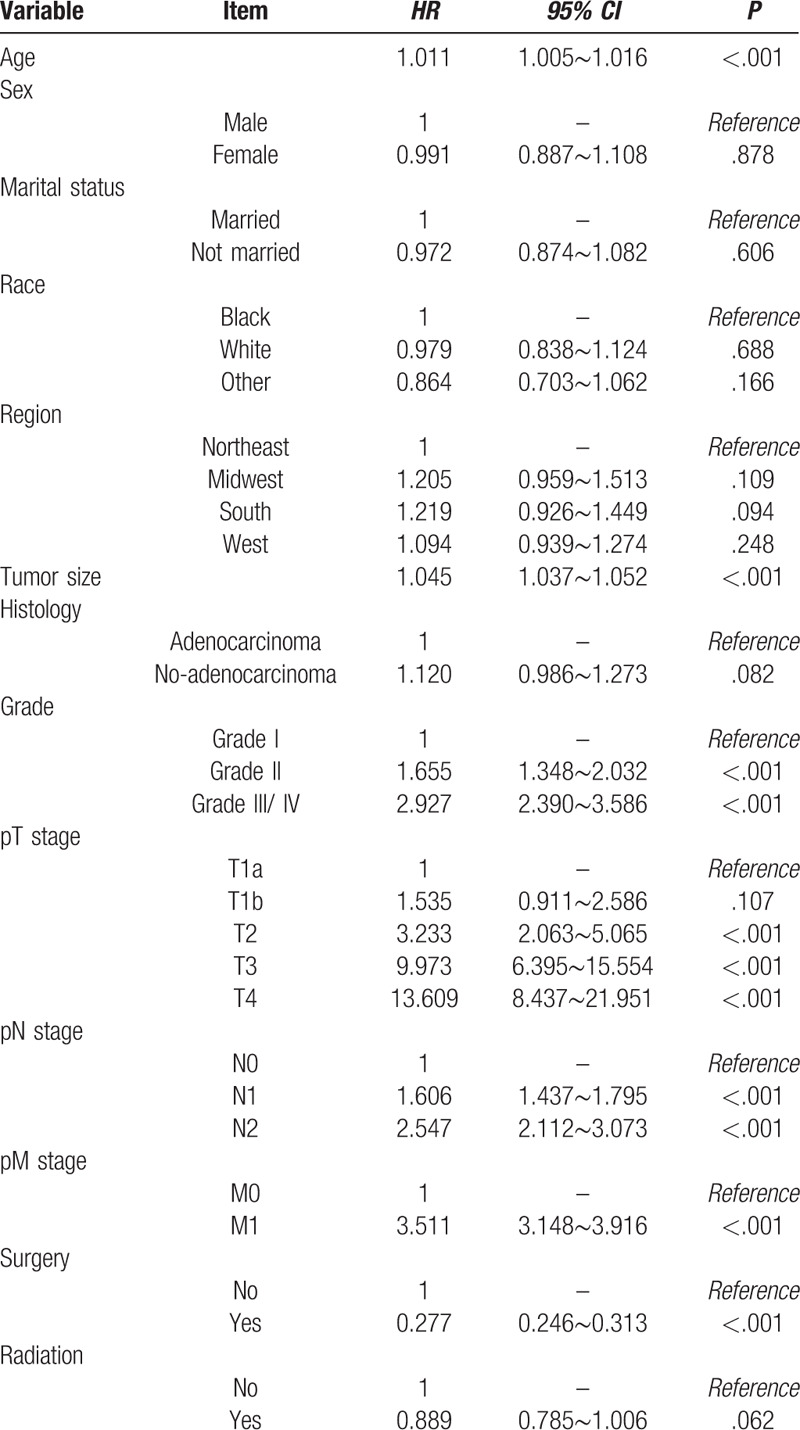
Table 4.
Relative hazards of death in PGC patients according to the Cox multivariate analysis.
4. Discussion
PGC is a fatal malignancy which displays considerable differences in certain ethnicities and sex. Differences in treatment may be a potential reason for the differences observed in mortality[13] which led to different survival status among different races/ethnic populations. Studies have suggested that a higher incidence of PGC in females may be affected by various female hormones.[14,15] However, there has been opposing studies, which have found no association between female hormones and incidence of PGC.[16] Therefore, more extensive studies are required to delineate the role of female hormones in PGC pathogenesis. PGC is also more prevalent in older individuals, and this may be due to the increased prevalence of gallstones caused by increased cholesterol secretion and infection of the biliary tract in elders. Meanwhile, a high percentage of patients were diagnosed with adenocarcinoma, which was consistent with other studies.[17,18] Adenocarcinomas are reported in 90.0% of PGC cases.[6] One key factor that can influence the treatment and outcome of surgery in PGC patients is the histologic subtype of the disease. As many gallbladder adenocarcinomas are detected incidentally in routine cholecystectomy specimens, systematic selective sampling of cholecystectomy specimens could be considered according to some study finding.[19] Moreover, there have been no documentation of a significant correlation between the prevalence of gallstones and histologic subtypes of PGC in this aspect further epidemiological research could be conducted.
PGC patients in advanced stages experience the lowest rates of survival. In this study, the overall median survival time was 19 months and the overall 5-year survival rate was 28.8%, similar to other published reports.[20,21] The study also showed that patients presented with pN2 stage or distant metastasis experienced very low survival rate. Symptoms and signs of PGC often appear late in the clinical course of the disease,[10] and over 40% of patients have been diagnosed with late-stage PGC. First, earlier diagnosis and treatment can facilitate better clinical outcomes for PGC patients. To improve early diagnosis of PGC, careful consideration should be given to PGC risk factors, such as sex, race, diet, bacterial infection and prevalence of gallstones. Some study has showed that a family history of gallstones, tobacco consumption, excessive intake of fried foods (reused oil), obesity, and blood type all increased the risk of PGC.[22,23] Familial databases such as the Swedish family-cancer database and Utah cancer registry have reported the first ever data on familial clustering of PGC.[24] Second, the AJCC cancer staging system is helpful in the selection of the type of surgical resection and evaluation of prognosis for PGC patients. In 2018, the AJCC 8th edition becomes the new global guideline for cancer diagnosis and treatment. The updates of the AJCC 8th edition for biliary tract cancer including gallbladder cancer placing particular emphases on precise definition of primary tumor (T) and regional lymph nodes (N), prognostic evaluation based on stage.[25] While in our study the AJCC 7th edition is still used to analyze the survival status and related factors due to the SEER data lacks of variables in the AJCC 8th edition till now.
Patients with PGC often has an overall dismal prognosis.[7] Prognosis can be enhanced using cancer screening tests.[26] To improve the prognosis of PGC patients, combination detection including biochemical detection, image examination and multigene detection has been mentioned in PGC diagnosis studies; for example, some study has showed that the relationship between RASSF1A with CyclinA2 protein and gallbladder can be used as the important index of PBC diagnosis.[27] Nevertheless, in the molecular levels of combination detection still needs further examination. Survival analysis by treatment showed that majority of the patients received surgery. Surgery is the curative treatment for PGC,[28] and surgical resection is the standard of care for PGC patients.[29] The type of surgery needed depends on the location and size of the tumor. Patients with stage I disease are ideal candidates for radical surgery. Although surgical resection is a popular treatment strategy for patients with stage II PGC, there is a high rate of distant metastasis, and a decreased 5-year survival rate. Therefore, adjuvant therapy is highly encouraged in this patient population.[30] An integrated therapeutic strategy is utilized in patients with stage III and IV PGC, and involves the combination of surgery and adjuvant therapy. Although adjuvant therapy is underused in patients with PGC,[31] there is evidence that postoperative adjuvant radiotherapy may be more effective than chemotherapy.[32] Notably, studies have shown that some operation such as routine EBD without bile duct infiltration is not associated with improved overall survival,[33] not to mention that intraoperative bile spillage and surgical drain placement at initial cholecystectomy are negatively associated with overall survival in gallbladder adenocarcinoma,[34] on this aspect systemic therapy are stronger consideration before definitive resection. However, owing to variations in incidence across different countries, it has been understudied, leading to variation in approaches to the initial pathologic evaluation, classification, and staging of the disease.[29] Further research on multidisciplinary approaches of PGC treatment is needed in patients particularly at advanced stages.
4.1. Limitation
One limitation of the study is that the SEER database does not code information on behavioral risk factors, which would allow for more extensive epidemiological studies. Additionally, although the SEER database is updated annually, the capture of radiotherapy data is not accurate. Despite these limitations, the SEER database represents 28% of the US population, and the findings should be given careful consideration.
5. Conclusion
Key groups including older individuals and female face high incidence rate of PGC which also linked to more complex risk factors. Early detection, standardized diagnosis and different treatment strategy in different stages are very helpful and positive in PGC treatment. Further research on histologic subtypes of PGC and treatment approach in patients at advanced stages needed to be conducted.
Acknowledgments
The authors would like to extend sincere gratitude to Professor. Xibin Sun at Henan Cancer Research and Control Office, Affiliated Tumor Hospital of Zhengzhou University/Henan Tumor Hospital, Dr Xiaonong Zou and Dr Wenjing Yang at National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College for their contributions and help in valuable suggestions for the article and final manuscript preparation.
Author contributions
Xiaolei Zhu and Xiaochang Zhang wrote the original draft. Xiao Hu, Hongyan Ren and Shenghui Wu performed the analysis. Jing Wu and Guoyi Wu conceived of and designed the study. Baohua Wang and Xiang Si supervised the study.
Footnotes
Abbreviations: PGC = primary gallbladder cancer, SEER = surveillance epidemiology and end results.
How to cite this article: Zhu X, Zhang X, Hu X, Ren H, Wu S, Wu J, Wu G, Si X, Wang B. Survival analysis of patients with primary gallbladder cancer from 2010 to 2015: A retrospective study based on SEER data. Medicine. 2020;99:40(e22292).
XZ and XZ are co-first authors.
This work was supported by grants from National Key Research and Development Program of Ministry of Science and Technology of China (grant no. 2016YFC1302600); Integrated Cancer Prevention and Control in the Huai River Program.
The authors declare no potential conflicts of interest.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Shukla SK, Singh G, Shahi KS, et al. Staging, treatment, and future approaches of gallbladder carcinoma. J Gastrointest Canc 2018;49:9–15. [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization. International Agency for Research on Cancer. Global Cancer Observatory. https://gco.iarc.fr/today/data/factsheets/cancers/12-Gallbladder-fact-sheet.pdf [access date June 2, 2020]. [Google Scholar]
- [3].Rahman R, Simoes EJ, Schmaltz C, et al. Trend analysis and survival of primary gallbladder cancer in the United States: a 1973-2009 population-based study. Cancer Med 2017;6:874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hori M, Saito E. Gallbladder cancer incidence rates in the world from the cancer incidence in five continents XI. Jpn J of Clin Oncol 2018;866–7. [DOI] [PubMed] [Google Scholar]
- [5].Lau CSM, Zywot A, Mahendraraj K, et al. Gallbladder carcinoma in the United States: a population based clinical outcomes study Involving 22,343 patients from the surveillance, epidemiology, and end result database (1973-2013). HPB Surg 2017;1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sharma A, Sharma KL, Gupta A, et al. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: Recent update. World J Gastroenterol 2017;23:3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Søreide K, Harrison EM, Wigmore SJ. Research gaps and unanswered questions in gallbladder cancer. HPB 2018;1–2. [DOI] [PubMed] [Google Scholar]
- [8].World Health Organization. International Agency for Research on Cancer. Cancer Incidence in Five Continents Time Trends. https://ci5.iarc.fr/CI5plus/Pages/online.aspx [access date June 2, 2020]. [Google Scholar]
- [9].Strom BL, Soloway RD, Rios-Dalenz JL, et al. Risk factors for gallbladder cancer. An international collaborative case-control study. Cancer 1995;76:1747–56. [DOI] [PubMed] [Google Scholar]
- [10].Randi G, Franceschi S, Vecchia CL. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer 2006;118:1591–602. [DOI] [PubMed] [Google Scholar]
- [11].Isambert M, Leux C, Métairie S, et al. Incidentally-discovered gallbladder cancer: when, why and which reoperation? J Visc Surg 2011;148:e77–84. [DOI] [PubMed] [Google Scholar]
- [12].Utsumi M, Aoki H, Kunitomo T, et al. Evaluation of surgical treatment for incidental gallbladder carcinoma diagnosed during or after laparoscopic cholecystectomy: single center results. BMC Res Notes 2017;10:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jaruvongvanich V, Assavapongpaiboon B, Wong L. Racial/ethnic disparities in gallbladder cancer receipt of treatments. J Gastrointest Oncol 2018;9:348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pilgrim CHC, Groeschl RT, Christians KK, et al. Modern perspectives on factors predisposing to the development of gallbladder cancer. HPB 2013;15:839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lyer P, Barreto SG, Sahoo B, et al. Non-typhoidal Salmonella DNA traces in gallbladder cancer. Infect Agents Cancer 2016;11:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barreto SG, Dutt A, Sirohi B, et al. Gallbladder cancer: a journey of a thousand steps. Future Oncol 2018;14:1299–306. [DOI] [PubMed] [Google Scholar]
- [17].Shen HX, Song HW, Xu XJ, et al. Clinical epidemiological survey of gallbladder carcinoma in northwestern China 2009–2013: 2379 cases in 17 centers. Chronic Dis Transl Med 2017;3:60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Alexander S, Lemmens VEPP, Houterman S, et al. Gallbladder cancer, a vanishing disease? Cancer Cause Control 2012;23:1705–9. [DOI] [PubMed] [Google Scholar]
- [19].Akki AS, Zhang W, Tanaka KE, et al. Systematic selective sampling of cholecystectomy specimens is adequate to detect incidental gallbladder adenocarcinoma. Am J Surg Pathol 2019;43:000. [DOI] [PubMed] [Google Scholar]
- [20].Wang TL. Analysis of prognostic factors of gallbladder cancer. Master thesis. 2017;Jilin: Jilin University, 35. [Google Scholar]
- [21].Dong MY. Analysis of prognostic factors of gallbladder cancer. Master thesis. 2016;Shanghai: Shanghai Jiao Tong University, 44. [Google Scholar]
- [22].Hsing AW, Gao YT, Han TQ, et al. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer 2007;97:1577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yagyu K, Kikuchi S, Obata Y, et al. for the J.A.C.C. Study Group. Cigarette smoking, alcohol drinking and the risk of gallbladder cancer death: A prospective cohort study in Japan. Int J Cancer 2007;122:924–9. [DOI] [PubMed] [Google Scholar]
- [24].Jackson HH, Glasgow RE, Mulvihill SJ, et al. Familial risk in gallbladder cancer. J Am Coll Surg 2007;205:S38.17916517 [Google Scholar]
- [25].Tang ZH, Tian XD, Wei MY, et al. Updates and interpretations of the 8th edition of AJCC cancer staging system for biliary tract carcinoma. Chin J Pract Surg 2017;37:248–54. [Google Scholar]
- [26].Henley SJ, Anderson RN, Thomas CC, et al. Invasive Cancer Incidence. 2004–2013, and Deaths. 2006–2015, in Nonmetropolitan and Metropolitan Counties — United States. Morbid Mortal Weekly Rep Surveil Summa 2017;66(SS-14):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhang XK. The expression and correlation of RASSF1A and CyclinA2 in Primary gallbladder carcinoma. Master thesis. 2016;Qinghai: Qinghai University, 34. [Google Scholar]
- [28].Zhu AX, Hong TS, Hezel AF, et al. Current management of gallbladder carcinoma. Oncologist 2010;15:168–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Aloia TA, Járufe N, Javle M, et al. Gallbladder Cancer: expert consensus statement. HPB 2015;17:681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhou D, Tang ZH, Quan ZW. Problems and reflections on the diagnosis and treatment of gallbladder cancer. Chin J Surg 2018;56:110–3. [DOI] [PubMed] [Google Scholar]
- [31].Mitin T, Enestvedt CK, Jemal A, et al. Limited use of adjuvant therapy in patients with resected gallbladder cancer despite a strong association with survival. J Natl Cancer Inst 2017;109:1–9. [DOI] [PubMed] [Google Scholar]
- [32].Kiran RP, Pokala N, Dudrick SJ. Incidence pattern and survival for gallbladder cancer over three decades—an analysis of 10301 patients. Ann Surg Oncol 2006;14:827–32. [DOI] [PubMed] [Google Scholar]
- [33].Nigri G, Berardi G, Mattana C, et al. Routine extra-hepatic bile duct resection in gallbladder cancer patients without bile duct infiltration: a systematic review. Surgeon 2016;14:337–44. [DOI] [PubMed] [Google Scholar]
- [34].Blakely AM, Wong P, Chu P, et al. Intraoperative bile spillage is associated with worse survival in gallbladder adenocarcinoma. J Surg Oncol 2019;1–8. [DOI] [PubMed] [Google Scholar]


