Abstract
The manifestations of borreliosis in the peripheral nervous system (PNS) remain poorly described. As the symptoms of neuroborreliosis can be reversed with timely introduction of antibiotics, early identification could avoid unnecessary axonal loss. Our aim was to describe the characteristics of confirmed neuroborreliosis cases involving the PNS diagnosed between 2007 and 2017 in our neuromuscular disease center in a nonendemic area (La Pitié-Salpêtrière Hospital, Paris, France).
Neuroborreliosis was defined as follows: compatible neurological symptoms without other cause of neuropathy; cerebrospinal fluid and serum analysis (positive serological tests with ELISA, confirmed by Western Blot); and improvement of symptoms with adapted antibiotherapy. All the patients consulting in our center between 2007 and 2017 underwent electrophysiological study.
Sixteen confirmed cases of neuroborreliosis involving the PNS were included: 10 cases of meningoradiculoneuritis, 4 of axonal neuropathy, and 2 of demyelinating neuropathy (one acute and one chronic). Only 4 (25%) patients reported tick bites. Meningoradiculoneuritis was characterized by lymphocytic meningitis, intense pain, cranial nerve palsy, and contrast enhancement of nerve roots on imagery. The patients with axonal neuropathy presented sensory symptoms with intense pain but no motor deficit and meningitis was rare. Nerve biopsy of 1 patient revealed lymphocytic vasculitis. Electrophysiological testing showed sensory or sensorimotor axonal neuropathy (3 subacute and 1 chronic) of the lower limbs, with asymmetrical neuropathy in 1 patients, symmetrical neuropathy in one and monomelic sensory mononeuritis multiplex in another. We also found 1 case of acute demyelinating neuropathy, treated with antibiotherapy and immunoglobulins, and 1 chronic demyelinating neuropathy. Overall, diaphragmatic paralysis was frequent (18.6%). Antibiotherapy (mostly ceftriaxone 3–4 weeks) resulted in symptom resolution.
This series gives an updated overview of the peripheral complications of neuroborreliosis to help identify this disease so that timely treatment could avoid axonal loss.
Keywords: axonal neuropathy, demyelinating neuropathy, electrophysiological study, meningoradiculoneuritis, mononeuritis multiplex, neuroborreliosis, peripheral nervous system
1. Introduction
Borreliosis is an infection caused by Borrelia burgdorferi which is transmitted by the tick Ixodes. The primary stage of the disease is characterized by erythema migrans which begins 3 to 30 days after the tick bite. After 1 week to 3 months, a secondary stage which affects the neurological or rheumatologic system (called the “early” neuroborreliosis stage) can occur and can persist for several weeks or months. The tertiary stage with neurological symptoms (“late” neuroborreliosis) can start 6 months after the tick bite and can last several years.[1]
The nervous system (central or peripheral) is involved in up to 15% of patients.[2,3] Neurological manifestations usually consist of lymphocytic meningitis, cranial neuropathy (especially seventh cranial nerve palsy), or painful radiculitis (association of neuropathic pain and muscle weakness). Chronic peripheral neuropathy, which is an asymmetrical axonal and predominantly sensory neuropathy, can occur during the late stage and is often associated with a chronic skin disorder: acrodermatitis chronica atrophicans.[1,3,4]
On suspicion of neuroborreliosis, diagnosis is confirmed by the presence of specific antibodies in the cerebrospinal fluid (CSF). The demonstration of the production of intrathecal specific antibodies has a high specificity and sensitivity, contrary to serological tests as 18% of healthy persons have Borrelia antibodies in their serum (false-positive).[1,5,6] Lymphocytic pleocytosis, an elevated protein content and oligoclonal bands, can also be detected in the CSF during the early neuroborreliosis stage.[1,3]
Once diagnosed, neuroborreliosis is usually treated by intravenous antibiotherapy (for example, a 3-week course of ceftriaxone) which typically results in the resolution of symptoms.[6]
The clinical manifestations of borreliosis in the peripheral nervous system (PNS) can mimic multiple diseases and are poorly described to date. Consequently, neuroborreliosis can be overlooked by the clinician especially in nonendemic areas of tick-borne diseases. However, as the symptoms can be reversed with timely antibiotherapy, it is important to identify this neurological disease as early as possible to introduce appropriate treatment before it results in axonal loss. The aim of this study was to describe the various characteristics of neuroborreliosis involving the PNS by reporting 16 confirmed cases diagnosed between 2007 and 2017 in our neuromuscular disease center in a non-endemic area (La Pitié-Salpêtrière Hospital, Paris, France).
2. Methods
2.1. Patients:
Consecutive patients presenting with neuropathy and Lyme borreliosis and followed up between 2007 and 2017 in our center (La Pitié-Salpêtrière Hospital, Paris, France) were selected from the authors’ files. Diagnosis of Lyme borreliosis was defined with the following criteria: peripheral neurological symptoms compatible with Lyme borreliosis without other cause of neuropathy; positive serological tests (with ELISA and Western Blot) in the CSF and/or serum; improvement of symptoms and signs with an adapted antibiotherapy.[7,8] Patients having another cause of neuropathy or with positive serological tests with ELISA but not with Western Blot were excluded. Informed consent was obtained from all the patients for the purpose of publication. According to French laws about observational studies, our work did not require ethical approval. All patients underwent a neurological and electrophysiological examination and were classified into different groups according to the characteristics of neuropathy based on both clinical and electrophysiological data.
Meningoradiculoneuritis was diagnosed on the basis of a proximal and/or distal sensorimotor deficit (with loss of deep tendon reflex) and spinal or radicular pain, with a root distribution, associated with meningitis in the CSF. The electrophysiological study had to show a neurogenic pattern with a root distribution, a decrease of distal compound muscle action potential (CMAP) amplitudes contrasting with a relative preservation of distal sensory potentials, compatible with a root distribution, without demyelinating features.
Axonal neuropathy was diagnosed on the basis of motor or sensory involvement, with a distal distribution and with a distal predominance of pain. The electrophysiological study had to show a decrease of distal CMAP amplitudes and sensory potentials, without conduction abnormalities or demyelinating features or prolonged F wave latencies. A diagnosis of mononeuritis multiplex was retained for cases of asymmetric axonal neuropathy with a nerve trunk distribution.
Demyelinating neuropathy was diagnosed on the basis of peripheral neuropathy, with weakness or sensory symptoms in at least 2 limbs, associated with demyelinating criteria on electrophysiological study: conduction blocks, reduced conduction velocities, prolonged distal latencies, abnormal temporal dispersion, and abnormalities of F-waves.[9] Onset was defined as “acute” if symptoms developed within <4 weeks, “subacute” if they developed within 4-8 weeks, and “progressive” if they developed after more than 8 weeks. Acute demyelinating neuropathy was defined as acute onset without relapse, and chronic demyelinating neuropathy as a progression of symptoms or relapses during the 6 months following the onset.
2.2. Electrophysiological study:
For all patients, the median, ulnar, tibial and peroneal nerves were examined on both sides. Distal latency, conduction velocity, CMAP amplitude (baseline to negative peak), and areas under negative phase and CMAP duration were measured. For each nerve segment, the reduction in CMAP amplitude or area on proximal versus distal stimulation was calculated. A conduction block was defined as a decrease in amplitude of 50% between proximal and distal sites of stimulation for any nerve. F-wave latency was recorded after distal supramaximal stimulation (at least 20 stimuli). Sensory nerve action potential amplitude (peak to baseline) was measured in the median, ulnar, radial, tibial, and superficial peroneal nerves on both sides, with surface recording and stimulating electrodes. A needle electromyographic examination was performed in all patients.
2.3. Evaluation procedures:
Neurological recovery was defined as a complete resolution of neurological signs. Neurological improvement was defined as an improvement of at least 2 Medical Research Council grades in any muscle or a decrease in pain and sensory symptoms.
Clinical worsening was defined if motor strength decreased by at least two Medical Research Council grades in any muscle or if weakness extended to other muscles, or if sensory and painful symptoms increased.
3. Results
Sixteen patients—13 men and 3 women—with confirmed neuroborreliosis involving the PNS were included. Ten patients (62.5%) had meningoradiculoneuritis, 4 (25%) axonal neuropathy, and 2 (12.5%) demyelinating neuropathy. The mean age at onset of neuroborreliosis was 54 years.
3.1. Meningoradiculoneuritis cases
The main characteristics of the patients with meningoradiculoneuritis, the most frequent manifestation in our series (10 cases), are shown in Table 1 . The mean age at onset of neuroborreliosis was 57.3 years in this group.
Table 1.
Main characteristics of meningoradiculoneuritis cases.
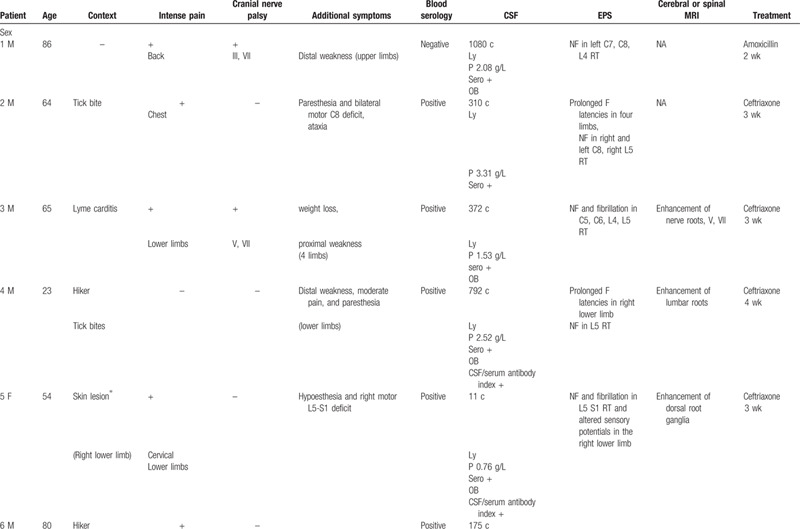
Only 3 of these patients reported tick bites although the context was suggestive of Lyme disease for 8 of the 10 patients: farmer, hiker, medical history (Lyme carditis), erythematous skin lesions, or tick bite. One patient had a history of Parsonage-Turner syndrome following a tick bite and another a history of Lyme carditis several years previously.
For the patients who reported tick bites or skin lesions, the neurological symptoms appeared within 15 days to 1 month. The onset of symptoms was acute in 9 patients and progressive in 1.
Nine of the patients had intense pain of the lower back and chest or radicular pain, with pain-related insomnia, and 4 of the 10 had cranial nerve involvement (III, V, IX, X, and most often VII). One patient had diaphragmatic paralysis. They also experienced sensory symptoms such as ataxic gait or paresthesia (6/10) and weakness (7/10). No patient had arthralgia.
All the patients had lymphocytic meningitis with specific antibodies in the CSF (confirmed by Western Blot analysis) and 5 also had oligoclonal bands. The blood serological tests were negative for 2 patients (blood and CSF serology was performed 3 weeks after symptom onset for the first of these patients and 4 months for the second patient).
Electromyography revealed radicular alteration, sometimes with proximal conduction abnormalities (for five patients), or secondary axonal loss. The majority of patients presented lumbosacral root involvement with neurogenic features in the L4, L5, and S1 root territories.
Magnetic resonance imaging (MRI) was performed in 5 patients, all of whom had contrast enhancement of nerve roots or of cranial nerves.
All the patients were treated with antibiotherapy (ceftriaxone, amoxicillin or doxycycline) for 21 to 28 days that resulted in rapid resolution of pain, and then of other signs and symptoms.
3.2. Axonal neuropathy cases
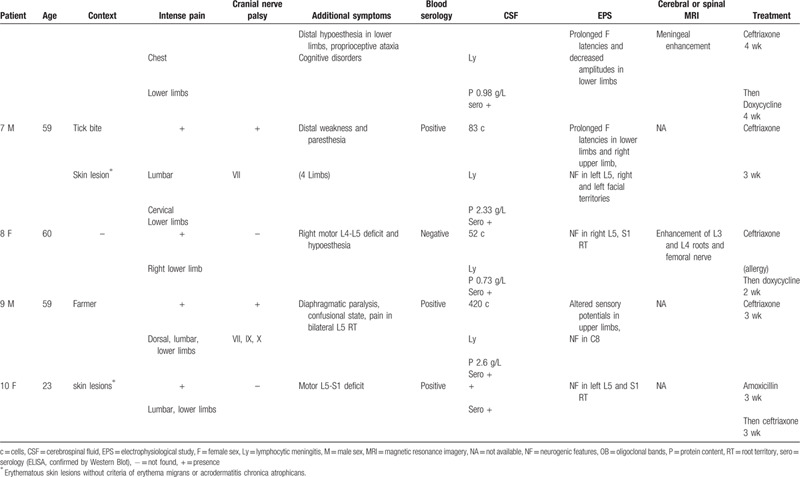
Four of the patients in our series had neuroborreliosis-related axonal neuropathy (Table 2).
Table 1 (Continued).
Main characteristics of meningoradiculoneuritis cases.
One patient (patient 1 in Table 2) was a 38-year-old man whose pastime was forest walking. One week after a sore throat and fever, he developed lower limb muscle ache followed by upper limb muscle ache and pain-related insomnia 3 days later. He also presented paresthesia and neuropathic pain in the 4 extremities before developing hypoesthesia in the back of his legs. One month later, he had erythematous skin lesions with concentric rings on the legs. Neuropathic pain was intense with burning and electrical shock sensations. On clinical examination, he was found to have hypoesthesia of the feet and in the back of the legs, and erythema migrans on the left thigh. There was no motor deficit and all osteotendinous reflexes were present.
The blood serology and serum western blot tests were positive for Lyme (Ig G and IgM). CSF analysis was normal (1 cell/mm3, normal CSF protein content (0.36 g/L) and serological tests were negative.
The first electromyography (carried out 1 month after the first symptoms) showed an alteration of CMAP amplitudes for the lateral popliteal nerves (right: 1.7 mV; left: 0.87 mV; normal lower limit [NLL]: 3 mV) with normal sensory potentials. The patient was treated with a 2-week course of doxycycline that cleared the skin lesions but the neuropathic pain increased during this time. The second electromyography (carried out 1 month after the first) found a deterioration of sensory potential amplitudes in the lower limbs (superficial fibular nerves: right: 5.2 μV; left: 2.6 μV; NLL:10 μV; sural nerves: 13-8.5 μV versus 37-45 μV 1 month before; NLL:10 μV). There was no damage evident in the upper limbs. Needle electromyographic examination showed active denervation (fibrillation) in the left lower limb. Subacute asymmetrical axonal neuropathy was diagnosed. The left superficial fibular nerve was biopsied and revealed axonal loss, with lymphocytic perivascular infiltrate (lymphocytic vasculitis) (Fig. 1). The patient was put on a 3-week course of ceftriaxone and corticosteroids at 1 mg/kg. The treatment resulted in resolution of neuropathic pain and denervation 2 months later, accompanied by an improvement in sensory responses (superficial fibular nerves: 9.2 μV; sural nerves: 16.2-19.2 μV) and motor responses (lateral popliteal nerves: right: 3.52 mV; left: 1.56 mV).
Figure 1.
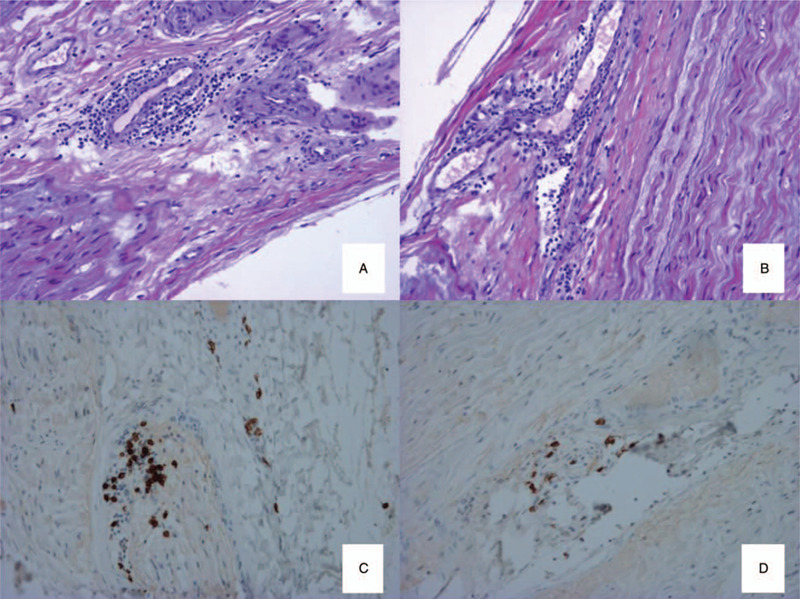
Biopsy of the left superficial fibular nerve: longitudinal sections, embedded in paraffin, stained with haematoxylin and eosin stain (A, B) (×270); immunolabelling of T-lymphocytes (anti-CD3) (C) (×540) and B-lymphocytes (anti-C20) (D) (×540): lymphocytic infiltrate with vascular tropism, with a majority of T-lymphocytes.
Overall, the context was suggestive for only 2 of the 4 patients with axonal neuropathy, both of whom who had a rash, and one who reported a tick. The neurological symptoms began at the same time as the skin lesions or tick bite. Two patients had intense pain with pain-related insomnia and all patients developed sensory symptoms in the lower limbs. There was no motor deficit and only 1 patient had meningitis in the CSF. Blood serological tests were positive. A nerve biopsy was performed for the patient with negative CSF serology. Electrophysiological study showed a symmetrical or asymmetrical sensory or sensorimotor axonal neuropathy of the lower limbs, without upper-limb damage. Three patients had subacute neuropathy (with symptoms appearing about 2 or 3 months before the first electromyography), and 2 of the 3 had pain; 1 patient had chronic neuropathy (with symptoms appearing about 2 years before the first electromyography). One patient experienced monomelic mononeuritis multiplex. The symptoms resolved with antibiotic therapy in all the patients.
3.3. Demyelinating neuropathy cases
Two of the patients in our series had neuroborreliosis-related demyelinating neuropathy (acute and chronic).
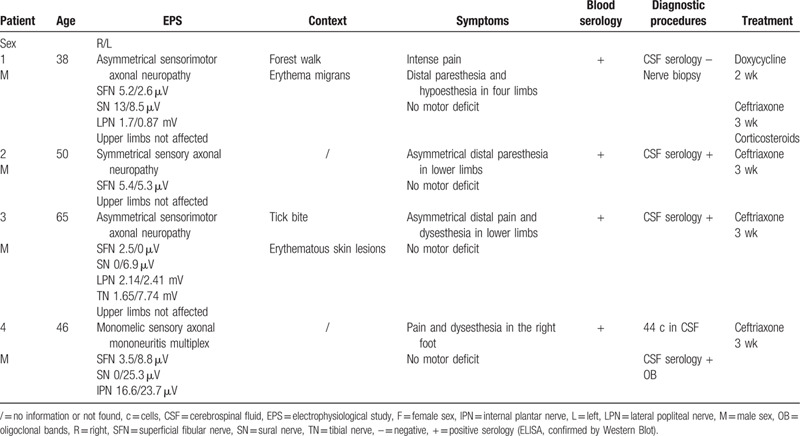
The first was a 44-year-old man, without a suggestive context for Lyme disease and who did not recall having been bitten by a tick, presenting neuropathic pain in both legs followed by headaches and vomiting 3 days later. CSF analysis showed lymphocytic meningitis (40 cells/mm3), elevated CSF protein content (0.83 g/L), oligoclonal bands, specific antibodies, and elevated CSF/serum antibody index. Blood serology was also positive. Four days later, the patient experienced hypoesthesia of the lower limbs and of the lower abdomen, with an ascending evolution. The clinical examination found absence of tendon reflexes, ataxia, mild tetraparesis and then bilateral facial paralysis, dysphagia and phrenic nerve palsy with dyspnea. He required intubation <1 month after the onset of symptoms.
Electrophysiological study showed signs of acute sensorimotor demyelinating neuropathy with axonal loss and damage of the phrenic nerve (Table 3): decreased CMAP and sensory potential amplitudes, conduction blocks, reduced conduction velocities, prolonged distal latencies, abnormalities of F-waves, and neurogenic features in the tibialis anterior muscles and the deltoid muscle, without signs of active denervation.
Table 2.
Main characteristics of axonal neuropathy cases.
This patient was treated with a 3-week course of intravenous ceftriaxone and immunoglobulins (2 g/kg), and the clinical symptoms and electrophysiological signs resolved. Electrophysiological study at 4 months showed normalization of CMAP amplitudes and an improvement in sensory potentials, without conduction block. At 6 months, the electromyography was normal.
The second patient, a 54-year-old hunter, developed chronic demyelinating neuropathy after meningitis due to neuroborreliosis. He first reported myalgia in the calves and lumbar pain which progressively worsened. Two months later, he had lower-limb weakness followed by predominantly distal weakness of the four limbs 1 month later, with absence of osteotendinous reflexes, paresthesia of the hands, and then paresthesia of the 4 limbs and circumoral paresthesia. He also presented with diaphragmatic paralysis. CSF analysis showed lymphocytic meningitis (100 cells/mm3, CSF protein content: 1 g/L) and serological tests were positive in both the blood and the CSF. He was treated with ceftriaxone 2 g/day, for 1 month, but neurological symptoms continued to worsen with the beginning of amyotrophy in the lower limbs.
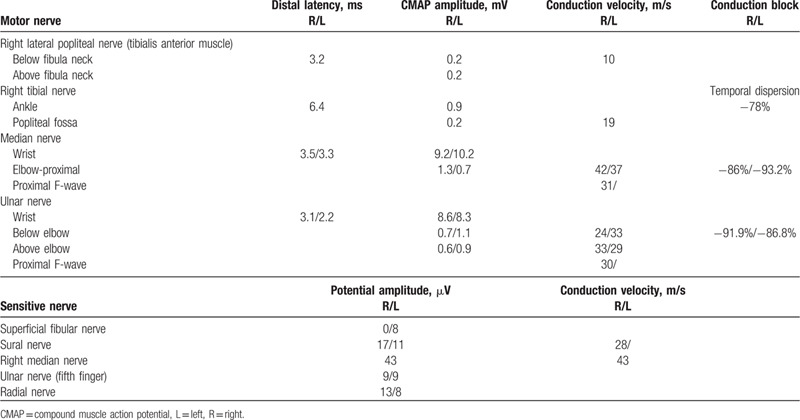
The first electromyography found decreased CMAP and sensory potential amplitudes, conduction blocks, reduced conduction velocities and reduced proximal conduction, severe active denervation (fibrillation) with neurogenic features in the 4 limbs (Table 4). The clinical picture first improved with several treatment sessions of immunoglobulins but then deteriorated (partial response and relapses). He was subsequently treated with corticosteroid therapy without effect, and then with plasma exchange and azathioprine which led to disease stability. Functional recovery was limited because of axonal loss.
Table 3.
First electrophysiological study of the patient with acute sensorimotor demyelinating neuropathy due to neuroborreliosis.
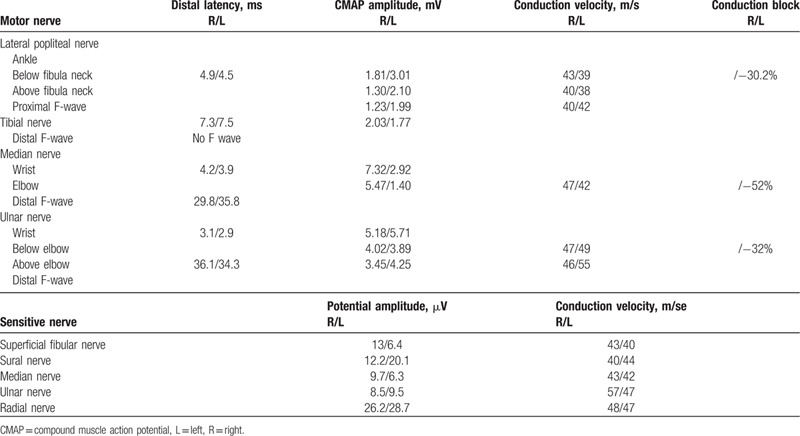
Table 4.
Electrophysiological study of the patient with a chronic demyelinating polyradiculoneuropathy secondary to neuroborreliosis.
4. Discussion
We report here 16 well documented cases of neuroborreliosis involving the PNS in our center located outside an endemic Lyme disease region. This series provides an updated overview of the peripheral complications of neuroborreliosis. Ten patients (62.5%) presented with meningoradiculoneuritis, 4 (25%) with axonal neuropathy, and 2 (12.5%) with demyelinating neuropathy.
To date, the few reports of neuroborreliosis involving the PNS have described small series.[10,11,12,13,14,15] One Spanish single-center series reports seven cases: one patient presented with meningoencephalitis, two with brachial plexopathy, two with radiculopathy, and one with cranial nerve palsy.[10] Another study collected patients with a positive serology and neurological symptoms in the South West of England: 22 cases were recorded during the 5-year study period including patients with facial palsy, radiculopathy and meningoencephalitis, and two patients experiencing peripheral sensory manifestations.[11] Isolated nerve palsy (facial palsy, left abducens palsy) has also been reported,[12,13,14] as well as Parsonage-Turner syndromes.[15]
As described in the literature, most of our patients (62.5%) experienced meningoradiculoneuritis. Thus, intense pain-related insomnia, cranial neuropathy and lymphocytic meningitis can be considered to be classic signs that should suggest a diagnosis of neuroborreliosis even in the absence of skin lesions or tick bite.[4,6,16,17,18] Meningoradiculitis was most commonly characterized by palsy of cranial nerve VII (in 40% of our cases) but also by involvement of the cranial nerves III, V, IX, X and phrenic nerves. All the patients in our series who underwent an MRI had contrast enhancement of nerve roots or cranial nerves.
The originality of our case series is that we report other clinical expressions including four cases of isolated axonal neuropathy. Chronic sensory axonal neuropathy is a late manifestation of neuroborreliosis, but is usually associated with acrodermatitis chronica atrophicans (a skin disorder due to chronic dermatoborreliosis). More than half of acrodermatitis chronica atrophicans cases are associated with a neuropathy, which is an asymmetrical distal sensory neuropathy that follows skin lesions (localized in the acral parts of the extremities) and that can be painful or painless.[1,6,19] On the contrary, in our study, 75% (3/4) of axonal neuropathies were subacute, and neurological symptoms and skin lesions were concomitant; 75% (3/4) were a painful asymmetrical neuropathy. Furthermore, it is important to note that the occurrence of meningitis in our patients with axonal neuropathy was infrequent in contrast to the cases of radiculonevritis. Nerve biopsy may be useful if vasculitis requiring corticosteroid is suspected: lymphocytic vasculitis was demonstrated by nerve biopsy for one of the patients in our series presenting a progressive painful neuropathy. Moreover, it is also known that neuroborreliosis can present as mononeuritis multiplex, but this remains a rare clinical expression.[3,6,20,21,22] One patient in our series presented monomelic sensory mononeuritis multiplex.
In addition, we observed 2 cases of demyelinating neuropathy: 1 Guillain-Barre syndrome and 1 case of a chronic demyelinating neuropathy which occurred subsequently to meningitis. We think that the 2 patients developed a real neuroborreliosis with symptoms of demyelinating neuropathy, rather than a classic Guillain-Barré syndrome and a classic chronic inflammatory demyelinating polyneuropathy with an infectious trigger. Indeed, the presence of a lymphocytic meningitis with an increased cellularity and the time sequence of symptoms onset would support the hypothesis of an active infection.[23] Antibiotic treatment combined with a specific treatment for the demyelinating neuropathy, such as immunoglobulins, is recommended in this case.[24,25]
Surprisingly, even if individual cases of diaphragmatic paralysis have been reported,[26,27] we observed a high frequency (18.6%) in our series and therefore believe that this symptom is suggestive of neuroborreliosis. Conversely, only one of our patients, with a history of myocarditis, developed organ involvement outside the PNS.
All but one of our patients (who was diagnosed by nerve biopsy) had a positive CSF serology, but two patients with meningoradiculoneuritis had a negative blood serology. Thus negative blood serology should not rule out a diagnosis of neuroborreliosis, as described in the literature.[6,8] The serological tests were performed for 1 of the 2 patients 3 weeks after symptoms onset whereas specific antibodies can only be detected after 4 to 6 weeks.[6] Sensitivity is low in the early stages of the disease, as the antibody response may be absent. However, serological tests can be positive in the CSF before seroconversion in the peripheral blood.[8] For the second patient, tests were performed 4 months after symptom onset, but seroconversion is sometimes absent after early antibiotic treatment that can block antibody production. For the only patient who had a negative CSF serology, the lumbar puncture was performed 3 weeks after symptom onset, and specific intrathecal IgG production is detectable only after 6 to 8 weeks in some cases.[8]
Furthermore, the presence of oligoclonal bands associated with a positive serology in the CSF would be an argument for the diagnosis of neuroborreliosis because oligoclonal bands could be an indicator of active infection.[28,29]
In our study, 81.3% (13/16) of the patients were initially treated with intravenous ceftriaxone (for 3–4 weeks), which led to a favorable outcome for 84.6% of patients (11/13). This treatment was ineffective for the patient with chronic demyelinating neuropathy because of axonal loss. For another patient (with meningoradiculoneuritis), symptoms disappeared with ceftriaxone but atypically reappeared at the end of the antibiotherapy. The patient was subsequently successfully treated with a 4-week course of doxycycline. One patient was switched to doxycycline after developing an allergy to ceftriaxone. Two patients initially received a 3-week course of amoxicillin (with one treatment failure, treated secondarily with ceftriaxone) and 1 patient failed to respond to an initial treatment with doxycycline and was treated secondarily with ceftriaxone. In summary, antibiotic treatment led to a favorable outcome in almost all cases, as reported in other series.[10,11] According to both European and American guidelines, patients with early neuroborreliosis (meningitis, radiculopathy) should be treated with intravenous ceftriaxone (2 g/for at least 2 weeks). If a patient is intolerant to b-lactam antibiotics, doxycycline can be used. Patients with late neuroborreliosis should be treated with intravenous ceftriaxone (for 2–4).[7,30]
The main limitation of our study was that data about the CSF/serum antibody index were limited, because of a retrospective data collection for biological results, and because this analysis was not performed for the oldest medical records. The CSF/serum antibody index is useful to exclude a passive transfer of antibodies from blood. However, diagnosis of neuroborreliosis was defined in our study with positive serological tests confirmed by Western Blot and based on a body of clinical arguments (improvement of symptoms with an adapted antibiotherapy, exclusion of other diseases, and a frequent occurrence of meningitis and pain for our patients).
Moreover, our cohort might not reflect all cases of neuroborreliosis because we included only patients who had an electrophysiological study in a neuromuscular disease center in a nonendemic area. We could not include a large number of patients, because an electrophysiological study is not systematically performed in case of neuroborreliosis, and we included only patients with a confirmed diagnosis.
Although it is known that Borrelia primarily affects the skin and then other tissues through the blood and lymphatic systems, the pathogenesis of neuroborreliosis in the various clinical presentations is not clearly understood.[31] It is thought that the bacteria are harbored by macrophages, causing degeneration of perineurial cells (which normally play a role of protective barrier) and increasing the permeability of the blood-nerve barrier, which leads to migration of inflammatory cells into the nerves.[32,33,34] This would explain the occurrence of interstitial lymphoplasmacytic infiltration forming clusters around the epineurial, endoneurial and perineurial capillaries with or without necrosis of the vessel walls, and complement and immunoglobulin deposition.[32] The local production of inflammatory and cytotoxic mediators induces apoptosis and the loss of neurons and glial cells. Matrix metalloproteinases then attack the myelin proteins and digest glycoproteins and proteoglycans leading to local demyelination. This ultimately results in multifocal axonal loss of the nerve roots and distal nerve segments, and loss of both myelinated and unmyelinated fibers.[19,24,34,35,36,37,38,39] This cell loss might also be due to the direct action of spirochetes and infiltrating cells.[40,41] Moreover, the presence of cross-reactive antibodies to self-antigens (antibodies directed against epitopes of Borrelia with cross-reaction with epitopes of peripheral nerve) has been described.[32,39,40]
It was difficult to know exactly where the axonal loss started for our patients, since the electrophysiological study mostly analysed distal nerves and the location of infection onset was often unknown.
For the neurologist, diagnosing neuroborreliosis is challenging as the clinical presentation is highly varied and the neurological symptoms often reveal borreliosis (as for 58% of our patients) without a reported history of tick bite or skin lesions. The occurrence of a subacute, painful, and asymmetric neuropathy should suggest a diagnosis of neuroborreliosis. A delayed diagnosis with subsequent axonal loss can have an adverse effect on the outcome, and symptom resolution is observed in most patients treated early with antibiotics. The objective must be to identify neuroborreliosis and atypical cases as early as possible, with the help of CSF analysis, to treat the patient rapidly, avoid the axonal loss and improve prognosis.
Acknowledgments
The authors thank Felicity Neilson (Matrix Consultants) for medical English editing services.
Author contributions
All authors contributed to the study conception. Data collection was performed by Anne-Laure Kaminsky. The first draft of the manuscript was written by Anne-Laure Kaminsky and all authors commented on previous versions of the manuscript, read and approved the final manuscript.
Footnotes
Abbreviations: CMAP = compound muscle action potential, CSF = cerebrospinal fluid, MRI = magnetic resonance imagery, NLL = normal lower limit, OP = oligoclonal bands, PNS: peripheral nervous system.
How to cite this article: Kaminsky AL, Maisonobe T, Lenglet T, Psimaras D, Debs R, Viala K. Confirmed cases of Neuroborreliosis with involvement of peripheral nervous system: Description of a cohort. Medicine. 2020;99:40(e21986).
There was no funding.
The authors report no conflicts of interest.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Mygland A, Skarpaas T, Ljøstad U. Chronic polyneuropathy and Lyme disease. Eur J Neurol 2006;13:1213–5. [DOI] [PubMed] [Google Scholar]
- [2].Akbik F, Matiello M, Piquet A, et al. Bibrachial plegia due to Lyme radiculopoliomyelitis-myelitis. J Neurol Sci 2017;378:1–2. [DOI] [PubMed] [Google Scholar]
- [3].Halperin JJ. Neuroborreliosis. J Neurol 2017;264:1292–7. [DOI] [PubMed] [Google Scholar]
- [4].Cuvelier ML, Léonard P, Rikir E, et al. Neuroborreliosis [Article in French]. Rev Med Liege 2008;63:349–53. [PubMed] [Google Scholar]
- [5].Schwenkenbecher P, Pul R, Wurster U, et al. Common and uncommon neurological manifestations of neuroborreliosis leading to hospitalization. BMC Infect Dis 2017;17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Koedel U, Fingerle V, Pfister HW. Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat Rev Neurol 2015;11:446–56. [DOI] [PubMed] [Google Scholar]
- [7].Mygland A, Ljøstad U, Fingerle V, et al. EFNS guidelines on the diagnosis and management of European Lyme neuroborreliosis. Eur J Neurol 2010;17:8–16. [DOI] [PubMed] [Google Scholar]
- [8].Stanek G, Fingerle V, Hunfeld KP, et al. Lyme borreliosis: Clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect 2011;17:69–79. [DOI] [PubMed] [Google Scholar]
- [9].Van den Bergh PY, Hadden RD, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society — First Revision. Eur J Neurol 2010;17:356–63. [DOI] [PubMed] [Google Scholar]
- [10].Gómez-Eguílaz M, Gómez-Cerquera J, Calvo-Pérez L, et al. Neuroborreliosis: a single-hospital series of 7 cases. Neurol Barc Spain 2016;31:137–9. [DOI] [PubMed] [Google Scholar]
- [11].Lovett JK, Evans PH, O’Connell S, et al. Neuroborreliosis in the South West of England. Epidemiol Infect 2008;136:1707–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kindler W, Wolf H, Their K, et al. Peripheral facial palsy as an initial symptom of Lyme neuroborreliosis in an Austrian endemic area. Wien Klin Wochenschr 2016;128:837–40. [DOI] [PubMed] [Google Scholar]
- [13].Sellam A, Fournier I, Biton-Ouayoun C. Isolated left abducens nerve palsy secondary to Lyme disease in an 11-year-old boy [Article in French]. J Fr Ophtalmol 2015;38:e155–6. [DOI] [PubMed] [Google Scholar]
- [14].Van Erp WS, Bakker NA, Aries MJ, et al. Opsoclonus and multiple cranial neuropathy as a manifestation of neuroborreliosis. Neurology 2011;77:1013–4. [DOI] [PubMed] [Google Scholar]
- [15].Schmitt M, Daubail B, Bohm A. Parsonage-Turner syndrome secondary to Lyme disease. Jt Bone Spine 2018;85:387–8. [DOI] [PubMed] [Google Scholar]
- [16].Rupprecht TA, Elstner M, Weil S, et al. Autoimmune-mediated polyneuropathy triggered by borrelial infection? Muscle Nerve 2008;37:781–5. [DOI] [PubMed] [Google Scholar]
- [17].Thaisetthawatkul P, Logigian EL. Peripheral nervous system manifestations of lyme borreliosis. J Clin Neuromuscul Dis 2002;3:165–71. [DOI] [PubMed] [Google Scholar]
- [18].Knudtzen FC, Andersen NS, Jensen TG, et al. Characteristics and clinical outcome of lyme neuroborreliosis in a high endemic area, 1995-2014: a retrospective cohort study in Denmark. Clin Infect Dis Off Publ Infect Dis Soc Am 2017;65:1489–95. [DOI] [PubMed] [Google Scholar]
- [19].Kindstrand E, Nilsson BY, Hovmark A, et al. Peripheral neuropathy in acrodermatitis chronica atrophicans - effect of treatment. Acta Neurol Scand 2002;106:253–7. [DOI] [PubMed] [Google Scholar]
- [20].Jalladeau E, Pradat PF, Maisonobe T, et al. Multiple mononeuropathy and inflammatory syndrome manifested in Lyme disease [Article in French]. Rev Neurol (Paris) 2001;157:1290–2. [PubMed] [Google Scholar]
- [21].Halperin JJ. Nervous System Lyme Disease. Clin Lab Med 2015;35:779–95. [DOI] [PubMed] [Google Scholar]
- [22].Elamin M, Alderazi Y, Mullins G, et al. Perineuritis in acute lyme neuroborreliosis. Muscle Nerve 2009;39:851–4. [DOI] [PubMed] [Google Scholar]
- [23].Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain–Barré syndrome in ten steps. Nat Rev Neurol 2019;15:671–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Patel K, Shah S, Subedi D. Clinical association: Lyme disease and Guillain-Barre syndrome. Am J Emerg Med 2017;35: 1583.e1-1583.e2. [DOI] [PubMed] [Google Scholar]
- [25].Tyagi N, Maheswaran T, Wimalaratna S. Neuroborreliosis: the Guillain-Barré mimicker. BMJ Case Rep 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Faul JL, Ruoss S, Doyle RL, et al. Diaphragmatic paralysis due to Lyme disease. Eur Respir J 1999;13:700–2. [DOI] [PubMed] [Google Scholar]
- [27].Djukic M, Larsen J, Lingor P, et al. Unilateral phrenic nerve lesion in Lyme neuroborreliosis. BMC Pulm Med 2013;13:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sindic CJ, Monteyne P, Laterre EC. Occurrence of oligoclonal IgM bands in the cerebrospinal fluid of neurological patients: an immunoaffinity-mediated capillary blot study. J Neurol Sci 1994;124:215–9. [DOI] [PubMed] [Google Scholar]
- [29].Ljostad U, Mygland A. Remaining complaints 1 year after treatment for acute Lyme neuroborreliosis; frequency, pattern and risk factors. Eur J Neurol 2010;17:118–23. [DOI] [PubMed] [Google Scholar]
- [30].Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis Off Publ Infect Dis Soc Am 2006;43:1089–134. [DOI] [PubMed] [Google Scholar]
- [31].Dubrey SW, Bhatia A, Woodham S, et al. Lyme disease in the United Kingdom. Postgrad Med J 2014;90:33–42. [DOI] [PubMed] [Google Scholar]
- [32].Schäfers M, Neukirchen S, Toyka KV, et al. Diagnostic value of sural nerve biopsy in patients with suspected Borrelia neuropathy. J Peripher Nerv Syst JPNS 2008;13:81–91. [DOI] [PubMed] [Google Scholar]
- [33].Créange A, Saint-Val C, Guillevin L, et al. Peripheral neuropathies after arthropod stings not due to Lyme disease: a report of five cases and review of the literature. Neurology 1993;43:1483–8. [DOI] [PubMed] [Google Scholar]
- [34].Moguelet P. Histopathologie de la Borréliose de Lyme. Médecine Mal Infect 2007;37:189–93. [DOI] [PubMed] [Google Scholar]
- [35].Hansen K, Crone C, Kristoferitsch W. Lyme neuroborreliosis. Handb Clin Neurol 2013;115:559–75. [DOI] [PubMed] [Google Scholar]
- [36].Halperin J, Luft BJ, Volkman DJ, et al. Lyme neuroborreliosis. Peripheral nervous system manifestations. Brain J Neurol 1990;113(Pt 4):1207–21. [DOI] [PubMed] [Google Scholar]
- [37].Halperin JJ. Nervous system Lyme disease. J R Coll Physicians Edinb 2010;40:248–55. [DOI] [PubMed] [Google Scholar]
- [38].Kindstrand E, Nilsson BY, Hovmark A, et al. Polyneuropathy in late Lyme borreliosis - a clinical, neurophysiological and morphological description. Acta Neurol Scand 2000;101:47–52. [DOI] [PubMed] [Google Scholar]
- [39].Perides G, Tanner-Brown LM, Eskildsen MA, et al. Borrelia burgdorferi induces matrix metalloproteinases by neural cultures. J Neurosci Res 1999;58:779–90. [DOI] [PubMed] [Google Scholar]
- [40].Ramesh G, Santana-Gould L, Inglis FM, et al. The Lyme disease spirochete Borrelia burgdorferi induces inflammation and apoptosis in cells from dorsal root ganglia. J Neuroinflammation 2013;10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Parthasarathy G, Philipp MT. The MEK/ERK pathway is the primary conduit for Borrelia burgdorferi-induced inflammation and P53-mediated apoptosis in oligodendrocytes. Apoptosis Int J Program Cell Death 2014;19:76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]


