ABSTRACT
We report a 52-year-old man who developed drug-induced liver injury after taking Alpha Bolic (contains RAD-140) and Alpha Elite (contains both RAD-140 and LGD-4033) supplements. Liver biopsy demonstrated diffuse centrilobular canalicular cholestasis, prominent ductular reaction, and mild lobular inflammation with rare non-necrotizing epithelioid granuloma suggestive of drug-induced liver injury. Liver enzymes returned to normal levels approximately 3 months after the patient stopped both supplements. We present the mechanism of drug-induced liver injury associated with 2 selective androgen receptor modulators, including RAD-140 and LGD 4033.
INTRODUCTION
RAD-140 (2-chloro-4-[[(1R,2S)-1-[5-(4-cyanophenyl)-1,3,4-oxadiazol-2-yl]-2-hydroxypropyl]amino]-3-methylbenzonitrile) and LGD-4033 (4-[(2R)-2-[(1R)-2,2,2-trifluoro-1-hydroxyethyl]pyrrolidin-1-yl]-2-(trifluoromethyl)benzonitrile) are nonsteroidal selective androgen receptor modulators (SARM) that bind as a ligand to the androgen receptors and express tissue-selective activities.1–4 SARMs have a highly selected anabolic effect on muscles and bones, with antagonistic features in productive organs such as the prostate.4,5 Therefore, SARMs have been investigated for the treatment of osteoporosis, sarcopenia, and cachexia; however, their use in clinical practice remains unapproved by the US Food and Drug Administration (FDA).6–13 Owing to potential misuse of their anabolic effects, they were added to the World Anti-Doping Agency's Prohibited List.14 However, they are sold as a dietary supplement over the counter and on the internet, causing a serious threat to public health. We present a case of severe drug-induced hepatotoxicity associated with RAD-140 and LGD-4033.
CASE REPORT
A 52-year-old white man without chronic medical problems presented to our hepatology clinic for an evaluation of elevated liver enzymes and jaundice. His symptoms included right upper quadrant pain, jaundice, pruritis, and diarrhea that started approximately 3 months before his presentation. Approximately 4 months before his presentation, he started taking 2 capsules of Alpha Bolic (1 capsule contains 10 mg of RAD-140, the bottle contains 60 tablets) daily for 4 weeks. After he finished Alpha Bolic, he started taking 2 capsules of Alpha Elite (1 capsule contains 7.5 mg of RAD-140 and 5 mg of LGD-4033, the bottle contains 60 tablets) daily for 3 weeks for muscle building. The total duration of the supplement consumption was 7 weeks. Soon after that he developed jaundice, right upper quadrant pain, pruritus, and greasy diarrhea. His medical, surgical, and family histories were noncontributory. He reported drinking bourbon and beer daily and using marijuana.
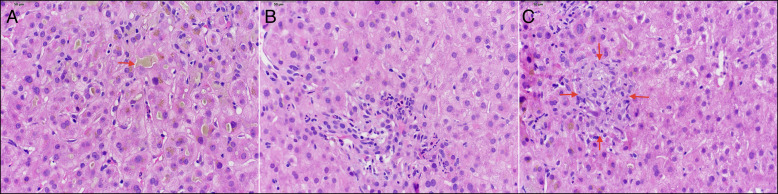
The vital signs were normal. The physical examination was normal except for scleral icterus and jaundice. The initial laboratory results were as follows: aspartate aminotransferase 36 U/L, alanine aminotransferase 46 U/L, alkaline phosphatase (ALP) 529 U/L, total bilirubin 34.5 mg/dL, international normalised ratio 1.0, gamma-glutamyl transferase 47 U/L, total protein 5.5 g/dL, albumin 3.8 g/dL, and globulin 1.7 g/dL. Serological markers for acute hepatitis secondary to A, B, and C, herpes simplex virus, cytomegalovirus, and Epstein-Barr virus were negative. Iron level was 181 μg/dL, total iron-binding capacity was313 μg/dL, iron saturation was 58%, ferritin was 342 ng/mL, ceruloplasmin was 45 mg/dL, and alpha-1 antitrypsin was 146 mg/dL. Serum antimitochondrial antibody, antismooth muscle antibody, and anti-liver-kidney microsomal antibody were negative. He was negative for C282Y and H63D mutations. He had a normal complete blood cell count. Abdominal magnetic resonance imaging showed no gallstone or biliary dilation. The patient underwent an ultrasound-guided liver biopsy that showed diffuse centrilobular canalicular cholestasis, prominent ductular reaction, and mild lobular inflammation with rare non-necrotizing epithelioid granuloma and mild portal and periportal fibrosis suggestive of drug-induced liver injury (Figure 1). The liver biopsy also showed mild to moderate hemosiderosis; however, the hepatic iron index was 0.6. His hemosiderosis was likely secondary to his history of alcohol use. The etiology of the liver disease was most consistent with a cholestatic drug-induced liver injury (R factor was 0.2).15 The patient was instructed to remain abstinent from taking any supplements and stop alcohol. Total bilirubin, ALP, and aminotransferase levels significantly improved approximately 3 months after the patient stopped supplements (aspartate aminotransferase 37 U/L, alanine aminotransferase 30 U/L, ALP 121 U/L).
Figure 1.
Liver biopsy showing (A) canalicular bile plugs (arrows), (B) portal tract with bile duct damage and cholangiolar proliferation with associated neutrophils, and (C) non-necrotizing epithelioid granuloma (arrows) in the lobule (hematoxylin and eosin, 400×).
DISCUSSION
The major ingredient of Alpha Bolic is RAD-140 (2-chloro-4-[[(1R,2S)-1-[5-(4-cyanophenyl)-1,3,4-oxadiazol-2-yl]-2-hydroxypropyl]amino]-3-methylbenzonitrile).2 Per the manufacturer, Alpha Bolic also contains gelatin (capsules) and rice flour. The discovery of RAD-140 and its SARM properties was first reported in 2011 by Miller et al.4 Alpha Elite contains 2 SARMs including RAD-140 (2-chloro-4-[[(1R,2S)-1-[5-(4-cyanophenyl)-1,3,4-oxadiazol-2-yl]-2-hydroxypropyl]amino]-3-methylbenzonitrile)2 and LGD-4033 (4-[(2R)-2-[(1R)-2,2,2-trifluoro-1-hydroxyethyl]pyrrolidin-1-yl]-2-(trifluoromethyl)benzonitrile).1,2 It also contains vitamin C (ascorbic acid), potassium nitrate, Bulbine Natalensis P.E., gelatin (capsules), magnesium stearate, and silicon dioxide.
Although SARMs are not approved by the FDA, they can still be purchased from various websites or over the counter without a prescription.9–13 A study evaluated the ingredients of the 44 dietary supplements sold as SARM.13 According to this study results, 39% of these dietary supplements contained unapproved drugs other than SARMs, including ibutamoren (a growth hormone secretagogue), GW501516 (a peroxisome proliferator-activated receptor-δ agonist), and SR9009 (Rev-Erbα [a circadian clock protein] agonist).13 The mass spectrometric analysis of these dietary supplements revealed that only 52% of these supplements sold in the market contained SARMs, and the remaining supplements were mislabeled.13
This case illustrates drug-induced liver injury in the setting of RAD-140 and LGD-4033 use. However, it is possible that both Alpha Bolic and Alpha Elite pills had another unapproved substance that has not been reported by the manufacturer in the drug label. In addition, Alpha Elite contains 5 mg of LGD-4033 per capsule in addition to RAD-140. The amount of LGD-4033 reported in the Alpha Elite is 5–50 times higher than the daily amount of LGD-4033 that was administered in the placebo-controlled trial of LGD-4033 conducted in 76 healthy men.3 Different doses of RAD-140 (0.01 mg/kg, 0.1 mg/kg and 1 mg/kg) were tested in male cynomolgous monkeys, and no elevation greater than 2-folds of the baseline levels was observed in the liver enzymes.4 Our patient took 20 mg of RAD-140 daily for 4 weeks and, after that, 15 mg of RAD-140 daily for 3 weeks. The weight-based RAD-140 doses that the patient took were approximately 0.25 mg/kg (Alpha Bolic) and 0.19 mg/kg (Alpha Elite) and higher than the weight-based RAD-140 doses of 0.01 mg/kg and 0.1 mg/kg given to the cynomolgous monkeys.4
Although the patient had a history of alcohol use, there were no histological features of acute alcoholic hepatitis noted in his liver biopsy. In contrast to the cholestatic phase of acute alcoholic hepatitis that usually presents with histological features including steatosis, very prominent ballooning degeneration with well-formed Mallory-Denk bodies, neutrophilic satellitosis around the Mallory hyaline, and pericellular fibrosis, along with cholestasis in the liver biopsy, this patient had no steatosis, ballooning degeneration with Mallory-Denk bodies, lobular neutrophils, or pericellular fibrosis in his liver biopsy. Specifically, he had a largely bland canalicular cholestasis, one focus of lobular non-necrotizing epithelioid granuloma, and ductular reaction. After ruling out other possible causes of acute liver injury, the diagnosis was made based on the timing of the administration of RAD-140 and LGD-4033, the improvement of liver enzymes after the patient stopped taking these supplements, and liver biopsy findings. Although there were no histological features of acute alcoholic hepatitis in the liver biopsy, alcohol use may have predisposed the patient to RAD-140- and LGD-4033-associated drug-induced liver injury. In conclusion, we reported severe drug-induced hepatotoxicity associated with RAD-140 and LGD-4033. Although RAD-140 and LGD-4033 are not FDA-approved for clinical use, they are freely sold over the counter and via the internet as a dietary supplement. A higher level of regulations for these products is required to protect public health.
DISCLOSURES
Author contributions: M. Barbara and AL Mindikoglu wrote the manuscript and revised the manuscript for intellectual content. S. Dhingra revised the manuscript for intellectual content. AL Mindikoglu is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
REFERENCES
- 1.National Center for Biotechnology Information. PubChem Database. CID=44137686 (https://pubchem.ncbi.nlm.nih.gov/compound/lgd-4033). Accessed January 25, 2020.
- 2.National Center for Biotechnology Information. PubChem Database. Testolone, CID=44200882 (https://pubchem.ncbi.nlm.nih.gov/compound/Testolone). Accessed May 24, 2020.
- 3.Basaria S, Collins L, Dillon EL, et al. The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men. J Gerontol A Biol Sci Med Sci. 2013;68(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller CP, Shomali M, Lyttle CR, et al. Design, synthesis, and preclinical characterization of the selective androgen receptor modulator (SARM) RAD140. ACS Med Chem Lett. 2011;2(2):124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon ZJ, Mirabal JR, Mazur DJ, Kohn TP, Lipshultz LI, Pastuszak AW. Selective androgen receptor modulators: Current knowledge and clinical applications. Sex Med Rev. 2019;7(1):84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann DB, Komrakova M, Pflug S, et al. Evaluation of ostarine as a selective androgen receptor modulator in a rat model of postmenopausal osteoporosis. J Bone Miner Metab. 2019;37(2):243–55. [DOI] [PubMed] [Google Scholar]
- 7.Dalton JT, Taylor RP, Mohler ML, Steiner MS. Selective androgen receptor modulators for the prevention and treatment of muscle wasting associated with cancer. Curr Opin Support Palliat Care. 2013;7(4):345–51. [DOI] [PubMed] [Google Scholar]
- 8.Morimoto M, Aikawa K, Hara T, Yamaoka M. Prevention of body weight loss and sarcopenia by a novel selective androgen receptor modulator in cancer cachexia models. Oncol Lett. 2017;14(6):8066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FDA analysis shows body-building products labeled to contain steroid and steroid-like substances continue to inflict serious liver injury (https://www.fda.gov/drugs/drug-safety-and-availability/fda-analysis-shows-body-building-products-labeled-contain-steroid-and-steroid-substances-continue). Accessed October 14, 2019.
- 10.FDA In Brief: FDA warns against using SARMs in body-building products (https://www.fda.gov/news-events/fda-brief/fda-brief-fda-warns-against-using-sarms-body-building-products). Accessed October 15, 2019.
- 11.Warning letter (https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/infantry-labs-llc-535333-10232017). Accessed October 15, 2019.
- 12.Warning letter (https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/panther-sports-nutrition-535341-10232017). Accessed October 15, 2019.
- 13.Van Wagoner RM, Eichner A, Bhasin S, Deuster PA, Eichner D. Chemical composition and labeling of substances marketed as selective androgen receptor modulators and sold via the internet. JAMA. 2017;318(20):2004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thevis M, Schanzer W. Detection of SARMs in doping control analysis. Mol Cell Endocrinol. 2018;464:34–45. [DOI] [PubMed] [Google Scholar]
- 15.Bénichou C. Criteria of drug-induced liver disorders: Report of an international consensus meeting. J Hepatol. 1990;11(2):272–6. [DOI] [PubMed] [Google Scholar]