Supplemental Digital Content is available in the text
Keywords: diabetes, hyperlipidemia, meta-analysis, silymarin
Abstract
Background:
To comprehensively evaluate the treatment efficacy and safety of silymarin for patients with glucose/lipid metabolic dysfunction using a meta-analysis.
Methods:
A systematic literature search in PubMed, EMBASE and Cochrane Library databases was performed up to October 1, 2019. STATA 13.0 software was used to estimate pooled standardized mean difference (SMD) and 95% confidence interval (95% CI).
Results:
Sixteen studies involving 1358 patients were identified. Overall meta-analysis showed that compared with control, silymarin significantly reduced levels of fasting blood glucose (SMD: −1.27, 95% CI = [−1.78, −0.76]; P < .001), homeostatic model assessment for insulin resistance (SMD: −0.41, 95% CI = [−0.70, −0.12]; P = .005), hemoglobin A1c (SMD: −1.88, 95% CI = [−2.57, −1.20]; P < .001), total cholesterol (SMD: −1.13, 95% CI = [−1.82, −0.77]; P < .001), triglyceride (SMD: −0.37, 95% CI = [−0.69, −0.05]; P = .025), low-density lipoprotein-cholesterol (SMD: −1.30, 95% CI = [−1.93, −0.67]; P < .001), C-reactive protein (SMD: −0.63, 95% CI = [−1.01, −0.27]; P = .001), and increased high-density lipoprotein-cholesterol (SMD: 0.17, 95% CI = [0.05, 0.29]; P = .005), but had no impacts on function indicators of liver and kidney (alanine transaminase, aspartate aminotransferase, creatinine phosphokinase, creatinine) and the complication rate. Subgroup analyses indicated that insulin (which was negative in overall analysis) was significantly decreased in patients undergoing silymarin monotherapy (SMD: −2.03, 95% CI = [−3.03, −1.04]; P = .044) for more than 3 months (SMD: −0.01, 95% CI = [−0.25, −0.24]; P = .035).
Conclusion:
Supplementation of silymarin may be effective and safe for the management of diabetes mellitus and hyperlipidemia.
1. Introduction
Diabetes mellitus (DM) and hyperlipidemia are both common, chronic metabolic diseases and their incidence has been continuously rising in recent years (especially the young people) partly due to excessive food consumption, physical inactivity, and longer period under psychosocial stress.[1,2,3] Individuals with DM and hyperlipidemia were associated with a higher risk for developing various kinds of cardiovascular diseases,[4] which are the leading cause of worldwide mortality annually. Thus, how to control the levels of blood glucose and lipid has been an important public health problem.
The conventional management of DM and hyperlipidemia mainly focuses on the administration of synthetic Western drugs (hypoglycemic: glibenclamide, repaglinide, rosiglitazone, metformin[5]; lipid-lowering drugs: statins, ezetimibe, fibrates, niacin[6]). However, several potential side effects (such as gastrointestinal discomfort, hepatotoxicity, and renal injury) frequently make the patients intolerant and decide to decrease or stop the use of them.[7,8] Therefore, the development of more effective and safe drugs for the treatment of DM and hyperlipidemia is urgently needed.
Traditional Chinese herbs usually have both edible and medicinal value and hereby, they are widely reorganized as alternative medications for the treatment of various diseases. Silymarin, a mixture of 3 flavonolignans silybin, silydianin, and silychristin, is the active component in the seeds of the milk thistle plant (Silybum marianum).[9] Recently, several animal model studies have shown that silymarin may exert potential antidiabetic and lipid-lowering roles.[10,11] Theoretically, silymarin may also be effective for the treatment of patients with DM and hyperlipidemia. This hypothesis has been demonstrated by some scholars. For example, Ebrahimpour-Koujan et al[12] found that supplementation of silymarin for 45 days significantly reduced fasting blood glucose (FBG), insulin (FBI), homeostatic model assessment for insulin resistance (HOMA-IR), triglyceride (TG), and triglyceride to high-density lipoprotein-cholesterol (HDL-C) ratio and increased HDL-C levels in type 2 DM (T2DM) patients compared to the placebo. Elgarf et al[13] also reported that FBG, hemoglobin A1c (HbA1c), HOMA-IR, total cholesterol (TC), TG, and low-density lipoprotein-cholesterol (LDL-C) were significantly different between the silymarin intervention group and the control group. However, the study of Ghorbani et al[14] showed that the level of FBG in diabetic patients with uncontrolled dyslipidemia was not significantly reduced after the use of an herbal compound that included silymarin compared with controls. Khalili et al[15] indicated that TC, LDL-C, and HDL-C were not significantly changed after 3 months of the intervention with a mixed herbal formulation (silymarin, olibanum, and nettle). Accordingly, whether silymarin is a promising herbal regimen to treat DM and hyperlipidemia remains unclear and needs to be further evaluated.
The goal of this study was to perform a meta-analysis to reassess the effects of silymarin on control of blood glucose and lipid. Previously, there have been meta-analyses conducted to confirm the roles of silymarin supplementation in patients with T2DM[16,17,18] and hyperlipidemic subjects.[19] Compared with these studies, the number of included literature was enlarged (16 vs 5–9), which increased the statistical power. Furthermore, some new indicators were evaluated, including HOMA-IR, function indicators of liver and kidney (alanine transaminase [ALT], aspartate aminotransferase [AST], creatinine phosphokinase [CPK], creatinine) and inflammation (C-reactive protein [CRP]). We believe that our study may provide powerful evidence to validate the roles of silymarin.
2. Materials and methods
2.1. Search strategies
This study was conducted following the protocols of Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA). PubMed, EMBASE, and Cochrane Library databases were searched using the following keywords
(“Silybum” OR “silymarin” OR “milk thistle” OR SIL) AND (“diabetes mellitus” OR hyperglycemia OR diabetic OR “hyperlipemia” OR dyslipidemic). The retrieval time was from the inception of the database to October 1, 2019. Furthermore, the reference lists of identified papers were manually checked to obtain potential eligible articles.
2.2. Inclusion and exclusion criteria
Studies were considered to be eligible if they fulfilled the following inclusion criteria: case-control studies that evaluated the treatment effects of silymarin for patients with glucose/lipid metabolic dysfunction; data of blood glucose (FBG, FBI, HOMA-IR, and HbA1c), blood lipids (TC, TG, LDL-C, and HDL-C), adverse events (complication rate, ALT, AST, CPK, and creatinine), inflammation (CRP) and oxidative stress (malondialdehyde [MDA]) related indicators were available; and duration and drug dose were explicitly described. The exclusion criteria were: duplicate publication; abstracts, reviews, case reports, or animal studies; studies lacking a control group; and studies without outcome data.
2.3. Data extraction and quality assessment
Data extraction and quality assessment processes were independently completed by 2 investigators. Any disagreement was resolved with the input of a third review researcher to make a final determination. The following data were extracted from each included article: first author's name, publication year, country, sample size, disease type, therapeutic regimens of case and control groups, silymarin dose, follow-up, study design, and outcomes.
The quality of the eligible studies was evaluated using the Cochrane criteria, which assessed the following aspects: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; adequate assessment of incomplete outcome; selective reporting avoided; and no other bias.
2.4. Statistical analysis
The mean and standard deviation of continuous outcomes or number (percentage) of categorical variables during the follow-up were extracted from the intervention and control groups. For each indicator, the unit was converted to be consistent. STATA 13.0 (STATA Corporation, College Station, TX) was used for all statistics. Cochrane's Q test and I2 tests were performed to determine the heterogeneity. A fixed-effects model was applied to compute the pooled results if an obvious heterogeneity was present (P < .10 and I2 > 50%); otherwise, a random-effects model was selected. Odds ratio (OR, categorical data) or standardized mean difference (SMD, continuous data) and its corresponding 95% confidence interval (95% CI) were estimated as pooled effect size for these outcomes. The overall effect was tested using the Z score, with P < .05 defined to be statistically significant. Moreover, a subgroup analysis was conducted based on intervention type (silymarin monotherapy or combination with others), control type (synthetic medicines or others [including placebo or other herbs]), duration (<3 months or ≥3 months), silymarin dose (<450 mg or ≥450 mg) and disease type (diabetes or dyslipidemia) to find the source of heterogeneity. Publication bias was checked by Egger linear regression test.[20] The trim and fill method was used to adjust for meta-analysis estimate in the presence of publication bias (P < .05).[21] Sensitivity analysis was performed by omitting 1 study at a time.
3. Results
3.1. Literature selection
The study selection process is shown in Figure 1. The electronic database search yielded 335 records. Of them, 114 were excluded because they were duplicates. After reading the title or abstract, 200 were further eliminated because they were animal studies (n = 99), meta-analysis (n = 15), case report (n = 1), descriptive study (n = 12), irrelevant studies (n = 70), or other diseases (n = 3). Then, 21 full-text articles were downloaded and carefully reviewed, after which 5 were found not to meet our inclusion criteria because of lack of control drugs (n = 3), animal study (n = 1), and data unavailable (n = 1). Finally, 16 studies fulfilled the eligibility criteria and were included for this meta-analysis.[12,13,14,15,22,23,24,25,26,27,28,29,30,31,32,33]
Figure 1.
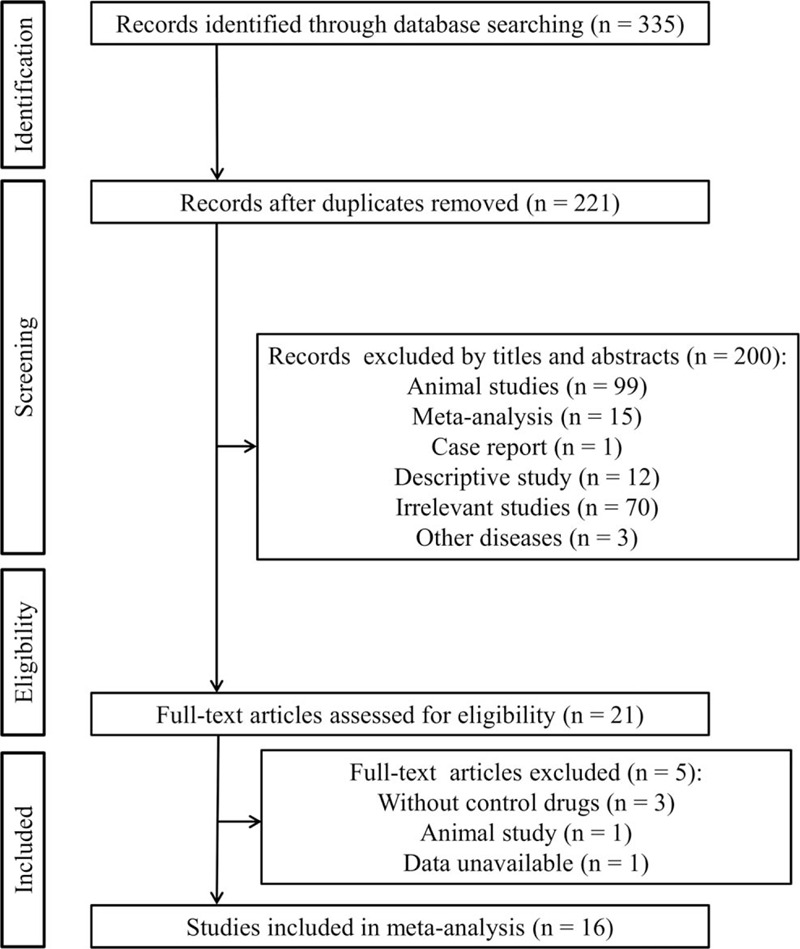
Flow diagram of the study selection procedure.
3.2. Characteristics of the included studies
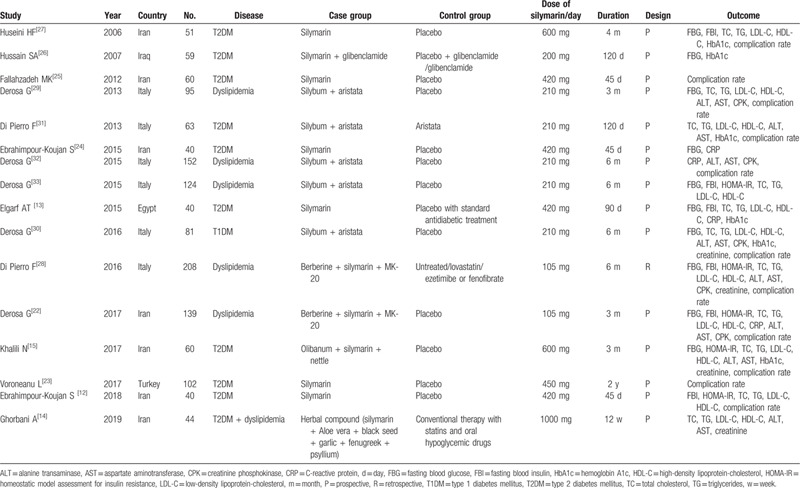
The characteristics of these 16 included studies are presented in Table 1. All the studies consisting of 1358 patients were published from 2006 to 2019. Seven studies were conducted in Iran, 6 were in Italy, and another 3 were performed in Iraq, Egypt, and Turkey. Ten studies investigated the therapeutic effects of silymarin for patients with DM and 5 reported the treatment outcomes for patients with dyslipidemia. In 1 study, dyslipidemia and T2DM coexisted in the patients. The used dose of silymarin ranged from 105 mg to 1000 mg per day and the follow-up period ranged from 45 days to 2 years. Six trials compared the treatment effects of silymarin monotherapy with control, while the remaining 10 trials combined with other Chinese medicine (Berberine, aristata, MK-20, Olibanum, nettle, Aloe vera black seed, garlic, fenugreek, psyllium) or hypoglycemic drugs (glibenclamide). Most of the studies (15/16) were designed as a prospective randomized controlled trial (RCT), with the control of placebo. The study of Hussain[26] included 2 control types (placebo + glibenclamide or glibenclamide) and thus, 2 datasets were involved. The controls of Elgarf et al[13] and Ghorbani et al[14] included standard antidiabetic treatment (such as statins). Furthermore, the study of Di Pierro et al[28] was a retrospective control trial that included 4 comparison groups (control was untreated, lovastatin or ezetimibe/fenofibrate) and thus, 4 datasets were involved. According to the above design description, the quality of eligible studies was considered to be high overall (Supplement Table 1).
Table 1.
Characteristics of included studies.
3.3. Meta-analysis
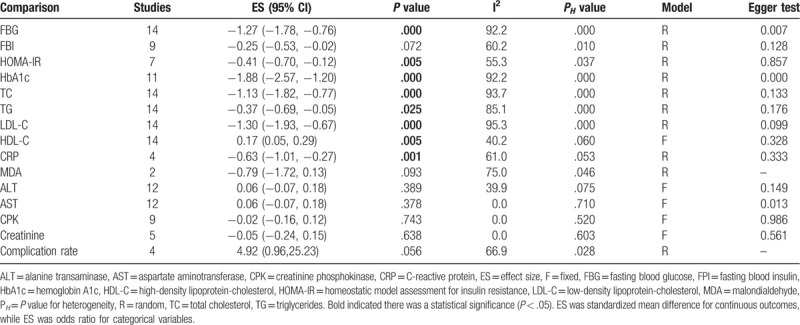
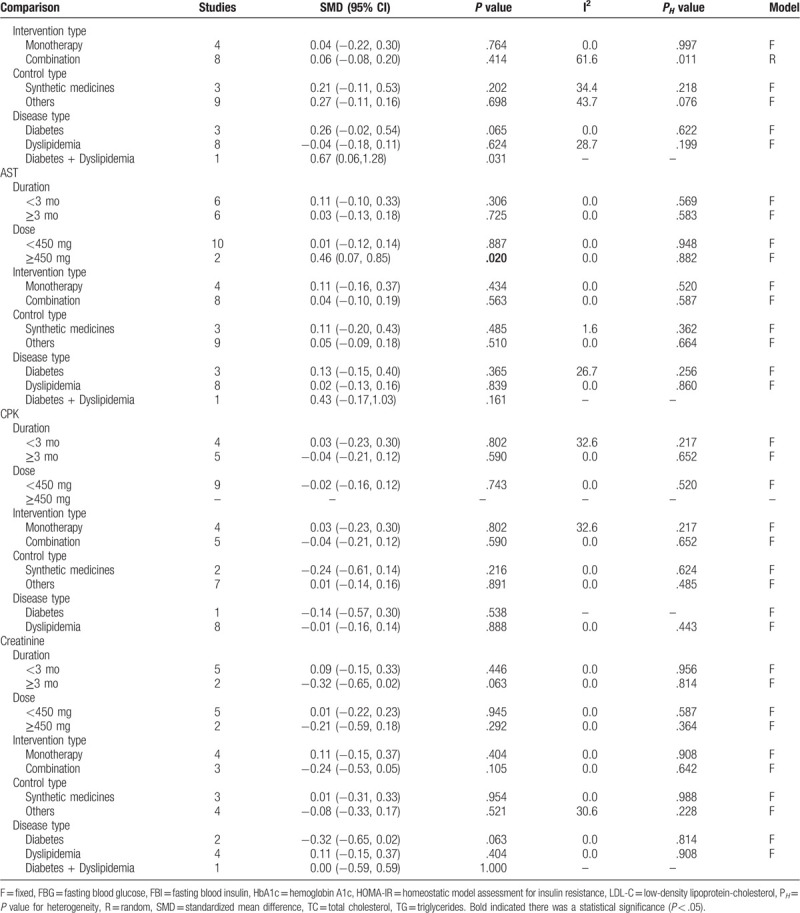
The effects of silymarin treatment on FBG, FBI, HOMA-IR, HbA1c, TC, TG, LDL-C, HDL-C, CRP, MDA, ALT, AST, CPK, creatinine and the complication rate for patients with glucose/lipid metabolic dysfunction were reported in 14, 9, 7 11, 14, 14, 14, 14, 4, 2, 12, 12, 9, 5, and 4 datasets, respectively. The pooled results showed that silymarin supplementation significantly reduced the concentration of FBG (SMD: −1.27, 95% CI = [−1.78, −0.76]; P < .001) (Fig. 2A), HOMA-IR (SMD: −0.41, 95% CI = [−0.70, −0.12]; P = .005) (Fig. 2B), HbA1c (SMD: −1.88, 95% CI = [−2.57, −1.20]; P < .001) (Fig. 2C), TC (SMD: −1.13, 95% CI = [−1.82, −0.77]; P < .001) (Fig. 3A), TG (SMD: −0.37, 95% CI = [−0.69, −0.05]; P = .025) (Fig. 3B), LDL-C (SMD: −1.30, 95% CI = [−1.93, −0.67]; P < .001) (Fig. 3C), CRP (SMD: −0.63, 95% CI = [−1.01, −0.27]; P = .001) (Fig. 4) and increased the level of HDL-C (SMD: 0.17, 95% CI = [0.05, 0.29]; P = .005) (Fig. 3D) compared with control (Table 2). However, there were no significant differences in FBI, MDA, ALT, AST, CPK, creatinine, and the complication rate between silymarin and control treatment groups (Table 2).
Figure 2.
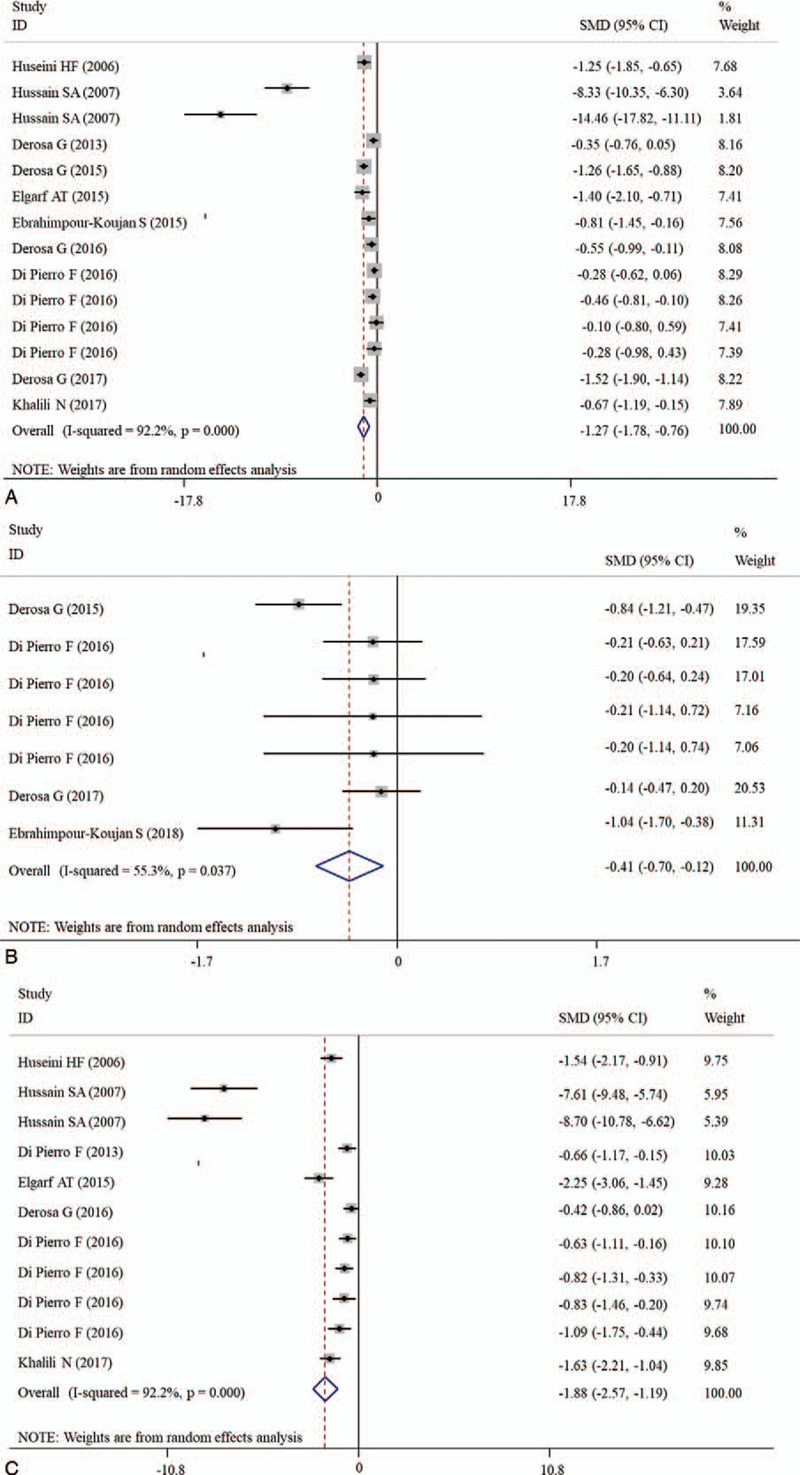
Forest plot showing the effects of silymarin on the levels of blood glucose indicators. A, FBG; B, HOMA-IR; C, HbA1c. CI = confidence interval, FBG = fasting blood glucose, HbA1c = hemoglobin A1c, HOMA-IR = homeostatic model assessment for insulin resistance, SMD = standardized mean difference.
Figure 3.
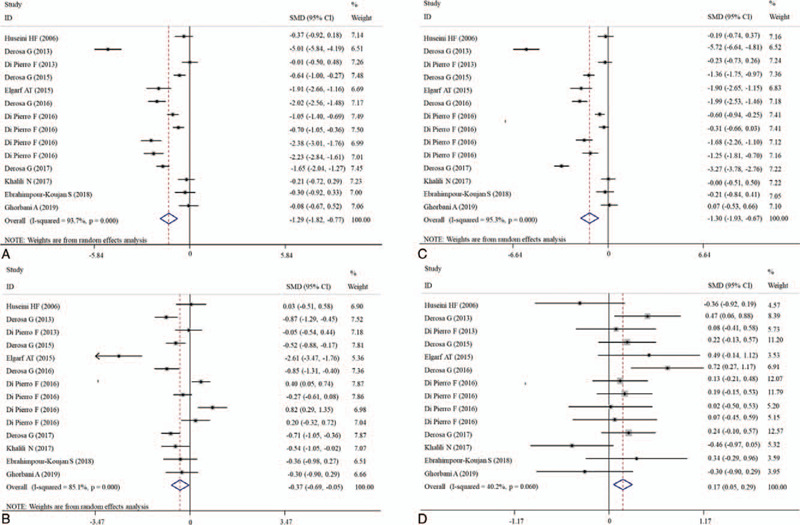
Forest plot showing the effects of silymarin on the levels of blood lipid indicators. A, TC; B, TG; C, LDL-C; D, HDL-C. CI = confidence interval, HDL-C = high-density lipoprotein-cholesterol, LDL-C = low-density lipoprotein-cholesterol, SMD = standardized mean difference, TC = total cholesterol, TG = triglycerides.
Figure 4.
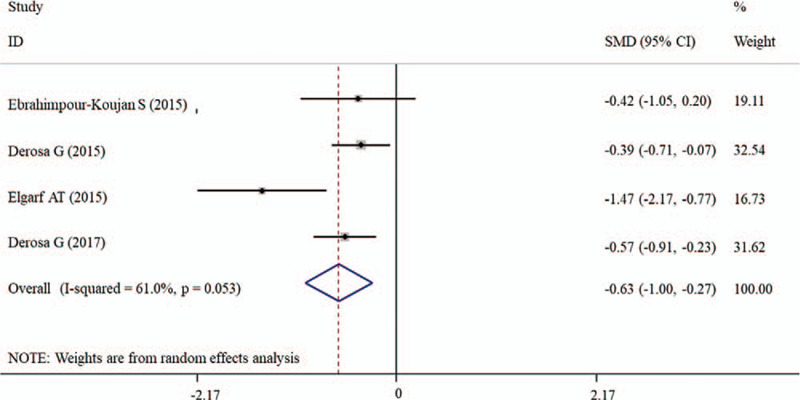
Forest plot showing the effects of silymarin on the level of CRP. CI = confidence interval, CRP = C-reactive protein, SMD = standardized mean difference.
Table 2.
Meta-analysis results.
3.4. Subgroup analyses
Subgroup analyses according to duration, daily dose, intervention type, control type, and disease type were performed for outcomes (FBG, FBI, HOMA-IR, HbA1c, TC, TG, LDL-C, HDL-C, ALT, AST, CPK, and creatinine). The results revealed that FBG and HbA1c were still significantly reduced after the administration of silymarin regardless of which subgroups compared with controls (Table 3 ). TC was demonstrated to be significantly reduced in most of the subgroups other than the high dose group (Table 3 ). Silymarin was found to be effective for reducing HOMA-IR mainly in DM patients who underwent silymarin monotherapy with the dose of less than 450 mg daily for less than 3 months compared with nonsynthetic medicine control, while its effects on TG were mainly observed in DM patients who underwent combination treatment with the silymarin dose of larger than 450 mg daily for longer than 3 months (Table 3 ). Silymarin administration could significantly induce a reduction in LDL-C and an increase in HDL-C for patients with dyslipidemia, especially the long-term follow-up (Table 3 ). For the FBI that was negative in overall analysis, we also found a statistical significance: FBI was shown to be significantly decreased in patients who underwent silymarin monotherapy (SMD: −2.03, 95% CI = [−3.03, −1.04]; P = .044) for longer than 3 months (SMD: −0.01, 95% CI = [−0.25, −0.24]; P = .035) (Table 3 ). The results of ALT, AST, CPK, and creatinine were not changed in most of subgroups, still showing no significance between silymarin and controls (including synthetic medicine and others) (Table 3 ).
Table 3.
Subgroup analysis.
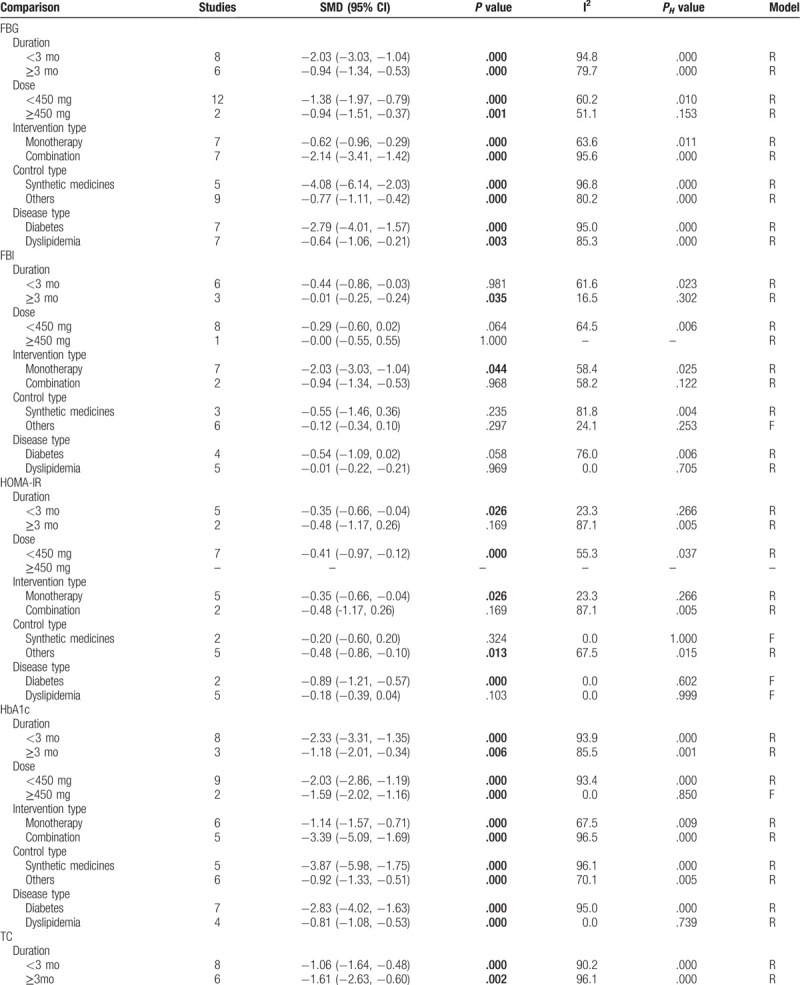
Table 3 (Continued).
Subgroup analysis.
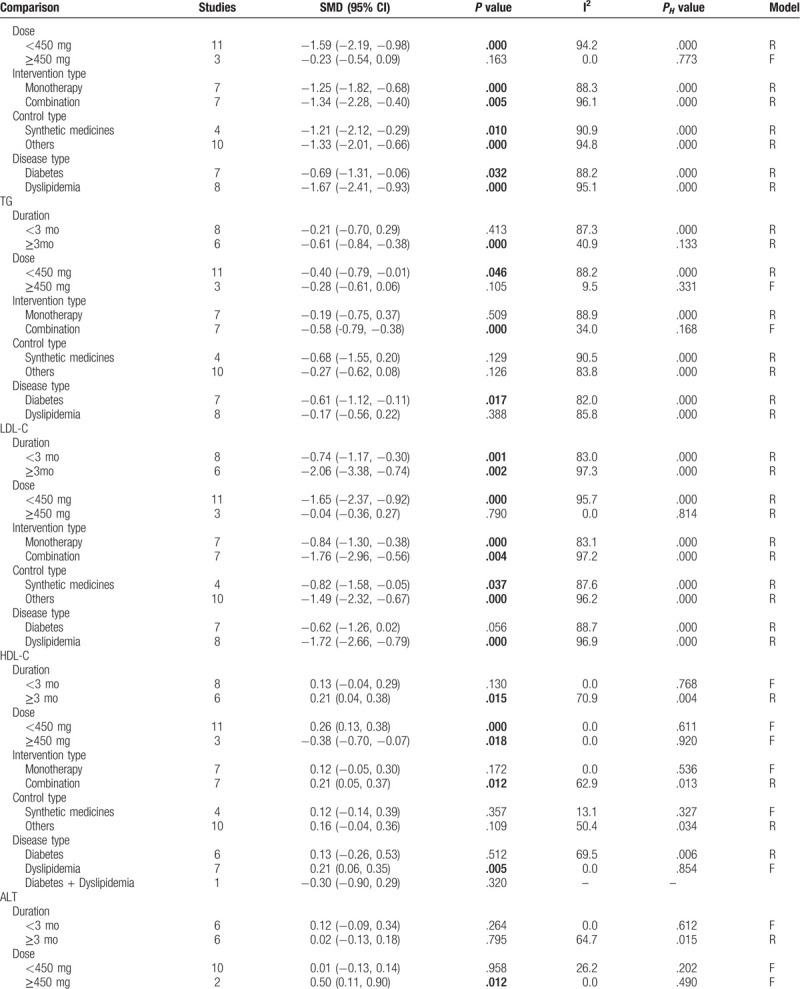
Table 3 (Continued).
Subgroup analysis.
3.5. Publication bias and sensitivity analyses
Egger test showed that there was a significant publication bias for estimating the effects on FBG (P = .007), HbA1c (P < .001), and AST (P = .013) (Table 2). Thus, trim and fill method was used to adjust them, however, after which significant results were still observed for FBG (P < .001) and HbA1c (P < .001), but nonsignificant was for AST (P = .883), indicating the analysis results were believable. The sensitivity analysis was also conducted to verify the reliability of our conclusions after the removal of any individual study (Fig. 5).
Figure 5.
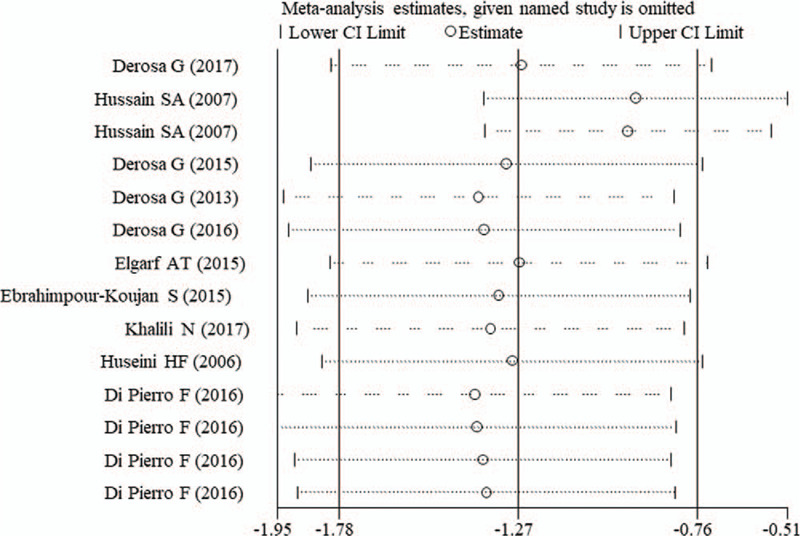
Sensitivity analysis for FBG. CI = confidence interval, FBG = fasting blood glucose.
4. Discussion
In the present study, we enrolled 16 articles to reassess the therapeutic effects of silymarin for patients with glucose/lipid metabolic dysfunction. Overall, the pooled results showed that compared with control treatment, silymarin supplementation significantly reduced levels of FBG, HOMA-IR, HbA1c, TC, TG, LDL-C, CRP, and increased HDL-C. Subgroup analyses indicated the FBI could also be significantly decreased in patients who underwent silymarin monotherapy for longer than 3 months. Silymarin did not influence ALT, AST, creatinine phosphokinase, creatinine, and the complication rate.
Our results on blood lipids (TC, TG, LDL-C, and HDL-C) were in line with the study of Mohammadi et al[19] who integrated 10 clinical trials. However, there were differences compared with the study of Hadi et al[16] who reported that silymarin supplementation did not significantly reduce FBG, TC, and TG using 7 trials and the study of Voroneanu et al[23] who used 5 RCTs to reveal silymarin supplementation had no effects on lipid profile (TC, TG, and HDL-C). These discrepancies may be resulted from an increased sample size in our study. Thus, our conclusion may be more believable.
In addition to the control for blood glucose and lipid, our study, for the first time, analyzed the potential side effects of silymarin on function indicators of liver and kidney (ALT, AST, CPK, and creatinine) and possible function mechanisms to inhibit inflammation (CRP). As expected, compared with placebo, ALT, AST, CPK, and creatinine were not significantly changed after supplementation of silymarin, indicating the safety to use the Traditional Chinese herbs for metabolic diseases.[34] The fact that no significant differences in ALT, AST, CPK, and creatinine between silymarin and synthetic medicines (those may be more toxic theoretically) may be resulted from the small sample size (2 or 3) for analysis of them. As an inflammatory biomarker, elevated baseline CRP was previously demonstrated to be independently associated with an increased risk for developing T2DM[35,36] and its related complications.[37,38] CRP was positively related to the increased levels of FBG, TC, TG, LDL-C, and decreased HDL-C.[36] In a high-fat high-carbohydrate diet-induced prediabetic rat model, CRP was also shown to be increased significantly accompanied by inflammatory cytokines (interleukin [IL]-6 and tumor necrosis factor [TNF]-α).[39] In vitro analysis further confirmed CRP may be involved in the inflammatory response by activation of the NF-kappa B signal pathway.[40] Silymarin was proved to have anti-inflammatory functions in several diseases. For example, Hussain et al[41] concluded that silymarin treatment for 8 weeks significantly reduced serum levels of IL-1 alpha and IL-8, C3, and C4 for patients with knee osteoarthritis compared to the pretreatment levels. Silymarin also exerted hepatoprotective actions through combating inflammatory conditions.[42,43] The inhibition targets of silymarin also included p65 NF-κB, and its downstream IL-1β and TNF-α.[44] Theoretically, CRP could also be reduced after the addition of silymarin for DM and dyslipidemia subjects, which was confirmed in our study.
However, some limitations should be taken into account. First, significant heterogeneity was detected between included studies, which may introduce some potential bias. Second, the included studies were only conducted in a few countries, including Iran, Italy, Iraq, Egypt, and Turkey (all were Asian). Whether it is also effective for other ethnic groups needs further confirmation. Third, the inflammatory inhibition effects of silymarin could only be evaluated according to the level of CRP. Its effects on IL-6 and TNF-α were only reported in 1 study,[22] which also should be further assessed in the future. Fourth, previous studies demonstrated silymarin may also induce potential toxicity (such as nausea, vomiting, headache, diarrhea, and so on).[45,46] However, rare study[28] compared the toxicity of silymarin and synthetic medicines during the treatment of DM and dyslipidemia. Thus, this also warrants further investigation.
5. Conclusion
Our findings suggest that silymarin may have a significant hypoglycemic and lipid-lowing effect and not induce obvious adverse reactions. Attenuation of inflammatory status may be the possible mechanism behind this observed efficacy.
Author contributions
Conceptualization: Fengyan Xiao.
Data curation: Fengyan Xiao, Feng Gao.
Formal analysis: Fengyan Xiao, Feng Gao.
Investigation: Shengxue Zhou.
Methodology: Feng Gao, Shengxue Zhou.
Project administration: Lina Wang.
Software: Shengxue Zhou.
Supervision: Lina Wang.
Writing – original draft: Fengyan Xiao.
Writing – review & editing: Lina Wang.
Supplementary Material
Footnotes
Abbreviations: ALT = alanine transaminase, AST = aspartate aminotransferase, CI = confidence interval, CPK = creatinine phosphokinase, CRP = C-reactive protein, DM = diabetes mellitus, FBG = fasting blood glucose, FBI = fasting blood insulin, HbA1c = hemoglobin A1c, HDL-C = high-density lipoprotein-cholesterol, HOMA-IR = homeostatic model assessment for insulin resistance, IL = interleukin, LDL-C = low-density lipoprotein-cholesterol, MDA = malondialdehyde, OR = odds ratio, PRISMA = Preferred Reporting Items for Systematic Review and Meta-analysis, RCT = randomized controlled trial, SMD = standardized mean difference, TC = total cholesterol, TG = triglyceride, TNF = tumor necrosis factor.
How to cite this article: Xiao F, Gao F, Zhou S, Wang L. The therapeutic effects of silymarin for patients with glucose/lipid metabolic dysfunction: a meta-analysis. Medicine. 2020;99:40(e22249).
All data in this meta-analysis could be available in previous published studies.
Ethical approval was unnecessary because only the published studies were used.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Read SH, Kerssens JJ, Mcallister DA, et al. Trends in type 2 diabetes incidence and mortality in Scotland between 2004 and 2013. Diabetologia 2016;59:2106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Karr S. Epidemiology and management of hyperlipidemia. Am J Manag Care 2017;23: 9 suppl: S139–148. [PubMed] [Google Scholar]
- [3].Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271–81. [DOI] [PubMed] [Google Scholar]
- [4].Fan D, Li L, Li Z, et al. Effect of hyperlipidemia on the incidence of cardio-cerebrovascular events in patients with type 2 diabetes. Lipids Health Dis 2018;17:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang SL, Dong WB, Dong XL, et al. Comparison of twelve single-drug regimens for the treatment of type 2 diabetes mellitus. Oncotarget 2017;8:72700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pahan K. Lipid-lowering drugs. Cell Mol Life Sci 2006;63:1165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol 2016;11:157–66. [DOI] [PubMed] [Google Scholar]
- [8].Simic I, Reiner Z. Adverse effects of statins – myths and reality. Curr Pharm Des 2015;21:1220–6. [DOI] [PubMed] [Google Scholar]
- [9].Milic N, Milosevic N, Suvajdzic L, et al. New therapeutic potentials of milk thistle (Silybum marianum). Nat Prod Commun 2013;8:1801–10. [PubMed] [Google Scholar]
- [10].Sheela N, Jose MA, Sathyamurthy D, et al. Effect of silymarin on streptozotocin-nicotinamide-induced type 2 diabetic nephropathy in rats. Iran J Kidney Dis 2013;7:117–23. [PubMed] [Google Scholar]
- [11].Kheiripour N, Karimi J, Khodadadi I, et al. Silymarin prevents lipid accumulation in the liver of rats with type 2 diabetes via sirtuin1 and SREBP-1c. J Basic Clin Physiol Pharmacol 2018;29:301–8. [DOI] [PubMed] [Google Scholar]
- [12].Ebrahimpour-Koujan S, Gargari BP, Mobasseri M, et al. Lower glycemic indices and lipid profile among Type 2 diabetes mellitus patients who received novel dose of Silybum marianum (L.) Gaertn. (silymarin) extract supplement: a triple-blinded randomized controlled clinical trial. Phytomedicine 2018;44:39–44. [DOI] [PubMed] [Google Scholar]
- [13].Elgarf AT, Mahdy MM, Sabri NA. Effect of silymarin supplementation on glycemic control, lipid profile and insulin resistance in patients with type 2 diabetes mellitus. Int J Adv Res 2015;3:812–21. [Google Scholar]
- [14].Ghorbani A, Zarvandi M, Rakhshandeh H. A randomized controlled trial of a herbal compound for improving metabolic parameters in diabetic patients with uncontrolled dyslipidemia. Endocr Metab Immune Disord Drug Targets 2019;19:1075–82. [DOI] [PubMed] [Google Scholar]
- [15].Khalili N, Fereydoonzadeh R, Mohtashami R, et al. Silymarin, olibanum, and nettle, a mixed herbal formulation in the treatment of Type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. J Evid Based Complementary Altern Med 2017;22:603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hadi A, Pourmasoumi M, Mohammadi H, et al. The effects of silymarin supplementation on metabolic status and oxidative stress in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of clinical trials. Complement Ther Med 2018;41:311–9. [DOI] [PubMed] [Google Scholar]
- [17].Suksomboon N, Poolsup N, Boonkaew S, et al. Meta-analysis of the effect of herbal supplement on glycemic control in type 2 diabetes. J Ethnopharmacol 2011;137:1328–33. [DOI] [PubMed] [Google Scholar]
- [18].Luminita V, Ionut N, Raluca D, et al. Silymarin in Type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Res 2016;2016:5147468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mohammadi H, Hadi A, Arab A, et al. Effects of silymarin supplementation on blood lipids: a systematic review and meta-analysis of clinical trials. Phytother Res 2019;33:871–80. [DOI] [PubMed] [Google Scholar]
- [20].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [22].Derosa G, D’Angelo A, Romano D, et al. Effects of a combination of berberis aristata, silybum marianum and monacolin on lipid profile in subjects at low cardiovascular risk; a double-blind, randomized, placebo-controlled trial. Int J Mol Sci 2017;18:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Voroneanu L, Siriopol D, Dumea R, et al. Addition of silymarin to renin–angiotensin system blockers in normotensive patients with type 2 diabetes mellitus and proteinuria: a prospective randomized trial. Int Urol Nephrol 2017;49:2195–204. [DOI] [PubMed] [Google Scholar]
- [24].Ebrahimpour koujan S, Gargari BP, Mobasseri M, et al. Effects of Silybum marianum (L.) Gaertn. (silymarin) extract supplementation on antioxidant status and hs-CRP in patients with type 2 diabetes mellitus: a randomized, triple-blind, placebo-controlled clinical trial. Phytomedicine 2015;22:290–6. [DOI] [PubMed] [Google Scholar]
- [25].Fallahzadeh MK, Dormanesh B, Sagheb MM, et al. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in Type 2 diabetic patients with overt nephropathy: a randomized, double-blind, placebo-controlled trial. Am J Kidney Dis 2012;60:896–903. [DOI] [PubMed] [Google Scholar]
- [26].Hussain SA. Silymarin as an adjunct to glibenclamide therapy improves long-term and postprandial glycemic control and body mass index in Type 2 diabetes. J Med Food 2007;10:543–7. [DOI] [PubMed] [Google Scholar]
- [27].Huseini HF, Larijani B, Heshmat R, et al. The efficacy of Silybum marianum (L.) Gaertn. (Silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Phytother Res 2006;20:1036–9. [DOI] [PubMed] [Google Scholar]
- [28].Di Pierro F, Putignano P, Villanova N. Retrospective analysis of the effects of a highly standardized mixture of Berberis aristata, Silybum marianum, and monacolins K and KA in diabetic patients with dyslipidemia. Clin Pharmacol 2016;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Derosa G, Bonaventura A, Bianchi L, et al. Berberis aristata/Silybum marianum fixed combination on lipid profile and insulin secretion in dyslipidemic patients. Expert Opin Biol Ther 2013;13:1495–506. [DOI] [PubMed] [Google Scholar]
- [30].Derosa G, D’Angelo A, Maffioli P. The role of a fixed Berberis aristata/Silybum marianum combination in the treatment of type 1 diabetes mellitus. Clin Nutr 2016;35:1091–5. [DOI] [PubMed] [Google Scholar]
- [31].Di Pierro F, Putignano P, Montesi L, et al. Preliminary study about the possible glycemic clinical advantage in using a fixed combination of Berberis aristata and Silybum marianum standardized extracts versus only Berberis aristata in patients with type 2 diabetes. Clin Pharmacol 2013;5:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Derosa G, Romano D, D’Angelo A, et al. Berberis aristata/Silybum marianum fixed combination (Berberol(®)) effects on lipid profile in dyslipidemic patients intolerant to statins at high dosages: a randomized, placebo-controlled, clinical trial. Phytomedicine 2015;22:231–7. [DOI] [PubMed] [Google Scholar]
- [33].Derosa G, Romano D, D’Angelo A, et al. Berberis aristata combined with Silybum marianum on lipid profile in patients not tolerating statins at high doses. Atherosclerosis 2015;239:87–92. [DOI] [PubMed] [Google Scholar]
- [34].Lan J, Zhao Y, Dong F, et al. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol 2015;161:69–81. [DOI] [PubMed] [Google Scholar]
- [35].Phosat C, Panprathip P, Chumpathat N, et al. Elevated C-reactive protein, interleukin 6, tumor necrosis factor alpha and glycemic load associated with type 2 diabetes mellitus in rural Thais: a cross-sectional study. BMC Endocr Disord 2017;17:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lainampetch J, Panprathip P, Phosat C, et al. Association of tumor necrosis factor alpha, interleukin 6, and c-reactive protein with the risk of developing Type 2 diabetes: a retrospective cohort study of rural Thais. J Diabetes Res 2019;2019:9051929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shaheer AK, Tharayil JK, Krishna PW. A comparative study of high sensitivity C-reactive protein and metabolic variables in Type 2 diabetes mellitus with and without nephropathy. J Clin Diagn Res 2017;11:BC01–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hermans MP, Ahn SA, Rousseau MF. Increased CRP: an extended biomarker of microvascular risk in men with type 2 diabetes. J Diabetes Complications 2019;33:107413. [DOI] [PubMed] [Google Scholar]
- [39].Mzimela NC, Ngubane PS, Khathi A. The changes in immune cell concentration during the progression of pre-diabetes to type 2 diabetes in a high-fat high-carbohydrate diet-induced pre-diabetic rat model. Autoimmunity 2019;52:27–36. [DOI] [PubMed] [Google Scholar]
- [40].Fei L, Yong-Jun H, Zhang-Min M, et al. Rosiglitazone attenuates memory impairment in aged rat with diabetes by inhibiting NF-kappa B signal pathway activation. Exp Clin Endocrinol Diabetes 2015;123:536–42. [DOI] [PubMed] [Google Scholar]
- [41].Hussain SA, Numan IT, Alkhalifa II, et al. Anti-inflammatory activity of silymarin in patients with knee osteoarthritis. Saudi Med J 2009;30:98–103. [PubMed] [Google Scholar]
- [42].Kumar N, Rai A, Reddy ND, et al. Improved in vitro and in vivo hepatoprotective effects of liposomal silymarin in alcohol-induced hepatotoxicity in Wistar rats. Pharmacol Rep 2019;71:703–12. [DOI] [PubMed] [Google Scholar]
- [43].Abdel-Moneim AM, Al-Kahtani MA, El-Kersh MA, et al. Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against CCl4 induced rat liver damage. PLoS One 2015;10:e0144509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tian MY, Fan JH, Zhuang ZW, et al. Effects of silymarin on p65 NF-κB, p38 MAPK and CYP450 in LPS-induced hoof dermal inflammatory cells of dairy cows. BMC Vet Res 2019;15:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Soleimani V, Delghandi PS, Moallem SA, et al. Safety and toxicity of silymarin, the major constituent of milk thistle extract: an updated review. Phytother Res 2019;33:1627–38. [DOI] [PubMed] [Google Scholar]
- [46].Chen C, Yu Z, Li Y, et al. Effects of berberine in the gastrointestinal tract – a review of actions and therapeutic implications. Am J Chin Med 2014;42:1053–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


