Abstract
This study aimed to investigate the associations between the sonographic findings and duration of symptoms in children with pilomatricoma.
This study included 86 children with 95 lesions confirmed to be pilomatricoma after pathological examination. The associations between symptom duration and sonographic observations, including the presence or absence of peritumoral hyperechogenicity, calcification, and vascularity were investigated. The internal echogenicity of each pilomatricoma was scored using a 5-point scale based on echogenic spots and calcification with posterior acoustic shadowing. The Mann–Whitney U and Kruskal–Wallis tests were used for statistical analysis.
We found that the absence of peritumoral hyperechogenicity and severity of calcification were associated with increased symptom duration. Calcification, (present, 19.19 ± 18.99 months vs absent, 4.31 ± 3.24 months; P < .01) and peritumoral hyperechogenicity (present, 5.02 ± 5.80 months vs absent, 16.17 ± 18.24 months; P < .01), and grade of internal echogenicity (grade 0/1/2/3/4 = 3 months [1 patient]/4.33 ± 3.26 months [range, 1–12]/4.57 ± 3.46 months [range, 2–12]/10.89 ± 9.17 months [range, 3–28]/35.27 ± 19.16 months [range, 9–60], respectively; P = .01 and <.01) were associated with significant differences in symptom duration. There were no significant between-group differences in vascularity (6.01 ± 7.24 months; range, 1–48 vs 15.50 ± 19.12 months; range, 1–60; P = .08).
Pilomatricomas with a relatively short symptom duration were more likely to exhibit peritumoral hyperechogenicity and calcification with less severe posterior acoustic shadowing compared to lesions with a longer symptom duration. These sonographic findings provided useful information that facilitated the correct and rapid diagnosis of pilomatricoma.
Keywords: calcifying epithelioma, pilomatricoma, pilomatrixoma, sonography, ultrasound
1. Introduction
Pilomatricoma, also known as pilomatrixoma or pilomatricoma of Malherbe, is a benign tumor arising from hair matrix cells.[1,2] It is one of the most commonly occurring superficial mass lesions in children.[3,4] Pilomatricoma usually presents as a lesion that develops slowly over several months or years, but occasionally demonstrates rapid growth,[1,3,5,6,7] similar to other soft tissue malignancies such as fibrosarcoma or malignant transformation of pilomatricoma.[8,9,10,11,12] Surgical resection is the treatment of choice for pilomatricoma, similar to other malignant diseases that usually need rapid surgical intervention.[8,13] However, pilomatricoma is a benign tumor and commonly occurs in cosmetically sensitive areas; thus, the decision to perform surgical intervention should be made after careful consideration.[13,14] Therefore, it is important to differentiate pilomatricoma from malignant diseases such as fibrosarcoma or malignant transformation of pilomatricoma.[8,9,10,11,12]
Knowledge of the imaging characteristics of pilomatricoma is essential for accurate diagnosis. Recent studies have investigated the accuracy of ultrasonography, computed tomography, and magnetic resonance imaging for identifying pilomatricoma.[15,16,17] They found that pilomatricoma was associated with calcification around the edematous stroma, but substantial variations were evident among the cases reported.[15,16,17]
Ultrasonography is usually the first imaging modality used for evaluating pediatric patients with a superficial mass.[18,19] To the best of our knowledge, no study has focused on the association between sonographic findings and the duration of symptoms of pilomatricoma. This knowledge could be useful in ensuring correct and rapid diagnosis of pilomatricoma. The purpose of the present study was to investigate the associations between the sonographic characteristics of pilomatricoma and duration of symptoms in pediatric patients.
2. Materials and methods
2.1. Patients
The relevant ethics committee approved the study. The requirement for informed consent was waived owing to the retrospective nature of the study. The medical records of 86 children with 95 lesions that were confirmed to be pilomatricomas upon pathological examination, who underwent ultrasonographic examination between April 2012 and November 2019 were included in the study. One patient was excluded from the analysis due to inadequate medical data.
2.2. Diagnosis, size, location, and duration of symptoms of pilomatricoma
The diagnoses of pilomatricoma were confirmed by pathological examination. The lesion volume of the surgically resected tissue was estimated using the following ellipsoid formula, where a, b, and c are the orthogonal radii:
The location of the lesion (head, face, neck, trunk, upper extremity, and lower extremity) and symptom duration were ascertained from the medical records. The duration of symptoms was defined as the time-interval between the observation of the lesion by the patients or parents and acquisition of the sonographic scan.
2.3. Ultrasonography
All sonograms were obtained using 9 to 15 MHz linear transducers (LOGIQ E9, and E10; GE Healthcare, Waukesha, WI). Sonographic examinations were conducted by 4 pediatric radiologists with 20, 15, 10, and 7 years of clinical experience in performing ultrasonography for children.
2.4. Evaluation of sonography results
The following sonographic parameters were evaluated, based on previous reports describing the sonographic, computed tomography, and magnetic resonance appearance of pilomatricoma[7,15,16,17,20]:
-
(1)
presence or absence of peritumoral hyperechogenicity,
-
(2)
presence or absence of calcification with acoustic shadowing, and
-
(3)
presence or absence of vascularity on Doppler examination.
An echogenic spot with posterior acoustic shadowing was defined as a calcification on ultrasound.
The internal echogenicity within the pilomatricoma was assessed using a 5-point scale based on echogenic spots and calcification, where grade 0 = no echogenic spot (Fig. 1), grade 1 = tiny echogenic spot without posterior acoustic shadowing (Figs. 2 and 3), grade 2 = echogenic spot with posterior acoustic shadowing occupying <50% of the lesion's width (Fig. 4), grade 3 = echogenic spot with posterior acoustic shadowing occupying >50% of the lesion's width but not 100% (Fig. 5), and grade 4 = echogenic spot with posterior acoustic shadowing indicating complete calcification (Fig. 6). Calcification is easily detected by sonography in the presence of acoustic shadowing, but tiny echogenic spots in the pilomatricoma were reported to be small calcifications in the pathological specimens, and these small calcifications were detected as calcification without posterior acoustic shadowing by sonography.[8,20,21] Therefore, the internal echogenicity of the pilomatricomas was classified into 2 groups as follows: grades 0 and 1 represented the absence of calcification with acoustic shadowing (Figs. 1–3), and grades 2, 3, and 4 represented calcification with acoustic shadowing (Figs. 4–6). The degree of vascularity observed on Doppler examination was classified into 4 categories, where 0 = absent (Figs. 1 and 6), 1 = peripheral vascularity only, 2 = central only (Fig. 3), and 3 = peripheral and central vascularity (Figs. 2 and 5). Associations between the above-mentioned sonographic parameters and the duration of symptoms were evaluated.
Figure 1.

Sonography of a 70-mo-old boy with a pilomatricoma located on the neck. The duration of symptoms was 3 mo. The power Doppler image is seen on the right panel. Transverse sonography shows the absence of peritumoral hyperechogenicity and calcification with posterior acoustic shadowing. Internal echogenicity was classified as grade 0: no echogenic spot within the lesion. Vascularity was classified as type 0 (absent).
Figure 2.

Sonography of a 55-mo-old girl with a pilomatricoma located on the trunk. The duration of symptoms was 1 mo. The color Doppler image is seen on the middle panel. Scanning view of pathologic specimen is seen on the right panel. Transverse sonography shows the presence of peritumoral hyperechogenicity (arrows) and echogenic spots without posterior acoustic shadowing. Therefore, calcification with acoustic shadowing was deemed to be absent. Internal echogenicity was classified as grade 1: echogenic spot without posterior acoustic shadowing. Vascularity was present and was classified as type 3: peripheral and central. The pathological specimen shows transition from basaloid cells to shadow cells. Dense calcium deposition such as bony transformation is not seen.
Figure 3.

Sonography of a 62-mo-old girl with a pilomatricoma located on the face. The duration of symptoms was 3 mo. The color Doppler image can be seen on the right panel. Peritumoral hyperechogenicity is absent, while calcification without posterior acoustic shadowing can be observed on transverse sonography. Therefore, calcification with acoustic shadowing was deemed to be absent. Internal echogenicity was classified as grade 1: echogenic spot without posterior acoustic shadowing. Vascularity can be observed on sagittal sonography, which was classified as type 2: central.
Figure 4.
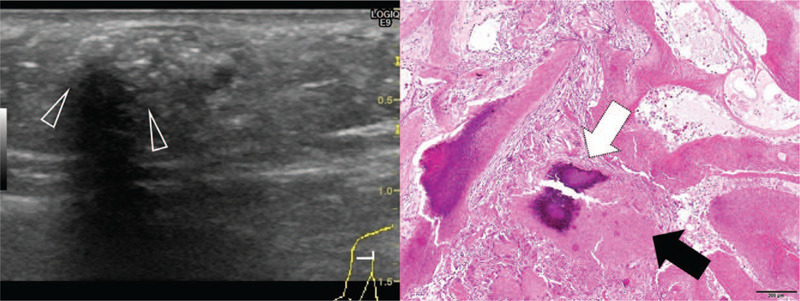
Sonography of a 132-mo-old girl with a pilomatricoma located on the upper extremity. Scanning view of pathologic specimen is seen on the right panel. The duration of symptoms was 4 mo shows the absence of peritumoral Hyperechogenicity is absent, while calcification with posterior acoustic shadowing (arrowheads) is observed on transverse sonography. Therefore, calcification with acoustic shadowing was deemed to be present. Internal echogenicity was classified as grade 2: echogenic spot with posterior acoustic shadowing occupying <50% of the lesion's width. The pathological specimen shows the transformation of most basaloid cells into shadow cells. Dense calcium deposition is observed.
Figure 5.

Sonography of a 52-mo-old girl with a pilomatricoma located on the trunk. The duration of symptoms was 6 mo. The color Doppler image is seen on the right panel. Transverse sonography shows the presence of both peritumoral hyperechogenicity (arrows) and calcification with posterior acoustic shadowing (arrowheads). Therefore, calcification with acoustic shadowing was deemed to be present. Internal echogenicity was classified as grade 2: echogenic spot with posterior acoustic shadowing occupying >50% of the lesion's width. Vascularity was classified as type 3: peripheral and central.
Figure 6.

Sonography of a 138-mo-old girl with pilomatricoma located on the head. The duration of symptoms was 60 mo. The power Doppler image is seen on the middle panel. Scanning view of pathologic specimen is seen on the right panel. Sagittal sonography reveals the absence of peritumoral hyperechogenicity and presence of calcification with posterior acoustic shadowing (arrowheads). Therefore, calcification with acoustic shadowing was deemed to be present. Internal echogenicity was classified as grade 4: echogenic spot with posterior acoustic shadowing extending across the complete width of the lesion. Vascularity was classified as type 0: absent. Few basaloid cells could be detected on the pathological specimen. Ossification is observed with the formation of typical trabecular bony spicules.
2.5. Review process
Two radiologists with 15 and 10 years of clinical experience reviewed all images on a 1600 × 1200 Picture Archiving and Communication System monitor (GE Healthcare). Any disagreements were resolved through discussion. Radiologists were blinded to the medical data or other imaging findings during the review process.
2.6. Statistical analysis
Continuous variables were expressed as the mean ± SD (range) and categorical variables were expressed as frequencies and percentages. Associations between the duration of symptoms, patient characteristics, and lesion volume were evaluated using Pearson correlation coefficient, the Mann–Whitney U test, and Kruskal–Wallis test, depending on the type of variable (continuous or categorical). The patient's age and volume of the pilomatricoma were continuous variables. Sex, lesion location, calcification, peritumoral hyperechogenicity, and vascularity (determined via Doppler examination) were categorical variables. The significance level adopted for all tests was 5% (2-sided). All data were analyzed using the commercially available SPSS statistical analysis software, version 24 (IBM, Armonk, NY).
3. Results
3.1. Participant characteristics
The participants’ mean age was 83.0 ± 47.0 months (range 6–231 months), and the mean duration of symptoms for all the lesions was 8.54 ± 12.36 months (range 1–60 months). Half of the lesions were located on the head. The patients’ characteristics are shown in Table 1.
Table 1.
Patient characteristics.

3.2. Associations between symptom duration and categorical or continuous variables
The results of the analyses for the associations between symptom duration and categorical variables are shown in Table 2. The absence of peritumoral hyperechogenicity and severe calcification were associated with increased symptom duration. There was no significant difference between symptom duration and sex (P = .27). No significant association was observed between symptom duration and age (r = 0.11, P = .29) and lesion location (P = .13). A weak association was observed between lesion volume and symptom duration (r = 0.22, P = .04).
Table 2.
Univariate analysis for the associations between duration of symptoms and categorical variables.

4. Discussion
Pilomatricomas with a relatively short history had a greater tendency to exhibit peritumoral hyperechogenicity and calcification with little posterior acoustic shadowing than those with a relatively long history. These sonographic findings are useful for the differentiation of pilomatricomas from other malignant diseases, which usually progress rapidly and require early surgical resection.
Earlier studies have suggest that peritumoral hyperechogenicity is caused by the surrounding edematous stroma,[16,17] and is associated with inflammation.[22,23,24,25,26] Some patients in the present study had a relatively longer symptom duration but exhibited peritumoral hyperechogenicity on sonography. Pilomatricomas are sometimes accompanied by chronic inflammation with foreign body reaction and proliferation of multinucleated giant cells and lymphocytes.[16,27] Therefore, inflammation may contribute to the variation in peritumoral echogenicity in pilomatricomas, which are usually located in subcutaneous tissue.
The degree of calcium deposition in pilomatricomas shows some variation.[16] Histologically, pilomatricomas consist of centrally located basophilic cells and shadow cells.[28] Basaloid cells are transformed into shadow cells. Chronic inflammation with foreign body reaction can be seen around the shadow cells and stroma, which may result in calcium deposition.[5,7,20,29] Therefore, calcium deposition may occur in patients with a long symptom duration. In this study, dense calcium deposition of greater severity was observed in the patient described in Figure 6 than those described in Figures 2 and 4, and the patient described in Figure 6 had a longer symptom duration than that of the other patients. Although there was no significant difference in symptom duration in lesions with or without vascularity, lesions with longer symptom duration tended to have decreased vascularity. Vascularity could not be detected in case of calcification with posterior acoustic shadowing or small lesions, which might have influenced this result.[7]
This study had some limitations. First, it included a small sample size and used only univariate analysis for the statistical analysis of the sonographic findings. Thus, additional studies with a larger population that include multivariate analysis of the sonographic findings are needed to confirm these preliminary results. Second, the quality of sonography depended on the child's cooperation and the sonographer's skill. Third, the duration of symptoms was based on the patients’ or parents’ observations. The lesion may have been present for some time before being noticed by the patients or parents. Thus, it is difficult to accurately define the time of onset of small subcutaneous lesions such as pilomatricomas. Fourth, we did not include malignant transformation of pilomatricoma in the present study. Further studies are needed to evaluate the differences in sonographic findings between various cutaneous lesions including malignant transformation of pilomatricoma, in order to confirm the utility of these sonographic findings.
5. Conclusion
Sonographic characteristics such as peritumoral hyperechogenicity and calcification with posterior acoustic shadowing were associated with symptom duration in the present study. The sonographic findings of pilomatricoma showed variations, depending on symptom duration. These sonographic findings were useful in establishing an accurate and rapid diagnosis of this disease.
Author contributions
Conceptualization: Takahiro Hosokawa, Saki Shibuki, Yutaka Tanami, Eiji Oguma.
Data curation: Takahiro Hosokawa, Saki Shibuki, Yutaka Tanami, Yumiko Sato, Eiji Oguma.
Formal analysis: Takahiro Hosokawa, Yutaka Tanami.
Methodology: Takahiro Hosokawa, Yutaka Tanami.
Writing – review and editing: Takahiro Hosokawa, Yumiko Sato, Eiji Oguma.
Footnotes
Abbreviation: SD = standard deviation.
How to cite this article: Hosokawa T, Shibuki S, Tanami Y, Sato Y, Oguma E. Sonographic characteristics of pilomatricomas and their association with symptom duration. Medicine. 2020;99:40(e22550).
This research was performed in accordance with the tenets of the Declaration of Helsinki.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Takahiro Hosokawa, Saki Shibuki, Yutaka Tanami, Yumiko Sato, and Eiji Oguma declare that they have no financial or personal relationships that could lead to a conflict of interest.
References
- [1].Kaddu S, Soyer HP, Cerroni L, et al. Clinical and histopathologic spectrum of pilomatricomas in adults. Int J Dermatol 1994;33:705–8. [DOI] [PubMed] [Google Scholar]
- [2].Forbis R, Jr, Helwig EB. Pilomatrixoma (calcifying epithelioma). Arch Dermatol 1961;83:606–18. [DOI] [PubMed] [Google Scholar]
- [3].Moehlenbeck FW. Pilomatrixoma (calcifying epithelioma). A statistical study. Arch Dermatol 1973;108:532–4. [PubMed] [Google Scholar]
- [4].Knight PJ, Reiner CB. Superficial lumps in children: what, when, and why? Pediatrics 1983;72:147–53. [PubMed] [Google Scholar]
- [5].Pirouzmanesh A, Reinisch JF, Gonzalez-Gomez I, et al. Pilomatrixoma: a review of 346 cases. Plast Reconstruct Surg 2003;112:1784–9. [DOI] [PubMed] [Google Scholar]
- [6].Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol 2018;40:631–41. [DOI] [PubMed] [Google Scholar]
- [7].Lin SF, Xu SH, Xie ZL. Calcifying epithelioma of malherbe (Pilomatrixoma): clinical and sonographic features. J Clin Ultrasound 2018;46:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kwee RM, Kwee TC. Calcified or ossified benign soft tissue lesions that may simulate malignancy. Skeletal Radiol 2019;48:1875–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Burdeny DA, Reed MH, Ferguson CA. Calcification of axillary lymph nodes following BCG vaccination. Can Assoc Radiol J 1989;40:92–3. [PubMed] [Google Scholar]
- [10].Kang HY, Kang WH. Guess what! Perforating pilomatricoma resembling keratoacanthoma. Eur J Dermatol 2000;10:63–4. [PubMed] [Google Scholar]
- [11].Mikhaeel NG, Spittle MF. Malignant pilomatrixoma with multiple local recurrences and distant metastases: a case report and review of the literature. Clin Oncol (R Coll Radiol) 2001;13:386–9. [DOI] [PubMed] [Google Scholar]
- [12].Otero MN, Trujillo CP, Parra-Medina R, et al. Metastatic malignant pilomatrixoma in an 8-year-old girl misdiagnosed as a recurrent pilomatrixoma. Am J Dermatopathol 2017;39:e41–3. [DOI] [PubMed] [Google Scholar]
- [13].Hassan SF, Stephens E, Fallon SC, et al. Characterizing pilomatricomas in children: a single institution experience. J Pediatr Surg 2013;48:1551–6. [DOI] [PubMed] [Google Scholar]
- [14].O’Connor N, Patel M, Umar T, et al. Head and neck pilomatricoma: an analysis of 201 cases. Br J Oral Maxillofac Surg 2011;49:354–8. [DOI] [PubMed] [Google Scholar]
- [15].Yi KM, Chen K, Wang L, et al. Pilomatricoma (calcifying epithelioma): MDCT and MR imaging findings in 31 patients with radiological-pathological correlation. Eur J Radiol 2018;106:92–9. [DOI] [PubMed] [Google Scholar]
- [16].Lim HW, Im SA, Lim GY, et al. Pilomatricomas in children: imaging characteristics with pathologic correlation. Pediatr Radiol 2007;37:549–55. [DOI] [PubMed] [Google Scholar]
- [17].Bulman JC, Ulualp SO, Rajaram V, et al. Pilomatricoma of childhood: a common pathologic diagnosis yet a rare radiologic one. AJR Am J Roentgenol 2016;206:182–8. [DOI] [PubMed] [Google Scholar]
- [18].Pirri C, Stecco C, Fede C, et al. Ultrasound imaging of the fascial layers: you see (only) what you know. J Ultrasound Med 2020;39:827–8. [DOI] [PubMed] [Google Scholar]
- [19].Hosokawa T, Takahashi H, Miyasaka Y, et al. Ultrasound evaluation of dermal sinuses/fistulas in pediatric patients. J Ultrasound Med 2019;38:3107–22. [DOI] [PubMed] [Google Scholar]
- [20].Hwang JY, Lee SW, Lee SM. The common ultrasonographic features of pilomatricoma. J Ultrasound Med 2005;24:1397–402. [DOI] [PubMed] [Google Scholar]
- [21].Choo HJ, Lee SJ, Lee YH, et al. Pilomatricomas: the diagnostic value of ultrasound. Skeletal Radiol 2010;39:243–50. [DOI] [PubMed] [Google Scholar]
- [22].Hosokawa T, Yamada Y, Tanami Y, et al. Associations between sonographic findings and operative time of transumbilical laparoscopic-assisted appendectomy for acute appendicitis in children. AJR Am J Roentgenol 2019;213:191–9. [DOI] [PubMed] [Google Scholar]
- [23].Lee MW, Kim YJ, Jeon HJ, et al. Sonography of acute right lower quadrant pain: importance of increased intraabdominal fat echo. AJR Am J Roentgenol 2009;192:174–9. [DOI] [PubMed] [Google Scholar]
- [24].Hosokawa T, Suzuki S, Tanami Y, et al. Ultrasound evaluation of complications after cardiovascular surgery in pediatric patients: a case series. Med Ultrason 2020;22:108–13. [DOI] [PubMed] [Google Scholar]
- [25].Hosokawa T, Tanami Y, Sato Y, et al. Comparison of sonographic findings between pediatric patients with mediastinitis and without mediastinitis after cardiovascular surgery. J Med Ultrason 2020;Online ahead of print; DOI: 10.1007/s10396-020-01029-3. [DOI] [PubMed] [Google Scholar]
- [26].Hosokawa T, Tanami Y, Sato Y, et al. Comparison of imaging findings between acute focal bacterial nephritis (acute lobar nephronia) and acute pyelonephritis: a preliminary evaluation of the sufficiency of ultrasound for the diagnosis of acute focal bacterial nephritis. Emerg Radiol 2020;27:405–12. [DOI] [PubMed] [Google Scholar]
- [27].Fujioka M, Gozo N, Osamu M, et al. Secondary anetoderma overlying pilomatrixomas. Dermatology (Basel, Switzerland) 2003;207:316–8. [DOI] [PubMed] [Google Scholar]
- [28].Lan MY, Lan MC, Ho CY, et al. Pilomatricoma of the head and neck: a retrospective review of 179 cases. Arch Otolaryngol Head Neck Surg 2003;129:1327–30. [DOI] [PubMed] [Google Scholar]
- [29].Yamaguchi S, Inui M, Takeoka T, et al. A case of old calcifying epithelioma processed Symptomless over 40 Years. Case Rep Dent 2013;2013:572372. [DOI] [PMC free article] [PubMed] [Google Scholar]


