Abstract
Children with end stage renal disease (ESRD) are liable to various health disorders that possibly impair their quality of life (QoL). Low dietary intake of Omega-3 fatty acids also called marine n-3 fatty acid (n-3 FA) may be associated with health problems which are among the leading causes of impaired QoL.
The objective of this study was to assess the effect of omega-3 Fatty acid (n-3 FA) supplements on quality of life among children on dialysis and to evaluate its use regarding adequacy of dialysis and inflammatory markers.
A prospective cohort study was conducted on 31 hemodialysis children. Quality of life was measured for patients and an equal number of matched controls using the PedsQL Inventory where the higher the score the poorer is the quality of life. n-3FA supplementation had been given to the patients for 3 months to study its effects on QoL. Laboratory investigations like hemoglobin, lipid profile, inflammatory markers, and tests for adequacy of dialysis had been carried out.
Patients had significantly higher QoL scores (42.22 ± 13.31) than controls (22.70 ± 1.31) (P < .001). Young ages showed higher score of physical functioning (18.23 ± 4.22) than older ones (13.92 ± 6.84) (P = .049). Females had significantly higher total QoL score (25.53 ± 6.61) than males (20.06 ± 7.09) (P = .010). The total QoL score was significantly lower post than pre administration of n-3FA (35.41 ± 10.36 vs 42.22 ± 13.31) (P < .001). Triglycerides and CRP were significantly lower post than pre n-3FA supplementation (160.64 ± 32.55 vs 169.35 ± 31.82) (P < .001) and (10.29 ± 4.39 vs 11.19 ± 4.83) (P = .006) respectively. Means of Kt/V and urea reduction ratio (URR) were significantly higher post (1.37 ± 0.09, 70.0 ± 5.99 respectively) than pre n-3FA (1.31 ± 0.07 and 65.25 ± 6.06 respectively) (P = .005, .001 respectively).
Quality of life and adequacy of dialysis get improved after n-3FA supplementation among children on dialysis which encourages its testing for more patients to evaluate its long term effects and support its routine use.
Keywords: dialysis, end stage renal disease, inflammatory markers, lipid profiles, omega 3 fatty acids, quality of life
1. Introduction
End stage renal disease (ESRD) changes the children patients entire life with the consequent exposure to maladjustment and psychological stress, school underperformance and peers social struggles They must follow dietary control and a lifelong dialysis to live Furthermore, dialysis treatment schedules are burdensome and interfere with school attendance and participation in peer-related activities, compromising opportunities for attaining academic and psychosocial potential.[1–3] Patients have a long-term survival because of advanced technology and medical care and this stimulate the need to measure how much the degree of improvement in survival can be reflected on the quality of life (QoL). Omega-3 fatty acids, also called marine n-3 fatty acid (n-3 FA), are a fatty acids family containing two or more double bonds. n-3 FA are mainly found in diet (i.e., they are “essential” fatty acids). Linolenic acid, the parent n-3 FA, is derived mostly from vegetable seed oils.[4] Enzymatic conversion of linolenic acid to longer chain fatty acids is limited, so individuals are likely to depend on dietary consumption like Coldwater fish to maintain optimal levels of Eicosapentaenoic Acid and Docosahexaenoic Acid levels that are formed in vivo from linolenic acid.[5,6] Generally, n-3 FA have shown promising results in changing disease processes involving the inflammatory and immune pathways. Although there is wide range of clinical applications, Lacking of general familiarity with the biochemical and clinical effects of n-3 FA therapy, is the reason behind no routine application of n-3 FA supplementation in the dialysis patients. But 2005 National Kidney Foundation Kidney Disease Outcomes Quality Initiative Clinical Practice Guidelines for Cardiovascular Disease in patients Dialysis Patients recommend further investigation in this area.[7] Also guidelines, nationally and internationally, have gathered on and recommended regular consumption of at least 250 mg/day of long-chain n-3 fatty acids or at least 2 servings/week of oily fish for the general population.[8] Manson et al[9] said that n 3 fatty acids supplementation did not lower the major cardiovascular events or cancer incidence in comparison to placebo while AlAmmar et al[10] reported that n-3 FA supplementations have beneficial effects on reducing the inflammatory markers, relapse rate, and getting better QoL for Multiple sclerosis patients. Children with ESRD are liable to various health and metabolic disorders that possibly impaired their QoL. Improving QoL gets great attention as an important outcome in research. Low dietary intake and a low status of n-3 FA may be linked to health problems which in turn impair QoL. Studies concerning QoL or Effect of n-3 FA supplements among ESRD children patients are few.[11] The aim of this study was to: -1. Measure the quality of life among children on dialysis and in comparison, to matched controls (Not on dialysis), 2. Study the effect of n-3 FA administration on QoL domains among studied patient group, (Primary outcome) and 3. Study the effect of n-3 FA administration on renal functions, lipid profile, and inflammatory markers among studied patient group (Secondary outcome).
2. Methods
Thirty-nine patients with ESRD were enrolled, 31 patients (17 males and 14 females, aged from 8–14 years) fit the inclusion criteria. Eight patients were excluded (6 of them due to their age as over 18 years and another 2 due to non-compliance). Estimated glomerular filtration rate (eGFR) was determined using the bedside Schwartz equation (0.413 × height]/serum creatinine concentration.[12] A control group with equal number matched for age, sex, residence, residence and socio-economic class had been taken from the families of the studied patients and the pediatrics outpatient clinics with paying attention to all mental, social and physical factors that may affect QoL.
2.1. Inclusion criteria
Children with ESRD with a glomerular filtration rate of < 10 ml/min/1.73 m2, Age < 18 years, and on regular hemodialysis (catheter) at least 3 sessions per week for at least 3 months before the study.
2.2. Exclusion criteria
Pre-dialysis stages of chronic kidney disease (CKD), patients on chronic peritoneal dialysis, regular hemodialysis < 3 months, and primary (non-uremic) cardiovascular disease, acute infection, hospitalization, febrile illness and non-compliance had been excluded from the study.
2.3. Data collection tools
The Pediatric quality of life Measurement model Inventory (PedsQL) is a modular approach used to measure health-related quality of life (HRQOL) in healthy or diseased children and adolescents. Twenty three-item PedsQL Scales were designed to measure the core dimensions of health as delineated by the World Health Organization and included physical functioning (8 items), emotional functioning (5 items), social functioning (5 items) and school functioning (5 items). The questionnaire involved questions about how much of each problem has been presented during the past month, using five-point Likert scale from zero (never) to four (almost always), where a score of zero if it is never a problem, one if it is almost never a problem, two if it is sometimes a problem, three if it is often a problem, or four if it is almost always a problem. This form includes parent and child reports. The questionnaire was filled by caregivers for those aged 8 years old and those aged >8 were able to respond well to all questions themselves to generalize the difference in parent-proxy and child-self reports among both studied groups and also before and after n-3FA supplementation. The questionnaire was filled in twice for the patients before and after supplementation of n-3FA, while it was filled in just once for the control group.
2.4. Intervention: Each n-3FA capsule contains
Fish Oil 1000 mg (Contains Eicosapentaenoic acid minimum (EPA) 13% & Docosahexaenoic acid minimum (DHA) 9%), and wheat germ oil (Linoleic Acid 52%–59%) (Omega-3 Plus; SEDICO, Giza, Egypt). Every patient enrolled in the study were given 2 n-3FA capsule daily for 3 months with regular follow up and recording of compliance to medications taken.[13,14] Side effects of n-3FA like a fishy taste in your mouth, fishy breath, stomach upset, Loose stools, nausea are informed to the patients. Risk of bleeding was guarded by taking 2 capsules/ day (Bleeding occur on taking more than 3 grams of fish oil daily).
2.5. Laboratory investigations
Laboratory investigations before and after n-3FA supplementation were carried out and included:
-
a)
Serum calcium and phosphorous, parathyroid hormone level (PTH).
-
b)
Hemoglobin, serum iron, ferritin
-
c)
Lipid profile including: - Serum triglycerides (TG), total cholesterol (TC), high density lipoprotein (HDL) and low density lipoprotein (LDL.
-
d)
Inflammatory factors including: - High sensitivity C - reactive protein, Tumor necrosis factor alpha (TNFα), Interlukin-6 (IL-6)
-
e)
Blood urea nitrogen
-
f)
Assessing the adequacy of dialysis using a single pool Kt/V formula: Kt/V is a number used to quantify hemodialysis and peritoneal dialysis treatment adequacy.(K – dialyzer clearance of urea, t – dialysis time, V – volume of distribution of urea, approximately equal to a patient total body water)
-
g)
The URR or Urea reduction ratio. This involves a direct comparison of pre- and post- dialytic urea concentrations and shows the percentage reduction of the urea concentration during dialysis treatment.
PedsQL Inventory Measurement Model was measured twice by the child before starting and after n-3FA supplementation course finishing. The higher the score to be a way from zero the poorer will be the quality of life. Also lab investigations were assessed before starting and after n-3FA supplementation course finishing.
2.6. Measurements
After completion of the dialysis session, blood pressure levels were taken. Height was measured in cm and BMI was calculated as weight (kg)/height (m2). Stunting and wasting were considered if Z scores below −2 SD For height and BMI respectively.
2.7. Ethical approval
An approval from local Ethics Committee in faculty of medicine was obtained. We received also written informed consent from the patient caregivers whether parent or primary guardian. The trial has been registered in International Clinical Trials Registry Platform (ICTRP), Pan African Clinical Trial Registration with identification number for the registry PACTR202001481782473/18-Jan-2020.
2.8. Statistical analysis
Results were statistically analyzed by SPSS version 20(SPSS Inc., Chicago, IL). Student's t-test and Paired t test were used for normally distributed quantitative variables. Mann-Whitney and Wilcoxon signed rank test were for not normally distributed quantitative variables. Spearman correlation was used to assess direction and strength of association. P value < .05 would have been significant.
3. Results
Table 1 shows that the study was carried out on 31 patients and 31 controls. The age ranged from 8 to 14 years in both groups. Male children represented a higher percentage in both groups in comparison with females. No significant difference detected between the studied groups regarding baseline characteristics (P > .05). Mean height was significantly higher among controls than patients while mean BMI was the reverse (P < .001 and 0.028 respectively). Mean arterial pressure (MAP) was significantly higher among patients than controls (P = .006). Antihypertensive drugs were given to seven patients. Monotherapy (calcium channel blockers or Angiotensin converting enzyme inhibitors) was given to four patients while combined therapy (calcium channel blockers with Beta blockers or calcium channel blockers with Angiotensin converting enzyme inhibitors) was given to three patients.
Table 1.
Characteristics of the studied groups.
Figure 1 shows that patients had total poor QoL scores (42.22 ± 13.31, R = 21–65) in comparison to controls (22.70 ± 1.31, R = 7–33) (P < .001). It is distributed as physical functioning (16.29 ± 5.87 (Range: 5–25) vs 9.09 ± 3.92 (R = 2–15) P < .001), emotional functioning (5.80 ± 3.85 (R = 0–13) vs 3.16 ± 1.52 (R = 1–7), P < .001), social functioning (8.35 ± 3.64 (R = 1–14) vs 4.61 ± 2.44,(0–10) P < .001) and school functioning (11.77 ± 3.97 (R = 5–18) vs 5.83 ± 2.93, (R = 0–11) (P < .001)
Figure 1.
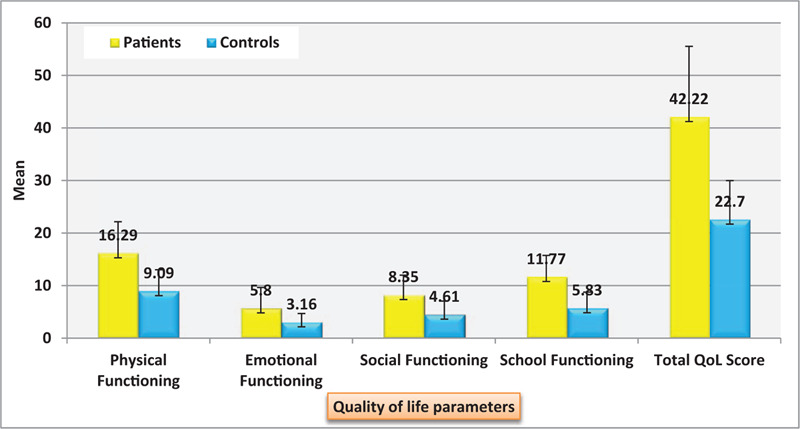
Distribution of the studied patients’ characteristics regarding pediatric quality of life inventory (PedsQL): Physical functioning, emotional functioning, social functioning and school functioning.
Table 2: Children aged < 12 years old showed higher score of physical functioning (18.23 ± 4.22) than ≥ those aged 12 years old (13.92 ± 6.84) (P = .049). Females had poorer total QoL score (25.53 ± 6.61) than males (20.06 ± 7.09) (P < .010), distributed as physical functioning (19.66 ± 4.18 vs 11.61 ± 4.55 (R = 2–15) P < .001), emotional functioning (7.61 ± 3.75 vs 3.30 ± 2.31, P = .002) and social functioning (10.27 ± 2.16 vs 5.69 ± 3.66, P = .001). Regarding duration of dialysis, children with ≤3 years duration of dialysis shows lower QoL than >3 years where physical functioning (20.17 ± 3.69 vs 11.57 ± 4.38, P < .001), emotional functioning (7.76 ± 3.75 vs 3.42 ± 2.40, P = .001) and social functioning (10.70 ± 1.68 vs 5.50 ± 3.34, P < .001).
Table 2.
Distribution of the studied patients’ characteristics regarding pediatric quality of life inventory (PedsQL): Physical functioning, emotional functioning, social functioning and school functioning.
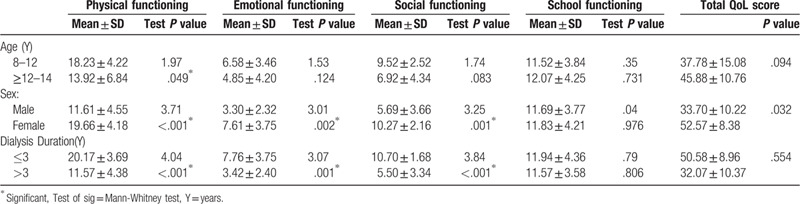
Figure 2 The total score was significantly lower after administration of n-3 FA (35.41 ± 10.36) than before (42.22 ± 13.31) (P < .001). It is distributed as physical functioning (16.29 ± 5.87 (Range: 5–25) vs 13.03 ± 5.10 (R = 3–22) P < .001), emotional functioning (5.80 ± 3.85 (R = 0–13) vs 5.09 ± 3.12 (R = 0–11), P = .025), and social functioning (8.35 ± 3.64 (R = 1–14) vs 7.45 ± 3.16,(0–13) P = .004) and school functioning (11.77 ± 3.97 (R = 5–18) vs 9.83 ± 3.40, (R = 5–16) P = .010)
Figure 2.
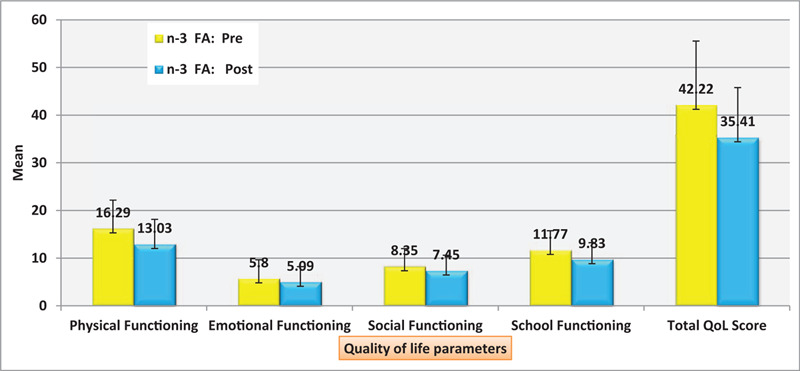
Distribution of the studied patients’ quality of life measurement model (PedsQL) regarding omega 3 pre and post intervention.
Figure 3 shows significant negative correlation between quality of life score and adequacy of dialysis (P < .05) (N.B: The higher the score to be a way from zero the poorer will be the quality of life).
Figure 3.
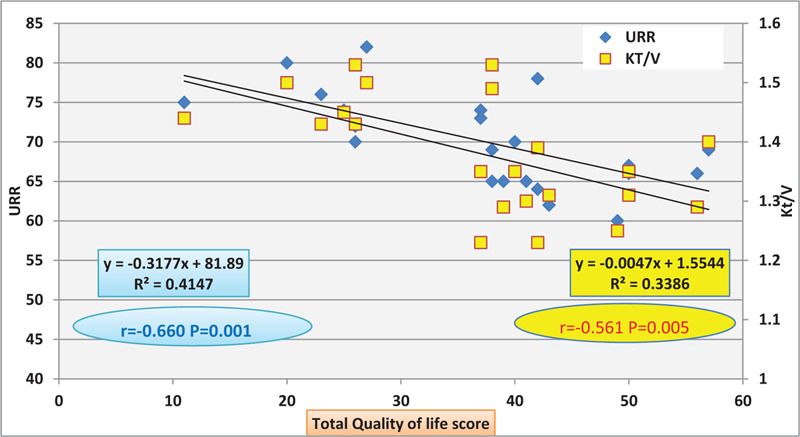
Negative Correlation between adequacy of dialysis represented in (Urea reduction ratio (URR) and Kt/V) and Total quality of life score.
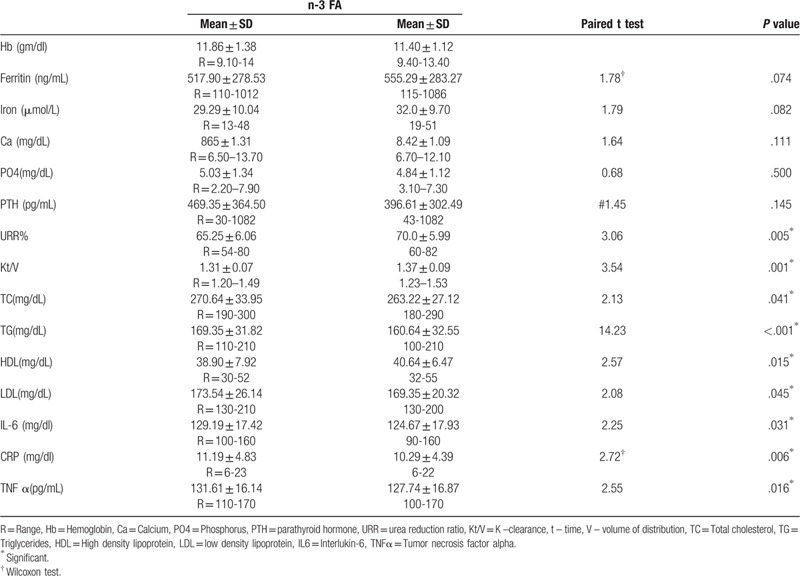
Table 3 shows the difference in lab investigations pre and post n-3FA supplementation, where TG (mg/dl) was significantly lower post (169.35 ± 31.82) than pre n-3FA (160.64 ± 32.55) (P < .001) also CRP (mg/dl) was significantly lower post (10.29 ± 4.39) than pre (11.19 ± 4.83) n-3 FA supplementation (P = .006). Inflammatory markers like Il6 (mg/dl) and TNF α (pg/mL) were significantly lower post (129.19 ± 17.42, 131.61 ± 16.14 respectively) than pre n-3 FA supplementation (124.67 ± 17.93, 127.74 ± 16.87) (P = .031 and 0.016 respectively) Kt/V and URR (adequacy of dialysis) were significantly higher post (1.37 ± 0.09, 70.0 ± 5.99 respectively) than pre n-3 FA (1.31 ± 0.07) and 65.25 ± 6.06 respectively) (P = .005, .001 respectively).
Table 3.
Distribution of the studied patients’ lab investigations regarding omega 3 (n-3 FA) pre and post intervention.
4. Discussion
Quality of life among ESRD population can be adversely affected by complications and comorbid conditions they are vulnerable to.[14] This study was carried out on two groups of thirty one children for each group, ESRD on hemodialysis for more than three months and controls. There was significant difference between patients and controls regarding their height and BMI. On Z score, 74.2% of patients had height below −2 SD (stunted), while 25.8% of them had BMI below −2 SD (wasted). This agrees with Lotfy et al[15] and Smith et al[16] Pediatric quality of life Measurement Model inventory (PedsQL) consisted of four items were evaluated at the beginning of study. In this study, The higher the score to be a way from zero the poorer will be the quality of life. Patients had higher scores in all domains of Health related quality of life (HRQOL) pediatric questionnaire, which was significantly higher than controls. Many studies support this finding (Arlene et al,[17] Goldstein et al,[18] Dotis et al,[19] and El Shafei et al,[20]). Kidney Disease Quality of Life Short Form (KDQOL-SF) scores was assessed also in this study before and after n-3 FA administration, and there was an improvement in quality of life domains. Moeinzadeh et al,[21] detected a significant improvement in the quality of life in the n-3 FA group without parallel increase in the controls. The current results revealed that < 12years old showed poor quality of life in comparison with the ≥ 12 specially regarding with the health activities and females had poorer quality of life in comparison with males where they scored higher regarding physical, emotional and social functioning. This agrees with Tjaden et al.[22] Taheri et al[23] and Baghayi et al[24] suggested that older hemodialysis patients had poor quality of life. However, Rafii et al[25] found that duration of dialysis played a role where the shorter duration of dialysis; the poorer will be the quality of life. In contrast, Barzegar,[26] found there was no significant relationship between the quality of life in patients and duration of hemodialysis but lower quality of life was reported in female patients compared to male patients; however, this difference was not statistically significant. There was no significant difference in the parathyroid hormone, calcium or phosphorus levels before and after n-3 FA supplementations. On the other hand, Allawi et al[27] found an insignificant difference in parathyroid hormone and phosphate level and significant increase in calcium level in patients treated by n-3 FA. It is strongly recommended monitoring serum levels of calcium, phosphorus, PTH, and alkaline phosphatase to begin at CKD stage III, and in children at CKD stage II. Serum calcium levels are usually 8.5 to 10.5 mg/dL (2.1–2.6 mmol/L).[28] Mean phosphorus level in this study is 5.03 (mild elevation than normal; 2.5 to 4.5 mg/dl), with no significant difference in the level before and after n-3 FA supplementations. Secondary hyperparathyroidism with resultant hypocalcemia, hyperphosphatemia, and increased Ca × Po4 product can result in children with CKD due to a marked decrease in glomerular filtration rate.[29] No significant changes were observed in HB level, serum ferritin, and iron before and after n-3 FA supplementation. This agrees with Gharekhani et al[30] Among patients with CKD, there is a relative deficiency in erythropoietin production, and this is the main reason for anemia to develop in addition to iron deficiency, blood loss, inflammation, hemolysis, and nutritional deficits. Patients on hemo-dialysis experience routine iron loss (approximately 1000 mg of iron per year) due to the dialysis treatment, frequent blood laboratory testing, surgical procedures, accidental (vascular access) and gastrointestinal blood loss.[31] Less consumption of fish or fish-derived products in this patient population causes significantly lower concentration of n-3 FA fatty acids in erythrocyte membrane phospholipids compared with the healthy controls.[6,28] In this study serum triglyceride concentration has been reduced after n-3 FA supplementation, this finding agrees with A. Kooshki et al,[32] as supraphysiologic n-3 FA doses (3 g/d) in humans can reduce triglyceride levels by 25% to 30%.[21] Significant reduction in inflammatory markers was detected in this study in relations to n-3 FA supplementations (IL-6, CRP, and TNF α). This is in agreement with Gharekhani et al,[28] Perunicic-Pekovic et al,[33] Saifullah et al,[34] and Bowden et al[35] who found significant reductions in inflammatory markers following daily supplement of n-3 FA which is suspected to produce an anti-inflammatory effect thus minimizes the platelet aggregation, chemotaxis, and cytokines productions.[6] On the other hand, Deike,[36] and Pluta et al,[37] detected no significant associations between n-3FA supplements and reduction in inflammatory markers. Similarly, no beneficial effects of fatty acids on the levels of serum CRP and IL-6 were shown in patients undergoing peritoneal dialysis and hemodialysis who received 8-week supplementation with n-3FA at a dose of 3 g/day.[38,39] Adequacy of dialysis represented by URR and Kt/V showed improvement before and after ω-3FA administration and this may be attributed to anti-inflammatory effects of n-3FA. The adequacy of dialysis is reflected on quality of life which by its turn showed improvement.
4.1. Strengths and limitations
The study assessed important parameters like quality of life and lab investigations and how improvement happened. This prospective cohort study could be considered also a pilot non randomized clinical trial study as it was conducted on just 31 patients in single center, and it allowed us to provide good observation to any change whether positive or negative, opening the doors to more large samples to work on.
5. Conclusion
Significant differences in quality of life scores were demonstrated between patients and control groups. Beneficial effects of n-3FA were detected in children on dialysis reflected on improved quality of life, and some laboratory parameters as lipid profiles, inflammatory markers, and renal functions which encourages its testing for more patients to evaluate its long term effects and support its routine use.
Acknowledgments
Special thanks to all children and controls who agreed to give us some of their time. Also great thanks to Alaa Meawad Abd Elhady, a student at faculty of medicine in Menoufia University, who helped in data collection.
Author contributions
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work. Zeinab A. Kasemy has the role of getting the idea, performing statistical analysis, writing the methodology and results sections, final revision and publishing. Hanan Hathout, has the role of writing Introduction and discussion. Wael A. Bahbah, Zein A. Omar and Mohamed A. Samir, the pediatricians, received, diagnosed and collected the data.
6. Correction
The exclusion criteria section was originally repeated in the inclusion criteria and has since been removed. When originally published, the details in this sentence were described as lower and should have been higher. It has been changed from “The higher the score to be a way from zero the poorer will be the quality of life. In this study, patients had low scores in all domains of Healthrelated quality of life (HRQOL) pediatric questionnaire, which also was significantly lower than controls. Many studies support this finding (Arlene et al,[17] Goldstein et al,[18] Dotis et al,[19] and El Shafei et al,[20]). “ to ” In this study, The higher the score to be a way from zero the poorer will be the quality of life. Patients had higher scores in all domains of Health related quality of life (HRQOL) pediatric questionnaire, which was significantly higher than controls. Many studies support this finding (Arlene et al,[17] Goldstein et al,[18] Dotis et al,[19] and El Shafei et al,[20]).
This sentence, "Figure 3 shows significant positive correlation between quality of life and adequacy of dialysis (P < .05)", was incorrect in the original publication and has since been updated to “Figure 3 shows significant negative correlation between quality of life score and adequacy of dialysis (P < .05) (N.B: The higher the score to be a way from zero the poorer will be the quality of life).”
Footnotes
Abbreviations: BMI = body mass index, CKD = chronic kidney disease, CRP = C - reactive protein, eGFR = estimated glomerular filtration rate, ESRD = end stage renal disease, HDL = high density lipoprotein, HRQO = health-related quality of life, IL-6 = Interlukin-6, KDQOL-SF = kidney disease quality of life short form, Kt/V = K –clearance t – time V – volume of distribution, LDL = low density lipoprotein, MAP = mean arterial Pressure, n-3 FA = Omega-3 Fatty acid, marine n-3 fatty acid, PedsQL = pediatric quality of life measurement model inventory, PTH = parathyroid hormone, QoL = quality of life, R = range, SPSS = statistical package of social science, TC = total cholesterol, TG = Triglycerides, TNFα = tumor necrosis factor alpha, URR = urea reduction ratio.
How to cite this article: Kasemy ZA, Hathout HM, Omar ZA, Samir MA, Bahbah WA. Effect of Omega-3 supplements on quality of life among children on dialysis: A prospective cohort study. Medicine. 2020;99:40(e22240).
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].López CA, Escribano AF, García EI, et al. Measurement of health-related quality of life in children with chronic kidney disease using a specific test. Influence of treatment. Nefrologia 2010;30:177–84. [DOI] [PubMed] [Google Scholar]
- [2].Neul KS, Minard CG, Currier H, et al. Health-related quality of life functioning over a 2-year period in children with end-stage renal disease. Pediatr Nephrol 2012;28:285–93. [DOI] [PubMed] [Google Scholar]
- [3].Kogon AJ, Stoep A, Vande Smith J, et al. Depression and its associated factors in pediatric chronic kidney disease. Pediatr Nephrol 2013;28:1855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kris-Etherton PM, Taylor DS, Yu-Poth S, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr 2000;71: Suppl: 179S–88S. [DOI] [PubMed] [Google Scholar]
- [5].Hussein N, Ah-Sing E, Wilkinson P, et al. Long-chain conversion of [13C] linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J Lipid Res 2005;46:269–80. [DOI] [PubMed] [Google Scholar]
- [6].Friedman A, Moe S. Review of the effects of omega-3 supplementation in dialysis patients. Clin J Am Soc Nephrol 2006;1:182–92. [DOI] [PubMed] [Google Scholar]
- [7].K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 2005;45:S91–5. [PubMed] [Google Scholar]
- [8].Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: effects on risk factors: molecular pathways, and clinical events. J Am Coll Cardiol 2011;58:2047–67. [DOI] [PubMed] [Google Scholar]
- [9].Manson JE, Cook NR, I-Min Lee I-Min, et al. Marine n–3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2019;380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].AlAmmar WA, Albeesh FH, Ibrahim LM, et al. Effect of omega-3 fatty acids and fish oil supplementation on multiple sclerosis: a systematic review. Nutr Neurosci 2019;28:1–1. Epub ahead of print. PMID: 31462182. [DOI] [PubMed] [Google Scholar]
- [11].Buyan N, Türkmen MA, Bilge I, et al. Quality of life in children with chronic kidney disease (with child and parent assessments). Pediatr Nephrol 2010;25:1487–96. Epub 2010 Apr 10. PMID: 20383649. [DOI] [PubMed] [Google Scholar]
- [12].Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009;20:629–37. pmid: 19158356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bos DJ, Oranje B, Veerhoek ES, et al. Reduced symptoms of inattention after dietary omega-3 fatty acid supplementation in boys with and without attention deficit/hyperactivity disorder. Neuropsychopharmacol 2015;40:2298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nagakura T1, Matsuda S, Shichijyo K, et al. Dietary supplementation with fish oil rich in omega-3 polyunsaturated fatty acids in children with bronchial asthma. Eur Respir J 2000;16:861–5. [DOI] [PubMed] [Google Scholar]
- [15].Lotfy HM, Sabry SM, Ghobrial EE, et al. The effect of regular hemodialysis on the nutritional status of children with end-stage renal disease. Saudi J Kidney Dis Transpl 2015;26:263–70. [DOI] [PubMed] [Google Scholar]
- [16].Smith JM, Stablein DM, Munoz R, et al. Contributions of the transplant registry: The 2006 annual report of the North American pediatric renal trials and collaborative studies (NAPRTCS). Pediatr Transplant 2007;11:366–73. [DOI] [PubMed] [Google Scholar]
- [17].Gerson AC, Wentz A, Abraham AG, et al. Health-related quality of life of children with mild to moderate chronic kidney disease. Pediatrics 2010;125:e349–57. doi:10.1542/peds.2009-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Goldstein SL, Graham N, Burwinkle T, et al. Health-related quality of life in pediatric patients with ESRD. Pediatr Nephrol 2006;21:846–50. [DOI] [PubMed] [Google Scholar]
- [19].Dotis J, Pavlaki A, Printza N, et al. Quality of life in children with chronic kidney disease”. Pediatr Nephrol 2016;31:2309–16. [DOI] [PubMed] [Google Scholar]
- [20].El Shafei AM, Hegazy IS, Fadel FI, et al. Assessment of quality of life among children with end-stage renal disease: a cross-sectional study. J Environ Public Health 2018;2018:8565498.doi:10.1155/2018/8565498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moeinzadeh F, Shahidi S, Mortazavi M, et al. Effects of Omega-3 fatty acid supplementation on serum biomarkers, inflammatory agents, and quality of life of patients on hemodialysis. Iran J Kidney Dis 2016;10:381–7. [PubMed] [Google Scholar]
- [22].Tjaden LA, Grootenhuis MA, Noordzij M, et al. Health-related quality of life in patients with pediatric onset of end-stage renal disease: state of the art and recommendations for clinical practice. Pediatr Nephrol 2016;31:1579–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Taheri N, Kamangar S, Cheraghian B, et al. Life quality of hemodialysis patients. Knowledge Health 2013;8:119–24. (Persian). [Google Scholar]
- [24].Baghaie Lake M, Rahimi S, Adib M, et al. Monfared A. redictive Personal factors of quality of life in hemodialysis patient. J Holistic Nurs Midwifery 2015;24:9–19. (Persian). [Google Scholar]
- [25].Rafii F, Rambod M, Hosseini AF. Quality of life in end stage renal disease and its related factors. Iran J Nurs 2010;23:35–42. (Persian). [Google Scholar]
- [26].Barzegar H, Jafari H, Yazdani Charati J, et al. Relationship between duration of dialysis and quality of life in hemodialysis patients. Iran J Psychiatry Behav Sci 2017;11:e6409.doi: 10.5812/ijpbs.6409. [Google Scholar]
- [27].Allawi AAD, Alwardi MAW, Altemimi HM. Effects of Omega-3 on vitamin D activation in iraqi patients with chronic kidney disease treated by maintenance hemodialysis. Pharm Sci & Res 2017;9:1812–6. [Google Scholar]
- [28].Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care 2008;35:215-vi.doi:10.1016/j.pop.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].El-Gamasy MA, El-Shehaby WA, Mabrouk MM. Early predictors of cardiac dysfunction in Egyptian children with chronic kidney disease. Ann Pediatr Cardiol 2019;12:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gharekhani A, Khatami MR, Dashti-Khavidaki S, et al. Potential effects of omega-3 fatty acids on anemia and inflammatory markers in maintenance hemodialysis patients. DARU J Pharm Sci 2014;22:11.doi:10.1186/2008-2231-22-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fishbane S, Spinowitz B. Update on anemia in ESRD and earlier stages of CKD: core curriculum. Am J Kidney Dis 2018;71:423–35. [DOI] [PubMed] [Google Scholar]
- [32].Kooshki A, Taleban FA, Tabibi H, et al. Effects of Omega-3 fatty acids on serum lipids, lipoprotein (a), and hematologic factors in hemodialysis patients. Ren Fail 2011;33:892–8. [DOI] [PubMed] [Google Scholar]
- [33].Perunicic-Pekovic GB, Rasic ZR, Pljesa SI, et al. Effect of n-3 fatty acids on nutritional status and inflammatory markers in haemodialysis patients. Nephrol 2007;12:331–6. PMID: 17635746. [DOI] [PubMed] [Google Scholar]
- [34].Saifullah A, Watkins BA, Saha C, et al. Oral fish oil supplementation raises blood omega-3 levels and lowers C-reactive protein in haemodialysis patients-a pilot study. Nephrol Dial Transplant 2007;22:3561–7. [DOI] [PubMed] [Google Scholar]
- [35].Bowden RG, Jitomir J, Wilson RL, et al. Effects of omega-3 fatty acid supplementation on lipid levels in endstage renal disease patients. J Ren Nutr 2009;19:259–66. [DOI] [PubMed] [Google Scholar]
- [36].Deike E, Bowden RG, Moreillon JJ, et al. The effects of fish oil supplementation on markers of inflammation in chronic kidney disease patients. J Ren Nutr 2012;22:572–7. [DOI] [PubMed] [Google Scholar]
- [37].Pluta A, Stró_zecki P, Kęsy J, et al. Beneficial effects of 6-month supplementation with omega-3 acids on selected inflammatory markers in patients with chronic kidney disease stages 1-3. Biomed Res Int 2017;2017:1680985.doi:10.1155/2017/1680985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tayyebi-Khosroshahi H, Houshyar J, Dehgan-Hesari R, et al. Effect of treatment with omega-3 fatty acids on C-reactive protein and tumor necrosis factor-alfa in hemodialysis patients. Saudi J Kidney Dis Transpl 2012;23:500–6. [PubMed] [Google Scholar]
- [39].Naini AE, Asiabi RE, Keivandarian N, et al. Effect of omega-3 supplementation on inflammatory parameters in patients on chronic ambulatory peritoneal dialysis. Adv Biomed Res 2015;10:167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


