Abstract
Viral respiratory infections are risk factors for cardiovascular disease (CVD). Underlying CVD is also associated with an increased risk of complications following viral respiratory infections, including increased morbidity, mortality, and health care utilization. Globally, these phenomena are observed with seasonal influenza and with the current coronavirus disease 2019 (COVID-19) pandemic. Persons with CVD represent an important target population for respiratory virus vaccines, with capacity developed within 3 large ongoing influenza vaccine cardiovascular outcomes trials to determine the potential cardioprotective effects of influenza vaccines. In the context of COVID-19, these international trial networks may be uniquely positioned to redeploy infrastructure to study therapies for primary and secondary prevention of COVID-19. Here, we describe mechanistic links between influenza and COVID-19 infection and the risk of acute cardiovascular events, summarize the data to date on the potential cardioprotective effects of influenza vaccines, and describe the ongoing influenza vaccine cardiovascular outcomes trials, highlighting important lessons learned that are applicable to COVID-19.
Key Words: acute myocardial infarction, cardioprotection, heart failure, influenza vaccination
Abbreviations and Acronyms: ACE2, angiotensin-converting enzyme 2; COVID-19, coronavirus disease 2019; CVD, cardiovascular disease; CVOT, cardiovascular outcome trial; HF, heart failure; MI, myocardial infarction; STEMI, ST-segment elevation myocardial infarction
Central Illustration
Highlights
-
•
Viral respiratory infections, such as seasonal influenza and COVID-19, are associated with elevated risks of cardiovascular events.
-
•
Several international CVOTs are investigating whether seasonal influenza vaccine reduces the risk of cardiovascular events among patients with HF or coronary artery disease.
-
•
Existing trial networks may provide an opportunity to assess primary and secondary prevention strategies for patients with CVD at risk of complications from COVID-19.
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a novel coronavirus, emerged in Wuhan in December 2019, becoming the primary agent driving a rapidly spreading outbreak of coronavirus disease 2019 (COVID-19). By March 11, 2020, COVID-19 was declared a pandemic by the World Health Organization (WHO), with an overall case fatality ratio of ∼2.3%. Its associated cardiopulmonary morbidity and mortality is likely to become more clear as we gain a better understanding of the true denominator of infected persons over time (1, 2, 3). Patients with or at risk of cardiovascular disease (CVD) have an increased case fatality ratio, including 6.0% for hypertension, 7.3% for diabetes, and 10.5% for CVD (4). With currently no proven vaccines or antiviral treatments, standard public health preventive efforts are being applied, based on lessons from combatting the 2012 MERS-CoV outbreak, the 2009 H1N1 influenza pandemic, the 2003 SARS-CoV epidemic, and the 1918 influenza pandemic. Increasing public and scientific attention has been given to early-phase vaccine development for COVID-19, which may be months away from being trialed in a broader number of high-risk patients. Here, we review the current state of influenza vaccine research in cardiovascular patients at high risk for cardiopulmonary morbidity and mortality, comparing what is known from influenza to what has been learned so far about COVID-19. We provide a background understanding of the association between influenza and influenza-like illness with development of cardiovascular complications, discuss the potential cardioprotective effect of standard seasonal influenza vaccines, and discuss 3 ongoing cardiovascular outcome trials (CVOTs) testing influenza vaccines in high-risk cardiovascular patients for cardiovascular risk reduction. These existing multinational trial networks may be positioned to redeploy clinical and data coordination along with enrollment site infrastructure to study strategies for primary and secondary prevention of COVID-19, or reducing its cardiopulmonary complications in patients with or at risk of CVD.
Association of Influenza Infection and Cardiovascular Disease
One of the earliest reports of an association between respiratory infection and higher cardiovascular mortality was during the 1918 influenza pandemic (5). Seasonal influenza epidemics have subsequently been associated with population-level increases in cardiovascular hospitalization and mortality (6, 7, 8). In a typical year, influenza infections are associated with ∼225,000 hospitalizations, 36,000 cardiopulmonary deaths, and 51,000 deaths in the United States (6,9). The prevalence of influenza-related deaths has significantly risen in the last 2 decades, although it remains unclear whether this is due to changes in the demographics of the U.S. population, altered virulence of circulating strains of influenza, limitations in vaccination (effectiveness or uptake), or increased sensitivity of influenza virus testing (9). For instance, the risk of cardiovascular complications from respiratory virus infections, including influenza, has become better recognized and characterized with improved methods of detection of subclinical and overt acute myocardial infarction (MI) and heart failure (HF) events associated with influenza-like illness among patients with or at risk of CVD (10).
A number of large population-based observational studies employing case-control or case-only study designs have shown an association between primary care physician visits for suspected acute respiratory virus infections and subsequent acute MI (11, 12, 13). Smeeth et al. (11) conducted a large self-controlled case series study to analyze 115,218 individuals hospitalized with MI or stroke. The investigators studied whether patients had previously been treated by their primary care practitioner for a respiratory infection or underwent influenza vaccination, using the United Kingdom General Practice Research Database. They found an ∼5-fold increase in rate of hospitalization for acute MI (incidence ratio: 4.95; 95% confidence interval [CI]: 4.43 to 5.53) and an ∼3-fold increase in rate of stroke (incidence ratio: 3.19; 95% CI: 2.81 to 3.62) in the first 3 days following presentation with symptoms of a respiratory tract infection (11). Incidentally, there was also a more modest time-dependent increase in cardiovascular events within the first 3 days following a diagnosis of urinary tract infection (MI incidence ratio: 1.66; 95% CI: 1.28 to 2.14; stroke incidence ratio: 2.72; 95% CI: 2.32 to 3.20). This weaker but significant temporal association may indicate that infection in general may be a precipitant for atherothrombotic cardiovascular events, but nevertheless, the accentuated pulmonary infection risk has been reproduced in other settings.
Warren-Gash et al. (14) further evaluated the time-dependent association between respiratory infection and cardiovascular risk in a time series analysis that additionally considered potential confounding by climate factors. After adjusting for seasonality and relevant environmental confounders (e.g., mean temperature and relative humidity), the temporally dependent seasonal associations between influenza and an increased risk of MI-associated deaths and hospitalizations were confirmed. Influenza accounted for a 3.1% to 3.4% population attributable risk of MI-associated deaths in England and Wales (p < 0.001) and 3.9% to 5.6% population attributable risk in Hong Kong (p = 0.018). The corresponding population attributable risk estimates for MI-associated hospitalizations were 0.7% to 1.2% (England and Wales) and 3.0% to 3.3% (Hong Kong). To further distinguish between ST-segment elevation myocardial infarction (STEMI) and non-STEMI, the investigators carried out a self-controlled case series analysis using the precise dates for acute MI events by linking the General Practice Research Database and the Myocardial Ischaemia National Audit Project, which is a comprehensive cardiac disease registry in the United Kingdom (15,16). Yet again, they found a comparable time-dependent incidence ratio for hospitalization with MI, with the highest risk within the first 3 days following an acute respiratory infection (incidence ratio: 4.19; 95% CI: 3.18 to 5.53). The risk then remained elevated while gradually diminishing until 28 days post-infection, at which time individuals reverted to their baseline risk.
The aforementioned studies evaluated nonspecific acute respiratory infection syndromes without a confirmed microbiologic diagnosis. Kwong et al. (17) addressed this in a subsequent study that evaluated the association between different laboratory-confirmed respiratory virus infections and acute MI hospitalizations via linkage of multiple health insurance claims databases with public health microbiology testing results. When a specific pathogen was identified, the authors found a ∼10-fold time-dependent increased incidence of acute MI within the first 7 days of testing positive for influenza B (incidence ratio: 10.11; 95% CI: 4.37 to 23.38) and ∼5-fold risk with influenza A (incidence ratio: 5.17; 95% CI: 3.02 to 8.84). They also found time-dependent increases in acute MI following infection with respiratory syncytial virus (incidence ratio: 3.51; 95% CI: 1.11 to 11.12) and other respiratory viruses, including typical coronavirus, parainfluenza virus, adenovirus, human metapneumovirus, and enterovirus infections (incidence ratio: 2.77; 95% CI: 1.23 to 6.24). Hence, influenza infection is associated with an increased risk of cardiovascular morbidity and mortality, but many other respiratory virus infections similarly trigger acute cardiovascular events.
Several proposed mechanisms support a potential causal association between influenza infection and cardiovascular risk. Most serious infections can destabilize patients with pre-existing CVD through increased metabolic demand (e.g., demand ischemia devolving into myocardial injury and plaque disruption via cytokine storm). When complemented by hypoxemia, influenza infection may exacerbate underlying CVD through increased vascular tone via activation of the sympathetic nervous system, inadequate coronary artery blood flow with fever and tachycardia, potential volume overload, and arrhythmia (13). Furthermore, influenza infection also predisposes patients to develop opportunistic infections like bacterial pneumonia, which in itself is associated with increased cardiovascular risk through a variety of effects on the cardiopulmonary system, from the vascular endothelium and peripheral vessels, to cardiac autonomic function and renal function (18,19). More directly, the influenza virus may precipitate acute cardiovascular events by stimulating a potent acute inflammatory response—a known trigger of acute plaque rupture and global myocardial depression (20, 21, 22). Influenza virus can also directly infect tissue in the heart, lungs, and blood vessels, as seen in murine models and human atherosclerotic plaques (10,23).
Association of COVID-19 and CVD
Accumulating data suggest that influenza infection and COVID-19 share a similar initial clinical presentation once symptoms develop, namely, fever, cough, and shortness of breath (24). However, although the virus’ true basic reproduction number (R0) is still under investigation, COVID-19 appears to be more transmissible (25). This may be due to significant viral shedding prior to symptoms or because many infected persons may be subclinical or have a mild course of illness, thus facilitating transmission in community settings. Most patients develop no more than a self-limiting mild-to-moderate influenza-like illness. However, a subgroup of patients develop substantial cardiac and pulmonary morbidity and mortality, typically observed within 2 to 4 weeks following symptom development of COVID-19, similar to the trajectory described following the onset of acute respiratory infections and influenza virus (26). Unlike influenza, COVID-19 is associated with a ∼10-fold higher hospitalization rate and a ∼5 to 10 times higher mortality rate (24,27,28).
Early reports suggest the comorbidities associated with the highest risk of requiring critical care among laboratory-confirmed COVID-19 patients are advanced age, diabetes, hypertension, heart failure, and atherosclerotic CVD (29, 30, 31, 32, 33). Many of these patients eventually died of acute cardiovascular and cardiopulmonary events, such as multiple organ failure, shock, acute respiratory distress syndrome, myocarditis, HF, and arrhythmias, with a reported ∼50% mortality rate among patients with cardiac injury, demonstrated by detectable circulating troponin levels (34,35). After multivariable adjustment, presence of cardiac injury was also associated with a ∼4-fold higher mortality risk (hazard ratio: 4.26; 95% CI: 1.92 to 9.49) (31). Other circulating markers that predict an adverse event include elevated D-dimer levels, which have been associated with higher odds of in-hospital death (odds ratio: 18.42; 95% CI: 2.64 to 128.55; p = 0.0033) (36).
Beyond standard pathways described in the previous text, in which influenza and other acute respiratory infections can destabilize CVD patients, the specific mechanisms by which SARS-CoV-2 leads to acute myocardial injury are still emerging. The potential role of angiotensin-converting enzyme 2 (ACE2) receptor has recently been suggested as a possible link, perhaps even allowing for direct viral infection of the myocardium and vascular endothelium (31,32,37). SARS-CoV-2 infection is triggered by binding of the spike protein of the virus to the ACE2 receptor, which is a highly expressed membrane-bound aminopeptidase in the heart and lungs. The ACE2 receptor has a vital role in the cardiovascular and immune systems, heart function, and the development of hypertension and diabetes mellitus (38). Perhaps because the ACE2 receptor is also found in alveolar epithelial and endothelial cells, this may be a potential mechanism for development of thrombosis, including large vessel clots, deep vein thrombosis/pulmonary embolism, and disseminated microvascular thrombosis in the heart, liver, and kidneys among patients with COVID-19 (39, 40, 41, 42, 43, 44). However, other immune activation pathobiology may be at play that is unique to COVID-19, such as the development of antiphospholipid antibodies among patients presenting with a hypercoagulable state and disseminated thrombosis (40).
As new evidence emerges, it is clear that atherosclerotic CVD and increased thrombogenicity intersect significantly with COVID-19, both as predisposing factors and downstream sequelae. This instantiates the need for evidence-based thromboprophylaxis and antithrombotic treatment in patients with or who are at risk of thrombotic disease during the COVID-19 pandemic, with several randomized controlled trials underway. A number of investigational treatments for COVID-19 may increase risk for thrombotic events or thrombocytopenia and have the potential for drug–drug interactions with commonly administered oral anticoagulants and antiplatelet agents, including lopinavir/ritonavir, remdesivir, and tocilizumab. This could be particularly concerning in patients who develop venous or arterial thrombosis following COVID-19, or those with pre-existing thrombotic disease (43). It is also unknown whether treatment with an anticoagulant or antiplatelet agent provides a net clinical benefit in patients with COVID-19, considering the risk of bleeding. Broadly, whether patients with lower to no baseline CVD risk who recover from COVID-19 have longer-term CVD sequelae as a result of a pathophysiological response to COVID-19 is unknown and warrants further study as well.
Vaccination Strategies to Prevent Influenza Infection
The general structure of an influenza virus consists of 7 to 8 single strands of ribonucleic acid (RNA) as its genome, surrounded by a lipid bilayer envelope (Table 1 ). Its genome is particularly susceptible to mutation and adaptation because RNA replication machinery does not have proofreading capabilities, as opposed to more stable deoxyribonucleic acid (DNA) viruses that replicate via DNA polymerase. This allows the virus to avoid the long-term adaptive immune responses in hosts (45). The viral replication process includes 3 stages: 1) viral entry into the host cell through neuraminidase binding; 2) viral ribonucleoprotein entry into the host nucleus to assemble the RNA polymerase replication machinery; and 3) transcription and replication of the viral genome (45).
Table 1.
A Summary of the Key Features of Influenza and SARS-CoV-2
| Influenza | SARS-CoV-2 | |
|---|---|---|
| Disease | Influenza infection | COVID-19 |
| Structure (45,133) | Segmented, negative-stranded RNA viruses; the envelope of influenza A is formed by HA, NA, M1, and M2 proteins, whereas influenza B is formed by NA, HA, NB, and BM2 proteins. | Positive, single-stranded RNA virus with glycoprotein spikes in the shape of a crown present in its envelope. |
| Global R0 (133,134) | 1.3 | 2.2 |
| Case fatality rate (133,135,136) | 0.2% | 1%–2% |
| Transmission | Respiratory droplets | |
| Incubation time (133,137) | 2–4 days | 3–7 days |
| Diagnosis (133,137) | Molecular test, serology, imaging | |
| Cardiovascular complications | Proinflammatory and prothrombotic effects that increase the risks of acute MI and heart failure. Patients with known CVD who get infected with influenza could also present with fever, tachycardia, potential volume overload, and arrhythmia. | Proinflammatory and prothrombotic effects leading to acute MI or myocardial injury and heart failure, disseminated thrombosis, hypotension, arrhythmia, sudden cardiac death, and pediatric inflammatory multisystem syndrome. |
BM2 = influenza B matrix ion channel 2; CVD = cardiovascular disease; HA = hemagglutinin; M1 = matrix ion channel 1; MI = myocardial infarction; NA = neuraminidase; NB = type III integral membrane protein; R0 = basic reproduction number; RNA = ribonucleic acid; SARS-CoV-2 = severe acute respiratory syndrome coronavirus-2.
Influenza viruses are divided into subtypes and are classified based on 2 main glycoprotein spikes present on the viral envelope surface, hemagglutinin (HA) and neuraminidase (NA). The most virulent subtypes belong to influenza virus type A, subtypes HA (H1, H2, and H3), and NA (N1 and N2) (46,47). Influenza virus type B has 2 well-known lineages: B/Yamagata/16/88-like, and B/Victoria, which also contribute to the variability in influenza virulence each year, but with a lower impact than type A (46,47).
The World Health Organization and the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices make annual recommendations for the composition of the influenza vaccine to manufacturers. These recommendations are usually based on the circulating strains observed in Australia and other countries in the Southern Hemisphere, which experience influenza seasons in discordant times of the year compared with the Northern Hemisphere (48,49). The viruses chosen for the seasonal influenza vaccines are then typically grown and propagated in chicken eggs, followed by inactivation—a process that has remained relatively unchanged for the past 40 years and remains the preferred method for influenza vaccine production, despite limitations described in the following text (50,51).
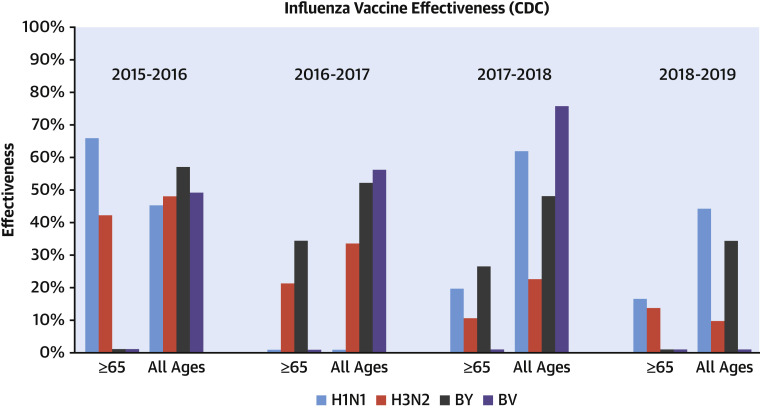
A key factor in determining vaccine effectiveness is how well the vaccine antigen formulation matches those of the circulating strains each year. Because each season can see the emergence of a new virulent strain of influenza without sufficient prior population immunity, it is recommended that every individual receive a new vaccine each season. However, antigen mismatch can occur due to diversity and drift in circulating viruses and their evolving antigenic distance from the corresponding vaccine component (52). As a result of this natural antigenic drift, predicting the viral strain makeup to include in the standard seasonal influenza vaccine is challenging and is to some extent an imprecise science (52). If the prediction is inaccurate, the effectiveness of the seasonal influenza vaccine may be low, as has been observed in the past 4 years in the United States (Figure 1 ) (53). The effectiveness of the influenza vaccine also depends on other clinical factors, such as age and comorbidities of the recipient, as discussed in the next section.
Figure 1.
Comparison of Seasonal Influenza Vaccine Effectiveness Between 2015 and 2019
There has been a modest decrease in overall influenza vaccine effectiveness between 2015 and 2019 among individuals of all ages and those age ≥65 years, with the largest reduction in vaccine effectiveness seen against H3N2. Zero-valued columns are included to denote cases of missing data from the Centers for Disease Control and Prevention (CDC) Influenza Effectiveness National Report (132). BV = B/Victoria lineage; BY = B/Yamagata lineage.
Vaccine mismatch can also occur due to mutations in hemagglutinin during influenza virus replication in eggs, termed egg-adaptation (50). The H3N2 influenza strain in particular is prone to glycosylation mutations in the antigenic sites of hemagglutinin (50). In the 2014 to 2015 season, circulating H3N2 virus was noted to have developed a new glycosylation mutation (K160T HA) that is not present in current egg-adapted versions of the H3N2 strains, leading to a mismatch between the 2 and poor effectiveness of the resultant vaccine (as seen in Figure 1) (54, 55, 56). It was also shown that this circulating glycosylation mutation cannot be adapted to grow in eggs as easily as in other cell-based culture systems, which gives credence to considering novel vaccine production techniques moving forward.
The low effectiveness against H3N2 has a pronounced impact among the elderly and other high-risk patients, who also experience the highest burden of influenza-like illness and related mortality (53). As a result, although the current egg-based mass production system is cost-effective, it has limitations that hinder its efficiency: 1) low effectiveness of the resultant vaccine due to egg-adaptation and antigenic drift; 2) a 6-month production lag following annual World Health Organization predictions; and 3) difficulties in changing a given strain mid-production when antigenic mismatch occurs between circulating and vaccine strains.
Emerging vaccine development techniques have the potential for superior efficacy, exploring higher doses, higher valences, and new vaccine production technologies beyond egg-based systems (57). For example, manufacturers have increasingly expanded toward producing quadrivalent in lieu of trivalent influenza vaccines, which as a result of their increased coverage are becoming the standard (48). Quadrivalent vaccines are composed of the same 2 influenza A strains and influenza B strains as the trivalent vaccine, but in addition contain the other most common circulating strain of influenza B (48). Another development is the utilization of recombinant DNA techniques to produce viral antigen using mammalian and insect cell culture systems (58). This method can result in a viable vaccine within 6 to 8 weeks compared with the 6-month horizon required with the egg harvest technique, and thus is touted as a possible solution to vaccine development time lags in novel pandemics (58). Although enhanced vaccine efficacy has been seen with innovative production technologies in preliminary studies, the Centers for Disease Control and Prevention does not recommend one formulation over the other, and more studies are needed to draw conclusive results.
Given the imprecision in current vaccine design, there is a demonstrated need for a different antigenic target that may yield a “universal” influenza vaccine. Such a vaccine would ideally induce cross-protective immunity against all influenza viruses for several years, potentially protecting against future influenza pandemics as well. Innovative vaccine technologies being explored toward this goal include nucleic acid-based delivery, alternate viral vectors, recombinant proteins (including DNA-/RNA-based), and the use of viral-like particles (57). However, most of these technologies have not been tested beyond phase I clinical trials (57). As production costs decrease, scalability increases, and efficacy and/or effectiveness is demonstrated, more manufacturers will invest in further innovative vaccine technologies.
Vaccination Strategies to Prevent COVID-19 Infection
With COVID-19, the first known vaccine candidate to be tested in a randomized controlled trial used innovative mRNA-based technology. Although this technology is reported to allow for faster production, it has never led to a licensed vaccine for sale (59). As of August 28, 2020, the World Health Organization listed 143 candidate vaccines in preclinical evaluation and 33 already in clinical trials (60). If any are successful, the challenge to implementation will likely be the time and infrastructure required to mass-produce a new vaccine during the COVID-19 pandemic. Beyond the challenges faced with influenza vaccine manufacturing, there are other challenges facing the rapid development of a COVID-19 vaccine. For instance, commonly circulating coronaviruses account for 10% to 30% of upper respiratory tract infections—none of which have existing vaccines (61). SARS-CoV-2 has also shown a similar profile to SARS-CoV for susceptible cell lines, which unfortunately suggests that embryonated eggs will not support SARS-CoV-2 replication as a culture system (62). Also, although not seen yet, it is unclear how stable the SARS-CoV-2 genome is with regard to antigenic drift, as RNA viruses like influenza tend to mutate. The current lack of immune pressure for SARS-CoV-2 works in favor of its genomic stability; therefore, the hope of developing a vaccine that could offer near-term protection is high (63).
There is also ongoing research to verify that immunity is established and can be prolonged following COVID-19 recovery, which is key for vaccine development. Recent evidence underscores the need for an effective vaccine because the production of neutralizing antibodies following COVID-19 infection has been suboptimal and short-lived, with levels declining substantially in <3 months, particularly in asymptomatic persons (64,65). This phenomenon raises significant issues about the risk of reinfection. In addition, the adaptive immune response in COVID-19 convalescent individuals may be via detectable neutralizing antibodies that correlate with the numbers of virus-specific T cells (66, 67, 68, 69, 70). T cell response-derived immunity may be more important than antibody response for COVID-19, but it remains unclear if T cell responses preclude recurrent infection (71). Given these immune correlates, experimental COVID-19 vaccine platforms are based on humoral and/or cell-mediated immunity (Table 2 ). In contrast, licensed seasonal influenza vaccines focus on humoral immunity because of evidence of its importance in protection against influenza (72); the cell-mediated responses produced by influenza vaccines are weak (73,74).
Table 2.
Comparison of Vaccine Platforms for Influenza and COVID-19
| Vaccine Technology/Platform | Influenza (138) | COVID-19 (60) |
|---|---|---|
| Licensed for use | ||
| Inactivated virus | ✓ | X |
| Live-attenuated virus | ✓ | X |
| Protein subunit | ✓ | X |
| Clinical evaluation | ||
| Inactivated virus | X | ✓ |
| Live-attenuated virus | ✓ | ✓ |
| Protein subunit | ✓ | ✓ |
| Virus-like particle | ✓ | ✓ |
| DNA | ✓ | ✓ |
| RNA | ✓ | ✓ |
| Nonreplicating viral vector | ✓ | ✓ |
| Replicating viral vector | X | ✓ |
| Preclinical evaluation | ||
| Inactivated virus | ✓ | ✓ |
| Live-attenuated virus | ✓ | ✓ |
| Protein subunit | ✓ | ✓ |
| Virus-like particle | ✓ | ✓ |
| DNA | ✓ | ✓ |
| RNA | ✓ | ✓ |
| Nonreplicating viral vector | ✓ | ✓ |
| Replicating viral vector | ✓ | ✓ |
COVID-19 = coronavirus disease 2019; DNA = deoxyribonucleic acid; RNA = ribonucleic acid.
Inflammaging and Immunosenescence
The highest-risk patients in need of an effective vaccine against influenza infection or COVID-19 are older individuals (typically age 60 years and older) and those with chronic comorbidities, including CVD. Paradoxically, as shown in Figure 1 for older individuals, such patients are also at high risk for showing reduced effectiveness from the standard seasonal influenza vaccine, as demonstrated by an inadequate immune response post-vaccination. This phenomenon may represent a complex pathobiological interplay between immunosenescence and inflammaging, seen in patients with CVD and other chronic diseases common among the elderly.
“Immunosenescence” refers to an altered immune response seen with increasing age and many chronic medical conditions, including cancer, autoimmune diseases, and CVD (75, 76, 77, 78). Inflammaging refers to the chronic, sterile, low-grade inflammation seen with increasing age, and implicated in the pathogenesis of chronic age-related diseases, like CVD (79). These phenomena may explain why older individuals and those with or at risk of CVD display an elevated risk for developing severe respiratory infections with associated adverse events, compared with milder presentations in younger people or those without multiple comorbidities (80, 81, 82). For instance, the worst prognosis and outcomes with COVID-19 infection have been among older patients and those with cardiovascular comorbidities. In Wuhan, patients age >60 years had more systemic symptoms and more severe pneumonia than younger patients (37,83), and the median age of patients requiring critical care is ∼60 years (29). Although a similar epidemiology has been observed with seasonal influenza and the prior 2009 H1N1 influenza pandemic, the lower rates of severe illness in neonates and young children with COVID-19 is a notable discrepancy that remains unexplained.
Regarding influenza, there are 2 currently approved vaccine formulations that were developed with the intent to boost the lackluster immune response seen among the elderly to standard influenza vaccine. One approach has been to add an adjuvant ingredient, namely MF59, to standard egg-based influenza vaccines to enhance antigen potency and cross-reactivity (84). Studies of adjuvant vaccines have demonstrated improved immunity; however, no definitive outcome trials have been reported (85). An alternative approach has been to inoculate older patients with higher amounts of inactivated viral particle antigen, considered a higher-dose influenza vaccine (86, 87, 88, 89, 90, 91, 92). Outcome data to support this strategy comes from a landmark randomized controlled trial that compared a 4-fold more concentrated high-dose trivalent influenza vaccine (60 μg/strain) with a standard-dose trivalent influenza vaccine (15 μg/strain) in 31,989 adults age 65 years and older during the 2011 to 2013 influenza seasons. Compared with standard-dose vaccine, patients treated with high-dose vaccine had a relative risk of 0.76 (95% CI: 0.63 to 0.90) for developing laboratory-confirmed influenza illness (86,87). Influenza hemagglutination inhibition assay titers and seroprotection rates were significantly higher in the high-dose vaccine group as well. As a result, the high-dose vaccine is currently indicated for individuals age 65 years and older, whereas standard-dose vaccine is indicated for all other individuals, starting from age 6 months, and is the North American standard of care at present (48).
To examine the broader real-world impact of this recommendation, Gravenstein et al. (93) conducted a cluster-randomized pragmatic trial in 823 Medicare-certified nursing homes in the United States, comparing the effect of the high-dose versus standard-dose vaccines on reducing hospital admissions related to pulmonary illness and influenza-like illness. The incidence of these hospital admissions was found to be significantly lower in nursing homes randomized to administer the high-dose vaccine (0.185 per 1,000 resident-days or 3.4% over 6 months vs. 0.211 per 1,000 resident-days or 3.9% over 6 months; adjusted relative risk: 0.87; 95% CI: 0.78 to 0.98; p = 0.023). The secondary outcome, which looked at all-cause hospital admissions, was also found to be lower in the high-dose vaccine group (1.021 per 1,000 resident-days vs. 1.113 per 1,000 resident-days; adjusted relative risk: 0.91; 95% CI: 0.86 to 0.97; p = 0.0028), which may be explained by reductions in the downstream consequences of influenza infection.
Beyond age, there are data showing that patients with established CVD, such as ischemic and nonischemic HF, have a reduced humoral and altered cell-mediated response to standard-dose influenza vaccine (90,91,94). This suggests that patients with CVD that are particularly at increased risk for influenza-related complications may not derive sufficient protection with a standard-dose vaccination. Van Ermen et al. (88) were among the first to conduct a randomized pilot study in patients with HF (age 54 to 74 years), using a double-dose of influenza vaccine (30 μg/strain), compared with the standard dose (15 μg/strain), investigating vaccine antibody titers at baseline, 2 to 4 weeks, and 4 to 6 months post-vaccination. Their results showed that at 2 to 4 weeks, double-dose hemagglutination unit changes were significantly higher than those of the standard dose (3.3 vs. 1.6 for influenza A/H3N2; p < 0.001; 1.9 and 1.1 for influenza A/H1N1; p = 0.009; and 1.7 and 1 for influenza B-type; p = 0.02). The increased humoral response seen with the higher dose vaccine in patients with pre-existing CVD may be associated with a lower risk of adverse outcomes, irrespective of age. This study provided further rationale for conducting sufficiently powered CVOTs.
Cardioprotective Effects of Influenza Vaccine
Although COVID-19 and other respiratory virus infections are associated with acute MI and other cardiovascular events, influenza has the best evidence of a safe vaccine option for cardiovascular risk reduction to date. Several observational (95, 96, 97, 98) and small randomized (99, 100, 101, 102, 103, 104) studies have suggested that influenza vaccination may serve as a preventative measure against adverse cardiovascular outcomes. In 2013, Udell et al. (105) conducted a meta-analysis of 6 randomized controlled trials assessing the benefit of influenza vaccine in reducing cardiovascular events or reported cardiovascular-related adverse events within trials of influenza vaccine. The included trials were either studying vaccine effectiveness and collected cardiovascular events as part of their safety evaluation or were studies intended to determine the potential efficacy of vaccination for cardioprotection. The primary outcome was a composite of major adverse cardiovascular events, including cardiovascular death, hospitalization for MI, unstable angina, stroke, HF, or urgent coronary revascularization. The authors found that influenza vaccination was associated with a lower risk of composite cardiovascular events than placebo (in the blinded studies) or standard care without vaccination (among open-label trials) (2.9% vaccine vs. 4.7% placebo/control; relative risk: 0.64; 95% CI: 0.48 to 0.86; absolute risk difference: 1.74%; 95% CI: 0.81% to 2.67%; p = 0.003). A treatment interaction was detected between participants with and without recent acute coronary syndrome (p interaction = 0.03), where vaccination was associated with a lower risk of major cardiovascular events among participants with recent acute coronary syndrome (10.25% vaccine vs. 23.1% placebo/control; relative risk: 0.45; 95% CI: 0.32 to 0.63; absolute risk difference: 12.9%; 95% CI: 7.75% to 18.0%; p < 0.001) compared with participants with stable coronary artery disease (6.9% vaccine vs. 7.4% placebo/control; relative risk: 0.94; 95% CI: 0.55 to 1.61; p = 0.81), suggesting perhaps that acute cardiovascular patients with higher risk (such as acute coronary syndrome) may benefit the most regarding cardiovascular risk reduction from a once-annual influenza vaccine. Several mechanisms may be at play to explain these findings. In addition to preventing infection and thus avoiding disruptions in homeostasis, the vaccine itself may interact with immune and inflammatory systems to promote plaque stabilization (106). Vaccine-induced antibodies may also interact with the bradykinin 2 receptor, leading to increased nitric oxide production (107).
Influenza vaccination may also have important implications for protecting high-risk cardiovascular patients in the context of the ongoing respiratory viral pandemic, particularly if patients with COVID-19 infection are at risk for superimposed secondary infections, such as influenza. Despite early reports from China suggesting that coinfection with other respiratory infections are rare (34), increasing examples of respiratory viral and bacterial coinfections are emerging in the published data (108, 109, 110, 111, 112, 113, 114, 115). Vaccination will reduce the risk of influenza and may offer some incremental cardiorespiratory protection until a definitive COVID-19 vaccination is available.
However, despite international guidelines recommending routine influenza and pneumococcal vaccination (4) for patients with cardiovascular disease, uptake is substantially lacking and is often deprioritized, including at the time of a cardiovascular hospitalization (116). In patients hospitalized with HF at centers in the GWTG (Get With The Guidelines)-HF registry from October 2012 to March 2017, approximately 1 of 3 patients were not vaccinated for influenza or pneumococcal disease. These rates failed to show any improvement over 5 years of study (117). These findings underscore that despite the emerging evidence from observational and trial data, there remains skepticism that an influenza vaccine can reduce cardiovascular risk. Specifically, trials to date have been considerably underpowered to demonstrate an effect of influenza vaccine for individual cardiovascular endpoints, especially all-cause and cardiovascular-related death, and some were even unblinded. More compelling data derived from larger double-blind outcome trials conducted over multiple consecutive seasons, among patients with or at high risk of developing cardiovascular events, could clarify remaining questions and provide convincing evidence for patients, providers, payers, and policy makers.
Ongoing Influenza Vaccine CVOTs
There are currently 3 large ongoing CVOTs examining the cardioprotective effects of varying formulations of influenza vaccine on a global scale (Table 3 ).
Table 3.
Overview of the Influenza Vaccine Cardiovascular Outcome Trials
| INVESTED | IAMI | RCT-IVVE | |
|---|---|---|---|
| Trial title | INfluenza Vaccine to Effectively Stop cardioThoracic Events and Decompensated heart failure | Influenza Vaccination After Myocardial Infarction | Influenza Vaccine to Prevent Adverse Vascular Events |
| NCT number | NCT02787044 | NCT02831608 | NCT02762851 |
| Trial design | Pragmatic, randomized, quadruple-masked, parallel-assignment, active-controlled trial | Prospective registry-based, randomized, quadruple-masked, parallel-assignment, placebo-controlled trial | Randomized, quadruple-masked, parallel-assignment, placebo-controlled trial |
| Recruitment started | September 2016 | October 2016 | June 2016 |
| Anticipated enrollment | 9,300 | 4,400 | 5,000 |
| Estimated study completion | February 2021 | September 2021 | May 2021 |
| Intervention | High-dose trivalent inactivated influenza vaccine (IIV3-HD) | Standard-dose trivalent inactivated influenza vaccine (IIV3) Standard-dose quadrivalent inactivated influenza vaccine (IIV4) |
Standard-dose trivalent inactivated influenza vaccine (IIV3) |
| Comparator | Standard-dose quadrivalent inactivated influenza vaccine (IIV4) | Placebo, intramuscular saline injection | Placebo, intramuscular saline injection |
| Key inclusion criteria | ≥18 yrs of age Documented history of either: Hospitalization for spontaneous (type 1) or secondary (type 2) MI within 1 yr of baseline visit, or HF hospitalization within 2 yrs of the baseline visit. 1+ additional risk factor, e.g.:
|
Meet study definition for either: STEMI, or NSTEMI Stable CAD ≥75 yrs of age undergoing angiography/PCI AND with 1+ additional risk factor AND ≥18 yrs of age A finalized coronary angiography/PCI (optional for sites in Bangladesh) |
|
| Recruitment time | 4 influenza seasons | 4 influenza seasons | 3 influenza seasons |
| Trial participants, n | 5,266 enrolled as of October 9, 2019 | 2,573 enrolled as of March 2, 2020 | 4,871 enrolled as of January 14, 2019 |
| Primary endpoints | Time to first occurrence of all-cause death or cardiopulmonary hospitalization up to 3 yrs | Composite endpoint of time to all-cause death, a new MI or stent thrombosis (first occurring, ICD-10 codes), at 1 yr | Composite of cardiovascular death, nonfatal MI, nonfatal stroke, and hospitalizations for HF at 6 months |
| Regions | North America (United States and Canada) | Europe, Australia, Asia (8 countries) | Asia, Middle East, and Africa (10 countries) |
| No. of sites | 190 | 30 | 10 |
| Substudy (Y/N) | Yes, vaccine immunogenicity | Yes, vaccine immunogenicity | Yes, serological substudy to assess influenza infection |
BMI = body mass index; CAD = coronary artery disease; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; HF = heart failure; IAMI = Study on the Effect of Influenza Vaccination After Heart Attack on Future Cardiovascular Prognosis; ICD-10 = International Statistical Classification of Diseases and Related Health Problems-10th Revision; INVESTED = INfluenza Vaccine to Effectively Stop Cardio Thoracic Events and Decompensated Heart Failure; LVEF = left ventricular ejection fraction; MI = myocardial infarction; NSTEMI = non–ST-segment elevation myocardial infarction; PCI = percutaneous coronary intervention; RCT-IVVE = Influenza Vaccine To Prevent Adverse Vascular Events; STEMI = ST-segment elevation myocardial infarction.
The IVVE (Influenza Vaccine to Prevent Adverse Vascular Events; NCT02762851) is a placebo-controlled randomized trial (n = 5,000) to test if standard-dose trivalent inactivated influenza vaccine, compared with intramuscular saline placebo, reduces a composite of adverse cardiovascular events in high-risk adults with New York Heart Association functional class II to IV HF (118). The trial is recruiting from clinical centers spanning Asia, the Middle East, and Africa, where the influenza vaccine is not standard of care, and will follow patients over 3 consecutive years. Given that patients with HF are generally recommended to receive annual influenza vaccination, this trial will permit background usage of influenza vaccine to preserve trial equipoise. This will result in a small proportion of participants receiving 2 vaccinations within the same season, which does not have any safety concerns. The primary outcome measure will be a composite of cardiovascular death, nonfatal MI, nonfatal stroke, and hospitalizations for HF using standardized criteria over 6 months. Cardiovascular death at 6 months will be a secondary outcome. Recruitment is still ongoing, with the estimated study completion date being May 2021.
The IAMI (Influenza Vaccination After Myocardial Infarction; NCT02831608) trial is a placebo-controlled, registry-based randomized clinical trial testing the in-hospital administration of standard-dose trivalent inactivated influenza vaccine in patients with STEMI or non-STEMI undergoing coronary angiography (n = 4,400) (119). IAMI uses a hybrid registry-based randomized clinical trial methodology, wherein baseline information will be collected from national heart disease registries in Sweden and Denmark, and from conventional electronic case report forms in Norway, the Czech Republic, Scotland, Latvia, Australia, and Bangladesh (120). Follow-up will be performed using both registries and a structured telephone interview, and events will be adjudicated. The primary endpoint is time to all-cause death, hospitalization for a new MI, or stent thrombosis (first occurring) within 1 year. Like IVVE, recruitment is still ongoing, with the estimated study completion date being August 2021.
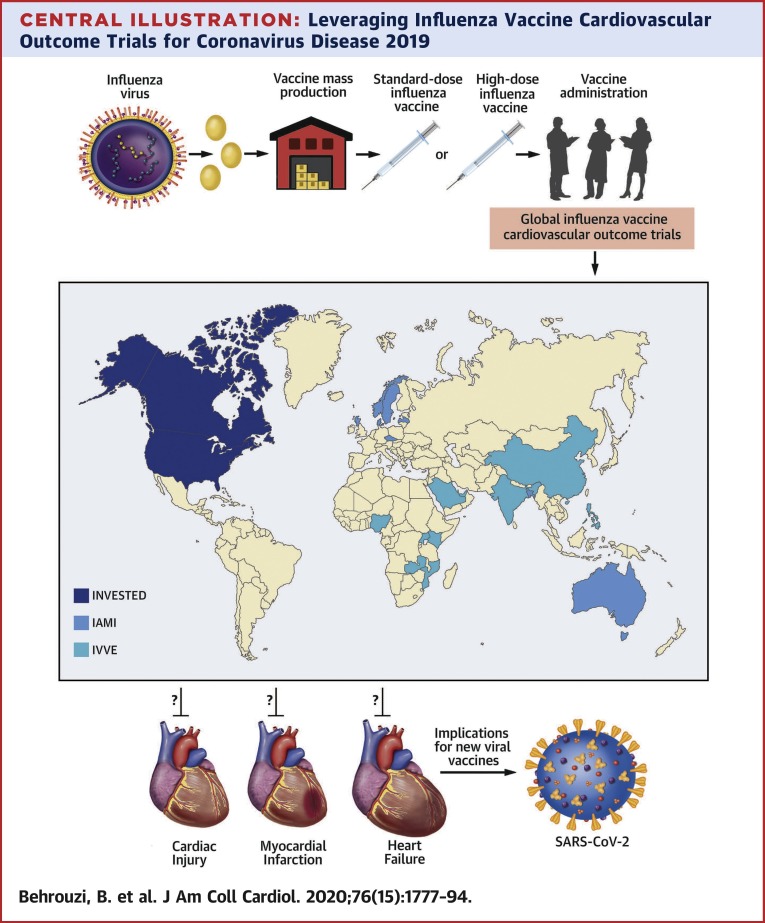
To address the issue of immunosenescence and identify whether one strategy of influenza vaccination is more cardioprotective, a third trial is being conducted in North America that also dispenses with concerns of equipoise by foregoing assignment to placebo. The INVESTED (INfluenza Vaccine to Effectively Stop cardioThoracic Events and Decompensated heart failure; NCT02787044) is the first and largest active-controlled randomized controlled trial (n = 9,300) comparing a high-dose trivalent inactivated influenza vaccine to a standard-dose quadrivalent influenza vaccine, over 4 influenza seasons, in high-risk cardiovascular patients with a recent history of MI or hospitalization for HF (75). The primary endpoint is time to first occurrence of all-cause death or cardiopulmonary hospitalization. Patients can participate for up to 3 consecutive years and remain assigned to the same therapy they received at baseline. Moreover, given the active comparator design of INVESTED with high-dose influenza vaccine, the trial provides a unique opportunity to explore the association among inflammaging, immunosenescence, and treatment effect within a substudy that will analyze participants’ immune response with outcomes (n ∼ 1,000). This will help address whether the high-dose vaccine produces a more pronounced humoral response in cardiovascular patients compared with the standard dose, as well as whether higher antibody concentrations correlate with a reduction in the composite endpoint of all-cause mortality and cardiopulmonary hospitalization. A genomic substudy will explore the potential association between carrier status for a genetic profile that predisposes individuals to develop a severe influenza infection and predilection for cardiopulmonary outcomes. For instance, the human interferon-inducible transmembrane protein-3 (IFITM3) plays a pivotal role in defense against influenza viral infection (98). There is a mutation (rs12252) in the IFITM3 gene with a highly variable minor allele (C allele) frequency (ranging in prevalence between 4% to 53%) that results in expression of a truncated protein rendering affected patients’ immune systems vulnerable to influenza (98). Having 1 (heterozygote) or 2 (homozygote) alleles has been found in multiple case-control studies to impact a patient’s ability to restrict virus replication transforming a mild infection into greater susceptibility for a severe influenza infection complicated by cardiopulmonary events and death (98,99). It has been proposed that pre-emptively screening for carriers of the IFITM3 genotype might predict which patients are most susceptible to cardiopulmonary complications from influenza. Moreover, such a strategy could influence decision making for preventive therapy, such as high-dose influenza vaccine. These findings may also have important implications for preventative or treatment strategies being designed for COVID-19, such as genetic screening to detect susceptibility for adverse events and vaccine development (Central Illustration ).
Central Illustration.
Leveraging Influenza Vaccine Cardiovascular Outcome Trials for Coronavirus Disease 2019
Lessons learned from seasonal influenza vaccine development and mass production as well as 3 international influenza vaccine cardiovascular outcome trials assessing its cardioprotective effects in high-risk patients (INVESTED [INfluenza Vaccine to Effectively Stop cardioThoracic Events and Decompensated heart failure], IAMI [Influenza Vaccination After Myocardial Infarction], and IVVE [Influenza Vaccine to Prevent Adverse Vascular Events]) can inform future efforts geared at developing and evaluating novel therapies, including vaccine strategies, for coronavirus disease 2019 (COVID-19). On the world map, regions in dark blue, teal, and light blue represent countries involved in the INVESTED, IVVE, and IAMI trials, respectively.
Perspectives and Future Directions
Although morbidity and mortality presently appear higher with COVID-19 compared with circulating influenza, it is still unclear how much similarity is shared between their transmission dynamics, including the extent and duration of immunity following infection (121). With growing herd immunity and the development of preventative strategies, including vaccination, recurrent waves of novel coronavirus may regress to a rate of morbidity and mortality that is similar to seasonal influenza. However, when compared with the public perception of COVID-19, individuals and policy makers may underappreciate the continuous, annual broad toll of seasonal influenza epidemics. In the United States alone, an estimated 35.5 million people were infected with influenza during the 2018 to 2019 season, 490,600 patients were hospitalized, and 34,200 died (53,54). Globally, the World Health Organization estimates that influenza kills as many as 650,000 people every year, citing it as a top 10 leading cause of death among people of all ages, especially those with 1 or more comorbidities like CVD (122). As a result, clinical guidelines recommend annual influenza vaccination for the general population for influenza-like illness risk reduction with special emphasis of its importance in high-risk individuals (Table 4 ).
Table 4.
Current Guidelines on Influenza Vaccine Administration
| Institution | General Population | High-Risk Population(s) |
|---|---|---|
| World Health Organization (WHO)∗ | Seasonal influenza vaccination is recommended for people of all ages to help prevent the spread of disease and associated complications (139) | Priority groups include children between the ages of 6 and 59 months, elderly, health care workers, and individuals with chronic medical conditions. Pregnant women are considered the highest-priority group and should receive seasonal inoculation when trying to conceive and during pregnancy (139) |
| Centers for Disease Control and Prevention (CDC) – Advisory Committee on Immunization Practices (ACIP)† | Routine annual influenza vaccination for all persons age ≥6 months who do not have contraindications (48) | Early inoculation at the beginning of the season, using a quadrivalent formulation like quadrivalent inactivated influenza vaccine or quadrivalent recombinant influenza vaccine, is recommended for people with asthma, HF, and diabetes (48) |
| European Centre for Disease Prevention and Control∗ | All Europeans who are recommended to get the influenza vaccine should be vaccinated, with emphasis on high-risk groups (140) | Strongly recommends seasonal influenza immunization for all groups considered high-risk, which include older adults (usually ≥65 yrs), persons with chronic health conditions (≥6 months of age), persons with conditions that may aggravate a respiratory illness, persons with immunocompromise or immune-suppression, pregnant persons, and health care workers (140) |
| National Advisory Committee on Immunization (NACI) – Canada† | Early inoculation at the beginning of the fall season for all persons older than 6 months (46) | Special emphasis on the importance of vaccination in high-risk populations (46) |
| American Heart Association (AHA)/American College of Cardiology (ACC)† | Strongly recommends the influenza vaccination in all patients with CVD (4,141) | |
| European Society of Cardiology (ESC)† | Annual influenza vaccination for patients with established CVD, especially the elderly (class IB recommendation) (142) | |
| American Diabetes Association (ADA)∗ | Annual vaccination against influenza is recommended for all people ≥6 months of age, especially those with diabetes (143) | |
| Diabetes Canada† | Annual inoculation against the influenza virus in all patients with diabetes (144) | |
| American College of Physicians (ACP)∗ | As per ACIP’s recommendations | As per ACIP’s recommendations |
| College of Family Physicians of Canada (CFPC)∗ | As per NACI’s recommendations (145) | As per NACI’s recommendations (145) |
CVD = cardiovascular disease, HF = heart failure.
Information obtained from public statements on the corresponding institutions websites.
Information obtained from publicly available and approved guidelines.
With the ever-looming threat of future global respiratory viral outbreaks and their potential for downstream cardiovascular consequences, it is increasingly recognized that: 1) it is impossible to predict the next pandemic and its agent of cause (123,124); and 2) our existing vaccine technologies and mass production infrastructures are not built to address pandemics in a timely manner to minimize human cost (125,126). It is also known that although the seasonal influenza vaccine is better than nothing, it is not nearly as effective as it can be, having ranged from 10% to 60% in estimated effectiveness in recent years (75). In 2018 to 2019, the adjusted vaccine effectiveness for all influenza vaccines across all age groups was only 29%, which has only served to fuel vaccine hesitancy among the general public (127). Hence, there is a need for a collaborative shift in focus toward developing and evaluating stronger formulations of the influenza vaccine, towards the goal of increased effectiveness and uptake, and perhaps ultimately, a universal influenza vaccine (128,129). Notwithstanding the research recommendations for influenza and COVID-19 provided throughout this review (summarized in Table 5 ), a unified, top-down policy approach will be integral to combatting outbreaks from both viruses. To reduce the hundreds of thousands of preventable deaths from influenza and COVID-19, it is necessary to leverage the global research investments made for both viruses to inform sustainable policy efforts that are driven through interdisciplinary collaboration among scientists, politicians, health care systems, and other key stakeholders.
Table 5.
A Summary of the Key Research Recommendations for Influenza and COVID-19 Vaccines
| Recommendation | Impact |
|---|---|
| Ongoing research efforts | |
| Influenza vaccine CVOTs in high-risk CVD populations, e.g., INVESTED, IAMI, and IVVE | Generating high-quality evidence on hard clinical outcomes that are important in these patient populations Ensuring evidence is externally valid and generalizable to global, diverse patient populations Evaluating a low-cost and ubiquitous intervention for a high morbidity and mortality disease phenotype Identifying the best influenza vaccine type for this high-risk patient population Arming cardiologists and CV specialists with evidence and patient education tools to properly combat vaccine hesitancy beyond the primary care setting |
| Observational studies looking at short-term cardiovascular outcomes of COVID-19 in real-world, high-risk patients, e.g., INVESTED-COVID-19 | Identifying clinical, social, and genetic determinants of incidence and severity of COVID-19–like illness in high-risk CVD patients Assessing the relationship between humoral response to influenza vaccination and susceptibility to COVID-19–like illness and its severity in high-risk CVD patients |
| Delineate and substantiate correlates of immunity for both viruses and promising new vaccines | Establishing the strength and longevity of the adaptive immune response following the use of promising universal vaccine candidates for influenza Outlining the relationship between humoral and cell-mediated immunity following natural infection, as well as following the use of promising vaccine candidates for COVID-19
|
| Future research efforts | |
| Ongoing influenza CVOTs being redeployed to study COVID-19 vaccines in high-risk CVD patient populations, e.g., INVESTED, IVVE, and IAMI | Leverage already existing global clusters of trialists and participants who meet the inclusion criteria for RCTs focused on high-risk CVD populations, who are also at high-risk for COVID-19 and downstream complications Potential vaccine candidates are first tested in healthy volunteers only, whereas potential treatment agents are immediately tested in current or high-risk patients; thus, it is important to also evaluate vaccine outcomes in high-risk populations Same as noted above for the impacts of ongoing influenza vaccine CVOTs, contextualized to COVID-19 vaccines |
| Observational studies looking at short- and long-term cardiovascular outcomes of COVID-19 in the general and high-risk populations, e.g., those with pre-existing CVD | Generate necessary evidence to fortify prevention, treatment, and supportive planning for patients facing vast uncertainties following a novel disease Generate necessary evidence on coinfections that may complicate disease and/or treatment course, e.g., influenza coinfection Generate necessary evidence on potential vaccination strategies and drug-drug interactions, e.g., between antithrombotic agents and COVID-19 treatments |
| Preclinical evaluation of the mechanisms underlying the potential cardioprotective effects of different influenza and/or COVID-19 vaccine strategies | Gain more information on the pathophysiology and molecular mechanisms underlying various CVD phenotypes Generate necessary evidence for further establishing a causal association Produce knowledge that can point to other potential prevention and/or treatment candidates for these infections in high-risk patients |
| Safety and efficacy/effectiveness clinical trials for newly developed universal influenza vaccines in high-risk patient populations, e.g., those with pre-existing CVD | Improve the cost-effectiveness of available vaccines for high-risk populations that have proven to show a poor response to currently licensed vaccines, e.g., high-risk CVD patients Same as noted above for the impacts of ongoing influenza vaccine CVOTs |
| Knowledge translation and implementation of evidence via unified and interdisciplinary global policy and decision-making approaches | Use lessons learned from informing global influenza vaccination guidelines and policies with the latest evidence to create guidance for COVID-19 vaccination Use lessons learned from global influenza vaccination guidelines and policies to catalyze implementation of corresponding guidance for COVID-19 vaccination Leverage momentum and investments made into combatting COVID-19 globally to also efficiently tackle other common viral respiratory epidemics and pandemics with high morbidity and mortality, especially in ubiquitous high-risk CVD patients |
CVOT = cardiovascular outcome trial; RCT = randomized controlled trial; other abbreviations as in Table 1.
The possibility to reduce cardiovascular morbidity and mortality via a simple and cost-effective strategy like the influenza vaccine is a compelling rationale for its investigation in the 3 large CVOTs: IVVE, IAMI, and INVESTED. It is hypothesized that the effect size from influenza vaccination is comparable to existing secondary cardiovascular prevention strategies (130). Collectively, these trials offer an opportunity to explore the cardioprotective effects of both the standard- and high-dose influenza vaccines in patients with varying CVD phenotypes. If the influenza vaccine is rigorously demonstrated to reduce adverse cardiovascular events, it will represent an important change in how prevention of these events is considered on a global scale. In the interim, given the current COVID-19 pandemic, these active multinational trial networks may be well-positioned to redeploy their clinical coordination and data management infrastructure to study investigational strategies for primary prevention of COVID-19 or reducing its subsequent cardiopulmonary complications in patients with or who are at risk of CVD. Ongoing efforts include analyzing the strength of the association of clinical, social, and genetic risk factors among study participants and the incidence and severity of developing COVID-19, including immune responses to investigational vaccine treatments. As a multinational-represented, deeply phenotyped cohort of cardiovascular patients at high risk of developing COVID-19 infection, surveillance of these trial patients for COVID-19–related outcomes may enable further evaluation of which factors are associated with the highest risk for complications, including baseline treatment with angiotensin inhibitor therapies, acetylsalicylic acid, and nonsteroidal anti-inflammatory drugs.
The participating site investigators and research coordinators of these influenza vaccine CVOTs are also front-line cardiovascular clinicians who are well-positioned to advocate for influenza vaccination in their high-risk patients to combat low rates of vaccine uptake. Looking to the future, when promising COVID-19 vaccine candidates are identified in trials of healthy volunteers, the INVESTED, IAMI, and IVVE trial networks can then serve as an international platform for evaluating the effectiveness of these vaccines among higher-risk patients with established CVD who have expressed a willingness to study vaccinations for cardiovascular risk reduction.
To conclude, the cardiac complications of COVID-19 are “approximately commensurate with SARS and influenza analogs” (4). There are rigorous epidemiological data linking influenza infection with subsequent cardiovascular complications and several ongoing investigations of the potential risk reduction derived from standard and innovative influenza vaccine strategies. Three large, ongoing influenza vaccine CVOTs have an opportunity to contribute further to our understanding of the underlying comorbidities in these patients that may be driving morbidity and mortality associated with COVID-19 infection. These cohorts may also be an opportunity to explore novel infection prevention therapies beyond influenza vaccination in patients who have already volunteered to participate in a respiratory virus vaccine cardiovascular outcome study. While developing new vaccines, we will also learn soon whether influenza vaccination is an effective, low-cost, widely available therapy that reduces cardiovascular risk, which may further help prevent fatal and nonfatal cardiovascular complications of COVID-19 (37,131).
Footnotes
Ms. Behrouzi has been supported in part by a University of Toronto MD/PhD studentship award and a Heart & Stroke/Richard Lewar Centre of Excellence in Cardiovascular Research and CANHEART SPOR Graduate Studentship Award. Mrs. Araujo Campoverde has been supported by Secretaría de Educación Superior, Ciencia, Tecnología e Innovación (SENESCYT) 2018 Programa de Becas Internacionales. Dr. Talbot has served on the Data Safety and Monitoring Board for Seqirus. Dr. Bogoch has consulted for BlueDot, a social benefit company that predicts the spread of infectious diseases of global health significance. Dr. McGeer has received research grants to her institution from Pfizer, Sanofi Pasteur, Merck, and Seqirus; and has consulted for Sanofi Pasteur, Seqirus, GlaxoSmithKline, Merck, Pfizer, Cidara, and Medicago. Dr. Fröbert has been supported by Örebro University, Faculty of Health, Department of Cardiology; and has received unrestricted grant support for the IAMI study from Sanofi Pasteur. Dr. Loeb has consulted for Sanofi Pasteur, Seqirus, and Medicago; has received research funding from Seqirus; and has received influenza vaccine in-kind from Sanofi Pasteur. Dr. Vardeny has received grant support from the National Institutes of Health and AstraZeneca; and has provided consulting for Sanofi Pasteur, Novartis, and Amgen. Dr. Solomon has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, Bristol Myers Squibb, Celladon, Cytokinetics, Eidos, Gilead, GlaxoSmithKline, Ionis, Lone Star Heart, Mesoblast, MyoKardia, National Institutes of Health/National Heart, Lung, and Blood Institute, Novartis, Sanofi Pasteur, and Theracos; and has consulted for Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, Gilead, GlaxoSmithKline, Ironwood, Merck, Myokardia, Novartis, Roche, Sanofi Pasteur, Takeda, Theracos, Quantum Genetics, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Dinaqor, and Tremeau. Dr. Udell has been supported by a Heart and Stroke Foundation of Canada National New Investigator-Ontario Clinician Scientist Award, an Ontario Ministry of Research, Innovation and Science Early Researcher Award, as well as by Women’s College Research Institute and the Department of Medicine, Women’s College Hospital; has received grant support to his institutions from AstraZeneca, Novartis, and Sanofi; has served as a consultant for Amgen, Boehringer Ingelheim, Janssen, Merck, Novartis, and Sanofi; and has received honoraria from Boehringer Ingelheim and Janssen. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
References
- 1.WHO (World Health Organization) 2020. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19).https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf Available at: [Google Scholar]
- 2.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of Covid-19—studies needed. N Engl J Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 3.Lipsitch M., Donnelly C.A., Fraser C. Potential biases in estimating absolute and relative case-fatality risks during outbreaks. Galvani A.P., editor. PLoS Negl Trop Dis. 2015;9:e0003846. doi: 10.1371/journal.pntd.0003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Cardiology . 2020. COVID-19 clinical guidance for the cardiovascular care team.https://www.acc.org/∼/media/665AFA1E710B4B3293138D14BE8D1213.pdf Available at: [Google Scholar]
- 5.Collins S. Excess mortality from causes other than influenza and pneumonia during influenza epidemic. Public Health Rep. 1932;47:2159–2179. [Google Scholar]
- 6.Thompson W.W., Shay D.K., Weintraub E. Influenza-associated hospitalizations in the United States. J Am Med Assoc. 2004;292:1333. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 7.Madjid M., Miller C.C., Zarubaev V.V. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34 892 subjects. Eur Heart J. 2007;28:1205–1210. doi: 10.1093/eurheartj/ehm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reichert T.A., Simonsen L., Sharma A., Pardo S., Fedson D., Miller M. Influenza and the winter increase in mortality in the United States, 1959-1999. Am J Epidemiol. 2004;160:492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 9.Thompson W.W., Shay D.K., Weintraub E. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen J.L., Yang W., Ito K., Matte T.D., Shaman J., Kinney P.L. Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol. 2016;1:274. doi: 10.1001/jamacardio.2016.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smeeth L., Thomas S.L., Hall A.J., Hubbard R., Farrington P., Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 12.Clayton T.C., Thompson M., Meade T.W. Recent respiratory infection and risk of cardiovascular disease: case-control study through a general practice database. Eur Heart J. 2007;29:96–103. doi: 10.1093/eurheartj/ehm516. [DOI] [PubMed] [Google Scholar]
- 13.Corrales-Medina V.F., Madjid M., Musher D.M. Role of acute infection in triggering acute coronary syndromes. Lancet Infect Dis. 2010;10:83–92. doi: 10.1016/S1473-3099(09)70331-7. [DOI] [PubMed] [Google Scholar]
- 14.Warren-Gash C., Bhaskaran K., Hayward A. Circulating influenza virus, climatic factors, and acute myocardial infarction: a time series study in England and Wales and Hong Kong. J Infect Dis. 2011;203:1710–1718. doi: 10.1093/infdis/jir171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrett E., Smeeth L., Walker L., Weston C. The Myocardial Ischaemia National Audit Project (MINAP) Heart. 2010;96:1264–1267. doi: 10.1136/hrt.2009.192328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warren-Gash C., Hayward A.C., Hemingway H. Influenza infection and risk of acute myocardial infarction in England and Wales: a CALIBER self-controlled case series study. J Infect Dis. 2012;206:1652–1659. doi: 10.1093/infdis/jis597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwong J.C., Schwartz K.L., Campitelli M.A. Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med. 2018;378:345–353. doi: 10.1056/NEJMoa1702090. [DOI] [PubMed] [Google Scholar]
- 18.Yende S., D’Angelo G., Mayr F. Elevated hemostasis markers after pneumonia increases one-year risk of all-cause and cardiovascular deaths. Bozza PT, editor. PLoS One. 2011;6:e22847. doi: 10.1371/journal.pone.0022847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corrales-Medina V.F., Musher D.M., Shachkina S., Chirinos J.A. Acute pneumonia and the cardiovascular system. Lancet. 2013;381:496–505. doi: 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- 20.Fagnoul D., Pasquier P., Bodson L., Ortiz J.A., Vincent J.-L., De Backer D. Myocardial dysfunction during H1N1 influenza infection. J Crit Care. 2013;28:321–327. doi: 10.1016/j.jcrc.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Takano T., Tajiri H., Kashiwagi Y., Kimura S., Kawashima H. Cytokine and chemokine response in children with the 2009 pandemic influenza A (H1N1) virus infection. Eur J Clin Microbiol Infect Dis. 2011;30:117–120. doi: 10.1007/s10096-010-1041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bermejo-Martin J.F., Ortiz de Lejarazu R., Pumarola T. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13:R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haidari M., Wyde P.R., Litovsky S. Influenza virus directly infects, inflames, and resides in the arteries of atherosclerotic and normal mice. Atherosclerosis. 2010;208:90–96. doi: 10.1016/j.atherosclerosis.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 24.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020:27. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verity R., Okell L.C., Dorigatti I. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruan S. Likelihood of survival of coronavirus disease 2019. Lancet Infect Dis. 2020;20:630–631. doi: 10.1016/S1473-3099(20)30257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region. Italy. JAMA. 2020;323:1574. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou F., Yu T., Du R., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American College of Cardiology Chinese Cardiovascular Association. Epidemiology, CVD treatment & management. ACC’s COVID-19 Hub 2020. Available at. https://www.youtube.com/watch?v=CjEhV68GcD8&feature=youtu.be
- 40.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perini P., Nabulsi B., Massoni C.B., Azzarone M., Freyrie A. Acute limb ischaemia in two young, non-atherosclerotic patients with COVID-19. Lancet. 2020;395:1546. doi: 10.1016/S0140-6736(20)31051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J Thromb Haemost. 2020;18:786–787. doi: 10.1111/jth.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guzik T.J., Mohiddin S.A., Dimarco A. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116:1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouvier N.M., Palese P. The biology of influenza viruses. Vaccine. 2008;26:D49–D53. doi: 10.1016/j.vaccine.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Health Canada . 2019. Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2019-2020.https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2019-2020.html#III Available at: [Google Scholar]
- 47.WHO (World Health Organization) 2018. Influenza (Seasonal)https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) Available at: [Google Scholar]
- 48.Grohskopf L.A., Alyanak E., Broder K.R., Walter E.B., Fry A.M., Jernigan D.B. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 Influenza Season. MMWR Recomm Reports. 2019;68:1–21. doi: 10.15585/mmwr.rr6803a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.WHO (World Health Organization) WHO recommendations on the composition of influenza virus vaccines. 2019. https://www.who.int/influenza/vaccines/virus/recommendations/en/ Available at:
- 50.Wu N.C., Zost S.J., Thompson A.J. A structural explanation for the low effectiveness of the seasonal influenza H3N2 vaccine. PLOS Pathog. 2017;13:e1006682. doi: 10.1371/journal.ppat.1006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barberis I., Myles P., Ault S.K., Bragazzi N.L., Martini M. History and evolution of influenza control through vaccination: from the first monovalent vaccine to universal vaccines. J Prev Med Hyg. 2016;57:E115–E120. [PMC free article] [PubMed] [Google Scholar]
- 52.Harding A.T., Heaton B.E., Dumm R.E., Heaton N.S. Rationally designed influenza virus vaccines that are antigenically stable during growth in eggs. Dermody TS, editor. mBio. 2017;8:e00669–e00717. doi: 10.1128/mBio.00669-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Centers for Disease Control Estimated Influenza Illnesses, Medical Visits, Hospitalizations, and Deaths in the United States - 2017-2018 influenza season. 2019. https://www.cdc.gov/flu/about/burden/2017-2018.htm Available at:
- 54.Centers for Disease Control About flu. 2019. https://www.cdc.gov/flu/about/index.html Available at:
- 55.Paules C.I., Sullivan S.G., Subbarao K., Fauci A.S. Chasing seasonal influenza — the need for a universal influenza vaccine. N Engl J Med. 2018;378:7–9. doi: 10.1056/NEJMp1714916. [DOI] [PubMed] [Google Scholar]
- 56.Zost S.J., Parkhouse K., Gumina M.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci. 2017;114:12578–12583. doi: 10.1073/pnas.1712377114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei C.-J., Crank M.C., Shiver J., Graham B.S., Mascola J.R., Nabel G.J. Next-generation influenza vaccines: opportunities and challenges. Nat Rev Drug Discov. 2020;19:239–252. doi: 10.1038/s41573-019-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milián E., Kamen A.A. Current and emerging cell culture manufacturing technologies for influenza vaccines. Biomed Res Int. 2015;2015:1–11. doi: 10.1155/2015/504831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jackson N.A.C., Kester K.E., Casimiro D., Gurunathan S., DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines. 2020;5:11. doi: 10.1038/s41541-020-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.WHO (World Health Organization) Coronavirus disease (COVID-2019) R&D blueprint. 2020. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/ Available at:
- 61.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. JAMA. 2020;323:707. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 62.Harcourt J., Tamin A., Lu X. Severe acute respiratory syndrome coronavirus 2 from patient with 2019 novel coronavirus disease, United States. Emerg Infect Dis. 2020;26:1266–1273. doi: 10.3201/eid2606.200516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minotta M., Heady J. Italian researchers identify new SARS-CoV-2 gene variants that provide clues to coronavirus’s epidemiology. Thermo Fisher Science Press Release. 2020 http://thermofisher.mediaroom.com/2020-03-25-Italian-Researchers-Identify-New-SARS-CoV-2-Gene-Variants-That-Provide-Clues-to-Coronaviruss-Epidemiology Available at: [Google Scholar]
- 64.Long Q.-X., Tang X.-J., Shi Q.-L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 65.Wang X., Guo X., Xin Q. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis. 2020 Jun 4 doi: 10.1093/cid/ciaa721. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suthar M.S., Zimmerman M.G., Kauffman R.C. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Reports Med. 2020;1:100040. doi: 10.1016/j.xcrm.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ni L., Ye F., Cheng M.-L. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52:971–977.e3. doi: 10.1016/j.immuni.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thevarajan I., Nguyen T.H.O., Koutsakos M. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grifoni A., Weiskopf D., Ramirez S.I. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu F., Wang A., Liu M. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv. 2020 Apr 20 [E-pub ahead of print] [Google Scholar]
- 71.Robbiani D.F., Gaebler C., Muecksch F. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020 Jun 18 doi: 10.1038/s41586-020-2456-9. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scheifele D.W., McNeil S.A., Ward B.J. Safety, immunogenicity, and tolerability of three influenza vaccines in older adults. Hum Vaccin Immunother. 2013;9:2460–2473. doi: 10.4161/hv.25580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trieu M.-C., Zhou F., Lartey S.L., Sridhar S., Mjaaland S., Cox R.J. Augmented CD4+ T-cell and humoral responses after repeated annual influenza vaccination with the same vaccine component A/H1N1pdm09 over 5 years. NPJ Vaccines. 2018;3:37. doi: 10.1038/s41541-018-0069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomas P.G., Keating R., Hulse-Post D.J., Doherty P.C. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006;12:48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vardeny O., Udell J.A., Joseph J. High-dose influenza vaccine to reduce clinical outcomes in high-risk cardiovascular patients: rationale and design of the INVESTED trial. Am Heart J. 2018;202:97–103. doi: 10.1016/j.ahj.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fulop T., Larbi A., Dupuis G. Immunosenescence and inflammaging as two sides of the same coin: friends or foes? Front Immunol. 2018;8 doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsai C.-Y., Shen C.-Y., Liao H.-T. Molecular and cellular bases of immunosenescence, inflammation, and cardiovascular complications mimicking “inflammaging” in patients with systemic lupus erythematosus. Int J Mol Sci. 2019;20:3878. doi: 10.3390/ijms20163878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turner J.E., Campbell J.P., Edwards K.M. Rudimentary signs of immunosenescence in Cytomegalovirus-seropositive healthy young adults. Age (Omaha) 2014;36:287–297. doi: 10.1007/s11357-013-9557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 80.Weyand C.M., Goronzy J.J. Aging of the immune system. mechanisms and therapeutic targets. Ann Am Thorac Soc. 2016;13:S422–S428. doi: 10.1513/AnnalsATS.201602-095AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hodes R.J., Hathcock K.S., Weng N. Telomeres in T and B cells. Nat Rev Immunol. 2002;2:699–706. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- 82.Xue Q.-L. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27:1–15. doi: 10.1016/j.cger.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chan J.F.-W., Yuan S., Kok K.-H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilson K.L., Xiang S.D., Plebanski M. Micro and Nanotechnology in Vaccine Development. Elsevier; New York: 2017. Inflammatory/noninflammatory adjuvants and nanotechnology—the secret to vaccine design; pp. 99–125. [Google Scholar]
- 85.Centers for Disease Control Flu vaccine with adjuvant. 2019. https://www.cdc.gov/flu/prevent/adjuvant.htm Available at:
- 86.DiazGranados C.A., Dunning A.J., Kimmel M. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371:635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 87.DiazGranados C.A., Robertson C.A., Talbot H.K., Landolfi V., Dunning A.J., Greenberg D.P. Prevention of serious events in adults 65 years of age or older: A comparison between high-dose and standard-dose inactivated influenza vaccines. Vaccine. 2015;33:4988–4993. doi: 10.1016/j.vaccine.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 88.Van Ermen A., Hermanson M.P., Moran J.M., Sweitzer N.K., Johnson M.R., Vardeny O. Double dose vs. standard dose influenza vaccination in patients with heart failure: a pilot study. Eur J Heart Fail. 2013;15:560–564. doi: 10.1093/eurjhf/hfs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Albrecht C.M., Sweitzer N.K., Johnson M.R., Vardeny O. Lack of persistence of influenza vaccine antibody titers in patients with heart failure. J Card Fail. 2014;20:105–109. doi: 10.1016/j.cardfail.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vardeny O., Moran J.J.M., Sweitzer N.K., Johnson M.R., Hayney M.S. Decreased T-Cell responses to influenza vaccination in patients with heart failure. Pharmacotherapy. 2010;30:10–16. doi: 10.1592/phco.30.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vardeny O., Sweitzer N.K., Detry M.A., Moran J.M., Johnson M.R., Hayney M.S. Decreased immune responses to influenza vaccination in patients with heart failure. J Card Fail. 2009;15:368–373. doi: 10.1016/j.cardfail.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kadoglou N.P.E., Bracke F., Simmers T., Tsiodras S., Parissis J. Influenza infection and heart failure—vaccination may change heart failure prognosis? Heart Fail Rev. 2017;22:329–336. doi: 10.1007/s10741-017-9614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gravenstein S., Davidson H.E., Taljaard M. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir Med. 2017;5:738–746. doi: 10.1016/S2213-2600(17)30235-7. [DOI] [PubMed] [Google Scholar]
- 94.McElhaney J.E., Herre J.M., Lawson M.L., Cole S.K., Burke B.L., Hooton J.W. Effect of congestive heart failure on humoral and ex vivo cellular immune responses to influenza vaccination in older adults. Vaccine. 2004;22:681–688. doi: 10.1016/j.vaccine.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 95.Siscovick D.S. Influenza vaccination and the risk of primary cardiac arrest. Am J Epidemiol. 2000;152:674–677. doi: 10.1093/aje/152.7.674. [DOI] [PubMed] [Google Scholar]
- 96.Nichol K.L., Nordin J., Mullooly J., Lask R., Fillbrandt K., Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348:1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 97.Gwini S.M., Coupland C.A.C., Siriwardena A.N. The effect of influenza vaccination on risk of acute myocardial infarction: self-controlled case-series study. Vaccine. 2011;29:1145–1149. doi: 10.1016/j.vaccine.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 98.Siriwardena A.N., Gwini S.M., Coupland C.A.C. Influenza vaccination, pneumococcal vaccination and risk of acute myocardial infarction: matched case-control study. Can Med Assoc J. 2010;182:1617–1623. doi: 10.1503/cmaj.091891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Govaert T.M., Thijs C.T., Masurel N., Sprenger M., Dinant G., Knottnerus J. The efficacy of influenza vaccination in elderly individuals. JAMA. 1994;272:1661. [PubMed] [Google Scholar]
- 100.Gurfinkel E.P., Leon de la Fuente R., Mendiz O., Mautner B. Influenza vaccine pilot study in acute coronary syndromes and planned percutaneous coronary interventions. Circulation. 2002;105:2143–2147. doi: 10.1161/01.cir.0000016182.85461.f4. [DOI] [PubMed] [Google Scholar]
- 101.Gurfinkel E., Leon De La Fuente R., Mendiz O., Mautner B. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) study. Eur Heart J. 2004;25:25–31. doi: 10.1016/j.ehj.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 102.Ciszewski A., Bilinska Z.T., Brydak L.B. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. Eur Heart J. 2008;29:1350–1358. doi: 10.1093/eurheartj/ehm581. [DOI] [PubMed] [Google Scholar]
- 103.Ciszewski A., Bilińska Z.T., Kępka C., Kruk M., Księżycka-Majczyńska E., Różyłło W. The protective effect of influenza vaccination on the clinical course of coronary disease in patients with acute coronary syndromes treated by primary PCI – a report from FLUCAD study. Adv Interv Cardiol. 2010;1:6–11. [Google Scholar]
- 104.Phrommintikul A., Kuanprasert S., Wongcharoen W., Kanjanavanit R., Chaiwarith R., Sukonthasarn A. Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur Heart J. 2011;32:1730–1735. doi: 10.1093/eurheartj/ehr004. [DOI] [PubMed] [Google Scholar]
- 105.Udell J.A., Zawi R., Bhatt D.L. Association between influenza vaccination and cardiovascular outcomes in high-risk patients. JAMA. 2013;310:1711. doi: 10.1001/jama.2013.279206. [DOI] [PubMed] [Google Scholar]
- 106.Bermúdez-Fajardo A., Oviedo-Orta E. Influenza vaccination promotes stable atherosclerotic plaques in apoE knockout mice. Atherosclerosis. 2011;217:97–105. doi: 10.1016/j.atherosclerosis.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 107.Veljkovic V., Glisic S., Veljkovic N. Influenza vaccine as prevention for cardiovascular diseases: possible molecular mechanism. Vaccine. 2014;32:6569–6575. doi: 10.1016/j.vaccine.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 108.Ding Q., Lu P., Fan Y., Xia Y., Liu M. The clinical characteristics of pneumonia patients coinfected with 2019 novel coronavirus and influenza virus in Wuhan, China. J Med Virol. 2020 Mar 20 doi: 10.1002/jmv.25781. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu X., Cai Y., Huang X. Co-infection with SARS-CoV-2 and influenza A virus in patient with pneumonia, China. Emerg Infect Dis. 2020;26:1324–1326. doi: 10.3201/eid2606.200299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khodamoradi Z., Moghadami M., Lotfi M. Co-infection of coronavirus disease 2019 and influenza A: a report from Iran. Arch Iran Med. 2020;23:239–243. doi: 10.34172/aim.2020.04. [DOI] [PubMed] [Google Scholar]
- 111.Cuadrado-Payán E., Montagud-Marrahi E., Torres-Elorza M. SARS-CoV-2 and influenza virus co-infection. Lancet. 2020;395:e84. doi: 10.1016/S0140-6736(20)31052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Azekawa S., Namkoong H., Mitamura K., Kawaoka Y., Saito F. Co-infection with SARS-CoV-2 and influenza A virus. IDCases. 2020;20:e00775. doi: 10.1016/j.idcr.2020.e00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Konala V.M., Adapa S., Gayam V. Co-infection with influenza A and COVID-19. Eur J Case Reports Intern Med. 2020;7:1. doi: 10.12890/2020_001656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim D., Quinn J., Pinsky B., Shah N.H., Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323:2085. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martines R.B., Ritter J.M., Matkovic E. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg Infect Dis. 2020:26. doi: 10.3201/eid2609.202095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Madjid M., Alfred A., Sahai A., Conyers J.L., Casscells S.W. Factors contributing to suboptimal vaccination against influenza: results of a nationwide telephone survey of persons with cardiovascular disease. Texas Hear Inst J. 2009;36:546–552. [PMC free article] [PubMed] [Google Scholar]
- 117.Bhatt A.S., Liang L., DeVore A.D. Vaccination trends in patients with heart failure. J Am Coll Cardiol HF. 2018;6:844–855. doi: 10.1016/j.jchf.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 118.Loeb M., Dokainish H., Dans A. Randomized controlled trial of influenza vaccine in patients with heart failure to reduce adverse vascular events (IVVE): rationale and design. Am Heart J. 2019;212:36–44. doi: 10.1016/j.ahj.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fröbert O., Götberg M., Angerås O. Design and rationale for the Influenza vaccination After Myocardial Infarction (IAMI) trial. A registry-based randomized clinical trial. Am Heart J. 2017;189:94–102. doi: 10.1016/j.ahj.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 120.Erlinge D., Koul S., Eriksson P. Bivalirudin versus heparin in non-ST and ST-segment elevation myocardial infarction—a registry-based randomized clinical trial in the SWEDEHEART registry (the VALIDATE-SWEDEHEART trial) Am. Heart J. 2016;175:36–46. doi: 10.1016/j.ahj.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 121.Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.WHO (World Health Organization) Influenza surveillance and monitoring. 2019. https://www.who.int/influenza/surveillance_monitoring/en/ Available at:
- 123.Neumann G., Kawaoka Y. Predicting the next influenza pandemics. J Infect Dis. 2019;219:S14–S20. doi: 10.1093/infdis/jiz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.The Lancet Infectious Diseases How to be ready for the next influenza pandemic. Lancet Infect Dis. 2018;18:697. doi: 10.1016/S1473-3099(18)30364-5. [DOI] [PubMed] [Google Scholar]
- 125.Graham B.S., Mascola J.R., Fauci A.S. Novel vaccine technologies. JAMA. 2018;319:1431. doi: 10.1001/jama.2018.0345. [DOI] [PubMed] [Google Scholar]
- 126.Graham B.S., Sullivan N.J. Emerging viral diseases from a vaccinology perspective: preparing for the next pandemic. Nat Immunol. 2018;19:20–28. doi: 10.1038/s41590-017-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Flannery B., Kondor R.J.G., Chung J.R. Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J Infect Dis. 2019 doi: 10.1093/infdis/jiz543. https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiz543/5609441 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Abbasi J. The search for a universal flu vaccine heats up. JAMA. 1942;2019:322. doi: 10.1001/jama.2019.16816. [DOI] [PubMed] [Google Scholar]
- 129.Eisenstein M. Towards a universal flu vaccine. Nature. 2019;573:S50–S52. doi: 10.1038/d41586-019-02751-w. [DOI] [PubMed] [Google Scholar]
- 130.MacIntyre C.R., Mahimbo A., Moa A.M., Barnes M. Influenza vaccine as a coronary intervention for prevention of myocardial infarction. Heart. 2016;102:1953–1956. doi: 10.1136/heartjnl-2016-309983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang T., Du Z., Zhu F. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395:e52. doi: 10.1016/S0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Centers for Disease Control Seasonal Influenza Vaccine Effectiveness, 2016-2019. 2018. https://www.cdc.gov/flu/vaccines-work/2016-2017.html Available at:
- 133.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. Features, evaluation and treatment coronavirus (COVID-19). 2020. http://www.ncbi.nlm.nih.gov/pubmed/32150360 Available at: [PubMed]
- 134.Centers for Diseases Control and Prevention (CDC) Key facts about influenza (Flu). 2020. https://www.cdc.gov/flu/about/keyfacts.htm Available at:
- 135.Paget J., Spreeuwenberg P., Charu V. Global mortality associated with seasonal influenza epidemics: new burden estimates and predictors from the GLaMOR Project. J Glob Health. 2019;9:020421. doi: 10.7189/jogh.09.020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sudharsanan N., Didzun O., Bärnighausen T., Geldsetzer P. The contribution of the age distribution of cases to COVID-19 case fatality across countries. Ann Intern Med. 2020 Jul 22 doi: 10.7326/M20-2973. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.European Centre for Disease Prevention and Control Factsheet about seasonal influenza. 2020. https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet Available at:
- 138.University of Minnesota The Center for Infectious Disease Research and Policy’s universal influenza vaccine technology landscape. 2020. https://www.cidrap.umn.edu/universal-influenza-vaccine-technology-landscape Available at:
- 139.WHO (World Health Organization) Vaccine use. 2020. https://www.who.int/influenza/vaccines/use/en/ Available at:
- 140.European Centre for Disease Prevention and Control Risk groups for severe influenza. 2020. https://www.ecdc.europa.eu/en/seasonal-influenza/prevention-and-control/vaccines/risk-groups Available at:
- 141.Smith S.C., Jr., Allen J., Blair S.N. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2006;47:2130–2139. doi: 10.1016/j.jacc.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 142.Knuuti J., Wijns W., Saraste A. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 143.American Diabetes Association/ADA Flu and pneumonia shots. 2020. https://www.diabetes.org/diabetes/medication-management/flu-and-pneumonia-shots Available at:
- 144.Ivers N.M., Jiang M., Alloo J. Diabetes Canada 2018 clinical practice guidelines: key messages for family physicians caring for patients living with type 2 diabetes. Can Fam Physician. 2019;65:14–24. [PMC free article] [PubMed] [Google Scholar]
- 145.Worrall G. Influenza. Can Fam Physician. 2008;54:415–416. [PMC free article] [PubMed] [Google Scholar]