Abstract
Background:
Insomnia is a common disease characterized by difficulty falling and/or staying asleep, and accompanied by irritability or fatigue during wakefulness. It is widely reported that insomnia is one of the most extensive mental disorders which the incidence rate is estimated to be about 10%. Insomnia can have serious influences on patients health and quality of life. Electro acupuncture (EA) is reported to be efficacious and widely used for the treatment of insomnia in China. This overview aims to summarize the available evidence from current systematic reviews for the efficacy of electroacupuncture therapy for insomnia.
Methods:
We will make a comprehensive retrieval in 7 databases as following:
-
1.
Embase;
-
2.
Cochrane Library;
-
3.
Pubmed;
-
4.
Chinese databases SinoMed (previously called the Chinese Biomedical Database);
-
5.
Chinese National Knowledge Infrastructure (CNKI);
-
6.
Chinese Scientific Journals Database (VIP);
-
7.
Wanfang Data (WF).
The time is limited from the construction of the library to August 2020. We will use the Assessment of Multiple Systematic Reviews-2 (AMSTAR-2) tool to evaluate methodological quality. Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols (PRISMA-P) will be used in the report checklist to assess the quality of reports in the study. The Grading of the Classification of Recommendations, Evaluation, Development and Evaluation (GRADE) will be used to evaluate the included SRs and meta-analysis. Our reviewers will conduct systematic reviews, qualification evaluation, data extraction, methodological quality and evidence quality screening in pairs. The outcomes of interest include: The Pittsburgh Sleep Quality Index (PSQI), The Insomnia Severity Index (ISI), Athens Insomnia Scale, Sleep parameters measured by either subjective or objective approaches, such as actigraphy, polysomnogram, and electroencephalogram. Or any other scale used to assess the level of illness. The evidence will be synthesized where appropriate based on patient subgroups and outcomes.
Results:
The results will be published in a peer-reviewed journal.
Trial registration number:
INPLASY202080087.
Conclusion:
This overview will provide comprehensive evidence of EA for patients with insomnia.
Keywords: electroacupuncture, insomnia, overview, protocol
1. Introduction
Insomnia is a high incidence of adults, and its therapeutic effects are often unsatisfactory.[1,2] The main clinical manifestations are difficulty falling asleep, low sleep quality, easy awakening, forgetfulness, and daytime sleepiness.[3] According to the survey, about 12% to 20% of people can be diagnosed with insomnia, and 35% to 50% of adults have related symptoms.[4,5] Recent studies have shown that insomnia can also lead to cardiovascular and cerebrovascular diseases, endocrine disorders, and mental illnesses.[6,7,8] Not only that, insomnia, and cause apparent economic losses to society.[9] Therefore, it is essential to research the treatment of insomnia better to relieve patients physical and mental health.
Modern medicine believes that old age, women, internal medicine, and mental illness can all cause insomnia.[10] Studies have shown that older adults with respiratory symptoms and physical disabilities increase insomnia risk.[11] Not only that, but taking some drugs can also cause insomnia, including beta-blockers, glucocorticoids, nonsteroidal anti-inflammatory drugs, decongestants, and antiandrogens.[12] The etiology and pathophysiology of insomnia are also inseparable from heredity and the environment.[13]
Western medicine treatment of insomnia is mainly divided into psychotherapy and drug treatment. The most commonly used methods of psychotherapy mainly include brief insomnia behavior therapy and cognitive insomnia behavior therapy. They can effectively improve sleep and have almost no side effects.[14,15] However, its clinical practice is limited by the lack of well-trained therapists and poor patient compliance.[16,17] Clinical drugs mainly include benzodiazepines, non-benzodiazepines, melatonin receptor agonists, orexin receptor antagonists, and antidepressants with hypnotic effects.[18] However, the dependence of drugs, the possibility of abuse, withdrawal syndrome, and the adverse effects of these drugs’ long-term use also bother doctors and patients.[19] These risk factors prompt patients to seek safer and more effective alternative therapies, such as EA, which is considered an excellent choice by the general public.
Traditional Chinese medicine(TCM) believes that the central disease of insomnia lies in the heart, closely related to the dysfunction of the liver, spleen, and kidneys.[20] Based on TCM's theory, its basic pathogenesis is the loss of support or disturbance of mind.[21] And the imbalance between “yin” and “yang” is considered to be the total pathogenesis of insomnia.[22] Not only that, the meridian theory of TCM believes that the dysfunction of Yinqiao meridian and Yangqiao meridian is also a significant cause of insomnia.[23]
EA is one of the most popular TCM techniques used routinely in Asian countries and has a long history of treating insomnia. Many literature reports believe that EA has a unique clinical effect on insomnia.[24,25,26] Studies have found that EA can alleviate the abnormal excitement of the sympathetic nerve epithelial medulla system of insomnia rats, thereby effectively treating insomnia.[27] Not only that, EA can also regulate the balance of neurotransmitters in the brain, such as GABA and Glu, the 2 most crucial amino acid neurotransmitters involved in sleep regulation.[28] In addition, EA can also reduce the levels of IL-1β, IL-2, and TNF-α in the hippocampus of the brain, thereby improving sleep quality.[29]
Many randomized controlled trials (RCTs) have confirmed the efficacy of EA in insomnia treatment.[30,31,32] Many meta-analyses also show that EA treatment has specific benefits for insomnia patients.[33,34] However, there has not been a rigorously designed overview to evaluate the systematic evaluation of insomnia by EA. Therefore, this study evaluates and summarizes the clinical research literature of EA in the treatment of insomnia at home and abroad to provide evidence-based medicine for clinical practice.
2. Methods
2.1. Study registration
This protocol was designed in accordance with the methodological guidelines for overviews provided by the Cochrane Handbook for Systematic Reviews of Interventions.[35] It is registered on the International Prospective Register of Systematic Reviews. (Registration number INPLASY202080087; https://inplasy.com/inplasy-2020–8-0087/.)
2.2. Inclusion and exclusion criteria
PICOS will be applied, including Population, Intervention, Comparison, Outcome, and Study.
2.2.1. Type of study
It only includes systematic reviews and meta-analysis of randomized controlled trials (RCT) on EA in insomnia patients published in English and Chinese.
2.2.2. Type of participants
It will include a systematic review of people diagnosed with insomnia. Regardless of gender, race, occupation, education, nationality, etiology, and severity, all insomnia study participants of all ages can be included.
2.2.3. Type of interventions
One therapeutic intervention used in the experimental group is that EA will be included.
2.2.4. Type of comparator (s)/control
The control group can be a blank control group, a placebo group, a psychological control group or a drug treatment group. If the combination therapy has the same 2 groups, it will also include the combination of EA and other therapies.
2.2.5. Types of outcome measurements
2.2.5.1. Primary outcomes
The Pittsburgh Sleep Quality Index (PSQI) is widely used to assess a persons sleep quality. It consists of 19 self-evaluation items and 5 other evaluation items. The score will indicate the level of sleep quality and the severity of the sleep disorder.[36]
2.2.5.2. Secondary outcomes
Secondary outcomes mainly include the following aspects:
-
1.
The Insomnia Severity Index (ISI).
-
2.
Athens Insomnia Scale.
-
3.
Sleep parameters measured by either subjective or objective approaches, such as actigraphy, polysomnogram, and electroencephalogram.
-
4.
Adverse effect, such as vomiting, nausea, or dizziness.
2.2.6. Study Design
SRs containing more than 1 RCT were included. There is no systematic reviews, no separate summary of RCT data, and abstracts without sufficient data will be excluded.
2.3. Search methods for identification of studies
We searched three foreign electronic databases (Cochrane Library, Embase, Pubmed) and 4 Chinese electronic databases (China National Knowledge Infrastructure (CNKI), WangFang Database, Chinese Biomedical Literature Database (CBM) and Chinese Scientific Journal Database (VIP) to collect potential systematic reviews (SRs) from their inceptions to August 2020. The language of publication is limited to Chinese or English. The following search terms will be used:Disorders of Initiating and Maintaining Sleep, Disorders of Initiating and Maintaining Sleep, Early Awakening, Nonorganic Insomnia, Insomnia, Primary Insomnia, Transient Insomnia, Rebound Insomnia, Secondary Insomnia, Sleep Initiation Dysfunction, Sleeplessness, Insomnia Disorder, Psychophysiological Insomnia, Electroacupuncture, Electroacupuncture, complementary therapy of electroacupuncture, Traditional Chinese therapy of electroacupuncture, systematic review, meta-analysis, et al. A draft search strategy using Pubmed, one of the planned electronic databases to be searched, is presented in Table 1.
Table 1.
The search strategy for Pubmed.

2.4. Studies selection
Studies will be identified using NoteExpress 3.2.0. After the initial removal of duplicate studies, 2 reviewers (ZHC and ZRL) will independently screen titles and abstracts based on the eligibility criteria. Full-text studies will be retrieved for all potentially includable SRs or SR protocols. If studies contain insufficient information to make a decision about eligibility, GMH will try to contact authors of the original reports to obtain further details. During the procedure, disagreements will be resolved by discussion or consensus with the third reviewer (RY). Study selection will be performed in accordance with the PRISMA flowchart (Fig. 1).
Figure 1.
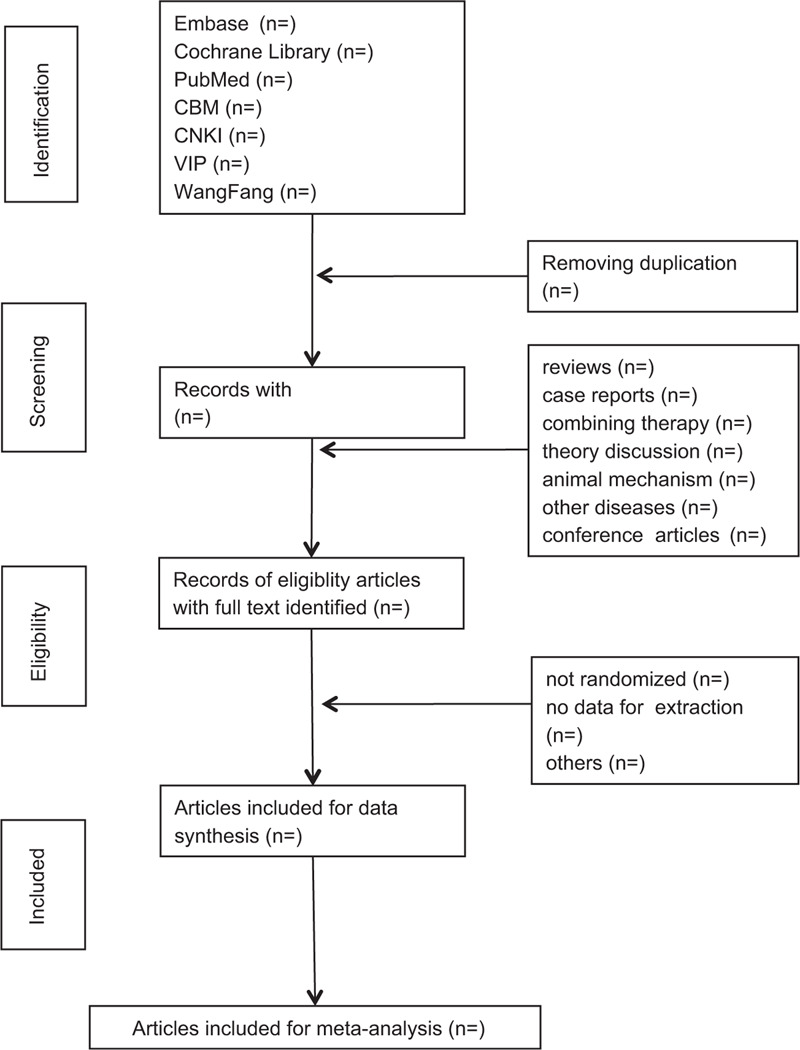
Flowchart of literature selection.
2.5. Data extraction
Two researchers (YNY and SSZ) extracted literature information based on inclusion and exclusion criteria, including the following:
-
1.
Study characteristics: author, year, study design, sample size and follow-up time;
-
2.
Patient characteristics: age, sex, and type of SP;
-
3.
Intervention: intervention measures in the experimental group, intervention measures in the control group);
-
4.
Outcome of the study: Two researchers (DSW and QJM) cross-checked the extraction results of the extracted documents. If there is any difference, you should consult a third party (ZHC) to resolve.
2.6. Evaluate the methodological quality of included studies
Two reviewers (XCZ and ZHC) will use the Assessment of Multiple Systematic Reviews 2 (AMSTAR-2) measurement tool to independently assess each SRs methodological quality that meets the inclusion criteria.[37] This is most commonly used to assess the quality of systematic reviews included in overviews. AMSTAR-2 is an update of AMSTAR, which can be used to appraise SRs of both randomized and non-randomized controlled trials. AMSTAR-2 includes 16 items, with each of the 16 criteria given a rating of “yes” (definitely done), “no” (definitely not done), “cannot report” (unclear if completed), or “not applicable” based on the information provided by the systematic review, when the standards are met, the evaluator will evaluate the evaluation. Disagreements will be resolved through discussions between them and arbitrated by the third general author (YS) if necessary.
2.7. Evaluation of the reporting quality of the included studies
The 2 authors of the overview (ZHC and JX) will independently evaluate the reports’ quality in each review to assess whether they meet the criteria specified in Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols (PRISMA-P).[38] If there are any differences, they will be resolved through discussion between them and arbitrated by the third general author (XCZ) if necessary.
2.8. Evaluation of the evidence quality of the included studies
The quality of evidence of the included SRs was assessed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.[39] This tool aims to assess the quality of evidence for each outcome indicator in the study. The 2 authors (ZRL and GMH) will independently evaluate the evidence of the results and should describe in detail the degradation or upgrade factors that affect the quality of the evidence to ensure the reliability and transparency of the results. Any disagreements will be resolved through discussion by 2 authors. The overall quality of evidence was judged as”high,” “moderate,” “low,” or “very low.”
2.9. Dealing with lost data
If there is no specific data or insufficient data in the published SRs, the author will be contacted by email or phone to provide the necessary information. If we cannot obtain enough data, the data will be discarded. The analysis will be based on the available data and the potential impact of missing data will be discussed.
2.10. Synthesis of data
Before data synthesis, the included SRs and meta-analysis should be considered. For different situations, different measures will be taken for overlapping basic research: whether the basic research completely overlaps, the comment with the highest quality will be selected. If the main research partially overlaps, when the lower quality reviews include more than one-third of the new research. If the basic research does not overlap, the 2 comments will remain. The quality of the review will be fully assessed Use ROBIS and AMSTAR-2. Besides, RevMan5.3.5 software will be used to calculate the standardized effect. The random-effects model (I 2 ≥ 50%) or fixed-effects model (I 2 < 50%) will be selected according to the heterogeneity levels of the included SRs and meta-analyses. If the I 2 value is higher than 75%, the clinical or methodological heterogeneity will be explored through discussion with the review team. When the meta-analysis is not possible, a narrative analysis will be performed. Indirect comparisons of different EA therapies will also be conducted using relative effectiveness outcomes including relative sensitivity and relative specificity.
3. Discussion
Insomnia is a disease that frequently occurs at any age. It not only seriously affects the work and life of patients, but also produces depression and anxiety. EA as an effective technique of TCM, has been accepted for insomnia in China. However, due to the lack of rigorous review evidence for EA treatment, clinicians cannot choose the best method. As a result, patients with insomnia are prone to delays. Therefore, the results of this overview will provide real and reliable research evidence for the treatment of insomnia with EA.
The study also has some defects as follows: low quality of original researches, the possible occurrence of false positive or false negative results, various duration of disease different dosage, and frequency of intervention, language restriction, etc. All of these will lead to some bias and influence the results of evaluation results, ultimately affecting this studys reliability.
Author contributions
Conceptualization: Xingchen Zhou, Zhenhai Chi.
Data curation: Xingchen Zhou, Jun Xiong, Guomin Huang, Ziru Li, Yanan Yang, Rui Yang, Shuisheng Zhou, Qiangjian Mao, Desheng Wu, Yang Shen.
Formal analysis: Xingchen Zhou, Jun Xiong.
Investigation: Xingchen Zhou, Zhenhai Chi, Jun Xiong.
Methodology: Xingchen Zhou, Guomin Huang, Ziru Li, Yanan Yang.
Software: Jun Xiong, Shuisheng Zhou.
Supervision: Zhenhai Chi, Jun Xiong.
Writing – original draft: Xingchen Zhou, Zhenhai Chi, Rui Yang, Qiangjian Mao.
Writing – review & editing: Zhenhai Chi, Jun Xiong, Desheng Wu, Yang Shen.
Footnotes
Abbreviations: AMSTAR-2 = Assessment of Multiple Systematic Reviews-2, EA = electroacupuncture, GRADE = grading of recommendations, assessment, development, and evaluation, PRISMA-P = Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols, RCTs = randomized controlled trials, SRs = systematic reviews, TCM = Traditional Chinese medicine.
How to cite this article: Zhou X, Chi Z, Xiong J, Huang G, Li Z, Yang Y, Zhou S, Yang R, Mao Q, Wu D, Shen Y. Effectiveness and safety of electroacupuncture for insomnia: a protocol for an overview of systematic reviews and meta-analysis. Medicine. 2020;99:40(e22502).
This work was supported by Project of Jiangxi University of Traditional Chinese Medicine 1050 youth talent project (Grant number: 5141900101), National Natural Science Foundation (Grant number: 81573835), Distinguished Young Talents Plan of Jiangxi Province (Grant No. 20171BCB23093), and Natural Science Youth Foundation Key Projects of Jiangxi Province (Grant No. 20192ACB21007).
This studys data are based on published systematic reviews, so ethical approval and patient consent are not required. The articles in this overview will be submitted to peer-reviewed journals for publication. The review will also be distributed in electronic and printed forms to help guide EA's clinical practice.
The authors have no conflicts of interest to declare.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Sutton EL. Insomnia. Med Clin North Am 2014;98:565–81. [DOI] [PubMed] [Google Scholar]
- [2].Siebern AT, Suh S, Nowakowski S. Non-pharmacological treatment of insomnia. Neurotherapeutics 2012;9:717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grandner MA, Perlis ML. Treating insomnia disorder in the context of medical and psychiatric comorbidities. JAMA Intern Med 2015;175:1472–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morin CM, LeBlanc M, Belanger L, et al. Prevalence of insomnia and its treatment in Canada. Can J Psychiatry 2011;56:540–8. [DOI] [PubMed] [Google Scholar]
- [5].Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; international statistical classifification of diseases and related health problems, tenth revision; and research diagnostic criteria/international classifification of sleep disorders, second edition criteria: results from the America insomnia survey. Biol Psychiatry 2011;69:592–600. [DOI] [PubMed] [Google Scholar]
- [6].Javaheri S, Redline S. Insomnia and risk of cardiovascular disease. Chest 2017;152:435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li M, Zhang XW, Hou WS, et al. Insomnia and risk of cardiovascular disease: a meta-analysis of cohort studies. Int J Cardiol 2014;176:1044–7. [DOI] [PubMed] [Google Scholar]
- [8].Dolsen MR, Asarnow LD, Harvey AG. Insomnia as a transdiagnostic process in psychiatric disorders. Curr Psychiatry Rep 2014;16:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kessler RC, Berglund PA, Coulouvrat C, et al. Insomnia and the performance of US workers: results from the America insomnia survey. Sleep 2011;34:1161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roehrs TA, Roth T. Hyperarousal in insomnia and hypnotic dose escalation. Sleep Med 2016;23:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Foley DJ, Monjan A, Simonsick EM, et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep 1999;22: Suppl 2: S366–72. [PubMed] [Google Scholar]
- [12].Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther 2016;38:2340–72. [DOI] [PubMed] [Google Scholar]
- [13].Riemann D, Nissen C, Palagini L, et al. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol 2015;14:547–58. [DOI] [PubMed] [Google Scholar]
- [14].Taylor DJ, Peterson AL, Pruiksma KE, et al. Impact of cognitive behavioral therapy for insomnia disorder on sleep and comorbid symptoms in military personnel: a randomized clinical trial. Sleep 2018;41.in press, DOI: 10.1093/sleep/zsy069. [DOI] [PubMed] [Google Scholar]
- [15].Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev 2016;27:20–8. [DOI] [PubMed] [Google Scholar]
- [16].Buford UJ, Nemeroff CB. Current treatment options for insomnia. Drugs Today (Barc) 2012;48:415–23. [DOI] [PubMed] [Google Scholar]
- [17].Matthews EE, Arnedt JT, McCarthy MS, et al. Adherence to cognitive behavioral therapy for insomnia: a systematic review. Sleep Med Rev 2013;17:453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sleep Disorder Section, Neurology Branch. Chinese Medical Association Chinese guideline for the diagnosis and treatment of insomnia in adults (2017). Chin J Neurol 2018;51:324–35. [Google Scholar]
- [19].Logan BK, Mohr ALA, Friscia M, et al. Reports of adverse events associated with use of novel psychoactive substances, 2013-2016: a review. J Anal Toxicol 2017;41:573–610. [DOI] [PubMed] [Google Scholar]
- [20].Yang Junxiong, Zhang Jianping, Yu Jianchun, et al. Study on the clinical efficacy of acupuncture and moxibustion in the treatment of insomnia. Chin General Pract 2013;16:466–8. [Google Scholar]
- [21].O’Brien K, Weber D. Insomnia in Chinese medicine: the heart of the matter. J Altern Complement Med 2016;22:684–94. [DOI] [PubMed] [Google Scholar]
- [22].Insomnia TCM Clinical Practice Guidelines Research Group. Insomnia TCM Clinical Practice Guidelines (WHO/WPO). World J Sleep Med 2016;3:8–25. [Google Scholar]
- [23].Liaoning University of Traditional Chinese Medicine, Bai Zhongguo. Study on Sleep Conditioning Mechanism and Application of “Huangdi Neijing”. 2019;doi:10.27213/d.cnki.glnzc.2019.000034. [Google Scholar]
- [24].Kim M, Kim JE, Lee HY, et al. Electroacupuncture for treating insomnia in patients with cancer: a study protocol for a randomised pilot clinical trial. BMJ Open 2017;7:e016269.Published 2017 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen L. Zhongguo Zhen Jiu 2005;25:687–8. [PubMed] [Google Scholar]
- [26].Ernst E, Lee MS, Choi TY. Acupuncture for insomnia? An overview of systematic reviews. Eur J Gen Pract 2011;17:116–23. [DOI] [PubMed] [Google Scholar]
- [27].Chengdu University of Traditional Chinese Medicine, Liu Ping Study on the effect of electroacupuncture on anxiety, depression and sympathetic-adrenal medulla system in insomnia rats. 2014. [Google Scholar]
- [28].Ruan Jiyuan. Effects of electroacupuncture on the content of glutamate and (-aminobutyric acid and the expression of (-aminobutyric acid type A receptor in the brain of insomnia rats. Chin J Traditi Chin Med 2013;28:3657–60. [Google Scholar]
- [29].Chen Lihua, Hu Youping, Luo Rong, et al. Effects of electroacupuncture at Baihui and Sanyinjiao on cytokines in rats with sleep deprivation. Shanghai J Acupuncture Moxibustion 2009;28:678–81. [Google Scholar]
- [30].Yin X, Dong B, Liang T, et al. Efficacy and safety of electroacupuncture on treating depression-related insomnia: a study protocol for a multicentre randomised controlled trial. BMJ Open 2019;9:e021484.Published 2019 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Xing J, Wu X, Liu H, et al. Effects of electroacupuncture therapy and cognitive behavioral therapy in chronic insomnia: a randomized controlled study. Evid Based Complement Alternat Med 2020;2020:5630130.Published 2020 Mar 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim SP, Kim JH, Kim BK, et al. Electroacupuncture for insomnia disorder: study protocol for a randomized controlled trial. Trials 2017;18:177.Published 2017 Apr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Li Z, Zhang Y, Wang Y, et al. Electroacupuncture for primary insomnia: protocol for a systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e11063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wei Xinran, Wei Gaowen, Chen Xiaoli, et al. Systematic evaluation of the effect of electroacupuncture on PSQI score in patients with primary insomnia. J Acupuncture Moxibustion 2016;32:60–4. [Google Scholar]
- [35].Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA 2015;313:1657–65. [DOI] [PubMed] [Google Scholar]
- [36].Buysse DJ, Reynolds CF, III, Monk TH, et al. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- [37].Zhang Fangyuan, Shen Aomei, Zeng Xiantao, et al. Interpretation of amstar 2, a quality evaluation tool for systematic evaluation methodology. Chin J Evidence Based Cardiovascular Med 2018;10:14–8. [Google Scholar]
- [38].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339. [PMC free article] [PubMed] [Google Scholar]
- [39].Deng Tong, Wang Yang, Huang Di, et al. Methods for formulating clinical practice guidelines - grade method theory. Chin J Evidence Based Cardiovascular Med 2018;10: 1441-1445 + 1449. [Google Scholar]


