Abstract
At present, glycated hemoglobin (HbA1c) and glycated albumin (GA) are used to evaluate glycemic control in diabetic patients, but they cannot reflect insulin deficiency and/or insulin resistance.
We investigated the feasibility of using estimated average glucose to fasting plasma glucose ratio (eAG/fPG ratio) to estimate insulin resistance in young adult diabetes. A total of 387 patients with type 2 diabetes were included and were stratified into 2 groups based on median values of the glycemic index ratio: the GA/A1c ratio <2.09 (n = 91) and ≥2.09 (n = 296); the eAG/fPG ratio <1.69 (n = 155) and ≥1.69 (n = 232). HbA1c, GA, fructosamine, insulin, and C-peptide levels were measured. The ratio of GA to HbA1c was calculated, and the homeostasis model assessment of β-cell function and insulin resistance were determined. The homeostasis model assessment of insulin resistance level was significantly associated with the eAG/fPG ratio, but not with the ratio of GA to HbA1c, GA, HbA1c, and fructosamine levels. The ratio of estimated average glucose to fasting plasma glucose level correlates with insulin resistance in young adult diabetes.
Keywords: estimated average glucose to fasting plasma glucose ratio, glycated hemoglobin, homeostasis model assessment of β-cell function, type 2 diabetes mellitus, young diabetes
1. Introduction
Diabetes is a group of disorders characterized by chronic elevations in blood glucose (hyperglycemia) resulting from insulin deficiency and/or insulin resistance.[1,2] Fructosamine, glycated albumin (GA), and glycated hemoglobin (HbA1c) are glycated proteins that are used to evaluate glycemic control in diabetic patients.[3] The lifespan of HbA1c is estimated to be about 90 to 120 days. Thus, it is known as an indicator of long-term glycemic control.[4,5] However, HbA1c is not suitable for the assessment of a patient's glycemic status in certain disorders including hemoglobinopathies and disorders with abnormal red blood cell turnover. For example, in patients with anemia, HbA1c is not accurate in reflecting the real plasma glucose burden.[6]
Fructosamine is an index of glycemic control in the past 2 weeks. Fructosamine is not affected by changes in hemoglobin metabolism, but can be influenced by disorders in protein turnover (ie, dysproteinemias). In addition, substances with low molecular weight such as uric acid and urea strongly influence fructosamine levels.[7]
GA, which is not influenced by changes in the lifespan of erythrocytes, is thought to be a good alternative indicator of glycemic control in diabetic patients.[8] GA is an index of glycemic control which is not affected by disorders of hemoglobin metabolism. Additionally, it reflects the short-term status of glycemic control compared with HbA1c. Furthermore, GA is not influenced by serum albumin concentration because it calculates the ratio of total serum albumin.[9]
GA/HbA1C ratio in patients with type 1 diabetes mellitus was shown to be significantly higher than that in patients with type 2 diabetes mellitus (T2DM).[10] There is a significant inverse correlation between GA/HbA1C ratio and homeostasis model assessment of β-cell function (HOMA-β)%, reflecting that the decreased endogenous insulin secretion is involved in an increased value of the GA/HbA1C ratio.
Few studies have closely examined the relationship between endogenous insulin production and the ratio of estimated average glucose to fasting plasma glucose levels (eAG/fPG ratio) in young adult diabetes. The present study investigated the usefulness of eAG/fPG ratio in estimating homeostasis model assessment of insulin resistance (HOMA-IR) in young adults with diabetes mellitus, and its difference with the ratio of GA to HbA1c (GA/A1c) ratio and the 3 glycated proteins.
2. Materials and methods
2.1. Subjects
A total of 387 patients with type 2 diabetes were studied, who came from the Tianjin Medical University Chu Hsien-I Memorial Hospital & Metabolic Diseases Hospital from October 2011 to January 2016. Their ages ranged from 10 to 25 (median age, 20 years). All patients fit the criteria for the diagnosis of diabetes by the American Diabetes Association.[11] Clinical and demographic data were collected retrospectively from a review of medical records. All patients signed at admission an informed consent, agreeing to partake in research projects as long as their confidentiality was respected. All procedures of this study respect the ethical standards of the Helsinki Declaration of 1975, as revised in 2008(5). This study was approved by the Institutional Review Board of Tianjin Medical University Chu Hsien-I Memorial Hospital & Metabolic Diseases Hospital (approval number: DXBYYhMEC2018-16).
2.2. Measurement of parameters
The following parameters were measured: glycated proteins (HbA1c, GA, and fructosamine), glucose levels (fPG and postprandial plasma glucose), glycemic index ratio (GA/A1c ratio and eAG/fPG ratio), β-cell function (C-peptide and insulin), anthropometric parameters (height, weight, and body mass index [BMI]), and glycemic index-associated parameters (serum albumin, hemoglobin, and serum creatinine). HOMA-β and HOMA-IR were determined by a HOMA calculator to assess the basal β-cell function and insulin resistance in patients with T2DM. BMI was calculated as weight in kilograms divided by the square of the height in meters. Blood specimens were collected before insulin treatment.
2.3. Calculation of eAG/fPG ratio
The eAG was calculated using the following equation: eAG (mmol/L) = 1.59 × HbA1c (%) – 2.59.[12] The eAG/fPG ratio was computed using the following formula: eAG/f PG ratio = eAG level (mmol/L)/f PG level (mmol/L). The estimated glomerular filtration rate was determined by the Schwartz formula[13]: estimated glomerular filtration rate (mL/min/1.73 m2) = proportionality constants × height (cm)/serum creatinine level (mg/dL). Patients with T2DM were stratified into 2 groups based on median values of the glycemic index ratio listed in Table 1: the GA/A1c ratio <2.09 (n = 91) and ≥2.09 (n = 296); the eAG/fPG ratio <1.69 (n = 155) and ≥1.69 (n = 232).
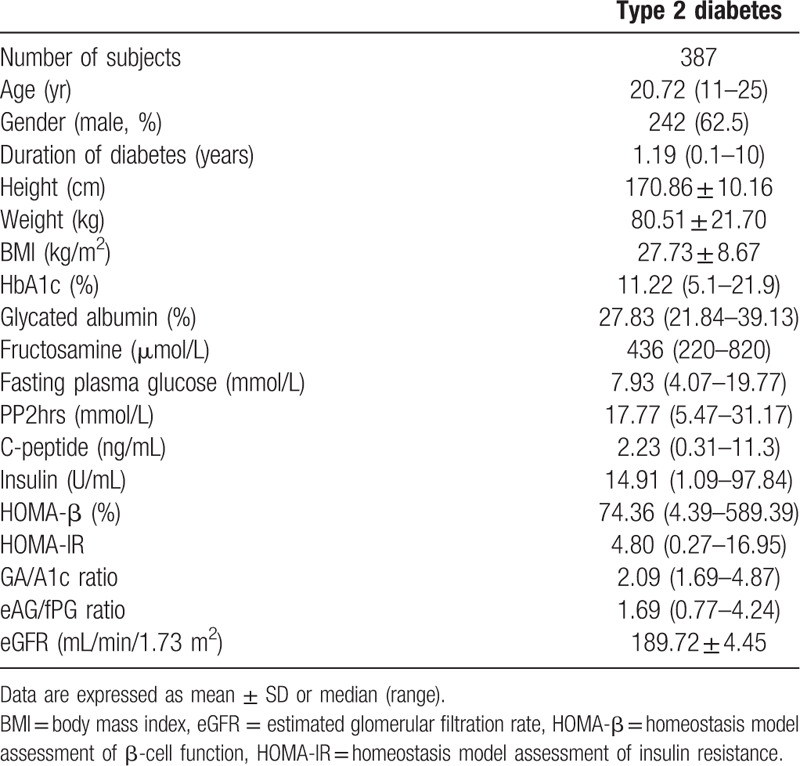
Table 1.
Baseline characteristics of the subject included in this study.
2.4. Statistical analysis
Data were presented as mean standard deviation if normally distributed and as median (range) if nonnormally distributed. Normality of the data distribution was tested by Kolmogorov–Smirnov 1-sample test. Categorical variables were expressed as frequencies and proportions. Mann–Whitney U test and Student t-test were used to analyze data between the 2 groups. A multivariate regression analysis of the eGA/fPG ratio, the GA/A1c ratio, GA, HbA1c, and fructosamine were conducted as a dependent variable in patients with T2DM. Adjustments for age, BMI, duration of diabetes, serum creatinine, and C-peptide levels were performed as independent variables. The P-value used to be considered significant as the following:
-
(1)
P > .05: nonsignificant
-
(2)
P < .05: significant
-
(3)
P < .01: highly significant.
3. Results
3.1. eAG/fPG ratio versus GA/A1c ratio
The baseline characteristics of all subjects are summarized in Table 1.
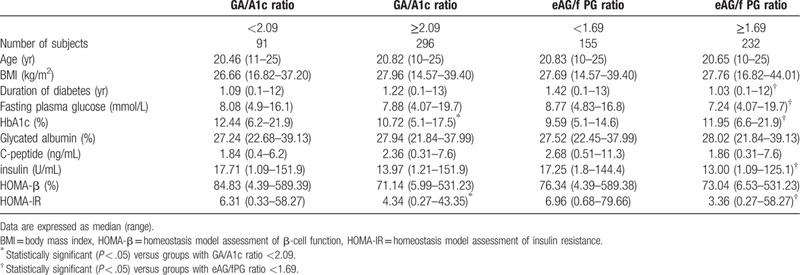
As shown in Table 2, duration of diabetes, fasting plasma glucose, HbA1c, insulin, and HOMA-IR levels in patients with eAG/fPG ratio ≥1.69 were significantly different from those with eAG/f PG <1.69 (1.03 vs 1.42 years, 7.24 vs 8.77 mmol/L, 11.95% vs 9.59%, 13.00 vs 17.25 U/mL, 3.36 vs 6.96; All <0.05). Similarly, HbA1c and HOMA- IR level was increased in patients with GA/A1c ratio <2.09 than in those with GA/A1c ratio ≥2.09 (12.44% vs 10.72%, 6.31 vs 4.34; All <0.05).
Table 2.
C-peptide, HOMA-β, and HOMA-IR levels according to the median values of the GA/A1c ratio and the eAG/fPG ratio in patients with T2DM.
3.2. Multivariate regression analysis
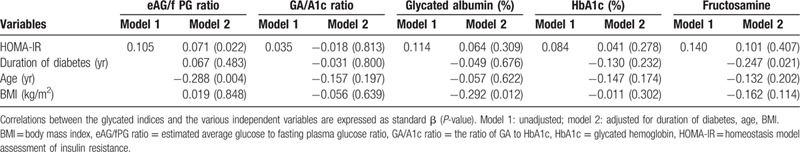
In a multivariate analysis adjusted for the independent variables, the HOMA-IR level was significantly associated with the eAG/fPG ratio, but not with the GA/A1c ratio, GA, HbA1c, or fructosamine levels (P < .05). There were significant correlations between the duration of diabetes and fructosamine; however, no significant correlation was observed between the eAG/fPG ratio, GA, HbA1c, GA/A1c ratio, and the duration of diabetes. The age was significantly associated with the eAG/fPG ratio, but not with the GA/A1c ratio, GA, HbA1c, or fructosamine levels. In contrast, BMI was significantly correlated to the GA, but was not correlated to the eAG/fPG ratio, GA/A1c ratio, fructosamine, or HbA1c levels (Table 3).
Table 3.
Multivariate regression analysis of the eGA/fPG ratio, the GA/A1c ratio, glycated albumin, HbA1c, and fructosamine as a respective dependent variable in patients with T2DM.
4. Discussion
With respect to the pathophysiology of type 2 diabetes, hyperglycemia is mainly influenced by dysfunction of pancreatic β-cells and increased insulin resistance.[14] Moreover, oxidative stress and inflammation have emerged as putative mechanisms in the development of cardiovascular disease in T2DM. Reactive oxygen species, via different metabolites and biomarkers, increase the activity of thrombocytes and lead to endothelial inflammation and proliferation of cells, as well as decrease the homeostasis in the vessel wall.[15] In this study, eAG/fPG ratio was determined using HbA1c-derived average glucose and fasting plasma glucose level. We used this parameter to estimate insulin resistance in patients with T2DM, and compared it with the well-known parameters, including GA/A1c ratio, HbA1c, GA, and fructosamine. Duration of diabetes, fasting plasma glucose, HbA1c, insulin, and HOMA-IR levels were significantly lower in patients with an increased eAG/fPG ratio than in those with a decreased eAG/f PG ratio. The eAG/f PG ratio was correlated with HOMA-IR, and this correlation was stronger than the correlation between GA/A1c ratio, HbA1c, GA, or fructosamine with HOMA-IR. These results suggest that the new parameter may reflect insulin resistance in young adult patients with T2DM.
Our data support the results of Huh et al,[16] who has found that the association between HOMA-β and GA/A1c ratio was not significant in diabetic patients. In contrast, Koga et al reported that HOMA-β had a significant inverse correlation with the GA/A1c ratio. In our study, the eAG/f PG ratio showed a negative correlation with the HOMA-IR level but showed no significant correlations with HOMA-β and C-peptide levels. Ji Eun Lee found that eAG/f PG was related with HOMA-β and BMI, but not with HOMA-IR. These discrepancies may reflect the differences in patient populations, the severity of disease, the status of glucose tolerance, or the number of subjects.[17]
The eAG infers an average glucose levels derived from HbA1c concentrations. Ji Eun Lee et al found that there was a relationship between eAG/fPG ratio and HOMA-β in children diabetes. In the present study, the eAG/fPG ratio was compared with the GA/A1c ratio to assess insulin resistance in child and adolescent diabetes. No similar study has been reported yet in this area.
After adjusting for age, BMI, duration of disease, the regression analysis consistently demonstrated a significant relationship between the eAG/fPG ratio and HOMA-IR. However, no significant correlation was observed between the GA/A1c ratio and HOMA-IR after adjusting for the corresponding parameters. These results suggest that eAG/fPG ratio is a better indicator of insulin resistance than GA/A1c ratio.
There are some limitations of our study. First, our study is an observational study not a cohort study. Second, the study was confined to young adult diabetes. The sample size of our study was too small to analyze the data in more detail. Further prospective studies with large sample size are needed especially in adult diabetic patients for the validation of the new parameter. Despite these limitations of our study, we believe that the eAG/f PG ratio is helpful for assessing HOMA-IR at least in young adult diabetes who has only the results of fasting plasma glucose and HbA1c tests.
5. Conclusions
This study shows that the eAG/fPG ratio is more closely associated with HOMA-IR than the GA/HbA1c ratio, suggesting that the eAG/fPG ratio has a more significant implication with Insulin resistance than the GA/HbA1c ratio. Further studies are required to determine the usefulness of eAG/fPG ratio as an indicator of Insulin resistance.
Acknowledgments
The authors thank the Tianjin Medical University Chu Hsien-I Memorial Hospital & Metabolic Diseases Hospital, for their support toward publishing this article. The authors also thank the staff and patients of the Tianjin Medical University Chu Hsien-I Memorial Hospital & Metabolic Diseases Hospital, for their contributions during the study.
Author contributions
Data analysis and statistics: Jun Guo, Sisi Lei, Yu Zhou.
Medical record collection and investigation: Sisi Lei, Jun Guo.
Writing – original draft: Jun Guo.
Writing – review & editing: Jun Guo, Congqing Pan.
Footnotes
Abbreviations: BMI = body mass index, eAG/fPG ratio = estimated average glucose to fasting plasma glucose ratio, GA = glycated albumin, GA/A1c ratio = the ratio of GA to HbA1c, HbA1c = glycated hemoglobin, HOMA-β = homeostasis model assessment of β-cell function, HOMA-IR = homeostasis model assessment of insulin resistance, T2DM = type 2 diabetes mellitus.
How to cite this article: Guo J, Lei S, Zhou Y, Pan C. The ratio of estimated average glucose to fasting plasma glucose level as an indicator of insulin resistance in young adult diabetes: An observational study. Medicine. 2020;99:40(e22337).
This work was supported by the National Natural Science Foundation of China (nos. 81273916) and the foundation of Tianjin Medical University Chu Hsien-I Memorial Hospital (nos. 2014DX01).
All authors declare that there is no conflicts of interests regarding the publication of this paper.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Parihar S, Tripathi R, Parihar AV, et al. Estimation of gingival crevicular blood glucose level for the screening of diabetes mellitus: a simple yet reliable method. J Oral Biol Craniofac Res 2016;6:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fahimipour F, Houshmand B, Alemi P, et al. The effect of He-Ne and Ga-Al-As lasers on the healing of oral mucosa in diabetic mice. J Photochem Photobiol B 2016;159:149–54. [DOI] [PubMed] [Google Scholar]
- [3].Furusyo N, Koga T, Ai M, et al. Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa Population Study (KOPS). Diabetologia 2011;54:3028–36. [DOI] [PubMed] [Google Scholar]
- [4].Roohk HV, Zaidi AR. A review of glycated albumin as an intermediate glycation index for controlling diabetes. J Diabetes Sci Technol 2008;2:1114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Guerin-Dubourg A, Catan A, Bourdon E, et al. Structural modifications of human albumin in diabetes. Diabetes Metab 2012;38:171–8. [DOI] [PubMed] [Google Scholar]
- [6].Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem 2001;47:153–63. [PubMed] [Google Scholar]
- [7].Schleicher ED, Olgemöller B, Wiedenmann E, et al. Specific glycation of albumin depends on its half-life. Clin Chem 1993;39:625–8. [PubMed] [Google Scholar]
- [8].Yazdanpanah S, Rabiee M, Tahriri M, et al. Evaluation of glycated albumin (GA) and GA/HbA1c ratio for diagnosis of diabetes and glycemic control: a comprehensive review. Crit Rev Clin Lab Sci 2017;54:219–32. [DOI] [PubMed] [Google Scholar]
- [9].Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J 2010;57:751–62. [DOI] [PubMed] [Google Scholar]
- [10].Yoshiuchi K, Matsuhisa M, Katakami N, et al. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J 2008;55:503–7. [DOI] [PubMed] [Google Scholar]
- [11].Bojanin D, Milenkovic T, Vekic J, et al. Effects of co-existing autoimmune diseases on serum lipids and lipoprotein subclasses profile in paediatric patients with type 1 diabetes mellitus. Clin Biochem 2018;54:11–7. [DOI] [PubMed] [Google Scholar]
- [12].Nathan DM, Kuenen J, Borg R, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 1987;34:571–90. [DOI] [PubMed] [Google Scholar]
- [14].Rhee SY, Woo JT. The prediabetic period: review of clinical aspects. Diabetes Metab J 2011;35:107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Găman MA, Cozma MA, Dobrică EC, et al. Dyslipidemia: a trigger for coronary heart disease in Romanian patients with diabetes. Metabolites 2020;10:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rhee EJ, Choi JH, Yoo SH, et al. The association of unintentional changes in weight, body composition, and homeostasis model assessment index with glycemic progression in non-diabetic healthy subjects. Diabetes Metab J 2011;35:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee JE, Lee JW, Fujii T, et al. The ratio of estimated average glucose to fasting plasma glucose level is superior to glycated albumin, hemoglobin A1c, fructosamine, and GA/A1c ratio for assessing β-cell function in childhood diabetes. Biomed Res Int 2014;2014:370790. [DOI] [PMC free article] [PubMed] [Google Scholar]


