Abstract
Objectives.
The purpose of this study was to evaluate the association between the follicle-stimulating hormone (FSH) receptor (c.-29G>A) and FSH beta chain (c.-280G>T) polymorphisms and endometriosis in Romanian women.
Material and methods.
We performed the polymorphic analysis of the FSH receptor gene and FSH beta chain in 44 patients with endometriosis and 34 controls. Genomic DNA was obtained from peripheral blood and polymorphisms were investigated using restriction fragment length polymorphism analysis (RFLP).
Results.
There were no significant differences in genotype frequencies of FSH receptor gene between endometriosis patients and controls. For the heterozygous type of the FSH receptor polymorphism (c.-29G>A) we did not find a significant difference in its frequency between patients with minimal/mild and moderate/severe endometriosis (p = 0.136). Also, the FSH beta chain (c.-280G> T) polymorphism frequency was not significantly associated with the severity of endometriosis (p = 0.966).
Conclusions.
FSH receptor and FSH beta chain polymorphisms do not seem to influence the severity of endometriosis, but they could be correlated with female infertility (primary or secondary), therefore further studies are required to debate this topic.
Keywords: FSH receptor, LH receptor, FSH receptor, endometriosis, infertility, polymorphism
INTRODUCTION
Endometriosis affects 1-2% of the general female population. It is considered a cosmopolitan disease, with a female phenotype including Caucasian, young, tall, normal weighted women, with an active social life. Also, it is affecting women of reproductive age, with a major impact on the patient’s daily life (1,2). Studies (3) affirm that the incapacity to conceive generates a high level of stress in young women, and their couple life. Alternative methods of treatment involve reproductive medicine, that comes with greater costs, possible complications, and life treating risks, without a full guarantee of a pregnancy (4). It is important to mention that, in Romania only after certain criteria for example anti-mullerian hormone (AMH)>1.2ng/mL in women, couples can apply for a national infertility funding program for in vitro fertilization (IVF).
Follicle stimulating hormone (FSH) is a glycoprotein hormone with a heterodimeric structure. It is formed by an alfa subunit, common to the structure of TSH, LH, hCG and another specific beta subunit (5). FSH binds to its receptor (FSHR), that is a G-protein coupled receptor. Through the interaction with its receptor, it acts on the endometrial tissue by signaling cAMP, therefore we can affirm that it is involved in mechanisms of development of endometriosis (6). FSHR is found in ovarian granulose cells and in testicular Sertoli cells, participating in the development of follicles and estradiol in women and spermatogenesis in man (5, 7). The FSHR gene contains more approximately 2000 single nucleotide variants (SNVs) (5). The most studied ones are: C.919G>A (rs6165); C.2039G>A (rs6166), and C.-29 G>A (rs1394205).
Endometriosis is a pathological condition characterized by the presence of endometrial tissue outside the uterine cavity, leading to a chronically inflammatory state of the pelvic organs (6). New theories about this disease claim that there are newer evidences that suggest immune cells, adhesion molecules, extracellular matrix metalloproteinase and pro-inflammatory cytokines can negatively influence the peritoneal microenvironment. In consequence, they create good conditions for the differentiation, adhesion, proliferation, and survival of ectopic endometrial cells, leading to further development of pelvic endometriosis (8).
The aim of this study was to find correlations between infertility and endometriosis with FSH polymorphism. We hypothesize that women affected by a certain degree of endometriosis, according to the American Society for Reproductive Medicine (ASRM), could have associated certain FSHR or FSH B type of polymorphisms.
PATIENTS AND METHOD
The study group consisted of 78 female patients, divided into two study groups, depending on the presence or absence of endometriosis associated with primary or secondary infertility. The study group consisted of 44 women diagnosed with endometriosis, inclusion criteria met the following aspects: women between 18-42 years of age diagnosed with different stages of endometriosis and infertility, defined as the incapacity of obtaining a pregnancy after more than a year of unprotected sexual intercourse, with an association of specific clinical symptoms such as menorrhagia, irregular menstrual cycles, dysmenorrhea, dyspareunia. We did not take into consideration the male partner cause for infertility, that was excluded from our research. The study did not include women with infertility of other causes than endometriosis, body mass index >40 Kg/m2, neoplasia, autoimmune diseases, diabetes mellitus, infectious disease, depression, and treatment for depressive disorders. Every patient enrolled into the study signed a written informed consent. The control group included 34 pregnant women, with no history of diagnosis with endometriosis, or previous clinical symptoms mentioned above, with more than 24 weeks of pregnancy, that delivered a viable baby at the end of pregnancy. Exclusion criteria consisted of history of diagnosis with pelvic endometriosis, prior to pregnancy, gynecological complaints of menorrhagia, irregular menstrual cycles, dysmenorrhea, dyspareunia in the past medical history. Every patient enrolled in the study signed a written informed consent. A form of the consent was approved by the Commission for Medical Ethics of the University.
Figure 1.
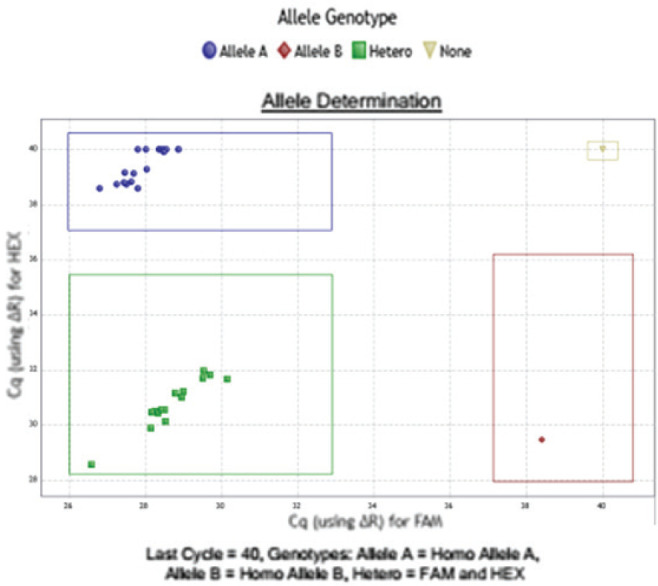
Real Time PCR aspect, with a scatter plot graph obtained during the interpretation of the FSHR polymorphism c.-29G>A with rhAmp™ SNP Genotyping (IDT Integrated DNA Technologies, Inc): Allele A- homozygote wild-type, Allele B -homozygote mutated, heterozygote status.
DNA testing
Genomic DNA was extracted from peripheral blood using Wizard Genomic DNA Purification Kit (Promega Corp., Madison, WI, USA).FSHR polymorphism c.-29G>A and FSHB polymorphism c.-280G>T detection was performed by real-time PCR based genotyping solution, with rhAmp™ SNP Genotyping (IDT Integrated DNA Technologies, Inc). Briefly, ten nanograms of genomic DNA were amplified in a total of five microliters PCR volume according to the manufacturer’s protocol in AriaMx Real-Time PCR System (Agilent, USA).
PCR conditions were: 10 minutes at 95°C for enzyme activation, followed by 45 cycles of 10 seconds at 95°C (denaturation), 30 seconds of 60°C (annealing), and extension at 60°C for 20 seconds, with fluorescence acquiring at the end of every cycle. g blocks gene fragments (1000 copies/reaction) were used as sample template, and heterozygous samples were obtained by mixing equal amounts of 2 different templates (wild type and mutant).
Statistical methods
In the statistical analysis, there were used both descriptive methods and analytical methods, with a threshold of significance of 95%. The results were centralized in SPSS18.0 databases and processed with the specific statistical functions: t-Student test, Chi2, mean, and standard deviation (sd). The severity of endometriosis correlation with FSH polymorphism was calculated using Kruskal-Wallis test.
RESULTS
Epidemiological characteristics
The age of the included patients, in the group of patients with endometriosis (Case group), had a mean of 31.3± 6.06 years (range 21-44 years) and a median value of 31 years, close to the average value. The skewness test result (p = 0.255) suggested the homogeneity of the value series. In the control group, consisting of patients with natural births or by caesarean section, the age had variations between 20 and 35 years, with a mean of 27.79 years ± 4.60 (sd) and a median value (28 years) close to the average value. The skewness test result (p = -0.245) suggested the homogeneity of the value series.
Genetic results
FSHR (c.-29G> A) rs1394205 test showed a slightly higher frequency of normal or wild type (W) response (GG) in the group of patients with endometriosis (59.1% vs. 55.9%) and frequency of heterozygous genotype (H) (AG) was slightly higher in the control group (36.4% vs. 41.2%) (p = 0.699). Of the test results rs10835638 (c.-280G> T) in FSHB, the frequency of response W (GG) (86.4% vs. 82.4%) and H (GT) (13.6% vs. 17.6%) was comparable in both study groups (p = 0.628).
From the total of 44 patients with endometriosis, we found 26 (59.1%) wild type (W) (GG) responses to FSHR (c.-29G> A) testing, and 38 (86.4%) W (GG) responses to FSHB (c.-280G> T) in the studied group. In the control group, formed by 34 patients, we had 19 (55.9%) W responses to the FSHR (c.-29G> A) gene test and 28(82.4%) to the FSHB (c.-280G>T) gene test.
In the studied group we found 16 H (GA) responses to FSHR (c.-29G> A) test and 38 W (GG) (86.4%) and 6 H (GT) (13.6%) responses in FSHB (c.-280G>T. In the control group, the polymorphism of the responses highlights the following aspects (p = 0.723): from the total of 34 pregnant patients, there were 19 patients (55.9%) that had W (GG) responses to FSHR testing (c.-29G> A), and there were 28 patients (82.4%) that had W (GG) responses to testing in FSHB (c.-280G> T). In 14 patients we found H (GA) results in FSHR (c.-29G> A), while 12 patients had W (GG) results in the FSHB (c.-280G> T) gene.
Table 1.
Comparative results between wild type (W), heterozygote (H) and mutant (M) of the FSH receptor and FSH beta chain polymorphism and the analyzed groups
| Results | Study group (n=44) | Control group (n=34) | p |
|---|---|---|---|
| FSH R (c.-29G>A) rs1394205 | |||
| W (GG) | 26 (59.1%) | 19 (55.9%) | 0.957 |
| M (AA) | 2 (4.5%) | 1 (2.9%) | 0.717 |
| H (GA) | 16 (36.4%) | 14 (41.2%) | 0.843 |
| FSH B (c.-280G>T) rs10835638 | |||
| W (GG) | 38 (86.4%) | 28 (82.4%) | 0.865 |
| M (TT) | - | - | - |
| H (GT) | 6 (13.6%) | 6 (17.6%) | 0.865 |
FSH R: follicle-stimulating hormone receptor, FSH B: follicle-stimulating hormone beta chain.
Mutant genotype noted as M (AA) response to FSHR (c.-29G> A) was found in only 2 patients from the study group and in only one patient from the control group. No mutant (M) form of response was noted in the FSHB (c.-280G> T) gene of both study and control group.
On FSHR (c.-29G> A) rs1394205, endometriosis was moderate-severe in 50% of patients with an H (GA) response (p = 0.136), suggesting no correlation. When testing rs10835638 (c.-280G> T) in FSHB, the severity of endometriosis was not associated with polymorphism (p = 0.966) (Table 2).
Table 2.
FSH receptor and FSH beta chain gene polymorphism results according to endometriosis degree (ASRM classification) and infertility status (primary or secondary)
| Parameters | Polymorphism W (GG) | M (AA) | H (GA) | p |
|---|---|---|---|---|
| FSH R (c.-29G>A) rs1394205 | ||||
| Control group (n=34) | 19 (55.9%) | 1 (2.9%) | 14 (41.2%) | 0.869 |
| Study group (n=44) | 26 (59.1%) | 2 (4.5%) | 16 (36.4%) | |
| Endometriosis severity | ||||
| Severity | ||||
| Minimal/Mild | 19 (73.1%) | 2 (100.0%) | 8 (50.0%) | 0.136 |
| Moderate/Severe | 7 (26.9%) | 0 (0.0%) | 8 (50.0%) | |
| Infertility history | ||||
| Primary | 19 (73.1%) | 1 (50.0%) | 9 (56.3%) | 0.479 |
| Secondary | 7 (26.9%) | 1 (50.0%) | 7 (43.8%) | |
| Polymorphism | p | |||
| W (GG) | M (TT) | H (GT) | ||
| FSH B (c.-280G>T) rs10835638 | ||||
| Control group (n=34) | 28 (82.4%) | - | 6 (17.6%) | 0.628 |
| Case group (n=44) | 38 (86.4%) | - | 6 (13.6%) | |
| Endometriosis symptoms | ||||
| Severity | ||||
| Minimal/Mild | 25 (65.8%) | - | 4 (66.7%) | 0.966 |
| Moderate/Severe | 13 (34.2%) | 2 (33.3%) | ||
| Infertility history | ||||
| Primary | 24 (63.2%) | - | 5 (83.3%) | 0.308 |
| Secondary | 14 (36.8%) | 1 (16.7%) | ||
FSH R: follicle-stimulating hormone receptor, FSH B: follicle-stimulating hormone beta chain.
Results in FSHR testing (c.-29G> A) rs1394205 showed that both mutants M (50%) and heterozygotes H (43.8%) were not significantly associated with secondary infertility (p = 0.479). Also, women with heterozygotes (H) genotype were mostly found to have primary infertility (83.3%).
DISCUSSION
We investigated the relationship between FSHR (c.-29G>A) rs1394205 and FSHB(c.-280G>T), rs10835638 polymorphisms and endometriosis disease in 44 Romanian women with endometriosis, regarding its correlation to infertility and in 34 control group women, with a good reproductive outcome. The findings showed that in FSHR (c.-29G>A) testing, endometriosis was not correlated with mutant forms (AA) response in moderate-severe endometriosis (p = 0.479). Also, in the FSHB (c.-280G>T) testing, endometriosis severity was not significantly associated with either W or H response in this polymorphism (p = 0.628).
Comparing to other articles, Wang et al. (3) described correlations between FSHR polymorphism and endometriosis, and concluded that mutant alleles of FSH receptor gene at the position 680 of amino acid (Asn680Ser) (GG genotype, 680Ser/Ser and GA genotype, 680Ser/Asn) could be associated with a lower risk of endometriosis, and with minimal/mild degrees of the disease in their analyzed population. In our study, we could not correlate endometriosis severity with our studied polymorphisms (FSHR (c.-29G>A)/rs1394205 and FSHB (c.-280G>T)/rs10835638).
Kerimoglu et al. (9) evaluated FSHR polymorphisms in Turkish women. The results showed no significant difference in genotype frequencies of Ala307Thr and Asn680Ser polymorphisms between endometriosis and controls. However, when the patients were divided according to disease severity, they found that the patients with the 680Ser/Ser (GG) or 307Ala/Ala (GG) genotypes were less likely to develop stage 3–4 endometriosis compared to the stage 1–2 endometriosis group.
FSH-R-29 G>A (RS1394205) is part of the intron area, in the 5′-untranslated region and is known to modulate ovarian response. We know that A allele influence has decreased mRNA transcriptional activity and a low number of proteins in the FSH-R (10). Achrekar et al. (11) in 2009, described women with variants AA that had fewer oocytes and consequently lower live birth rates compared with GG variants in the field of reproductive medicine.
Later, Desai et al. (12), described in 2013, in women with FSHR position-29, with AA type, an increased risk of low ovarian response (OR 8.63, 95% CI 1.84–45.79; P = 0.001).
On the other hand, other studies did not encounter a correlated significant lower response with ovarian stimulation (13-15).
Using fifty-seven studies, Professor Carlo Alviggi mentioned FSH-R-29 G>ARS1394205 in a recent meta-analysis and demonstrated the need for increased exogenous FSH doses, in homozygotes for the A allele compared in women with the G allele in reproductive medicine protocols (16).
LH receptor (LHR) gene is formed by 11 exons, as compared to FSHR (that is formed by only 10) (5,17). In contrast to LH beta subunit, FSH-B appears to be highly conserved (17). Indeed, fewer variants of the gene encoding for FSH-B subunit have been identified so far. The first variant was identified in 1993 in a woman with primary amenorrhea, poorly represented secondary sexual characteristics and infertility (18).
In time, other variants were discovered, the majority produce modifications of the cysteine component of FSH, that interfere with its biological activity (19). Therefore, inactivating FSH-B polymorphisms can lead to infertility, sexual infantilism, with subsequent altered breast development and puberty modifications (according to Tanner classification) (20).
From 24 SNP polymorphisms that are known in the FSH B (18), we know that one in the promoter chain area (C.-221G>T, RS10835638) has clinical impact in both female and male sexual function (21, 22).
Regarding infertility, a study noticed a decreased day three FSH value in infertile women, with normal menstrual cycles, in those with FSH-B and respectively FSH-R polymorphisms GT genotype (RS10835638) and AA ( RS6166) genotype, compared with the FSH-B GG/FSH-R GG genotype (23).
Now, we know that T allele modifications are correlated with increased FSH and LH levels and infertility (21). T allele of FSH-B (C-211 G>T, RS10835638) can induce a reduced gene transcriptional activity. There is also evidence which suggests that another variant of FSH-B subunit (C.228 C>T, RS6169) might be implicated in the development of polycystic ovarian syndrome (24).
We believe that the main clinical impact of FSH R and FSH B gene polymorphisms is their influence in the modulation of the reproductive system, that can result in a low ovarian response when ovarian stimulation is started.
Study limits
One major limitation of the study is the fact that we did not recruit a control group of women diagnosed with idiopathic infertility, thus we could not appreciate possible correlations with polymorphisms of the FSHR and FSH B gene on them. This happened because we wanted to include in the study only women with endometriosis and infertility and compare them with pregnant women, without prior history of infertility or endometriosis.
The FSHR gene polymorphisms have been incriminated to have an impact on FSH basal levels, ovarian reserve, and ovarian response to exogenous gonadotropins (2). Studies also showed an influence in women diagnosed with endometriosis when compared with women without endometriosis (18). Often, endometriosis is a significant cause of infertility, besides its most known clinical signs like menstrual irregularities, chronic pelvic pain (CPP), dysmenorrhea, dyspareunia (22). When associated these severe symptoms have an important psychological and social impact on the daily life and social activities of affected women. For this reason, endometriosis is considered as a disabling condition that may significantly compromise social relationships, sexuality, and mental health. Its incidence is often underestimated, worldwide impact being considered around 2%. If we focus only on Caucasian women, like in Romania, we believe that this percentage is actually much higher, but the low addressability to a gynecologist when mild symptoms occur, the absence of a screening program, makes it harder to treat in advanced stages.
In conclusion, FSH receptor and FSH beta chain polymorphisms do not seem to influence the severity of endometriosis, but they could be correlated with female infertility (primary or secondary), therefore further studies are required to debate this topic.
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
Daniela-Roxana Matasariu provided patient history information, Roxana Popescu analyzed genetically the FSHR and FSH B polymorphisms, Adina-Elena Tanase, Dragos Nemescu and Mircea Onofriescu performed statistical analysis and review from literature. All authors gave their consent for the publishing of the data. Adina-Elena Tanase wants to thank Professor Carlo Alviggi for allowing an Erasmus visit in his clinic in Napoli, where she learned more about FSH polymorphism.
References
- 1.Malutan A, Drugan T, Georgescu C, Ciortea R, Bucuri C, Bobric A, Rada MP, Mihu D. Vascular Endothelial Growth Factor serum levels in women with advanced endometriosis. Acta Endo (Buc). 2016;12(1):7–13. doi: 10.4183/aeb.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rižner TL. Noninvasive biomarkers of endometriosis: myth or reality? Expert Rev Mol Diagn. 2014;14(3):365–385. doi: 10.1586/14737159.2014.899905. [DOI] [PubMed] [Google Scholar]
- 3.Wang HS, Wu HM, Cheng BH, Yen CF, Chang PY, Chao A, Lee YS, Huang HD, Wang TH. Functional analyses of endometriosis-related polymorphisms in the estrogen synthesis and metabolism-related genes. PLoS One. 2012;7:e47374. doi: 10.1371/journal.pone.0047374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitale SG, Capriglione S, Peterlunger I, La Rosa VL, Vitagliano A, Noventa M, Valenti G, Sapia F, Angioli R, Lopez S, Sarpietro G, Rossetti D, Zito G. The Role of Oxidative Stress and Membrane Transport Systems during Endometriosis: A Fresh Look at a Busy Corner. Oxid Med Cell Longev. 2018(2018):7924021. doi: 10.1155/2018/7924021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gheorghiu ML. Actualities in Mutations of Luteinizing Hormone (LH) and Follicle stimulating Hormone (FSH) Receptors. Acta Endocrinologica (Buc). 2019;15(1):139–142. doi: 10.4183/aeb.2019.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robin B, Planeix F, Sastre-Garau X, Pichon C, Olesen TK, Gogusev J, et al. Follicle-stimulating hormone receptor expression in endometriotic lesions and the associated vasculature: an immunohistochemical study. Reprod Sci. 2016;23:885–891. doi: 10.1177/1933719115623647. [DOI] [PubMed] [Google Scholar]
- 7.Sprengel R, Braun T, Nicolics K, Segaloff DL, Seeburg PH. The testicular receptor for follicle-stimulating hormone: structure and functional expression of the cloned cDNA. Mol Endocrinol. 1990;4:525–530. doi: 10.1210/mend-4-4-525. [DOI] [PubMed] [Google Scholar]
- 8.Young VJ, Brown JK, Saunders PT, Horne AW. The role of the peritoneum in the pathogenesis of endometriosis. Human Reproduction Update. 2013;19(5):558–569. doi: 10.1093/humupd/dmt024. [DOI] [PubMed] [Google Scholar]
- 9.Kerimoglu OS, Yılmaz SA, Pekin A, Nergiz S, Incesu F, Dogan NU, Acar H, Celik C. Follicle-stimulating hormone receptor gene polymorphisms in women with endometriosis. Arch Gynecol Obstet. 2015;291(6):1411–1416. doi: 10.1007/s00404-014-3562-4. [DOI] [PubMed] [Google Scholar]
- 10.Casarini L, Santi D, Marino M. Impact of gene polymorphisms of gonadotropins and their receptors on human reproductive success. Reproduction. 2015;150:R175–184. doi: 10.1530/REP-15-0251. [DOI] [PubMed] [Google Scholar]
- 11.Achrekar SK, Modi DN, Desai SK, Mangoli VS, Mangoli RV, Mahale SD. Poor ovarian response to gonadotrophin stimulation is associated with FSH receptor polymorphism. Reprod Biomed Online. 2009;18:509–515. doi: 10.1016/s1472-6483(10)60127-7. [DOI] [PubMed] [Google Scholar]
- 12.Desai SS, Roy BS, Mahale SD. Mutations and polymorphisms in FSH receptor: functional implications in human reproduction. Reproduction. 2013;146:R235–248. doi: 10.1530/REP-13-0351. [DOI] [PubMed] [Google Scholar]
- 13.García-Jiménez G, Zariñán T, Rodríguez-Valentín R, Mejía-Domínguez NR, Gutiérrez-Sagal R, Hernández-Montes G, Tovar A, Arechavaleta-Velasco F, Canto P, Granados J, Moreno-Macias H, Tusié-Luna T, Pellicer A, Ulloa-Aguirre A. Frequency of the T307A, N680S, and -29G>A single-nucleotide polymorphisms in the follicle-stimulating hormone receptor in Mexican subjects of Hispanic ancestry. Reprod Biol Endocrinol. 2018;16(1):100. doi: 10.1186/s12958-018-0420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong Y, Liao WX, Roy AC, Ng SC. Association of AccI polymorphism in the follicle-stimulating hormone beta gene with polycystic ovary syndrome. FertilSteril. 2000;74:1233–1236. doi: 10.1016/s0015-0282(00)01616-2. [DOI] [PubMed] [Google Scholar]
- 15.Zamaniara T, Taheripanah R, Ghaderian SMH, Zamaniara E, Aghabozorgi SSA. Polymorphism FSHR (-29G/A) as a genetic agent together with ESRI (XbaIG/A) in women with poor response to controlled ovarian hyperstimulation. Hum Antibodies. 2017;26:143–147. doi: 10.3233/HAB-180332. [DOI] [PubMed] [Google Scholar]
- 16.Alviggi C, Conforti A, Santi D, Esteves SC, Andersen CY, Humaidan P, Chiodini P, De Placido G, Simoni M. Clinical relevance of genetic variants of gonadotrophins and their receptors in controlled ovarian stimulation: a systematic review and meta-analysis. HumReprod Update. 2018;24:599–614. doi: 10.1093/humupd/dmy019. [DOI] [PubMed] [Google Scholar]
- 17.Dufau ML. The luteinizing hormone receptor. Ann Rev Physiol. 1998;60:461–496. doi: 10.1146/annurev.physiol.60.1.461. [DOI] [PubMed] [Google Scholar]
- 18.Matthews CH, Borgato S, Beck-Peccoz P, Adams M, Tone Y, Gambino G, Casagrande S, Tedeschini G, Benedetti A, Chatterjee VKK. Primary amenorrhoea and infertility due to a mutation in the β–subunit of follicle–stimulating hormone. 5:83–86. doi: 10.1038/ng0993-83. HYPERLINK “ https://www.nature.com/ng”Nature Genetics.1993. [DOI] [PubMed] [Google Scholar]
- 19.Huhtaniemi IT, Themmen AP. Mutations in human gonadotropin and gonadotropin-receptor genes. Endocrine. 2005;26:207–217. doi: 10.1385/ENDO:26:3:207. [DOI] [PubMed] [Google Scholar]
- 20.Layman LC, Porto AL, Xie J, da Motta LA, da Motta LD, Weiser W, Sluss PM. FSH beta gene mutations in a female with partial breast development and a male sibling with normal puberty and azoospermia. J Clin Endocrinol Metab. 2002;87:3702–3707. doi: 10.1210/jcem.87.8.8724. [DOI] [PubMed] [Google Scholar]
- 21.Schuring AN, Busch AS, Bogdanova N, Gromoll J, Tuttelmann F. Effects of the FSH-beta-subunit promoter polymorphism–211G->T on the hypothalamic pituitary- ovarian axis in normally cycling women indicate a gender-specific regulation of gonadotropin secretion. J Clin Endocrinol Metab. 2013;98:E82–86. doi: 10.1210/jc.2012-2780. [DOI] [PubMed] [Google Scholar]
- 22.Grigorova M, Punab M, Ausmees K, Laan M. FSHB promoter polymorphism within evolutionary conserved element is associated with serum FSH level in men. Hum Reprod. 2008;23:2160–2166. doi: 10.1093/humrep/den216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.La Marca A, Papaleo E, Alviggi C, Ruvolo G, De Placido G, Candiani M, Cittadini E, De Michele F, Moriondo V, Catellani V, Volpe A, Simoni M. The combination of genetic variants of the FSHB and FSHR genes affects serum FSH in women of reproductive age. Hum Reprod. 2013;28:1369–1374. doi: 10.1093/humrep/det061. [DOI] [PubMed] [Google Scholar]
- 24.Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: Biochemistry, molecular biology, physiology, and pathophysiology. Endocrinol Rev. 1997;18:739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]


