Abstract
Context.
Vitamin D is a steroid hormone that acts by binding to the vitamin D receptor (VDR) found in many tissues. According to the long-term mechanism, vitamin D causes the proliferation and differentiation of muscle cells by gene transcription.
Objective.
We aimed to evaluate the relationship between muscle strength and serum vitamin D levels in elderly men.
Design.
Cross-sectional study.
Subjects and Methods.
Male patients over age 50 were included in the study. Study population was divided into 2 groups with handgrip strength according to body mass index, either as subjects with weak or with normal handgrip strength test (HGST). Vitamin D levels and other variables compared between weak and normal groups.
Results.
Vitamin D level of weak and normal groups were 7.5 (3-19.9) μg/L, and 11.6 (11.6-34.9) μg/L, which means significant reduced vitamin D levels in weakness group (p=0.01). Vitamin D levels were significantly correlated with HGST levels (r:0.362, p=0.001). Vitamin D levels were found to be an independent predictor of weakness according to HGST in logistic regression analysis (OR: 0.453, 95% Cl:0.138-0.769, p=0.05).
Conclusions.
Low vitamin D level is an independent risk factor for muscle weakness in men aged more than 50 years. Therefore, vitamin D levels should be screened and early replacement should be initiated for the sake of improvement of muscle strength in elderly subjects that vulnerable for frailty.
Keywords: vitamin D, muscle strength, handgrip stress test
INTRODUCTION
Vitamin D is a steroid hormone that acts by binding to the vitamin D receptor (VDR) found in many tissues (1). VDR has been shown to be present in skeletal muscle. Vitamin D causes proliferation and differentiation in muscle cells through VDR (2). Vitamin D acts on skeletal muscle by two different mechanisms, short and long-term effect (3). According to the long-term mechanism, vitamin D causes the proliferation and differentiation of muscle cells by gene transcription (4). According to the short-term mechanism, it affects skeletal muscle contraction through regulation of calcium-mediated secondary messengers (5). It is assumed that gradual loss of muscle mass and loss of muscle function in the elderly may also be associated with decreased VDR expression in skeletal muscle cells (6). The incidence of vitamin D deficiency increases in the elderly with less sun exposure, decreased intake of vitamin D-containing nutrients, and decreased skin’s ability to synthesize vitamin D.
Muscle weakness is associated with increased functional limitations, hospitalization and increased risk of death in older adults (7-9). Prospective studies among elderly adults have reported muscle strength loss rates of 1.5 to 3.6% per year in men and the elderly (10-12). The hand grip strength used to estimate the total muscle strength measures the strength of the hand muscles. It determines weakness according to the body mass index, for instance, HGST equal to or lower than 29 kg in subjects with a BMI ≤24 kg/m2 is defined as weakness. Determination of muscle weakness by measuring hand grip strength, which is also a marker of nutritional status, is important in the diagnosis of sarcopenia. Therefore, hand grip strength test is an easy way to assess muscle weakness.
We aimed to evaluate the relationship between muscle strength and serum vitamin D levels in elderly men. We also aimed to evaluate the relationship of muscle strength with anthropometric measurements such as waist circumference, triceps and biceps skin thickness and calf circumference.
PATIENTS AND METHODS
This cross-sectional study was performed with male patients who presented to Abant Izzet Baysal University Medical Faculty Hospital, Internal Medicine Department. Ethics committee approval of the institution was obtained (Decision no: 2019/171). 110 male patients over 50 years were included in the study with their consent forms. Active infection, rheumatoid arthritis and inflammatory disease, previous cerebrovascular event, acute coronary syndrome in the last 3 months, upper extremity deformity and malignancy were excluded from the study (22 patients). Patients on vitamin D supplements and subjects with peripheral neuropathy or peripheral vascular diseases were also excluded. The study group was divided into 2 groups with handgrip strength according to body mass index (BMI) either as subjects with weak or normal HGST. Patients’ age, gender, chronic disease and medications, demographic characteristics (age, sex, height, waist circumference, weight, middle arm circumference, triceps and biceps skinfold thickness) and laboratory data were recorded. Height and weight of the patients were measured, and BMI was calculated by weight (kg)/ height (m2) formula. Hand grip tests were performed with CARMY brand electronic hand dynamometer in order to evaluate muscle strength.
Middle arm circumference (cm), Triceps skinfold thickness (mm), Weight (kg), Body mass index (kg/m2), serum vitamin D (μg/L), Albumin (mg/dl), Height (cm), Diastolic blood pressure (mmHg), Systolic blood pressure (mmHg), Biceps skinfold thickness (mm), Waist circumference (cm), Age (year), Total protein (mg/dL), HGST (kg) and serum creatinine were recorded.
Measurements
The patient was in sitting position, shoulder in adduction and neutral rotation, elbow in 90 degrees flexion, forearm in neutral rotation, wrist in neutral position. This process was repeated 3 times with 30 second intervals and the average of 3 measurements was taken as a result. In addition, hand grip strength measurement was evaluated as weakness and normal strength according to BMI Weakness criteria are as follows; for HGST≤29 kg with BMI ≤24 kg/m2; for HGST≤30 kg with 24.1≤BMI≤26 kg/m2; for HGST≤30 kg with 26.1≤BMI≤28 kg/m2; for HGST≤32 kg with BMI>28 kg/m2 (13). Skinfold thickness was measured with Body Fat Caliper.
Statistical analysis
All data were analyzed by spss software (IBM SPSS 16.0). Kolmogorov-Smirnov test was used to examine the distribution of study parameters between the groups. Comparison of homogeneous parameters was performed by independent samples t test (Mean±StD) and non-homogeneous parameters by Mann-Whitney U test (Median±{Min-max}). X2 test was used to compare categorical variables between the groups. The correlation of variables was evaluated by Pearson correlation analysis. Variables affecting HGST were evaluated in the linear regression analysis. These variables were vitamin D, albumin, height, waist circumference, middle arm circumference, biceps skinfold thickness, triceps skinfold thickness and weight. P <0.05 was determined as the level of statistical significance.
RESULTS
A total of 88 male subjects; 45 (51%) weakness, and 43 (49%) in normal groups were included in the study after exclusion of the subjects that fall into exclusion criteria. The ages of weakness and normal groups were 66 (51-88) and 61 (51-71), respectively (p=0.001). Weakness group was significantly shorter than normal subjects (166.5 cm [142-179] in weakness group and 170 cm [150-183] in normal group) (p=0.02, Table 1). There were no significant differences between weakness and normal groups according to the waist circumference (p=0.6), and body weight (p=0.6). Similarly, systolic and diastolic blood pressures of the subjects are not statistically different (p=0.2 in both, Table 1). Middle arm circumference, biceps skinfold thickness and triceps skinfold thickness which all used for nutritional status were similar between study groups (Table 1, all p >0.05). Another nutritional parameter, albumin, was also similar in study groups (p>0.05). Vitamin D levels of weakness and normal groups were 7.5 (3-19.9) μg/L, and 11.6 (11.6-34.9) μg/L, which means significant reduced vitamin D levels in weakness group (p=0.01). Body mass indexes of the weak and normal groups were 29.8±5.8 kg/m2 and 31.1±5.2 kg/m2, respectively (p=0.6). Study parameters were summarized in Table 1.
Table 1.
General characteristics of the study groups according to the HGST
| Weakness to HGST | Normal to HGST | p | |
|---|---|---|---|
| Mean ±St.D. | |||
| Middle arm circumference (cm) | 28.6±4.2 | 29.9±3 | 0.8 |
| Creatinine (mg/dL) | 0.9±0.15 | 0.8±0.15 | 0.8 |
| Triceps skinfold thickness (mm) | 14.1±5.9 | 13.9±4.2 | 0.5 |
| Weight (kg) | 81.6±16.4 | 88.9±15.1 | 0.6 |
| Body mass index (kg/m2) | 29.8±5.8 | 31.1±5.2 | 0.6 |
| Median (min-max) | |||
| Vitamin D (μg/L) | 7.5 (3-19.9) | 11.6 (11.6-34.9) | 0.01* |
| Albumin (mg/dL) | 4.3 (2.8-5) | 4.3 (3.3-4.9) | 0.07 |
| Height (cm) | 166.5 (142-179) | 170 (150-183) | 0.02* |
| Diastolic blood pressure (mmHg) | 80 (70-100) | 90 (60-100) | 0.2 |
| Systolic blood pressure (mmHg) | 130 (100-180) | 140 (100-180) | 0.2 |
| Biceps skinfold thickness (mm) | 16 (8-45) | 16 (6-45) | 0.3 |
| Waist circumference (cm) | 110.5 (88-142) | 115 (88-149) | 0.9 |
| Age (year) | 66 (51-88) | 61 (51-71) | 0.001* |
| Total protein (mg/dL) | 7.2 (5.2-8.2) | 7.1 (5.7-8.6) | 0.7 |
| HGST (kg) | 23.4 (8.7-30.7) | 38.6 (30.2-56.2) | <0.001* |
HGST: hand grip strength test.
HGST levels of weakness and normal groups were 23.4 (8.7-30.7) kg, and 38.6 (30.2-56.2) kg, respectively (p<0.001). Vitamin D levels were significantly correlated with HGST levels (r:0.362, p=0.001, Fig. 2). Vitamin D levels were found to be an independent predictor of weakness according to HGST in logistic regression analysis (OR: 0.453, 95% Cl:0.138-0.769, p=0.05, Table 2).
Figure 1.
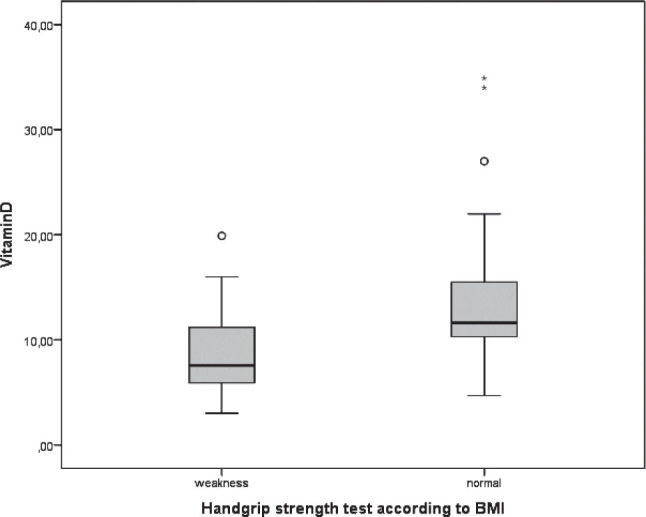
Vitamin D levels in study groups.
Figure 2.
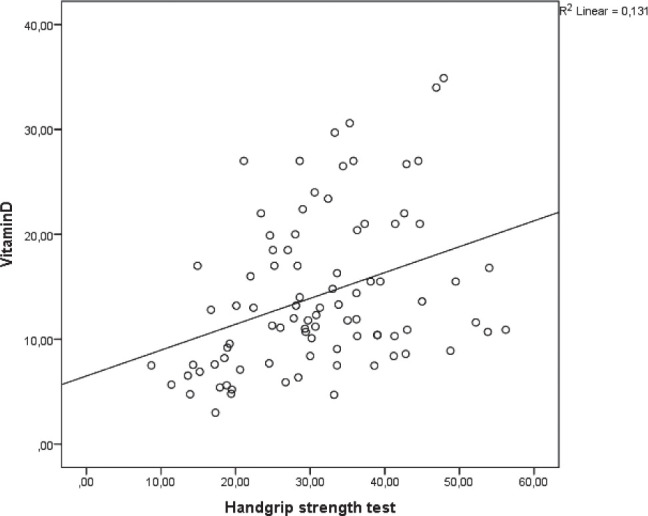
Correlation between vitamin D levels and Handgrip strength test (r:0.362, p=0.001).
Table 2.
Variables that affect HGST in linear regression analysis
| Variable | OR | 95% Cl | p |
|---|---|---|---|
| Vitamin D | 0.453 | 0.138-0.769 | 0.049* |
| Albumin | 3.06 | -2.17- 8.29 | 0.25 |
| Height | -0.23 | -0.393-0.348 | 0.9 |
| Waist circumference | -0.366 | -0.732- (-0.001) | 0.05 |
| Middle arm circumference | -0.051 | -1.042-0.941 | 0.9 |
| Biceps skinfold thickness | 0.088 | -0.238-0.415 | 0.59 |
| Triceps skinfold thickness | -0.205 | -0.769-0.360 | 0.47 |
| Weight | 0.350 | -0.064-0.765 | 0.09 |
HGST: hand grip strength test.
DISCUSSION
The main finding of the present study is significantly lower vitamin D levels in weakness group compared to normal group. Another important finding of the present study was that patients in weakness group were significantly shorter and significantly older than the patients in normal group. Therefore, weakness could be related with the reduced vitamin D levels.
Frailty is a geriatric syndrome and its incidence increases by aging. Moreover, vitamin D producing capacity of the skin reduces in the elderly. Moreover, daylight exposure of the older population is lower than of younger individuals. These consequences contribute to lower vitamin D levels in older population. Low vitamin D levels are associated with bone and muscular disorders (2,6). Musculoskeletal disorders induce frailty in elderly. Frailty is a cause of low HGST in this population (14).
The HGST is a simple, non-invasive and useful diagnostic test in detection of upper extremity weakness. HGST reduces by aging (14). Accordingly, weakness group was older than normal group in the present study.
Reduced testosterone is an important cause of muscle weakness in men. Testosterone reduces by advanced age, therefore, muscle weakness is more prevalent in elderly men compared to younger counterparts. Higher testosterone levels were suggested to be correlated with elevated HGST levels in the literature (15). Lower HGST in the weak compared to normal group could be a consequence of lower testosterone levels, since the subjects in the weak group were older than their counterparts. However, we did not study serum levels of testosterone in the present study.
Muscle mass is strongly correlated with the serum levels of vitamin D in medical literature (2,4,6). On the other hand, low vitamin D levels were associated with reduced muscle mass (16). Similarly, we found that vitamin D levels of the weakness group were significantly lower than that of the normal group. Moreover, vitamin D was suggested to be a predictor of muscle weakness in the present study.
The association between vitamin D status and HGST is well established. Several studies stated the positive correlation between vitamin D and HGST (17,18). On the contrary, a study from Asia reported no association between these two parameters (19). We found a significant correlation between serum vitamin D and HGST in the present study.
Limitations of the present study are relatively small study population and lack of serum testosterone level measurements. However, results of present study that pointed out a significant correlation between muscle weakness and serum vitamin D levels are important for current medical literature.
In conclusion, low vitamin D level is an independent risk factor for muscle weakness in men aged more than 50 years. Therefore, vitamin D levels should be screened and early replacement should be initiated for the sake of improvement of muscle strength in elderly subjects that are vulnerable for frailty.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Heaney RP, Armas LA. Screening for vitamin D deficiency: is the goal disease prevention or full nutrient repletion? Annals of internal medicine. 2015;162(2):144–145. doi: 10.7326/M14-2573. [DOI] [PubMed] [Google Scholar]
- 2.Olsson K, Saini A, Strömberg A, Alam S, Lilja M, Rullman E, Gustafsson T. Evidence for vitamin D receptor expression and direct effects of 1α, 25 (OH) 2D3 in human skeletal muscle precursor cells. Endocrinology. 2016;157(1):98–111. doi: 10.1210/en.2015-1685. [DOI] [PubMed] [Google Scholar]
- 3.Christakos S, Hewison M, Gardner DG, Wagner CL, Sergeev IN, Rutten E, Pittas AG, Boland R, Ferrucci L, Bikle DD. Vitamin D: beyond bone. Ann NY Acad Sci. 2013;1287:45–58. doi: 10.1111/nyas.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int. 2002;13(3):187–194. doi: 10.1007/s001980200012. [DOI] [PubMed] [Google Scholar]
- 5.Dirks-Naylor AJ, Lennon-Edwards S. The effects of vitamin D on skeletal muscle function and cellular signaling. J Steroid Biochem Mol Biol. 2011;125:159–168. doi: 10.1016/j.jsbmb.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari HA, Borchers M, Gudat F, Dürmüller U, Stähelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19:265–269. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 7.Chan OY, van Houwelingen AH, Gussekloo J, Blom JW, den Elzen WP. Comparison of quadriceps strength and handgrip strength in their association with health outcomes in older adults in primary care. Age (Dordr). 2014;36(5):9714. doi: 10.1007/s11357-014-9714-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taekema DG, Gussekloo J, Maier AB, Westendorp RG, de Craen AJ. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing. 2010;39(3):331–337. doi: 10.1093/ageing/afq022. [DOI] [PubMed] [Google Scholar]
- 9.Hicks GE, Shardell M, Alley DE, Miller RR, Bandinelli S, Guralnik J, Lauretani F, Simonsick EM, Ferrucci L. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2012;67(1):66–73. doi: 10.1093/gerona/glr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly RM, Rosengren BE, Alwis G, Ahlborg HG, Sernbo I, Karlsson MK. Gender specific age-related changes in bone density, muscle strength and functional performance in the elderly: a-10 year prospective population-based study. BMC Geriatr. 2013;6(13):71. doi: 10.1186/1471-2318-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassey EJ, Harries UJ. Normal values for handgrip strength in 920 men and women aged over 65 years, and longitudinal changes over 4 years in 620 survivors. Clin Sci (Lond). 1993;84(3):331–337. doi: 10.1042/cs0840331. [DOI] [PubMed] [Google Scholar]
- 12.Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM. Grip strength changes over 27 yr in Japanese-American men. J Appl Physiol. (1985) 1998;85(6):2047–2053. doi: 10.1152/jappl.1998.85.6.2047. [DOI] [PubMed] [Google Scholar]
- 13.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 14.Goh VH, Hart WG. Associations of physical exercise as a lifestyle habit with lean and fat body mass and handgrip strength and age in Asian men. Aging Male. 2014;17(3):131–135. doi: 10.3109/13685538.2014.925441. [DOI] [PubMed] [Google Scholar]
- 15.Chiu HT, Shih MT, Chen WL. Examining the association between grip strength and testosterone. Aging Male. 2019:1–8. doi: 10.1080/13685538.2019.1632282. [DOI] [PubMed] [Google Scholar]
- 16.Gunton JE, Girgis CM. Vitamin D and muscle. Bone Rep. 2018;8:163–167. doi: 10.1016/j.bonr.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhanwal DK, Dharmshaktu P, Gautam VK, Gupta N, Saxena A. Hand grip strength and its correlation with vitamin D in Indian patients with hip fracture. Arch Osteoporos. 2013;8:158. doi: 10.1007/s11657-013-0158-8. [DOI] [PubMed] [Google Scholar]
- 18.von Hurst PR, Conlon C, Foskett A. Vitamin D status predicts hand-grip strength in young adult women living in Auckland, New Zealand. J Steroid Biochem Mol Biol. 2013;136:330–332. doi: 10.1016/j.jsbmb.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Kim BJ, Kwak MK, Lee SH, Koh JM. Lack of Association Between Vitamin D and Hand Grip Strength in Asians: A Nationwide Population-Based Study. Calcif Tissue Int. 2019;104(2):152–159. doi: 10.1007/s00223-018-0480-7. [DOI] [PubMed] [Google Scholar]


