Abstract
Context.
Follicular thyroid carcinomas (FTC) represent 6-10 % of all thyroid carcinomas; the evolution of FTC is quite controversial, partly due to frequent changes of the histopathological definition (minimally invasive–MIFTC or widely invasive carcinoma–WIFTC) and treatment strategies adjustments.
Objective.
This research aims to examine the diagnostic procedure, therapeutic attitude and survival rates of patients with FTC, over a period of 16 years in the same institution, with a follow-up of at least 4 years, by analyzing correlations between histology subtype, treatments and the rate of recurrent disease.
Subjects and methods.
We have studied 5891 patients with thyroid carcinomas who have undergone surgical or oncological treatment within the institution, between 1st January 2000 – 31st December 2015; among them we found 133 patients (2.25%) with “pure” follicular thyroid carcinoma: 114 (86%) women and 19 (14%) men, with a female-male ratio of 6:1. The age of the patients ranged from 10 to 76 years, with an average of 47.8 years. Statistical analysis was done comparing differences among groups of MIFTC and WIFTC.
Results.
There was an unexpected high percentage of WIFTC and also an increased number of biochemically persistent and/or recurrent disease in patients with MIFTC. A stronger correlation was observed with the tumour dimensions, rather than with the histopathological subtype.
Conclusions.
This research observed that overall survival was associated with tumour size rather than histopathological subtype and there is an important need to perform further studies to assess the effectiveness of treatment strategies.
Keywords: Minimally invasive, follicular carcinoma, thyroid, prognosis, widely invasive
INTRODUCTION
Thyroid carcinomas are one of the most frequent malignant endocrine tumors, with an increasing incidence in the world in recent years (1–4). Even though there are many described subtypes, most originate from follicular thyroid cells and are divided into well-differentiated papillary thyroid carcinomas (PTC) and follicular thyroid carcinomas (FTC) (5). PTC is the most common, followed by FTC, the latter having a maximum incidence between the fourth and sixth decades (6). There is a female predominance in reproductive years, which could be explained by the expression of estrogen receptors (7).
Follicular thyroid carcinomas account for 6–10% of thyroid carcinomas, low iodine diet being a risk factor. According to the World Health Organization (WHO) classification from 2017, three subtypes of FTC can be described (8):
1. Minimally invasive follicular carcinoma — characterized only by capsular invasion. This histopathological type is rare representing only 3%–13% of differentiated thyroid carcinomas (8,9);
2. Encapsulated angioinvasive carcinoma – otherwise formerly classified by invasion in less than 4 vessels (limited vascular invasion), usually having a good prognosis, or more than 4 vessels (extensive vascular invasion);
3. Widely invasive follicular carcinoma – which involves extensive invasion of the gland and sometimes extra-thyroidal tissue. The long-term mortality of this subtype is almost 50% (8,10).
The incidence of thyroid carcinomas in Romania is 1.5% according to Globocan 2018, with a mortality rate of 0.33% for both sexes, however, there are no national data regarding the frequency of histopathological subtypes of this disorder in Romania (10–13).
The purpose of this research is to analyse the diagnostic value, therapeutic decision, and to make correlations between different characteristics of patients with FTC throughout a period in which diagnostic criteria have been constantly evolving.
PATIENTS AND METHODS
This is an analytical observational study spanning across a period of 20 years (16 years of data collection and at least 4 years of follow-up). It analyses patients with follicular thyroid carcinoma who have undergone surgical or oncological treatment within the IOCN, between 1st January 2000 – 31st December 2015. Several parameters were followed including the type of surgical treatment, detailed histopathological features of the tumour, and the necessity of radioiodine treatment. Knowing the prevalence of thyroid pathology in areas characterized by iodine deficiency, or with respect to irradiation factors, this study also emphasizes the importance of the areas patients inhabit (2,14,15). We registered also the patients with multiple malignancies associated with follicular thyroid cancers, being in the evidence of the mentioned institution. For a correct classification and staging, we assessed the age, size of the tumour, capsular or vascular invasion, radioactive iodine treatment and the total dose received. To follow the evolution of the disease we considered 4 possibilities: 1. Cured– without clinical, biological or imagistic signs of malignancy; 2. Evolving disease–the presence of clinical, biological and imaging signs; 3. Recurrent disease–recurrence of clinical, biological and imaging signs following a period of absence; 4. Persistent biochemical disease–without clinical and imaging signs, but with above-the-limit blood sample counts, with the imminence of recurrence. We also recorded the disease-free survival rates.
All patients received informed consent forms for diagnostic and therapeutic procedures, as well as for participation in studies. The study was conducted with the approval of the Ethics Committee of both the hospital and “Iuliu Hațieganu” University of Medicine and Pharmacy of Cluj-Napoca.
To reveal the most accurate data we excluded from this research the patients with other types of thyroid carcinomas such as papillary (including follicular variant), medullary, anaplastic, Hürthle cell carcinoma or other subtypes. At the same time, patients whose medical records were not included in the archive, or with missing essential data such as a significant part of the histopathological examination or oncological treatment, were also excluded. Hürthle cell carcinoma is more aggressive than “pure” follicular thyroid carcinoma, even though it has been considered for many years a subtype thereof (16).
Given the fact that the classification has been changed several times in the latest years, and that our analysis included older histopathological descriptions, some from 19 years ago, where we have divided FTCs into two prognostic groups – a good general prognosis (equivalent to minimally invasive - MIFTC) or a poor prognosis (equivalent to widely invasive - WIFTC), according to the postoperative events and invasion pattern.
Statistical analysis
Statistical analysis was carried out using GraphPad Prism 6.0 software. We calculated normal distribution using the Shapiro-Wilk test. To compare differences among groups, we calculated ANOVA with Bonferroni correction. Survival analysis was performed using the Kaplan Meier method. The results were considered statistically significant if the adjusted p-value was less than 0.05.
RESULTS
Socio-demographic attributes
We have searched through 5891 patients with thyroid carcinomas, recorded by IOCN during the 16 years covered by our study.
After applying the inclusion and exclusion criteria, a number of 133 patients (2.25%) with “pure” follicular thyroid carcinoma was retained in the study. Of these, 114 (86%) are women and 19 (14%) men, with a female-male ratio of 6:1. The age of the patients at the time of diagnosis ranged from 10 to 76 years, with an average of 47.8 years. After this analysis, we found 88 cases of MIFTC (66%) and 45 cases of WIFTC (34%).
Pathological features
We reviewed all histologies of the selected cases. One of the macroscopic features we want to highlight is the location of the tumour. Most of them developed in the right lobe (n=66; 49.6%), followed by left lobe (n=49; 36.8%). One lesion was located in the isthmus and 5 were bilateral. In 12 of the cases, there were no available data about the site.
A fibrous capsule of variable thickness covered the tumours. The size of the nodules ranged from 0.2 to 9.5 cm, with a median of 3 cm. We focused on the division according to size categories, highlighting the 21 identified cases of small carcinomas (maximum diameter 1 cm). Most cases (24.8%) fell in the 2.1–3 cm category (Fig. 1). The term microcarcinoma refers in the literature to cases with the greatest size of 1 cm and classically is associated to papillary thyroid carcinomas; we consider all these cases (≤1 cm) as follicular small carcinoma due to the fact that there is no international agreement about the use of the term microcarcinoma; we underline that the disorder is by far less frequent than the papillary form and also the studies referring to this category of tumours are limited.
Figure 1.
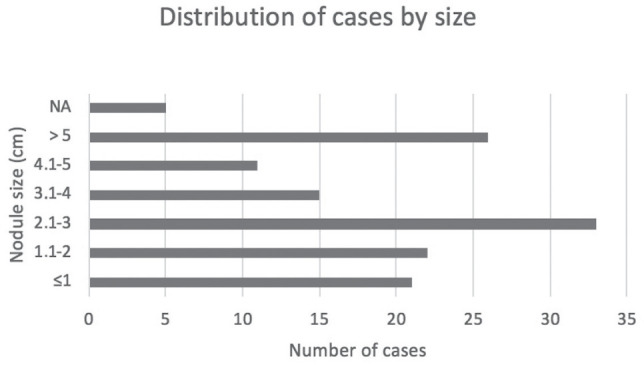
Distribution according to the size of tumours.
We quantified the details of the capsular and vascular tumour invasion in our study. Based on these capsular details we also looked at possible differences between cases C1 and C1+. Even though there are some controversies regarding the degree of capsular invasion (17), we could distinguish two types of invasion on histopathological findings: total capsule transgression (C1+) and partial invasion (C1). Mushroom-shaped tumor invasion was found in most of the cases, even if present just inside of the thick fibrous capsule or transgressing it. Cases with satellite nodules outside the tumor capsule were also included in the C1+ Group. Vascular invasion status is also a very important factor, which is why two groups were created: with no vascular invasion (V0), with vascular invasion (V1). We considered this classification necessary because in a few cases the pathologists mentioned the number of observed vascular invasion points. They were also included in Group V1. For a correct analysis of survival data we have associated the types of capsular and vascular invasion for each patient, obtaining the following variants: C1/V0, C1/V1, C1+/V0, C1+/V1, non-encapsulated/V0 and C0V1, the last one being explained by the presence of the malignant cells into some vessels, but without an evidence of the capsular penetration and probably due to the lack of serial sections to highlight any capsular lesion. Parameters of vascular and capsular invasion were as follows: C0/V1–2 patients, C1/V0–34; C1/V1–20; C1+/V0–33; C1+/V1–43; non-encapsulated/V0–1.
Nine patients (6.7%) had clinical adenopathies at the time of diagnosis, even if this is an unusual presentation for FTC; the lymph node involvement in FTC is far less frequent than in papillary thyroid cancers, being estimated at 8–10% of cases (18). This exceptionally rare occurrence, also suggested us, to analyse in our cohort, the patients with multiple malignancies associated with FTC, that might lead to lymph node metastases. Nine patients had other oncological pathologies with different locations, 1 synchronous and 8 metachronous, for which they received separate surgical and/or oncological treatment. These pathologies were: cervical (4 cases), breast (2 cases), bladder (1 case, laryngeal (1 case - synchronous), prostate (1 case) and liposarcoma (1 case). One patient had 2 additional cancers, apart from thyroid cancer (breast and cervix cancers). In 6 cases radiotherapy was performed, but none appeared before thyroid disease, so a link between the irradiation and the occurrence of thyroid cancer cannot be established. There were 12 patients with confirmed metastases, some of which from 2–4 different locations (lymph nodes, lungs, bones, liver or skin). Of these 12 patients with confirmed regional and distant metastases, only 11 patients among all 133 patients (8.2%) had thyroid carcinomas as origin of the metastases, while 1 patient with MIFTC and breast cancer had lung metastases from breast cancer and not from FTC. Among the 11 metastatic patients, 6 patients had multiple sites of metastatic disease (4.5%), of which 4 were WIFTC and one MIFTC, and 5 presented a single metastatic site (3.7%) of which 1 was MIFTC and 4 were WIFTC.
Treatment and follow-up
All patients received surgical treatment, and the majority also required oncological therapy (radioiodine and in some limited metastatic cases external beam radiotherapy or chemotherapy; none of these patients received systemic therapy with tyrosine kinase inhibitors). The patients were operated in different locations, by different surgeons and the most common technique was total thyroidectomy (TT). Some patients with TT were operated in 2 stages: initially with lobectomy (excision of the lobe where the lesion was described), followed by the completion of the bilateral surgery. Other types of interventions were subtotal thyroidectomy (TS) or thyroidectomy associated with lymphadenectomy, or other excisions. TT was performed in 117 cases; TT+Lymphadenectomy 3 cases; TT+mandibular resection–1 case; TT and laryngectomy in 1 case; TT+ bone metastasis resection-1 case; TT+internal jugular vein resection-1 case; near total thyroidectomy–9 cases.
Radioiodine therapy (RAIT) followed after surgical treatment. Of the total patients included in this research, 126 received radioiodine treatment, 42 of the patients (33.3%) receiving repeated doses of I-131 (2–8 doses), as needed. The total radioiodine activity in these patients ranges from 67–1057 mCi. The maximum dose was administered to a patient with evolving disease, with multiple lymph node, bone, lung and skin metastases, because of the widely invasive carcinoma. Regarding other additional oncological treatments for thyroid disorders, only one patient required chemotherapy and two other patients external radiotherapy, all 3 cases having bone, lung, skin or lymph node metastases.
The follow-up varied according to the date of diagnosis, but the minimum was 4 years. At the end of the research, most of the patients were considered cured (n=117; 87.9%). The others had either recurrent disease (n = 6), persistent biochemical disease (n= 4) or evolving disease (n=6). The relapse-free survival rate was calculated according to the status of the disease updated at each visit. This varied between 1 and 19 years, based on the last date of patient presentation and excluding patients with evolving disease.
We analysed the relation between the FTC tumor size and the sites of metastases. Among patients with lung metastases, two cases had only lung metastasis and two had multiple organs metastases. The tumour size of these patients was mean (± SD) = 6.12 ± 1.4 cm. There were 4 patients without pulmonary metastases (3% of total cases), but with lymph node metastases– 2 cases (1 WIFTC, the greatest tumour diameter 0.8cm and 1 MIFTC, greatest tumour diameter 6 cm), 1 case with bone metastases (WIFTC, greatest tumour diameter 2 cm) and 1 case with multiple sites of metastatic disease (bone, liver, cutaneous metastases) (WIFTC, greatest tumour diameter 6 cm). The tumour size of these patients was mean (± SD) = 3 ± 2.7 cm. The patients with pulmonary metastases are found to show significantly larger nodules than those without metastases on this site (p=0.02, 6.12 cm vs. 3.04 cm).
Patients without bone metastases were 5 cases (3.7% of total cases), mean (± SD) =5.3 ± 3.5 cm. The presentation was as follows: 4 with single site – 2 cases with lymph nodes metastases (1 WIFTC, the greatest tumour diameter 0.8 cm and 1 MIFTC, the greatest tumour diameter 6 cm), 2 cases with pulmonary metastases ((1 WIFTC, the greatest tumour diameter 6 cm and 1 MIFTC, the greatest tumour diameter 9.5 cm respectively); 1 case with multiple sites (lymph and pulmonary), (WIFTC, dimension – N/A).
In case of bone metastases the statistical significance is not preserved, so that, there are no differences between the tumour dimensions in those with bone metastases versus those without bone metastases (p=0.09, 5.22 cm vs. 3.08 cm).
The evolving disease status has associated cases with significantly larger tumour sizes than in the other patients (p=0.0001, 5.3 cm vs. 3.1 cm), compared with all other groups, in relation with tumour size > 1cm. On the other hand, we could not find any statistically significant difference between the nodular dimensions of patients with persistent biochemical disease and those of the other groups (p=0.16, 4.3 cm vs. 3.15 cm), compared with all other groups, in relation with tumour size > 1cm. The diameter of the thyroid tumour is significantly larger in men than in women (p=0.03). The disease-free survival rate was higher in women (p=0.01). Table 1 shows the described results as well as their statistical relevance.
Table 1.
The results of the statistical analysis
| Average (± SD) | Average (± SD) | ||
|---|---|---|---|
| Parameter | Tumour ≤ 1 cm | Nodule>1 cm | P value (Student test) |
| Pulmonary metastases | 0 | 6.12 ± 1.93 | 0.02 |
| Bone metastases | 0 | 5.22 ± 2.1 | 0.09 |
| Evolving disease | 0 | 5.3 ± 0.46 | 0.0001 |
| Biochemical persistence | 0 | 4.3 ± 1.2 | 0.16 |
| Women (n=114) | Men (n=19) | ||
| Tumour dimension (cm) | 3.35 ± 1.81 | 4.18 (± 1.97) | 0.03 |
| Disease-free survival (months) | 118 ± 50.6 | 85.8 ± 46 | 0.01 |
| Cured (n=117) | Uncured (n=16) | ||
| Lymphadenopathy | 3 | 6 | 0.013 |
| Pulmonary metastases | 0 | 7 | 0.003 |
| Lymph node metastases | 0 | 6 | 0.0089 |
| Liver metastases | 0 | 1 | 0.33 |
| Cutaneous metastases | 0 | 2 | 0.16 |
| Bone metastases | 0 | 6 | 0.009 |
| Metastases (other) | 0 | 11 | 0.00004 |
Another finding was that in the case of patients with MIFTC, 6.8 % of patients had recurrent or persistent biochemical disease compared to patients with WIFTC among whom only 8.8 % had a recurrent or persistent biochemical disease (after we excluded patients with evolving disease). It was observed that metastases were not found in the cured patients, which is to be expected in oncological pathology. Figure 2 shows a separate relationship between invasion type, relapse-free survival and total dose of radioiodine therapy, where “cv” are considered the patients who present both capsular and vascular invasion, without quantifying the depth of the capsular invasion . Thus, in patients with V0 status, the dose of radioiodine therapy was the lowest, and in those with vascular, capsular or both, a higher dose of radioiodine therapy was required.
Figure 2.
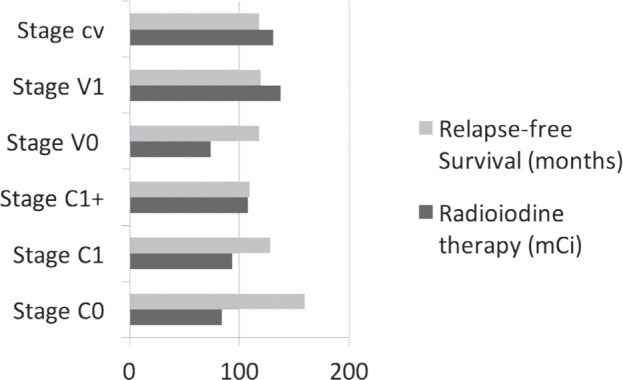
Relationship between invasion type, relapse-free survival and total dose of radioiodine therapy.
At the same time, relapse-free survival rate was the highest in patients with C1 status and, as we expected, the patients with C1+ status have a lower relapse-free survival than those with limited capsular invasion (C1).
There is no correlation between the total dose of radioiodine treatment and relapse-free survival rate (Pearson coefficient=–0.09).
If we consider the survival rate according to the combined invasion status (capsular and vascular), it is easy to see that patients with C1 / V0 stage had better survival than those with C1 + V0, even if in both cases there were pathological events during the follow-up period (Fig. 3).
Figure 3.
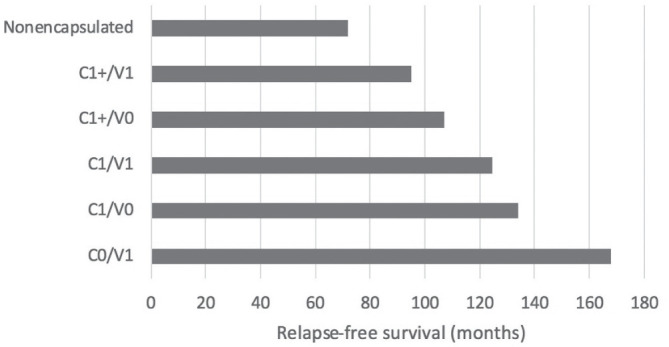
Relapse-free survival rate according to the combined invasion status.
Furthermore, as expected, patients with stage C1+/V0 were associated with nodules with the smallest sizes (maximum mean of 2.5 cm), such that the maximum tumour size was observed in patients with vascular and extended capsular invasion at the same time (maximum mean of 4 cm).
A curve exemplifying the relationship between the tumour size and the relapse-free survival rate was made, based on small or macrocarcinoma status (Fig. 4).
Figure 4.
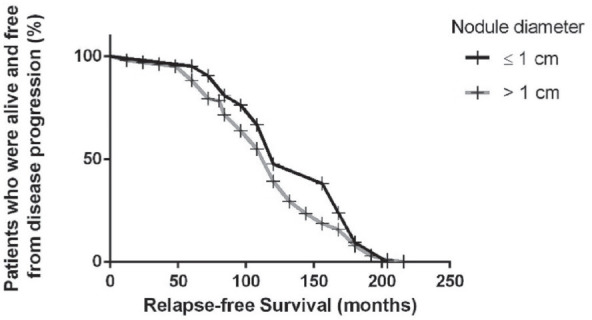
Relapse-free survival curve based on tumour size.
In the group with vascular and extended capsular invasion, only 79.6% of patients were considered cured, with persistent disease in 4.6% of cases. C0/V1 were cured in 100%, C1/V0 in 97.1%, C1/V1 in 85%. Recurrent disease was more common in patients with C1+/V0 (6%) and C1+/V1 (6.9%) and the evolving disease appeared in patients with vascular invasion (11.6%), According to data presented in Table 2, C1/V0 were cured in 97.1% vs. C1+/V0 cured in 94% and C1/V1 cured in 85% vs. C1+/V1 cured in 79.6%.
Table 2.
Disease status according to the invasion stage
| Stage | Cured (%) | Recurrence (%) | Evolving disease (%) | Biochemical persistent disease (%) |
|---|---|---|---|---|
| C0/V1 | 100 | 0 | 0 | 0 |
| C1/V0 | 97.1 | 0 | 0 | 2.9 |
| C1/V1 | 85 | 5 | 5 | 5 |
| C1+/V0 | 94 | 6 | 0 | 0 |
| C1+/V1 | 79.6 | 6.9 | 11.6 | 4.6 |
| Non-encapsulated/V0 | 100 | 0 | 0 | 0 |
DISCUSSION
Generally, thyroid carcinoma is a condition with a good prognosis, due to the increased frequency of the papillary type and current methods of diagnosis. At the same time, the diagnostic and therapeutic challenges come to light in particular forms, such as for follicular thyroid carcinoma, which, although not as common, has had its diagnostic criteria or classification changed over time. In our study, we observed the predominance of this disease in women, as in most studies. The mean (± SD) age of patients was 47.7 ± 14.45 (range 10–76) years; the age was similar to that reported by other authors from the literature (19).
It was unexpected to find such a large number of WIFTC, even though over time the classification regarding the invasive behaviour has changed, pathologists made this diagnosis primarily based on the extensive vascular invasion and/or tumour infiltration. Their number being about half compared to those with a minimally invasive carcinoma could explain the favourable evolution of the majority of patients. This increased number of cases with widely invasive carcinoma can also be explained by the late presentation of patients to the medical services, which is common in this region. The division by tumour size into groups has proven useful in quantifying the prognosis of this pathology. Even though most patients with small carcinomas were cured (20 out of 21 patients), only one case presented pathological events (local recurrent disease) during the follow-up period. This can be explained by the presence of C1+ stage and a surgical resection R1, which were submitted to oncological treatment.
One of the most important features of this type of carcinoma is the ability to realise invasion. It declares the histopathological subtype and correlates, in most studies, with the survival rate of patients. Accordingly, O’Neill et al. showed that disease-free survival rate at 40 months was 97% for MIFTC, 81% for encapsulated angioinvasive and 46% for widely invasive (20). In our study, almost all patients had a capsular invasion, but the relapse-free rate of patients was relatively good, probably explained by the fact that the associated vascular invasion was present in only half of the patients. In clinical practice, the degree of invasion is highly polymorphic, especially in the case of capsular invasion. There are opinions that support full capsule penetration for a diagnosis of carcinoma, others do not, being described different types of possible invasion (21). At the same time, the WHO classification 2017 describes the same lack of consensus regarding the complete invasion of the capsule and frequently describes cases in which the tumour cells, that initially have completely perforated the capsule, can be restricted by a newly formed fibrous band that limits their progression to the thyroid tissue. This event could give us the appearance of an incomplete invasion, or of an irregularity of the capsule or even the presence of intravascular cells in the absence of the capsular invasion (17). Therefore, in our study, we did not exclude the cases in which an incomplete invasion was described (together with no vascular invasion–C1/V0) where the pathologist considered a diagnosis of carcinoma, especially since some of these patients had clinical events (serological, biochemical persistent disease) during the follow-up period. Even more, we wanted to demonstrate a difference in the evolution between these two types of invasion (C1 vs. C1+).
Furthermore, judging by the multiple classifications of thyroid follicular carcinoma, in which a great deal of attention was given to the vascular invasion, we can consider this criterion as a decisive factor in the subsequent evolution of patients. Vascular invasion can induce metastasis (22), that is why we initiated a separate, parallel study in January 2015, with the aim of quantifying the prognostic value of the presence of circulating tumour cells (CTCs) in patients with MIFTC. It was a pilot study whose results were published in 2018. Although a small number of patients were considered, we demonstrated the feasibility of this serum biomarker. We adjusted the postoperative treatment according to the blood count of these cells and observed that none of the CTC negative patients had any recurrences during the follow-up period (9). Also, we previously succeeded in demonstrating the importance of detecting these biomarkers by conducting a review in which different molecular techniques for CTC detection were used (23). A study conducted in Korea, on patients with MIFTC, showed a higher metastasis rate than in our research (6.7% vs. 4.1%), in a study enrolling almost twice the number of patients, but with a similar follow-up period (19).
There were also patients with other oncological disorders that required external radiotherapy, but all emerged after the discovery of the thyroid disease, therefore, we cannot incriminate this treatment in the development of thyroid cancer for these patients. Although there is a tendency for limited surgical treatment, in our study, the majority of patients underwent radical interventions (24,25). Many of the patients who have been treated in this way have had two-stage surgery. Similarly, to results from the literature, we were able to demonstrate the importance of the tumour size: patients with macrocarcinomas were more frequently associated with the presence of persistent biochemical disease, the manifestation of recurrences and lung or bone metastases than those with small carcinomas (26). The poorer prognosis in men, which is also cited in most studies, can be attributed to significantly larger tumour sizes in men than in women (27).
A less expected outcome was the significant rate of recurrences and persistent biochemical diseases in patients with MIFTC, almost similar to those in the WIFTC group. This may be due primarily to less aggressive oncological treatment, especially in patients initially considered to be at minimal risk, including the fact that surgical treatment was somewhat extensive for most patients (according to the new treatment standards). Another argument for this situation is precisely the lack of a constant classification over time.
The novelty of this study is given by the fact that our research represents the only statistical analysis made on a large number of patients with strictly thyroid follicular cancer, in Romania. An important remark made from this study is the fact that the relapse-free survival rate is rather more associated with the size of the tumour, than the histological subtype, from an observational point of view meaning that a larger tumour can have a worse prognosis than a small one with extensive invasion. On the other hand, it is true, that in general, a large tumour is more susceptible to have a well-developed invasion. The necessity of introducing another prognostic marker, such as the CTC, was given by the observations made in our study in which MIFTC had almost similar clinical or biochemical recurrences, compared with WIFTC. The explanation for this behaviour is due mainly to the fact that MIFTC was treated in a more conservative way, avoiding radioiodine therapy in some situations. Another reason that supports the introduction of new prognostic factors is the fact that in our study no statistically significant differences were found regarding the tumour size of patients with persistent biochemical disease compared to other groups - although this condition is likely to generate recurrence. Also, noting that the recurrences were similar in the group of C1 + / V0 patients with those of the C1 + / V1 group, we can consider CTC detection useful in the early diagnosis of possible metastases. Introducing this technique leads to a personalised approach, selecting the positive cases for a more aggressive therapy.
In conclusion, follicular thyroid carcinoma is a disorder that by its subtypes still represents a challenge for proper diagnosis and treatment. The changes in recent years regarding its classification express the importance of the invasion pattern, especially the vascular one. We observed a stronger relation between relapse-free survival rate and tumour size, compared with relapse-free survival rate and invasion type.
This research reflects the need for a unitary histopathological description, permanently adapted to the new criteria of classification for follicular carcinomas and, last but not least, to carry out survival studies to verify the effectiveness of the diagnosis and treatment.
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding
The study was partially supported by PN-III-P1-1.2-PCCDI2017-0737: Genomic population mapping of radioactively and heavy metals contaminated areas in order to increase national security-ARTEMIS and by research grant no 7690/6/ 15.04.2016 of the “Iuliu Hațieganu” University of Medicine and Pharmacy Cluj-Napoca.
Author contributions
C.I.B., A.P., M.B and D.P. contributions to the acquisition, analysis and interpretation of data. D.A.-statistical analysis. M.B. responsive for anatomical images.
References
- 1.Cipriani NA, Nagar S, Kaplan SP, White MG, Antic T, Sadow PM, Aschebrook-Kilfoy B, Angelos P, Kaplan EL, Grogan RH. Follicular Thyroid Carcinoma: How Have Histologic Diagnoses Changed in the Last Half-Century and What Are the Prognostic Implications? Thyroid. 2015;25(11):1209–1216. doi: 10.1089/thy.2015.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piciu D. Thyroid cancer incidence 25 years after Chernobyl, in a Romanian cancer center: is it a public health problem? Curr Radiopharm. 2013;6(4):249–252. doi: 10.2174/1874471006666140109114218. [DOI] [PubMed] [Google Scholar]
- 3.Piciu D, Irimie A, Piciu A. Investigation of thyroid carcinoma over 40 years, using the database of the Ion Chiricuta Institute of Oncology Cluj-Napoca. J BUON. 2014;19(2):542–549. [PubMed] [Google Scholar]
- 4.La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, Negri E. Thyroid cancer mortality and incidence: a global overview. Int J Cancer. 2015;136(9):2187–2195. doi: 10.1002/ijc.29251. [DOI] [PubMed] [Google Scholar]
- 5.Fagin JA, Wells SA Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N Engl J Med. 2016;375(11):1054–1067. doi: 10.1056/NEJMra1501993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grani G, Lamartina L, Durante C, Filetti S, Cooper DS. Follicular thyroid cancer and Hürthle cell carcinoma: challenges in diagnosis, treatment, and clinical management. Lancet Diabetes Endocrinol. 2018;6(6):500–514. doi: 10.1016/S2213-8587(17)30325-X. [DOI] [PubMed] [Google Scholar]
- 7.Kawabata W, Suzuki T, Moriya T, Fujimori K, Naganuma H, Inoue S, Kinouchi Y, Kameyama K, Takami H, Shimosegawa T, Sasano H. Estrogen receptors (alpha and beta) and 17beta-hydroxysteroid dehydrogenase type 1 and 2 in thyroid disorders: possible in situ estrogen synthesis and actions. Mod Pathol. 2003;16(5):437–444. doi: 10.1097/01.MP.0000066800.44492.1B. [DOI] [PubMed] [Google Scholar]
- 8.Wei S. Follicular carcinoma. PathologyOutlines.com website. http://www.pathologyoutlines.com/topic/thyroidfollicular.html accessed on 27 December 2019.
- 9.Badulescu CI, Marlowe RJ, Piciu A, Buiga R, Barbos O, Bejinariu NI, Chereches G, Barbus E, Bonci EA, Piciu D. Circulating tumor cells in minimally invasive follicular thyroid carcinoma and benign thyroid tumors with a follicular pattern:pilot experience. Acta Endocrinol (Buchar). 2018;14(1):1–10. doi: 10.4183/aeb.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Agency for Research on Cancer. Globocan 2018. Available online: https://gco.iarc.fr/today/data/factsheets/populations/642-romania-fact-sheets.pdf (accessed on 8 February 2020)
- 11.Stanciu M, Racz IC, Bera L, Rotaru M, Popa F. Thyroid Cancer Incidence in Sibiu County. Acta Medica Transilv. 2015;20:16–18. [Google Scholar]
- 12.Cătană R, Boilă A, Borda A. Thyroid cancer profile in Mures County (Romania): a 20 years study. Rom J Morphol Embryol. 2012;53(4):1007–1012. [PubMed] [Google Scholar]
- 13.Piciu D, Irimie A. Diagnosis and treatment guidelines in thyroid. Carcinoma American and European consensus, adapted to Romania. Acta Endocrinol (Buchar). 2007;3(1):103–115. [Google Scholar]
- 14.Gabora K, Bărbuș E, Peștean C, Larg MI, Bonci EA, Bădulescu IC, Piciu A. Radiation induced thyroid carcinoma in Romania – effects of the Chernobyl fallout, a systematic review of observational studies. Clujul Med. 2018;91(4):372–375. doi: 10.15386/cjmed-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White MG, Cipriani NA, Abdulrasool L, Kaplan S, Aschebrook-Kilfoy B, Kaplan EL, Grogan RH, Onel K. Radiation-Induced Differentiated Thyroid Cancer Is Associated with Improved Overall Survival but Not Thyroid Cancer-Specific Mortality or Disease-Free Survival. Thyroid. 2016;26(8):1053–1060. doi: 10.1089/thy.2015.0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmadi S, Stang M, Jiang XS, Sosa JA. Hürthle cell carcinoma: Current perspectives. OncoTargets and Therapy. 2016;9:6873–6884. doi: 10.2147/OTT.S119980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lioyd RV, Osamura RY, Kloppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs, 4th Edition. Lyon: IARC. 2017:92–95. [Google Scholar]
- 18.Parameswaran R, Shulin HJ, Min EN, Tan WB, Yuan NK. Patterns of metastasis in follicular thyroid carcinoma and the difference between early and delayed presentation. Ann R Coll Surg Engl. 2017;99(2):151–154. doi: 10.1308/rcsann.2016.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YM, Song DE, Kim TY, Sung TY, Yoon JH, Chung KW, Hong SJ. Risk factors for distant metastasis in patients with minimally invasive follicular thyroid carcinoma. PLoS ONE. 2016;11(5):1–10. doi: 10.1371/journal.pone.0155489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Neill CJ, Vaughan L, Learoyd DL, Sidhu SB, Delbridge LW, Sywak MS. Management of follicular thyroid carcinoma should be individualised based on degree of capsular and vascular invasion. Eur J Surg Oncol. 2011;37(2):181–185. doi: 10.1016/j.ejso.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Ozgur M, Raja RS, Sylvia LA, Martin JB, Sally EC, Steven PH, Jonathan BM, Yuri EN, Jason P, Richardson MS, Jatin S, Lester DRT. Protocol for the Examination of Specimens From Patients With Carcinomas of the Thyroid Gland. Available online: https://documents.cap.org/protocols/cp-endocrine-thyroid-19-4200.pdf (accessed on 12 April 2020)
- 22.De Crea C, Raffaelli M, Sessa L, Ronti S, Fadda G, Bellantone C, Lombardi CP. Actual incidence and clinical behaviour of follicular thyroid carcinoma: an institutional experience. Scientific World Journal. 2014;952095:1–7. doi: 10.1155/2014/952095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bădulescu IC, Bărbuş E, Piciu D. Circulating tumor cells in thyroid carcinoma - the prognostic role of this biomarker. Review of the literature. Clujul Med. 2017;90(3):256–261. doi: 10.15386/cjmed-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermann M, Tonninger K, Kober F, Furtlehner EM, Schultheis A, Neuhold N. Minimal-invasives follikuläres Schilddrüsenkarzinom. Eine Thyreoidektomie ist nicht obligat. Chirurg. 2010;81(7):627–635. doi: 10.1007/s00104-009-1884-8. [DOI] [PubMed] [Google Scholar]
- 25.Delbridge L, Parkyn R, Philips J, Barraclough B, Robinson B. Minimally invasive follicular thyroid carcinoma: completion thyroidectomy or not? ANZ journal of surgery. 2002;72(11):844–845. [PubMed] [Google Scholar]
- 26.Podda M, Saba A, Porru F, Reccia I, Pisanu A. Follicular thyroid carcinoma: differences in clinical relevance between minimally invasive and widely invasive tumors. World J Surg Oncol. 2015;13:193. doi: 10.1186/s12957-015-0612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilubol N, Zhang L, Kebebew E. Multivariate analysis of the relationship between male sex, disease-specific survival, and features of tumor aggressiveness in thyroid cancer of follicular cell origin. Thyroid. 2013;23(6):695–702. doi: 10.1089/thy.2012.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]


