Abstract
Objective
In this study, we aimed to evaluate the prognostic value of axillary lymph node ratio (LNR) for disease-free survival (DFS) in node positive breast cancer (BC) patients with long term follow-up.
Materials and Methods
A total of 179 stage II to III female BC patients, who were followed between December 2001 and January 2019 at the department of medical oncology, were included in this study. Patients were classified into 3 groups based on the LNR as follows; LNR<0.21, LNR=0.21–0.65, and LNR>0.65. SPSS 22 for windows was used for statistical analysis.
Results
The median age was 49 (range, 24–83) years. The numbers of patients with stage II and stage III disease were 81 (45.3%) and 98 (54.7%), respectively. The median number of lymph node (LN) resected and positive LN were 15 (range, 3–48) and 3 (range, 1–29), respectively. There were 90 patients (50.3%) with LNR <0.21, 62 (34.6%) with LNR=0.21–0.65, and 27 (15.1%) with LNR >0.65. The median disease-free survival (DFS) was not reached in patients with LNR <0.21, 81 months in patients with LNR=0.21–0.65, and 43 months in patients with LNR>0.65 (p<0.001). Overall survival (OS) was found to be significantly related to LNR (p=0.042). In patients with LNR<0.21 and LNR=0.21–0.65, the median OS was not reached. In patients with LNR >0.65, the median OS was 101 months. In multivariate analysis, LNR=0.21–0.65 (Hazard ratio [HR], 6.99), LNR>0.65 (HR, 28.99), and HER-2 negativity (HR, 4.64) were the factors associated with DFS (p<0.05).
Conclusion
LNR is a more useful prognostic factor than the pathological lymph node staging for predicting survival in patients with nod-positive BC.
Keywords: Breast cancer, lymph node ratio, survival
Introduction
Globally, breast cancer (BC) is the most commonly diagnosed cancer in women as well as being one of the most common cancer-related deaths, particularly in patients aged 40–49 years (1,2). Its treatment requires a multidisciplinary team approach which includes surgical oncology, medical oncology, and radiation oncology. The majority of BC patients are diagnosed at non-metastatic stage. Approximately 5% of patients have metastatic disease at the time of diagnosis (2, 3). For BC patients, the treatment decision depends on some factors including disease stage, hormone receptor status, and human epidermal growth factor receptor-2 (HER-2) status at presentation (1–3).
It is well-known that locoregional radiotherapy to axillary lymph nodes (ALN) has decreased local recurrence and improved survival in node-positive BC. The ALN status has been considered a possible indication for post-surgical adjuvant radiotherapy. However, it may depend on the degree of ALN resected. Moreover, in some cases, the decision regarding whether radiotherapy is necessary depends on the physician (4–6).
The lymph node ratio (LNR) is described as the ratio of number of positive ALN to total number of ALN resected. Truong et al. (7) included 80 BC patients with 1 to 3 ALN positive and reported that LNR was related to an increased locoregional recurrence and also a stronger prognostic factor than the number of positive ALN. Similarly, Han et al. (8) included 130 BC patients with N1 stage and reported that LNR was associated with an increased risk of recurrence, especially in younger BC patients.
A study performed by Kuru (9) who analyzed 801 BC patients showed that the number of ALN resected >15 or number of negative ALN >15 improved survival. ALN status continues to be one of the main prognostic factors guiding the adjuvant radiotherapy decision. pN stage is based on the number of ALN resected. However, the accuracy of the approach is affected by the number of ALN resected, which may cause undesirable results (10, 11). In this retrospective study, we aimed to evaluate the prognostic value of LNR in ALN-positive BC patients on long-term outcomes.
Materials and Methods
Study population
A total of 179 stage II to III female BC patients, who were followed between December 2001 and January 2019 at the department of medical oncology, Yüzüncü Yıl University Faculty of Medicine, were included in this study. All patients had unilateral BC, with non-metastatic disease at initial presentation. ALN dissection was performed in all patients at the time of diagnosis. Patients were restaged based on the 8th edition of the American Joint Committee on Cancer staging system. Patients with any of the following criteria were excluded from the study; <18 years of age, metastatic stage, unoperated patients, receiving neoadjuvant treatment, history of a second primary cancer, histologic subtypes other than invasive ductal carcinoma (IDC) and invasive lobular carcinoma (ILC), and patients whose data were not available. The study was performed in accordance with the Helsinki Declaration and was approved by the Yüzüncü Yıl University Faculty of Medicine Ethics Committee (22/05/2020-2020/03-54).
Data collection
Demographic data including age, menopausal status (perimenopause vs. postmenopausal), type of surgery (breast-conserving surgery [BCS] vs. modified radical mastectomy [MRM]), hormone-receptor status, HER2-status, histology (IDC or ILC), stage, grade, perineural invasion, lymphovascular invasion, pathological tumor stage (pT), number of ALN resected, number of positive ALN, margin status, adjuvant chemotherapy, hormonotherapy and radiotherapy, recurrence and site of recurrence, and final status (exitus or alive) were obtained from the written archive files. LNR was calculated by the ratio of number of positive ALN to total number of ALN resected. The classifications of LNR were based on the previous studies which divided the LNR into 3 categories as follows; LNR <0.21, LNR=0.21–0.65, and LNR >0.65 (12, 13). pT stage was stratified into 2 groups as pT1–2 and pT3–4. Tumor grade was grouped into 2 categories as grade 1–2 and grade 3. Disease-free survival (DFS) was calculated as the time from the date of diagnosis to the date of recurrence or last control. Overall survival (OS) was estimated from the date of diagnosis to the date of death or last follow-up.
Statistical analysis
IBM Statistical Package for Social Sciences (IBM SPSS Corp.; Armonk, NY, USA) 22 for windows was used. Chi-square analysis was carried out to compare the ratios in the groups. Survival analysis was performed using Kaplan-Meier method, and Log-rank test was used for comparison of survival time. The independent prognostic factors for survival were identified by Cox Regression Analysis. Forward stepwise model was used for the factors with p<0.150, which were determined in univariate analysis. Statistical significance value was accepted as p<0.05.
Results
The median age of the patients were 49 years (range, 24–83). Of the 179 patients, 171 (84.4%) were hormone-receptor positive and 30 (17.3%) were HER-2 positive. Nine (5%) patients were triple negative. Eighty-one patients (45.3%) had stage II disease and 98 patients (54.7%) had stage III disease. The median number of ALN resected and positive ALN were 15 (3–48) and 3 (1–29), respectively. There were 90 (50.3%) patients with LNR <0.21, 62 (34.6%) with LNR 0.21–0.65, and 27 (15.1%) with LNR >0.65 (Table 1).
Table 1.
Patient characteristics
| Variable | n | % | |
|---|---|---|---|
| Age | Median (Min-Max) | 49 (24–83) | |
| Menopausal status | Post- | 92 | 51.4 |
| Pre- | 87 | 48.6 | |
| Surgical status | MRM | 114 | 63.7 |
| BCS | 65 | 36.3 | |
| Hormone-receptor status | Positive | 152 | 80.4 |
| HER2-status | Positive | 30 | 17.3 |
| TNCB | Yes | 9 | 5.0 |
| Histology | IDC | 165 | 92.2 |
| ILC | 14 | 7.8 | |
| Stage | II | 81 | 45.3 |
| III | 98 | 54.7 | |
| Grade | I+II | 125 | 69.8 |
| III | 54 | 30.2 | |
| Perineural invasion | Negative | 135 | 75.4 |
| Positive | 44 | 24.6 | |
| Lymphovascular invasion | Negative | 73 | 40.8 |
| Positive | 106 | 59.2 | |
| pT-stage | T1–2 | 134 | 74.9 |
| T3–4 | 45 | 25.1 | |
| Lymph node removed | Median (Min-Max) | 15 (3–48) | |
| Metastatic lymph node | Median (Min-Max) | 3 (1–29) | |
| pN- stage | 1–3 | 91 | 50.8 |
| 4–9 | 60 | 33.5 | |
| ≥10 | 28 | 15.6 | |
| LNR | <0.21 | 90 | 50.3 |
| 0.21–0.65 | 62 | 34.6 | |
| >0.65 | 27 | 15.1 | |
| Margin status | Positive | 9 | 5.0 |
| Adjuvant Chemotherapy | Doxorubicin | 38 | 21.2 |
| Doxorubicin +Taxane | 137 | 76.5 | |
| Taxane | 4 | 2.2 | |
| Adjuvant trastuzumab | Yes | 30 | 16.8 |
| Adjuvant hormonotherapy | Yes | 152 | 84.9 |
| Tamoxifen | 76 | 50.0 | |
| Aromatase inhibitors | 76 | 50.0 | |
| Adjuvant Radiotherapy | Yes | 171 | 95.5 |
| Recurrence and localization | Yes | 32 | 17.9 |
| locoregional | 3 | 9.4 | |
| Bone | 20 | 62.5 | |
| Liver | 3 | 9.4 | |
| Lung | 4 | 12.5 | |
| Brain | 2 | 6.3 | |
| Final status | Exitus | 8 | 4.5 |
| Alive | 171 | 95.5 |
BCS: Breast-conserving Surgery; HER2: Human Epidermal Growth Factor Receptor 2; ILC: Invasive Lobular Carcinoma; IDC: Invasive Ductal Carcinoma; LNR: Lymph Node Ratio; MRM: Modified Radical Mastectomy; pT: Pathologic Tumor Stage; pN: Pathologic Lymph Node Stage; SD: Standard Deviation; TNBC: Triple-Negative Breast Cancer
All patients received adjuvant chemotherapy following surgery. A total of 137 (76.5%) patients received doxorubicin + taxane-based chemotherapy regimen, 38 (21.2%) patients received doxorubicin-based chemotherapy regimen, and 4 (2.2%) patients received taxane-based chemotherapy regimen. Thirty (16.8%) patients received trastuzumab. Radiotherapy was given to 171 (95.5%) patients. Hormone receptor-positive patients received adjuvant endocrine therapy after the completion of chemotherapy and radiotherapy. During the median follow-up time of 36 months, 32 (17.9%) patients developed recurrence, 8 (4.5%) of whom died (Table 1).
In Kaplan Meier analysis, the LNR was found to be significantly associated with DFS (log rank p<0.001). The median DFS was not reached in the patients with LNR<0.21, 81 months (95% confidence interval [CI], 59.0–102.9) in patients with LNR=0.21–0.65 patients, and 43 months (95 % CI, 12.6–74.4) in patients with LNR >0.65 (Figure 1).
Figure 1.
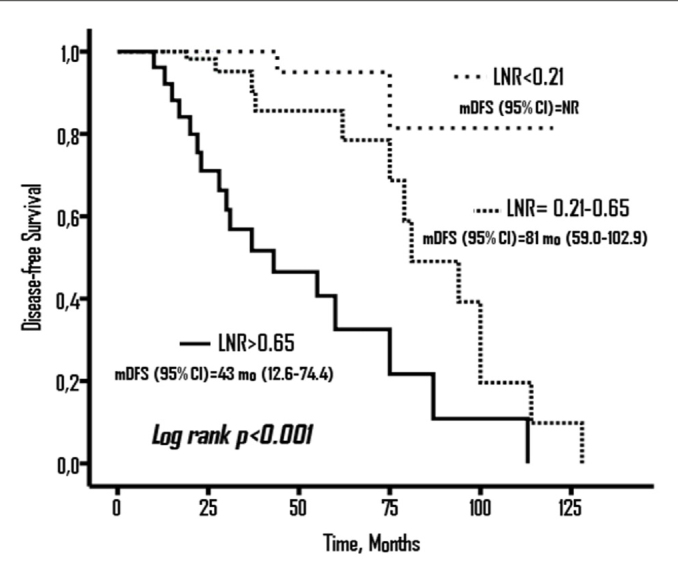
Disease- free Survival (DFS) according to LNR groups
OS was significantly associated with LNR (log rank p=0.042). The median OS was not reached in the patients with LNR<0.21 and LNR=0.21–0.65; however, it was 101 months (95% CI, 41–160.8) in the patients with LNR> 0.65 (Figure 2).
Figure 2.
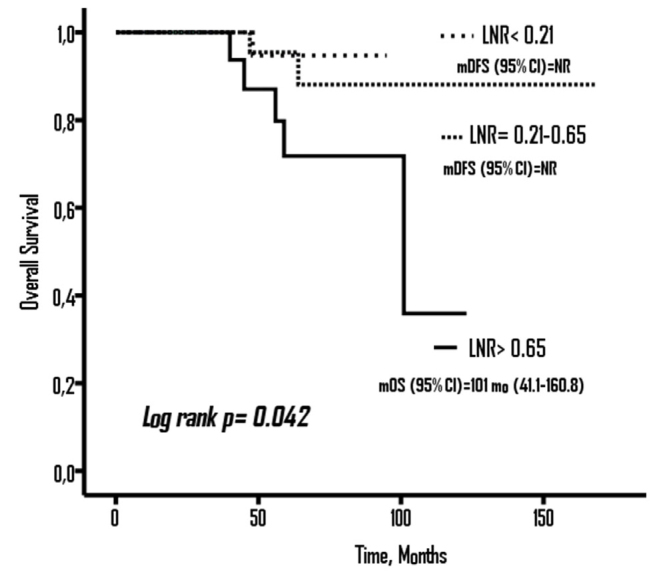
Overall Survival (OS) according to LNR groups
In univariate analysis; type of surgery (BCS vs. MRM), HER-2 status (negative vs. positive), disease stage (III vs. II), pT stage, pN stage, number of positive ALN and LNR, and trastuzumab therapy were the predictive factors associated with DFS (p<0.05) (Table 2). The factors with p≤0.150 identified in univariate analysis were then assessed in multivariate analysis with forward stepwise model. LNR and HER-2 status were found to be independent prognostic factors for DFS (p<0.05) (Table 3).
Table 2.
Univariate analysis for DFS
| Variable | HR | 95% CI for HR | p | |
|---|---|---|---|---|
| Age (year) | Median | 0.991 | 0.960–1.022 | 0.556 |
| Menopausal status | Pre vs. Post | 1.205 | 0.588–2.468 | 0.610 |
| Surgical status | BCS vs. MRM | 0.417 | 0.178–0.973 | 0.043 |
| Hormone-Receptor Status | Negative vs. Positive | 1.246 | 0.374–4.141 | 0.720 |
| HER2-Status | Negative vs. Positive | 2.669 | 1.049–6.785 | 0.039 |
| TNBC | Yes vs. No | 1.944 | 0.952–13.939 | 0.131 |
| Histology | ILC vs. IDC | 1.103 | 0.260–4.678 | 0.894 |
| Stage | III vs. II | 4.408 | 1.685–11.532 | 0.002 |
| Grade | III vs. I+II | 2.106 | 0.954–4.647 | 0.065 |
| Perineural invasion | Negative vs. Positive | 0.535 | 0.217–1.312 | 0.172 |
| Lymphovascular invasion | Negative vs. Positive | 0.854 | 0.418–1.741 | 0.664 |
| pT-Stage | 3+4 Vs 1+2 | 3.166 | 1.514–6.617 | 0.002 |
| pN-Stage | 1 | <0.001 | ||
| 2 | 2.246 | 0.686–7.346 | 0.181 | |
| 3 | 11.134 | 3.723–33.292 | <0.001 | |
| Lymph Node Removed | 1.000 | 0.955–1.047 | 0.997 | |
| Metastatic Lymph Node | 1.089 | 1.029–1.152 | 0.003 | |
| LNR | <0.21 | <0.001 | ||
| 0.21–0.65 | 6.180 | 1.377–27.734 | 0.017 | |
| >0.65 | 23.628 | 5.430–102.806 | <0.001 | |
| Surgical Margin | Negative vs. Positive | 0.656 | 0.088–4.867 | 0.680 |
| Adjuvant Treatment | Doxorubicin | 0.916 | ||
| Doxorubicin +taxane | 0.948 | 0.437–2.055 | 0.893 | |
| taxane | 0.647 | 0.083–5.026 | 0.677 | |
| Adjuvant Trastuzumab | Yes vs No | 0.364 | 0.143–0.922 | 0.033 |
| Adjuvant Hormonotherapy | Yes vs No | 0.726 | 0.274–1.920 | 0.518 |
| Adjuvant Hormonotherapy | Aromatase inhibitors vs Tamoxifen | 0.528 | 0.239–1.165 | 0.114 |
| Adjuvant Radiotherapy | No vs Yes | 1.350 | 0.318–5.717 | 0.683 |
BCS: Breast-conserving Surgery; HER2: Human Epidermal Growth Factor Receptor 2; ILC: Invasive Lobular Carcinoma; IDC: Invasive Ductal Carcinoma; LNR: Lymph Node Ratio; MRM: Modified Radical Mastectomy; pT: Pathologic Tumor Stage; pN: Pathologic Lymph Node Stage; SD: Standard Deviation; TNBC: Triple-Negative Breast Cancer; DFS: Disease-free Survival
Table 3.
Multivariate analysis for DFS
| Variable | HR | 95 % CI for HR | p | |
|---|---|---|---|---|
| HER2-Status | Negative vs. Positive | 4.641 | 1.614–13.340 | 0.004 |
| LNR | <0.21 (Ref.) | 1 | <0.001 | |
| 0.21–0.65 | 6.996 | 1.545+31.660 | 0.012 | |
| >0.65 | 28.997 | 6.512–129.106 | <0.001 |
HER2: Human Epidermal Growth Factor Receptor 2; LNR: Lymph Node Ratio; DFS: Disease-free Survival
Discussion and Conclusion
In this study, we evaluated the prognostic value of LNR in ALN-positive BC patients with long term follow-up and real-life data. We demonstrated that the LNR can better predict tumor recurrence and survival than nodal staging in operated-stage II–III BC patients. The risk of recurrence almost increased by 30 folds in BC patients with LNR>0.65.
Danish Breast Cancer Cooperative Group Randomized Trials Subgroup Analysis evaluated the locoregional recurrence rate and survival regarding the number of positive ALN. In that analysis, the 15-year OS rates were observed to be 39% and 29% with and without radiotherapy, respectively. In the study, radiotherapy reduced the 15-year locoregional recurrence rate from 51% to 10% in patients with ≥4 positive ALN and from 27% to 4% in those with 1–3 positive ALN. They concluded that the survival benefit after adjuvant radiotherapy was substantial and similar to patients with 1–3 positive ALN and ≥4 positive ALN. However, in that study few lymph nodes were removed in most patients (median number of ALN resected was 7) (14). This situation may have affected the study results.
Nagao et al. (15) included 789 pN1–3 BC patients in whom the median number of ALN resected was 18.6 and reported that adjuvant radiotherapy did not significantly improve the outcomes. In another study, the median number of ALN resected was 23. In this study, the authors concluded that hormone receptor-positive patients treated by mastectomy and complete axillary dissection had a low risk of locoregional recurrence, even if four or more positive ALN were involved, thus, giving rise to doubts about the use of adjuvant radiotherapy in this subset of patients (16). These results suggest that sufficient number of ALN resected is helpful to reduce recurrence, hence affecting treatment decision-making in adjuvant radiotherapy.
In our study, the median number of ALN resected and the number of positive ALN was 15 and 3, respectively. Radiotherapy was given to 171 (95.5%) patients. Recurrence developed in 32 (17.9%) patients, only 3 (9.4%) of whom were locoregional. The lower rate of locoregional recurrence was due to the fact that almost all patients received adjuvant radiotherapy.
Kim et al. (17) performed a multicenter study with N1-stage BC patients and reported that high LNR was an independent prognostic factor for pN1 BC patients treated with BCS followed by adjuvant radiotherapy. It can also be helpful in deciding whether to irradiate supraclavicular lymph nodes in order to improve DFS. In a study designed by Wu et al. (13) including stage II–III BC patients who were not treated with adjuvant radiotherapy, it was reported that LNR was a better prognostic factor than pN staging in ALN positive BC. LNR should be used as an indication for adjuvant radiotherapy. Similarly, Ataseven et al. (18) reported that LNR was a prominent prognostic factor for survival and can potentially provide more information than the pN staging in different molecular subtypes of BC patients. In our study, the LNR is an independent prognostic factor of DFS and pN staging lost its significance when LNR was added to the multivariate analysis. These results suggest that LNR has a better prognostic value than pN staging.
Our study had some limitations. The study had a retrospective nature; hence, the results might be inherently flawed by selection bias. In addition, the number of samples was relatively low. Since it was a single-centered study, the outcomes may not reflect the results of a general population. However, most of the previous studies regarding this issue have enrolled only pN1-stage BC patients (8, 13, 17, 18), whereas our study also included pN1-3 stage BC patients.
In conclusion, our study showed that LNR was a better prognostic factor than nodal staging to predict recurrence and survival in stage II–III operated-BC patients. More importantly, the risk of recurrence almost increased by 30 folds in patients with LNR>0.65. However, there is no consensus on the optimal cut off value for LNR. Therefore, further prospective multicenter studies are required to assess the effect of LNR on prognosis in node-positive BC patients.
Key Points.
Axillary lymph nodes (ALN) status has been considered a possible indication for post-surgical adjuvant radiotherapy. However, the accuracy of the approach is affected by the number of ALN resected, which may cause undesirable results.
The lymph node ratio (LNR) is described as the ratio of the number positive ALN to the total number of ALN resected. In this study, we evaluated the prognostic value of LNR in ALN-positive breast cancer (BC).
Disease-free survival and overall survival were found to be significantly related to LNR.
The LNR is an independent prognostic factor of disease-free survival and pN staging lost its significance when LNR was added to the multivariate analysis.
LNR is a more useful prognostic factor than the pathological lymph node staging for predicting survival in operated stage II–III BC patients.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Yüzüncü Yıl University (22/05/2020-2020/03-54).
Informed Consent: The patients were not required to give informed consent for this study because the study utilized the anonymous retrospective data obtained after each patient accepted the treatment by a written consent.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.S., M.N.A.; Design – A.S., M.N.A.; Supervision – A.S., M.N.A.; Resources – A.S., M.N.A.; Materials – A.S., M.N.A.; Data Collection and/or Processing – M.N.A.; Analysis and/or Interpretation – A.S., M.N.A.; Literature Search – M.N.A.; Writing Manuscript – A.S.; Critical Review – A.S., M.N.A.; Other – A.S., M.N.A.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Kesson EM, Allardice GM, George WD, Burns HJ, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ. 2012;344:e2718. doi: 10.1136/bmj.e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 4.Van de Steene J, Soete G, Storme G. Adjuvant radiotherapy for breast cancer significantly improves overall survival: the missing link. Radiother Oncol. 2000;55:263–272. doi: 10.1016/S0167-8140(00)00204-8. [DOI] [PubMed] [Google Scholar]
- 5.Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, Kjaer M, Gadeberg CC, Mouridsen HT, Jensen MB, Zedeler K. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 6.Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, Knowling MA, Coppin CML, Weir L, Gelmon K, Le N, Durand R, Coldman AJ, Manji M. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 7.Truong PT, Woodward WA, Thames HD, Ragaz J, Olivotto IA, Buchholz TA. The ratio of positive to excised nodes identifies high-risk subsets and reduces inter-institutional differences in locoregional recurrence risk estimates in breast cancer patients with 1–3 positive nodes: an analysis of prospective data from British Columbia and the M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 2007;68:59–65. doi: 10.1016/j.ijrobp.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Han TJ, Kang EY, Jeon W, Kim SW, Kim JH, Kim YJ, Park SY, Kim JS, Kim IA. The prognostic value of the nodal ratio in N1 breast cancer. Radiat Oncol. 2011;6:131. doi: 10.1186/1748-717X-6-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuru B. Prognostic significance of total number of nodes removed, negative nodes removed, and ratio of positive nodes to removed nodes in node positive breast carcinoma. Eur J Surg Oncol. 2006;32:1082–1088. doi: 10.1016/j.ejso.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Beenken SW, Urist MM, Zhang Y, Desmond R, Krontiras H, Medina H, Bland KI. Axillary lymph node status, but not tumor size, predicts locoregional recurrence and overall survival after mastectomy for breast cancer. Ann Surg. 2003;237:732–739. doi: 10.1097/01.SLA.0000065289.06765.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang N, Choi JE, Kang SH, Bae YK. Validation of the pathological prognostic staging system proposed in the revised eighth edition of the AJCC staging manual in different molecular subtypes of breast cancer. Virchows Arch. 2019;474:193–200. doi: 10.1007/s00428-018-2495-x. [DOI] [PubMed] [Google Scholar]
- 12.Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, Deglise C, Usel M, Lutz JM, Bouchardy C. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009;27:1062–1068. doi: 10.1200/JCO.2008.18.6965. [DOI] [PubMed] [Google Scholar]
- 13.Wu SG, Chen Y, Sun JY, Li FY, Lin Q, Lin HX, He ZY. Using the lymph nodal ratio to predict the risk of locoregional recurrence in lymph node-positive breast cancer patients treated with mastectomy without radiation therapy. Radiat Oncol. 2013;8:119. doi: 10.1186/1748-717X-8-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007;82:247–253. doi: 10.1016/j.radonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Nagao T, Kinoshita T, Tamura N, Hojo T, Morota M, Kagami Y. Locoregional recurrence risk factors in breast cancer patients with positive axillary lymph nodes and the impact of postmastectomy radiotherapy. Int J Clin Oncol. 2013;18:54–61. doi: 10.1007/s10147-011-0343-y. [DOI] [PubMed] [Google Scholar]
- 16.Gentilini O, Botteri E, Rotmensz N, Intra M, Gatti G, Silva L, Peradze N, Sahium RC, Gil LB, Luini A, Veronesi P, Galimberti V, Gandini S, Goldhirsh A, Veronesi U. Is avoiding post-mastectomy radiotherapy justified for patients with four or more involved axillary nodes and endocrine-responsive tumours? Lessons from a series in a single institution. Ann Oncol. 2007;18:1342–1347. doi: 10.1093/annonc/mdm182. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Park W, Kim JH, Choi DH, Kim YJ, Lee ES, Shin KH, Kim JH, Kim K, Kim YB, Ahn SJ, Lee JH, Chun M, Lee HS, Kim JS, Cha J. Clinical Significance of Lymph-Node Ratio in Determining Supraclavicular Lymph-Node Radiation Therapy in pN1 Breast Cancer Patients Who Received Breast-Conserving Treatment (KROG 14–18): A Multicenter Study. Cancers (Basel) 2019;11:680. doi: 10.3390/cancers11050680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ataseven B, Kummel S, Weikel W, Heitz F, Holtschmidt J, Lorenz-Salehi F, Kümmel A, Traut A, Blohmer J, Harter P, du Bois A. Additional prognostic value of lymph node ratio over pN staging in different breast cancer subtypes based on the results of 1,656 patients. Arch Gynecol Obstet. 2015;291:1153–1166. doi: 10.1007/s00404-014-3528-6. [DOI] [PubMed] [Google Scholar]


