Abstract
The following case report features a middle-aged female patient, previously diagnosed with Cowden syndrome, who presented to the hospital with symptoms of headaches and changes in vision that began with no apparent cause and persisted for almost a month. MRI of the head confirmed a diagnosis of dysplastic cerebellar gangliocytoma, also known as Lhermitte-Duclos disease. This cerebellar tumor, while extremely rare in incidence, is classified as the most common type of brain lesion in adult patients with Cowden syndrome. This report will also include a comprehensive literature review of Cowden syndrome and Lhermitte-Duclos disease, with greater emphasis on the radiologic characteristics of Lhermitte-Duclos disease.
Keywords: Dysplastic Cerebellar Gangliocytoma, Lhermitte-Duclos, Cerebellar Tumor, Cowden Disease, Multiple Hamartoma-Neoplasm Syndrome, MRI brain
CASE REPORT
A 56-year-old female patient with a complicated medical history of Cowden syndrome, Hashimoto’s thyroiditis, nephrolithiasis, and ductal carcinoma in situ came to the hospital with gradual onset of diplopia, flashing lights, and intractable headaches of four-weeks’ duration with no apparent cause. These clinical findings were suggestive of increased intracranial pressure and required further evaluation with Magnetic Resonance Imaging (MRI) of the brain. MRI was performed with Siemens Verio 3- Tesla 8-channel scanner. 10CC Gadavist IV contrast was utilized. Post-contrast imaging was performed in the venous phase.
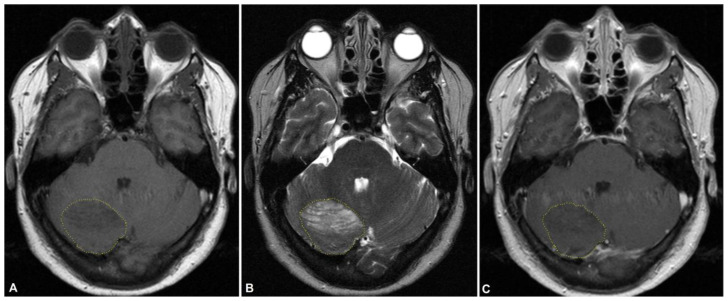
Axial MRI of the brain revealed predominantly T2 and FLAIR hyperintense signal in the right cerebellar hemisphere (Figures 1 and 2). T1 non-contrast imaging demonstrated T1 low signal corresponding to the findings noted on the heterogenous T2 weighted imaging. This nonenhancing cerebellar lesion was well-circumscribed and measured 4.9×4.0×2.0cm. There was mild associated localized mass effect.
Figure 1.
56-year-old female with dual diagnosis of Cowden syndrome and Lhermitte Duclos disease.
Findings: MRI brain performed on the day of initial diagnosis with non-contrast axial T1w (A), non-contrast axial T2w (B), and post-contrast T1w (C) shows predominantly T2 hyperintense signal in right cerebellar hemisphere. Lesion (outlined in yellow) measures 4.9×4.0×2.0cm and is well-circumscribed. There is mild associated localized mass effect. T1 non-contrast imaging demonstrates T1 low signal corresponding to the findings noted on the heterogenous T2 weighted imaging. There is mild heterogenous enhancement on post-contrast imaging.
Technique: MRI, Siemens Verio 3-Tesla 8-channel; (A): Axial T1w non-contrast: TR 599.999, TE 13.8094; (B): Axial T2w non-contrast: TR 5758.58, TE 110; (C) Axial T1w post-contrast: TR 599.999, TE 13.8094
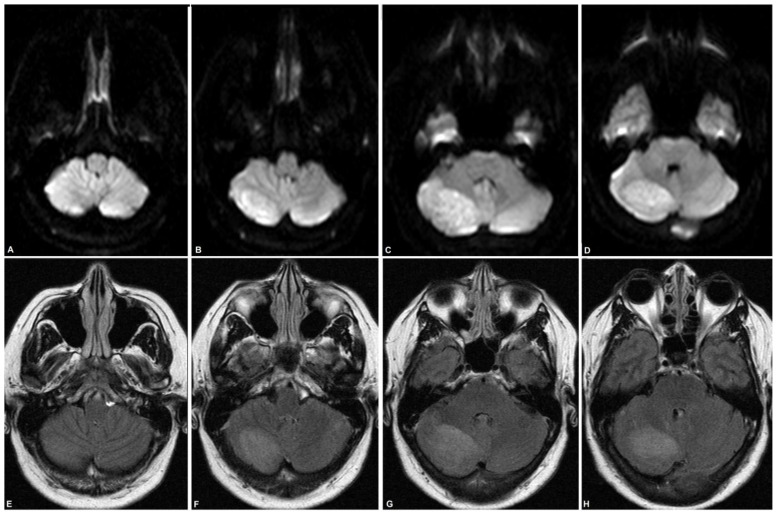
Figure 2.
56-year-old female with dual diagnosis of Cowden syndrome and Lhermitte Duclos disease.
Findings: MRI brain performed on the day of initial diagnosis with axial DWI/ADC (A–D, from inferior to superior) and axial FLAIR (E–H, from inferior to superior) shows well-circumscribed, hyperintense signal in right cerebellar hemisphere. Dimensions of the lesion were measured as 4.9×4.0×2.0cm. Mild associated local mass effect was noted.
Technique: MRI, Siemens Verio 3-Tesla 8-channel; (A–D): Axial DWI/ADC: TR 3955.46, TE 74; (E–H): Axial FLAIR: TR 11000, TE 140
These imaging findings, along with the patient’s previous medical history of Cowden syndrome, strongly supported a diagnosis of Cowden-Lhermitte-Duclos syndrome (COLD syndrome). The cerebellar lesion was diagnosed as dysplastic cerebellar gangliocytoma (ie. Lhermitte-Duclos disease) (Table 1).
Table 1.
Summary table of Lhermitte-Duclos disease (LDD)
| Etiology | PTEN mutation that leads to dysplastic gangliocytoma of the cerebellum. |
| Incidence | Highly rare-only 222 known cases of LLD have been published in PubMed-indexed literature as of March 20, 2020, with the first case published in 1975. |
| Gender ratio | No gender predilection |
| Age predilection | Most frequently diagnosed in 2nd or 3rd decade |
| Risk factors | Genetic correlation between LDD and Cowden syndrome, an autosomal dominant syndrome characterized by multi-organ hamartomatous tumors |
| Treatment | Observation, unless mass effect symptoms warrant surgical intervention. Complete surgical resection is curative. |
| Prognosis | This is a slow-growing, benign tumor, so the prognosis is generally favorable. |
| Findings on imaging | (MRI is usually sufficient for diagnosis) Gross: focal, well-circumscribed lesion restricted to one of the cerebellar hemispheres with folia hypertrophy T1: hypointense signal T2: “tiger stripes” appearance of alternating low and high signal |
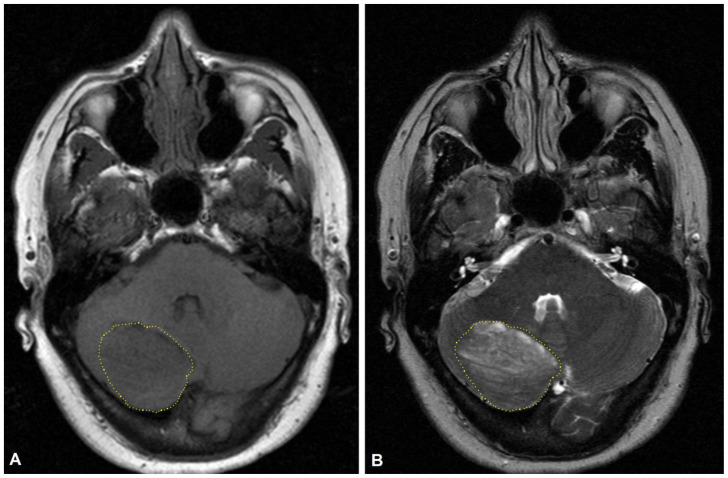
The patient was followed-up with a second MRI after six months. The new brain MRI was stable as compared to the prior study (Figure 3). The right cerebellar lesion was stable in appearance and dimensions.
Figure 3.
56-year-old female with dual diagnosis of Cowden syndrome and Lhermitte Duclos disease.
Findings: MRI brain performed six months after initial diagnosis with non-contrast axial T1w (A) and non-contrast axial T2w (B) shows the right cerebellar lesion (outlined in yellow) stable in appearance and gross size.
Technique: MRI, Siemens Verio 3-Tesla 8-channel; (A): Axial T1w non-contrast: TR 599.999, TE 13.8094; (B): Axial T2w non-contrast: TR 5758.58, TE 110
DISCUSSION
Etiology & Demographics
Cowden Syndrome (CS) is a rare genetic disorder of an autosomal dominant inheritance pattern with an estimated prevalence of 1 in 200,000 to 1 in 250,000 [1]. CS is associated with a germline mutation in the phosphatase and tensin homolog (PTEN) gene, which codes for a multi-functional tumor suppressor involved in signaling pathways that are responsible for cell proliferation and cell cycle control [2]. Thus, CS may be seen as a cancer predisposition syndrome.
The distinguishing phenotype of CS is the presence of multiple hamartomatous tumors across different tissues and organ systems, with hamartomatous ductal breast cancer as the most common type of malignancy [3]. The most common type of brain tumor arising in adult CS patients is dysplastic gangliocytoma of the cerebellum, also referred to as Lhermitte-Duclos disease (LDD). This benign tumor is found in up to 35 percent of CS patients, and research suggests it may be an important indicator of CS in adults [4].
Because of the high frequency of LDD in CS patients, these two pathologies are sometimes referred together as a phakomatosis termed Cowden-Lhermitte-Duclos syndrome (COLD syndrome) [5]. COLD syndrome usually arises in young to middle-aged adults in the second or third decade but has also been diagnosed in patients aged anywhere from 4 to 75[6].
Lhermitte-Duclos disease is a very rare type of posterior fossa tumor that can either arise as an isolated condition or as a part of COLD syndrome. The disease is extremely rare, with a literature search in March 2020 resulting in only 222 confirmed cases. It has no gender predilection. Clinical symptoms generally [arise from/are related to] mass effect changes in the posterior fossa and can include headache, nausea, and visual disturbances. The severity of symptoms can vary depending on the size of the lesion, and patients with isolated LDD may be asymptomatic for years. If the lesion grows large enough, patients may also exhibit signs of cerebellar dysfunction and obstructive hydrocephalus [7]. Because of LDD’s benign, slow-growing nature, prognosis is mostly favorable and surgical intervention may not be necessary in asymptomatic patients. There have also been no documented reports of malignant transformation.
Imaging & Clinical findings
LDD typically presents with a focal, well-circumscribed lesion restricted to one of the cerebellar hemispheres. PET scan typically shows increased uptake in the vicinity of the lesion. Non-contrast MRI is preferred over CT for diagnosis due to limitations of CT in evaluating the posterior fossa. An MRI of the brain shows gross cerebellar folia hypertrophy due to thickening of the molecular layer and infiltration of the granule cell layer with dysplastic ganglionic cells [8]. There is also thinning of medullary white matter with the loss of the Purkinje cell layer [6]. Noncontrast T1 typically shows a hypointense signal while non-contrast T2 shows an alternating pattern of high and low signal with a classic striated appearance that is said to resemble “tiger stripes” [9]. This tigroid appearance on T2 imaging distinguishes LDD from other more common primary cerebellar gliomas that destroy the delicate folial pattern of the cerebellum. MRI findings alone are usually sufficient for diagnosis without further histopathological workup [10].
Because of the strong association between LDD and CS, imaging of other organ systems such as the breast and colon should be considered in patients with LDD to rule out occult neoplasms in other organ systems [3]. For pre-symptomatic diagnosis of CS, genetic testing for PTEN germline mutations in the patient’s progeny may also be cautiously recommended.
Treatment & Prognosis
Suggested treatment of LDD is observation with symptom management, unless the mass effect symptoms are problematic enough to warrant surgical resection [10]. Complete surgical resection is associated with low rates of post-operative recurrence [6].
Differential Diagnoses
While rare, a classic presentation of LDD on MRI leaves very little room for differential diagnoses, especially if the patient has a previous diagnosis of Cowden syndrome or a cancer of hamartomatous origin. The striated, non-enhancing posterior fossa lesion limited to one of the cerebellar hemispheres is characteristic of LDD.
Other diagnoses that may mimic LDD based on neuroimaging studies alone include nodular medulloblastoma in adults and pseudotumoral hemicerebellitis in children [11, 12] (Table 2). Each of these diagnoses may present with a hypointense T1 and hyperintense T2-weighted lesion limited to one of the cerebellar hemispheres, or similar mass effect symptoms of headache and nausea [11, 12].
Table 2.
Differential diagnosis table for unilateral lesion with tiger stripe appearance in cerebellar hemisphere
| Etiology | Medical management and prognosis | MRI-T2 findings | |
|---|---|---|---|
| Lhermitte-Duclos disease | Primary hamartomatous tumor | Observation if patient is asymptomatic; slow-growing tumor with no malignant transformation | “Tiger stripes” appearance of alternating hyper- and hypo-intense striations |
| Nodular medulloblastoma | Primary neuroectodermal tumor | Urgent total surgical resection, followed by chemotherapy and radiotherapy | Cortical hyperintensity ± necrosis, cyst formation, and calcifications with surrounding edema |
| Pseudotumoral hemicerebellitis | Inflammatory process secondary to infection | Observation if patient is asymptomatic; self-resolving inflammatory process | Cortical hyperintensity ± cerebellar edema |
Nodular medulloblastoma is a malignant primary neuroectodermal neoplasm that warrants total excision followed by radiotherapy and chemotherapy. It is more common in children than adults generally, but the variant of medulloblastoma that mimics LDD is typically seen in older patients. Adult medulloblastoma cases in particular are more likely to be hemispheric than midline in the cerebellum [11]. Medulloblastoma usually presents with cystic or necrotic components with calcifications, but there may be variants that mimic the nonenhancing tiger stripe pattern on MRI that need to be biopsied and diagnosed via histopathology [11]. Surrounding edema is also commonly seen in medulloblastoma, whereas the surrounding parenchyma is mostly undisturbed in LDD.
Pseudotumoral hemicerebellitis is an inflammatory complication of infection. This is the most common cause of acute ataxia in children, and should therefore be higher on the differential than Lhermitte-Duclos for younger patients. The disease is self-limited, and surgical intervention is not recommended except in rare cases of severe cerebellar edema [12]. Regression of the pathological findings in MRI over time – in the absence of specific treatment – will also help differentiate pseudotumoral hemicerebellitis from LDD [12].
Clinical presentations and subtle differences in neuroimaging studies help distinguish the proper diagnosis of LDD (Table 2). If the imaging is highly suggestive of LDD but the patient does not have any other symptoms of Cowden syndrome, a biopsy may rule out other posterior fossa lesions that require different clinical management.
TEACHING POINT
Dysplastic gangliocytoma of the cerebellum, also known as Lhermitte-Duclos disease, is an uncommon hamartomatous tumor associated with Cowden syndrome that typically grows unilaterally in one of the cerebellar hemispheres. On MRI, Lhermitte-Duclos is characterized as a nonenhancing cerebellar mass with a distinct, striated appearance.
ABBREVIATIONS
- CS
Cowden Syndrome
- COLD
Cowden-Lhermitte-Duclos syndrome
- CT
Computed tomography
- FLAIR
Fluid-attenuated inversion recovery
- LDD
Lhermitte-Duclos Disease
- MRI
Magnetic resonance imaging
REFERENCES
- 1.Nelen MR, Kremer H, Konings IB, Schoute F, van Essen AJ, Koch R, Woods CG, Fryns JP, Hamel B, Hoefsloot LH, Peeters EA, Padberg GW. Novel PTEN mutations in patients with Cowden disease: absence of clear genotype-phenotype correlations. Eur J Hum Genet. 1999;7(3):267. doi: 10.1038/sj.ejhg.5200289. [DOI] [PubMed] [Google Scholar]
- 2.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95(1):29. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 3.Pilarski R. PTEN Hamartoma Tumor Sundrome: A Clinical Overview. Cancers (Basel) 2019 Jun;11(6):844. doi: 10.3390/cancers11060844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lok C, Viseux V, Avril MF, Richard MA, Gondry-Jouet C, Deramond H, Desfossez-Tribout C, Courtade S, Delaunay M, Piette F, Legars D, Dreno B, Saïag P, Longy M, Lorette G, Laroche L, Caux F. Brain magnetic resonance imaging in patients with Cowden syndrome. Medicine (Baltimore) 2005 Mar;84(2):129–36. doi: 10.1097/01.md.0000158792.24888.d2. [DOI] [PubMed] [Google Scholar]
- 5.Tortori-Donati P, Rossi A. Pediatric Neuroradiology. [Google Scholar]
- 6.Masmoudi A, Chermi ZM, Marrekchi S, et al. Cowden syndrome. J Dermatol Case Rep. 2011;5(1):8–13. doi: 10.3315/jdcr.2011.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kulkantrakorn K, Awwad EE, Levy B, Selhorst JB, Cole HO, Leake D, Gussler JR, Epstein AD, Malik MM. MRI in Lhermitte-Duclos disease. Neurology. 1997 Mar;48(3):725–31. doi: 10.1212/wnl.48.3.725. [DOI] [PubMed] [Google Scholar]
- 8.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klisch J, Juengling F, Spreer J, Koch D, Thiel T, Büchert M, Arnold S, Feuerhake F, Schumacher M. Lhermitte-Duclos disease: assessment with MR imaging, positron emission tomography, single-photon emission CT, and MR spectroscopy. AJNR Am J Neuroradiol. 2001 May;22(5):824–30. [PMC free article] [PubMed] [Google Scholar]
- 10.Savardekar A, Salunke P, Ahuja CK, Rane S, Singla N. Unusual presentation in adult medulloblastomas: imaging features mimicking cerebellar dysplastic gangliocytoma (Lhermitte-Duclos disease) Neurol India. 2012 Sep-Oct;60(5):555–7. doi: 10.4103/0028-3886.103225. [DOI] [PubMed] [Google Scholar]
- 11.Chen KS, Hung PC, Wang HS, Jung SM, Ng SH. Medulloblastoma or cerebellar dysplastic gangliocytoma (Lhermitte-Duclos disease)? Pediatr Neurol. 2002 Nov;27(5):404–6. doi: 10.1016/s0887-8994(02)00455-1. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Cruz PM, Janet-Signoret S, Miranda-Herrero MC, Barredo-Valderrama E, Vazquez-Lopez M, Ruiz-Martin Y, Castro-De Castro P. Acute hemicerebellitis in children: case report and review of literature. Eur J Paediatr Neurol. 2013 Sep;17(5):447–53. doi: 10.1016/j.ejpn.2013.03.013. [DOI] [PubMed] [Google Scholar]