Abstract
Posttraumatic pulmonary artery pseudoaneurysm is a very rare, yet potentially lethal complication after thoracic trauma. Pulmonary artery pseudoaneurysm is associated with high mortality. Still literature highlights that untreated, lesions can enlarge, rupture, and lead to exsanguination and death. We present a case of a posttraumatic peripheral pulmonary artery pseudoaneurysm with complete disappearance after one year. This case confirms that conservative treatment can be an effective option in asymptomatic and stable patients.
Keywords: Penetrating chest trauma, pulmonary artery, pseudoaneurysm, non-operative treatment, spontaneous resolution, contrast enhanced computed tomography
CASE REPORT
A 49-year-old man was admitted to the emergency department after having attempted suicide by stabbing himself with a kitchen knife in the left upper anterior chest. On admission, the patient complained about chest pain and progressive shortness of breath, was hemodynamically stable and conscious. Physical examination showed a 2 cm skin wound over the 2nd intercostal space of the left parasternal region and diminished breath sounds on this side. The assessment of the patient according to the advanced trauma life support protocol indicated a high probability of the patient having a pneumothorax. Consequently, a left chest tube thoracostomy (28 French) was performed immediately. The patient’s chest tube initially drained 900 mL of blood during the first 30 minutes, after which the tube output decreased markedly. Since the patient showed further stable condition under optimization of the coagulation system, imaging of the thorax was possible.
Imaging findings and diagnosis
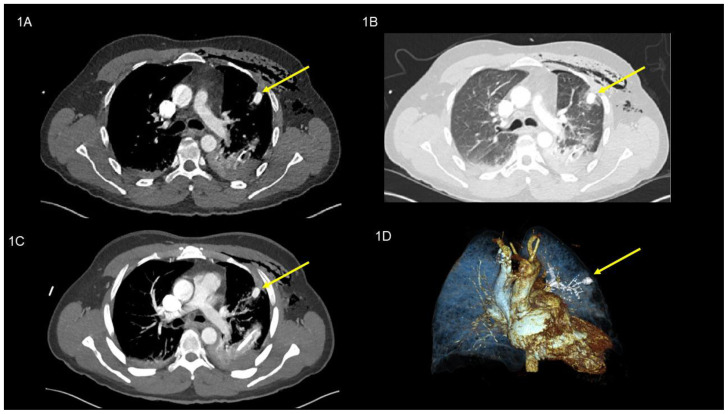
Contrast-enhanced computed tomography scan (CT scan) revealed a (16 × 11 mm), hyperdense (135 Hounsfield units (HU); (equivalent to contrast agent density) rounded lesion in the left upper lobe (anterior segment S III), located sub-pleural at the end of the radiologically suspected penetration channel as well as a residual left-sided hemothorax and extended subcutaneous/intramuscular emphysema. Early arterial contrast enhancement without venous pooling in the late phase suggested an injury of the pulmonary sub-segmental artery in segment III of the left upper lobe. Accordingly, this lesion was interpreted to be a post-traumatic peripheral pulmonary artery pseudoaneurysm (PAP); (Image 1 A / B / C / D). After interdisciplinary evaluation, we decided on conservative treatment, as the clinical condition remained stable without onset of further complications as e.g. hemoptysis. At day three after injury, the chest drain was removed because of minimal serous drainage and resolved air leak. After psychiatric evaluation the compliant patient was instructed for self-monitoring at home and discharged. Clinical and radiological follow up was planned three weeks later.
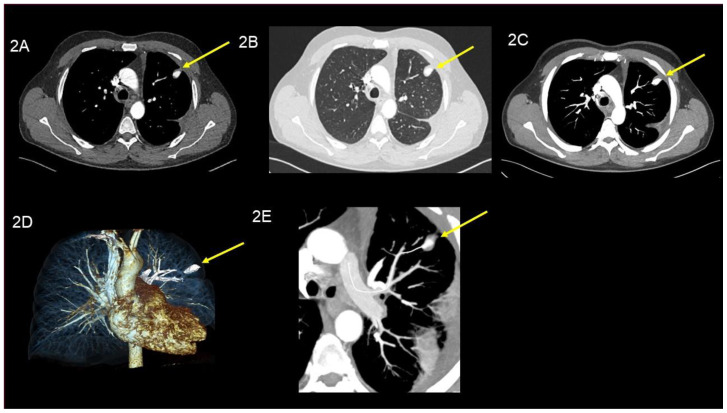
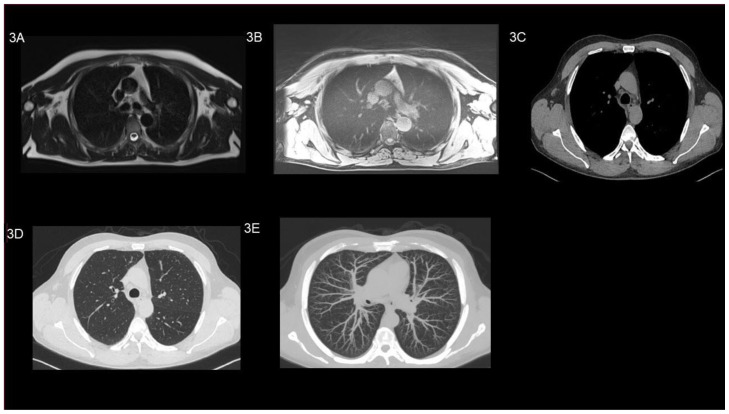
At the follow up visit at our daily outpatient clinic, patient’s history and physical examination were unremarkable without signs associated with the PAP. CT scan of the chest showed a stable situation of the PAP unchanged in its size in segment III (160 Hounsfield units (HU); (equivalent to contrast agent density) (Image 2 A / B / C / D and E). The patient was planned for imaging follow-up after one year. Firstly, we performed a non contrast MRI of the lung (T2-axial Haste and T1-star vibe axial) which did not show a PAP anymore. To reassure that this was not due to the low resolution of the MRI image, we additionally performed a CT-scan without any contrast agent. Surprisingly, the high-resolution image confirmed the complete disappearance of the PAP (Image 3 A / B / C / D and E).
DISCUSSION
Etiology & Demographics
With regards to Laplace’s law, the risk of wall stress or rupture increases with pressure and vessel radius. Because wall thickness is a protective factor and peripheral arteries have thinner walls, peripheral lesions are at higher risk of rupture than greater vessels [1]. A pseudoaneurysm does not involve all three arterial wall layers and thus poses a higher risk of rupture than a true aneurysm including all of them.
Pulmonary artery pseudoaneurysms (PAPs) are rare, and are either congenital or acquired. Most frequently, PAPs are acquired and associated with cardiovascular disease. Further causes are infections (e.g. mycotic pseudoaneurysm) and chronic inflammations such as Behçet’s disease and Takayasu arteritis. Iatrogenic PAP can be seen for example as complication after pulmonary catheter insertion and is even more frequent than posttraumatic PAP [2].
Still, a posttraumatic peripheral PAP of the lung is a very rare, yet potentially lethal complication after chest trauma [3]. Literature states that early diagnosis and treatment are decisive for patient survival and optimal outcome [3]. It has been reported that a PAP rupture carries a mortality of 50–100% [4].
With regards to demographics of a PAP, literature is rare and only few cases have been published [5].
Clinical & Imaging findings
Clinical presentations range from life-threatening hemorrhage to ‘silent’, asymptomatic lesions, which potentially enlarge over days, months, or years. The formation of an arterial pseudoaneurysm has been reported to occur between minutes to months after arterial injury. However, in the lung, the time interval between injury and diagnosis varies from a few days to years, maybe as a consequence of the rarity of this finding [6]. Symptoms of PAP may include chest pain, hemoptysis, and dyspnea. Hemoptysis, due to communication of the pseudoaneurysm to the bronchial system is the most common symptom and requires rapid work up and treatment [7]. Multi-slice CT angiography is the gold standard for diagnosis and shows early arterial contrast enhancement without venous pooling in the late phase as well as a contrast filled pouch with persistent communication to the damaged artery.
Treatment & Prognosis
Treatment options include pulmonary resection, endovascular embolization or conservative treatment with close clinical and radiological monitoring depending on patients stability and onset of complications [8]. Literature postulates that surgical repair is necessary if the aneurysm involves the main pulmonary artery vessel and if the patient has any symptoms, regardless of the aneurysm size[9]. Peripheral aneurysm are mostly treated with endovascular coil embolization.
In our patient, the complete disappearance of the PAP after one year can be caused by the low pressure within peripheral pulmonary artery vessels which let smaller lesions tamponade by surrounding clots more easily [10]. Detailed information on complications rates after conservative treatment of PAP are missing in the literature. Therefore, there is no guidelines helping to choose between the different treatment options. It seems that treatment should depend on location and size of PAP and on presence of symptoms, but there is a lack of evidence [11]. We assume that PAP location is relevant because of the correlating diameter of the involved pulmonary artery branch. In this stable patient, the decision for a conservative approach was driven by the assessment that the risk of further complication caused by any intervention outweighed any potential benefits.
Differential Diagnosis
True Aneurysm
A pulmonary artery aneurysm is a focal dilatation of all three layers of the vessel wall. Although pulmonary artery aneurysm is a rare finding, several conditions can be associated with it. The most common risk factors for developing a pulmonary artery aneurysm are infection, structural cardiac anomalies, structural vascular anomalies, and pulmonary hypertension [12]. Chest CT scan shows abnormal dilatation of the artery with no evidence of extravascular contrast.
Hematoma
Pulmonary hematoma consists of hemorrhage into the alveolar and interstitial spaces, mostly associated with surrounding intra-parenchymal hemorrhage. Hematoma typically develops into a discrete mass with distinct margins and normally tends to distinguish after two to four weeks [13].
Vascular malformation
Vascular malformation are characterized by an abnormal leash of vessels allowing for arteriovenous shunting [14]. Pulmonary arteriovenous malformations (PAVMs) are rare, and is mostly due to abnormal low resistance vascular structures that connect a pulmonary artery to a pulmonary vein, thereby bypassing the normal pulmonary capillary bed and resulting in an intrapulmonary right-to-left shunt. Appearance of ground glass can be seen by CT scan. Gold standard imaging test for PAVM is CT scan.
Tumor or metastases
CT scan has high sensitivity for detecting lung cancer or lung metastases. Further modalities e.g Positron emission tomography (PET) scan can be used as an alternative to distinguish the lesion from viable tumor tissue or from surrounding atelectasis and/or consolidation. Image-guided biopsy or bronchoscopy is used to obtain tissue diagnosis [15].
TEACHING POINT
With this case we would like to raise awareness amongst clinicians involved in the acute care of patients after penetrating trauma. At CT scan, hyperdense lung lesions in direct association with a pulmonary artery branch can be a caused by pulmonary artery pseudoaneurysm (PAP). After exclusion of other possible diagnosis, it is a valuable option to consider conservative treatment of a size stable posttraumatic PAP in asymptomatic and stable patients. As we have seen, it may completely resolve after one year without any intervention or treatment.
Figure 1.
49-year-old man with peripheral pulmonary artery pseudoaneurysm in the left upper lobe. Initial images after the trauma (all images are at the same level).
Findings:
Axial contrast enhanced chest CT in the dual-phase showed initially hemopneumothorax and extended subcutaneous/intramuscular emphysema and a round lesion (pulmonary artery pseudoaneurysm), yellow arrow, (16 × 11 mm), (135 Hounsfield units; equivalent to contrast agent density), in the left upper lobe (anterior segment S III), (a) axial soft tissue window, (B) axial lung window, (c) MIP 10 mm, and (d) Image reconstruction.
Technique: CT SIEMENS (Somatom Definition Edge 128) 142 mAs, 120kV, 1mm slice thickness, 90 ml Iomeron 400 KM i.v.
Figure 2.
49-year-old man with peripheral pulmonary artery pseudoaneurysm in the left upper lobe. Images performed three weeks after initial trauma (all images are at the same level).
Findings:
Axial contrast enhanced chest CT in the arterial phase showed a stable situation of the round lesion in the left upper lobe after three weeks. Yellow arrow, points out the pulmonary artery pseudoaneurysm, (160 Hounsfield units; equivalent to contrast agent density) unchanged in its size, (16 × 11 mm), (a) axial soft tissue window, (B) axial lung window, (c) MIP 10 mm, (d) Image reconstruction, (e) Image reconstruction with a center line.
Technique: CT SIEMENS (Somatom Definition Edge 128) 125mAs, 125kV, 1mm slice thickness, 110 ml Ultravist 350 KM i.v.
Figure 3.
49-year-old man after peripheral pulmonary artery pseudoaneurysm in the left upper lobe. Images performed one year after initial trauma (all images are at the same level).
Findings:
Non-contrast MRI (a, b) and non-contrast CT (c, d, e) imaging of the lung, after one year after initial trauma, showed normal tissue as well as normal vascularization of the lung
(a) axial T2-haste, (b) axial T1-star vibe (c) axial soft tissue window, (d) axial lung window and (e) axial MIP 10 mm.
Technique: Non-contrast 1.5 Tesla MRI SIEMENS (Aera) of the lung 5mm slices (a) axial T2-haste (TE= 95 ms, TR=600 ms), (b) axial T1-star vibe (TE= 1.98 ms, TR= 4.27 ms);
Non-contrast CT SIEMENS (Somatom Definition Flash 128), 95 mAs, 120 kV, 1 mm slice thickness.
Table 1.
Summary table for posttraumatic pulmonary artery pseudoaneurysm.
| Etiology | Chest trauma |
| Incidence | Insufficient information/unknown |
| Gender ratio | Commonly associated with acquired etiologies. |
| Age predilection | Commonly associated with acquired etiologies. |
| Treatment | Pulmonary resection, angiographic embolization or conservative treatment with close clinical and radiological monitoring |
| Prognosis (of PAP) | Endovascular treatments carry similar risks to other endovascular embolization procedures throughout the body. |
| Imaging findings | Early arterial contrast enhancement without venous pooling in the late phase. Contrast filled pouching with persistent communication to the damaged artery. |
Table 2.
Differential diagnosis table for posttraumatic pulmonary artery pseudoaneurysm.
| CT | T1/T2-MRI | |
|---|---|---|
| Hematoma | Mass with increased density extravascular (depending on age of the hematoma). | Perivascular mass with complex T1/T2 signal depending on the chronicity of the thrombus. No contrast flow would be seen in the mass. |
| True Aneurysm | Bulbous or fusiform abnormal dilatation of the aorta/artery. Contains all 3 layers of the vessel wall. Abnormally dilated artery with no evidence of contrast extravasation. | Abnormally dilated artery with no evidence of contrast extravasation. The true lumen is opacified with contrast. |
| Vascular malformation | Contrast CT scan shows enhancement of the feeding artery, the aneurysmal part, and the draining vein on early-phase sequences. | Rapidly flowing blood results in absent or minimal MR sign. Regions of slow blood flow may result in intermediate signal intensity |
| Tumor and/or metastases | Depending on the origin of the tumor imaging can vary between high density or low density. Sometime vessels are connecting with the lesion. | Size <5 mm pulmonary nodule can hardly be detected in MRI. |
| Pseudoaneurysm | Early arterial contrast enhancement without venous pooling in the late phase. Contrast filled pouching with persistent communication to the damaged artery. | Abnormally dilated artery. The true lumen is opacified with contrast. |
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to Prof. Dr. med. Dr. h. c. Ralph Alexander Schmid, Prof. Dr. med. Peter Vock, Dr. med. Christoph Schröder, Dr. med Ante Katunaric, Dr. med Rubén Encinas, Dr. phil. nat. Thomas Michael Marti, and all the nurses and radiology assistants at the Department of Radiology at the University Hospital Bern for their patient care and support in measurements.
ABBREVIATIONS
- CT
Contrast-enhanced computed tomography
- HU
Hounsfield units
- PAP
Pulmonary artery pseudoaneurysm
- PAVMs
Pulmonary arteriovenous malformations
- PET
Positron emission tomography
REFERENCES
- 1.Fowler NO. Law of Laplace. N Engl J Med. 1971 Nov;285(19):1087–8. doi: 10.1056/NEJM197111042851917. [DOI] [PubMed] [Google Scholar]
- 2.Boyd KD, Thomas SJ, Gold J, Boyd AD. A prospective study of complications of pulmonary artery catheterizations in 500 consecutive patients. Chest. 1983 Sep;84(3):245–9. doi: 10.1378/chest.84.3.245. [DOI] [PubMed] [Google Scholar]
- 3.Lafita V, Borge MA, Demos TC. Pulmonary artery pseudoaneurysm: etiology, presentation, diagnosis, and treatment. Semin Intervent Radiol. 2007 Mar;24(1):119–23. doi: 10.1055/s-2007-971202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park HS, Chamarthy MR, Lamus D, Saboo SS, Sutphin PD, Kalva SP. Pulmonary artery aneurysms: diagnosis & endovascular therapy. Cardiovasc Diagn Ther. 2018 Jun;8(3):350–361. doi: 10.21037/cdt.2018.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bao M, Zhou Y, Jiang G, Chen C. Pulmonary artery pseudoaneurysm after a left upper sleeve lobectomy. World J Surg Oncol. 2013 Oct;11(1):272. doi: 10.1186/1477-7819-11-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jonge I, Vahl A, van der Hulst V. Coil embolization of a left pulmonary artery pseudoaneurysm after penetrating injury. J Endovasc Ther. 2003 Jun;10(3):681–3. doi: 10.1177/152660280301000342. [DOI] [PubMed] [Google Scholar]
- 7.Quartey B, Jessie E. Pulmonary artery and vein pseudoaneurysm after gunshot wound to the chest. Journal Emerg Trauma Shock. 2011 Apr;4(2):313. doi: 10.4103/0974-2700.82235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelage J-P, et al. Pulmonary artery interventions: an overview. Radiographics. 2005 Nov-Dec;25(6):1653–1667. doi: 10.1148/rg.256055516. [DOI] [PubMed] [Google Scholar]
- 9.Reade CC, Jenkins NL, Bard MR, Kuszyk BS, Koutlas TC, Rotondo MF. Immediate diagnosis and nonoperative treatment of a pulmonary artery pseudoaneurysm after blunt traumatic injury. J Trauma. 2006 Apr;60(4):894–896. doi: 10.1097/01.ta.0000214598.48638.b5. [DOI] [PubMed] [Google Scholar]
- 10.Wagenvoort CA, Mooi WJ. Dial DH and Pulmonary pathology. 2nd ed. New York: Springer; 1994. Vascular diseases; pp. 985–1026. [Google Scholar]
- 11.Mohan B, Singal S, Bawa AS, Mahindra P, Yamin M. Endovascular management of traumatic pseudoaneurysm: Short & long term outcomes. J Clin Orthop Trauma. 2017 Jul-Sep;8(3):276–280. doi: 10.1016/j.jcot.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farber HW, McDermott S, Witkin AS, Kelly NP, Miloslavsky EM, Stone JR. Case 11–2018: A 48-Year-Old Woman with Recurrent Venous Thromboembolism and Pulmonary Artery Aneurysm. N Engl J Med. 2018 Apr;378(15):1430–1438. doi: 10.1056/NEJMcpc1800323. [DOI] [PubMed] [Google Scholar]
- 13.Trinkle JK, Richardson JD, Franz JL, Grover FL, Arom KV, Holmstrom FM. Management of flail chest without mechanical ventilation. Ann Thorac Surg. 1975 Apr;19(4):355–63. doi: 10.1016/s0003-4975(10)64034-9. [DOI] [PubMed] [Google Scholar]
- 14.Gossage JR, Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med. 1998 Aug;158(2):643–61. doi: 10.1164/ajrccm.158.2.9711041. [DOI] [PubMed] [Google Scholar]
- 15.Purandare NC, Rangarajan V. Imaging of lung cancer: Implications on staging and management. Indian J Radiol Imaging. 2015 Apr-Jun;25(2):109–20. doi: 10.4103/0971-3026.155831. [DOI] [PMC free article] [PubMed] [Google Scholar]