Abstract
The damage and injury that ground level ozone (O3) causes vegetation has become increasingly evident over the past half century with a large body of observational and experimental evidence demonstrating a variety of effects at ambient concentrations on crop, forest and grassland species and ecosystems. This paper explores the use of experimental data to develop exposure-response relationships for use in risk assessment studies. These studies have typically identified the USA mid-West, much of Europe, the Indo Gangetic Plain in South Asia and the Eastern coastal region of China as global regions where O3 is likely to threaten food supply and other ecosystems. Global risk assessment modelling estimates yield losses of staple crops between 3 to 16% causing economic losses of between US$14 to 26 billion in the year 2000. Changes in anthropogenic emissions of O3 precursors in recent decades have modified O3 concentration profiles (peaks versus background O3) and global distributions with the Northern Hemisphere seeing increases in O3 levels of between 1 and 5 ppb/decade since the 1950s and the emergence of Asia as the region with the highest O3 concentrations. In the future, O3 mitigation could focus on methane (CH4) and nitrogen oxide (NOx) emissions; these will differentially influence global and local/regional O3 concentrations and influence daily and seasonal profiles. The consequent effects on vegetation will in part depend on how these changes in O3 profile alter the exceedance of detoxification thresholds for plant damage. Adaptation options may play an important role in enhancing food supply while mitigation strategies are being implemented. An improved understanding of the mechanisms by which O3 affects plants, and how this might influence detoxification thresholds and interactions with other environmental variables such as water stress and nutrients, would help develop O3 deposition and impact models to support the development of crop, land-surface exchange and ultimately earth system models for holistic assessments of global change.
This article is part of a discussion meeting issue ‘Air quality, past present and future’.
Keywords: ozone pollution, vegetation damage, flux-based metrics, process-based modelling, air quality policy
1. Introduction
Tropospheric ozone (O3) occurs naturally in the environment but industrialization has seen a steady increase in O3 pollution as anthropogenic emissions of nitrogen oxides (NOx), carbon monoxide (CO), non-methane volatile organic compounds (NMVOCs) and methane (CH4) have led to the formation of O3 through photochemical reactions. This has caused O3 levels to increase from concentrations of less than 20 ppb in pre-industrial times [1] to current levels that have increased Northern mid-latitude average O3 concentrations to 30–50 ppb [2]. Since the 1950s mean O3 concentrations have been growing at a rate of 1–5 ppb/decade in the Northern Hemisphere (NH) and by 2 ppb/decade in the Southern Hemisphere (SH) [2]. As these concentrations have risen, evidence of O3 injury and damage to vegetation due to the phyto-toxic nature of O3, has also increased substantially over the last half-century [1]. The fact that O3 is a secondary pollutant leads to significant geospatial and temporal variability in O3 concentrations caused by conditions such as downwind proximity to large-scale emission sources, meteorological patterns (such as seasonality, land-coastal processes and monsoon systems) and stratosphere–troposphere exchange. This means that O3 concentrations tend to be highest in rural and semi-rural areas downwind of urban and industrial sources of pre-cursor emissions, i.e. in those locations important for agriculture, forestry and grasslands, along with the ecosystem services they provide [3]. O3 and its precursors are also transported around the world in air masses; when O3 degradation (by chemical transformation and deposition) is slower than O3 generation, background O3 concentrations will be enhanced. These global processes influence local-to-regional O3 concentrations (including the duration, frequency and magnitude of O3 levels), all of which are important in determining vegetation response.
The earliest records of O3-induced injury to crops were in the 1950s as ‘oxidant stipple’ on grapevines near San Bernardino, California [4] and ‘weather fleck’ on tobacco plants in the eastern USA [5]. Injury was also observed on a mixed conifer forest in southern California [6]; these observed injuries coincided with rapid increases in population and industry in the Los Angeles basin post World War II [1]. Since these first recordings, a large body of observational and experimental evidence has been collected demonstrating a variety of O3 impacts on ecosystems in North America, Europe and more recently Asia [2]. This wealth of data provides ever-greater opportunity to understand and predict the cascade of effects that occur when O3 exposure damages vegetation over extended periods. This review sets out what we know of the impacts of O3 to crops, forests and grasslands including physiological, individual and ecosystem level responses. The review describes the evolution of metrics to identify target levels of atmospheric O3 exposure, which has resulted in the development of new, more biologically relevant, flux-based methods to assess O3 risk and damage to vegetation to inform emission reduction policy. The knowledge obtained from conducting these assessments is considered in relation to opportunities for mitigation and adaptation, providing insight on future research priorities to develop an improved understanding of those plant processes (from physiology to ecosystem level) that are influenced by O3 and may have wider ecosystem-level implications. This will support the development of future risk assessment methods to determine the scale and magnitude of O3 effects on a variety of ecosystems services that include crop yield, carbon sequestration, water provision and biodiversity. It will also inform the development of crop, land-surface exchange and earth system models that can assess the effects of stresses such as O3 in the context of broader, global environmental change.
2. O3 effects on agriculture, forests and grasslands
(a). Agriculture
Arable agriculture, particularly the staple crops, is the most studied vegetation type for O3 impacts. This is in part due to crops having been the focus of two coordinated regional efforts: the North America Crop Loss Assessment Network (NCLAN) [7] and the European Open Top Chamber programme [8]. These programmes used open-top chambers with standardized experimental protocols to assess the effects of a targeted range of pre-industrial, ambient and elevated O3 exposures on crop growth, development and yield. The results from these programmes, coupled with additional studies that have been conducted since the 2000s in Asia, describe crop responses to O3 which have been widely analysed and reviewed, e.g. [9–11]. These reviews show that O3 causes a variety of responses including visible injury, reductions in Rubisco activity, chlorophyll content and photosynthesis and alterations in stomatal conductance (gsto), alterations to carbon (C) allocation including decreased root:shoot ratios, and reductions in biomass and yield quantity and quality. A meta-analysis of studies on the effects of ambient versus pre-industrial O3 levels found wheat yield losses of 8.4% demonstrating a significant and consistent crop response to current ambient O3 concentrations [12]. In recent years, O3 effects on crop quality have received more attention; the importance of these effects for global protein production were highlighted through estimates that current day O3 levels could be reducing soya bean seed protein yield by 200 kg protein per hectare compared with pre-industrial levels [13].
Epidemiological studies detected the influence of O3 in national level agricultural production statistics [14,15]. These studies use statistical multiple linear regression models to analyse 5–30-year time series of historical data to explore the relationship between past crop yield outcomes and trends or inter-annual variations in weather variables (e.g. monthly temperature and precipitation) and O3 pollution (described by a particular vegetation O3 metric, see §3a). A study on soya bean growing across the mid-west USA over a 5-year period was able to detect a 10% reduction in yield [14]. By contrast, only a 0.54% decrease in yield with a 10% increase in O3 concentrations (characterized using the AOT40 metric (see §3a)) was found at wheat trial sites across the UK over a 13-year period, lower than would be expected according to experimental data [15]. Understanding such discrepancies is important and may be due to the influence of confounding factors (e.g. high temperatures and reduced soil water that tend to co-occur with O3 and themselves cause yield losses), the inadvertent selection of resistant crop cultivars, and the use of O3 metrics that may not estimate damage accurately.
Finally, studies have not only been confined to arable agriculture, O3 effects on the yield and quality of component species of productive grasslands used for animal grazing have also been identified (e.g. [16]). Impacts on grasslands arise both from an O3-induced reduction in the legume fraction [17] as well as from alterations in the feed quality of pasture, often measured as ‘Relative Feed Value’ [18].
However, even for agriculture there are substantial gaps in our knowledge with many species, especially those more commonly grown in tropical countries, having been less widely studied, even though evidence suggests growing conditions in these global regions may enhance O3 sensitivity [19]. There is also substantial variability in the response to O3 between cultivars of the same species, the reasons for which are not fully understood [20,21]. Studies have also suggested that O3 can reduce the grain yield return from nitrogen (N) fertilizer applications which may increase the risk of N loss to the environment [22]. Finally, there is uncertainty as to how O3 may influence crop response to abiotic (e.g. temperature, soil water, soil fertility [23], and biotic stresses (pests and disease [9])).
(b). Forests
Our understanding of O3 effects on temperate and boreal forests is also relatively strong, based primarily on studies conducted in North America and Europe [1,24]. Observational studies found substantial O3 impacts in the forest ecosystems of the San Bernardino Mountains and Sierra Nevada of southern California, the Appalachian mountains of the eastern USA and forests of Germany and eastern France [1,25]. This led to more focused and coordinated open-top chamber experimental studies on tree seedlings and saplings in the USA [26] and Europe [25] during the 1990s.
Together, observational and experimental studies have identified a substantial variety of O3 impacts on forests, trees and ecosystems which have been extensively reviewed (e.g. [1,26,27]). These reviews show visible injury symptoms including chlorophyll degradation, chlorotic mottling and premature senescence. O3 also affects physiology including reductions on photosynthesis and gsto, and can lead to accelerated leaf senescence and foliar loss of macro- and micro-nutrients and shifts in allocation to enhance above-ground biomass. These effects impact whole tree growth and productivity [28] and cause increased mortality. Wider ecological consequences include changes in forest stand composition and community structure (with O3-tolerant species being favoured), alteration in forest successional pathways, and changes in species dominance of understory vegetation influencing drought and fire suppression. Ozone has also been found to increase forest susceptibility to drought, wind throw, as well as insect and pest attack (e.g. bark beetle, wood borer, fungal infection). Finally, O3 was found to reduce late season stream-flow at six forested watersheds in the southeastern USA by as much as 23% based on analysis of 18–26-year data records [29]. The authors primarily attributed this reduced stream flow to increased transpiration through other factors such as reduced root biomass and subsequent soil water uptake may have contributed. Were such changes in transpiration to occur over sufficiently large areas, there may be implications for surface energy balance [30].
A meta-analysis of 263 peer-reviewed articles explored the effect of elevated O3 (an average of 64 ppb) on northern temperate and boreal trees and found a decrease in tree biomass of 11% compared with trees grown at ambient O3 [31]. In addition, significant reductions in root:shoot ratio, leaf area, Rubisco and chlorophyll content, transpiration rates, tree height and stem diameter were also found. Empirical evidence also suggests that deciduous tree species may be more sensitive to O3 than evergreen coniferous species [32,33], which may be due to the higher rates of gas exchange [34] or reduced detoxification ability [35] of deciduous species. There is far less evidence to robustly quantify impacts of O3 on forest types that fall outside the category of NH temperate and boreal forests, although recent studies have identified sensitive tree species of Mediterranean regions [33] and subtropical Chinese forests [36].
There have been two important long-term experimental studies on forest trees that have used free air concentration enrichment (FACE) systems. These systems allow studies on more mature trees, covering longer lifespans and on plant competition and multi-level trophic effects. Aspen FACE exposed hardwood tree species (trembling aspen, paper birch and sugar maple) of the northern USA to 50% elevated O3 and/or CO2 concentrations over 11 years [37]. This study found that O3 exposure initially led to reductions in total tree biomass and ecosystem carbon content by 16% and 9%, respectively, under elevated O3. The study also found that O3 offset CO2-stimulated growth of both trembling aspen [38] and paper birch [39]. However, after 12 years the long-term effect of elevated O3 on net primary productivity (NPP) may be smaller than expected, possibly due to altered tree community composition in favour of O3-tolerant genotypes [40]. The second major experimental study was ‘Kranzberger FACE’, a study in southern Germany which exposed trees in a mature stand (50–70 years old) of European beech and Norway spruce (five trees of each species) to twice ambient O3 concentrations over 8 years [41]. Elevated O3 was found to decrease stem volume growth in European beech by 44%, and to shift resource allocation from stem diameter to height growth in European beech and Norway spruce [42].
There remain substantial gaps in our knowledge of O3 effects on forest trees. Many of the experimental studies have been conducted on young trees (seedlings or saplings) leading to large uncertainty on how O3 effects on young trees might scale to mature trees [43]. These scaling issues further confound our understanding from empirical data on how O3 affects C allocation and architectural structure of the roots, stem and canopy. Additionally, one of the key challenges to understanding forest ecosystem response is the lack of clarity on how O3 affects competition between species and how this might vary by life stage, species composition, tree density and leaf area distribution in space and time [44]. Finally, we are still unable to explain the mechanisms and processes behind the variability in the sensitivity of different forest tree genotypes, species and functional types to elevated O3, although unifying concepts have been put forward suggesting the importance of gas exchange, leaf lifespan and leaf mass [34,43,45].
(c). Ecosystems
The vast majority of research investigating grassland responses to O3 comes from Europe, with little experimentation done in the USA, even less in Asia and none in the tropics [24]. Thus, compared to trees and arable crops, much less is known about how grasslands are impacted by current and future O3 concentrations. Grasslands can be highly diverse, multi-species communities, with a wide range of productivities. Therefore, predicting a general response of grasslands to O3 is complex, dependent upon the stress history of the community, the sensitivities of individual species, the mutualistic and/or competitive interactions between species and the specific microclimatic conditions to which individual species are exposed that will influence O3 exposure and sensitivity. While experiments have shown decreases in productivity from elevated O3 [46,47], other experiments on permanent temperate [48], calcareous [49] and alpine grasslands [50] have shown that NPP of these systems is relatively resilient to elevated O3. For example, one of the longest running (7 years) experiments currently performed explored the combined effects of N deposition and O3 free air concentration enrichment on the species composition of a subalpine Geo-Montani-Nardetum pasture at 2000 m a.s.l. in the Central Alps. This study found that elevated O3 in the presence or absence of additional N exposure had no detectable effect on functional group composition and productivity [51,52]. Grassland species have also been shown to respond differently to O3 depending on competition [53] and O3 can have carry-over effects on growth and overwintering of species [54]. Ozone can also have more subtle changes on C assimilation, leaf longevity, the growth and flowering of plants, pollination efficiency, biomass partitioning of grassland species and species composition and richness [24]. Effects that influence at the ecosystem level include changes in water flux regulation and plant pathogen development. Recent research is leading to a better understanding of effects below ground, including changes in soil invertebrates, plant litter quantity and quality, decomposition, and nutrient cycling and C pools [55]. All of these changes are likely to be slow and may take decades to become detectable [24].
3. Methods to assess O3 impacts
Regional controls on precursor emissions in Europe and North America have led to reductions in peak O3 concentrations over recent decades; however, O3 episodes can still occur under particular climatic and O3 precursor emission conditions that are high enough to cause damage to vegetation. Since the 1990s, a large portion of the anthropogenic O3 precursor emissions are now coming from Asia [2,56]. The influence of these changes in O3 precursor emissions was apparent in the NH distribution of prevailing O3 concentrations explored in a study of approximately 3300 O3 monitoring sites [57]. This study found that between 2010 and 2014 the highest mean O3 values were in the mid-latitudes of the NH, including southern USA, the Mediterranean basin, northern India, north, northwest and east China, the Republic of Korea and Japan. Furthermore, trend analyses conducted between 1995 and 2014 using various vegetation-relevant O3 metrics found that the North America region was dominated by a significant decrease in O3, while in Europe there was no change and in East Asia there was a significant increase in O3 concentrations [57]. The findings from this study are consistent with other studies which show that vegetation exposure to O3 in China in recent years is now greater than in any other region of the world [58].
Crucial to our ability to assess the injury, damage or risk caused by these ever-changing air pollution concentrations and regional distributions has been the development of robust O3 vegetation damage metrics—essentially a means of characterizing those aspects of O3 exposure that are most important in determining O3 damage. These metrics have been used to develop exposure-response relationships (ERRs) for different species, with regressions between the O3 exposure metric and the response (most often defined as visible injury, yield or biomass), providing a useful statistical indication of which metrics most reliably capture O3 injury and damage [59,60]. Experimental studies that control the O3 concentration to which a receptor is exposed over a defined growth period have been widely used to define robust O3 metrics and associated ERRs.
(a). Ozone metrics and exposure-response relationships
Exposure-response relationship data from multiple experiments, locations and years can be pooled from studies that use a common approach to defining pollutant exposures and plant response allowing the development of robust ERRs. The experimental programmes that were conducted in North America and Europe during the 1980s and 1990s provided an excellent source of data for establishing ERRs [7,8] since they were conducted using standardized filtration and fumigation experiments at multiple locations and focused on key response parameters (i.e. yield and biomass). The development of these EERs over recent decades has seen metrics that describe daylight and growing season O3 characteristics evolve from the use of concentration-based to flux-based approaches [61]. Some of the common O3 concentration-based metrics include: (i) daylight hour (7 h (M7) or 12 h (M12)) growing season average ozone concentrations; (ii) accumulated daylight O3 concentrations above thresholds (e.g. AOT40 and SUM06) or; (iii) continuously weighted growing season averages (W126) to emphasize the higher O3 concentrations. Weibull (M7, M12, SUM06 and W126) or linear (AOT40) ERRs have commonly been used to relate the metrics to damage (for further details see [57]). It is important to understand the capabilities of different metrics in capturing the effects of different O3 concentration profiles. The seasonal mean metrics such as M7 and M12 work well when there is not too much variation in O3 concentration profile (i.e. when O3 concentrations consistently fall close to a mean growing season value) and when other environmental conditions (e.g. water stress, heat stress) are not limiting O3 uptake. However, these metrics will confer less emphasis to the damaging effect of elevated O3 concentrations during pollution episodes. Here, metrics such as AOT40 and SUM06 that accumulate concentrations above a threshold will be more suitable, again so long as environmental conditions are optimum for O3 uptake. For these reasons, the use of flux-based metrics is now encouraged since they capture concentrations that are likely to cause damage to vegetation (i.e. that occur when O3 concentrations will lead to effective O3 uptake) and consider both chronic and acute O3 concentrations that contribute equally to O3 damage.
The flux-based approach estimates a species-specific gsto and combines this with an estimate of the leaf/canopy O3 concentration to estimate stomatal O3 flux over the sensitive part of a species-growing season. Stomatal O3 flux is considered to provide a more biologically meaningful characterization of O3 exposure to vegetation as it describes the O3 dose (uptake) experienced by the plant [62] but also requires more detailed datasets (describing hourly meteorology), which limit the number of empirical datasets that can be reanalysed to provide flux-response data. The phytotoxic ozone dose above a threshold ‘y’ (PODy) is the flux-based metric that accumulates stomatal O3 flux, above a threshold that is considered to cause no plant damage.
The ICP Vegetation Task Force of the UNECE Convention on Long-Range Transboundary Air Pollution (CLRTAP) has established a number of PODy ERRs through (re-)analysis of empirical datasets [63]. The development of these ERRs requires the use of a gsto model. Currently, multiplicative models are used to estimate gtso (e.g. [61]) as a function of species, phenology and environmental conditions (i.e. irradiance, temperature, vapour pressure deficit and soil moisture). Importantly, these flux-based ERRs have been shown to have stronger statistical relationships between PODy and response than concentration-based metrics such as AOT40 [33,59,60]. There is also some evidence that presenting flux on a leaf mass, rather than a leaf area basis, may further improve the tightness of the relationship between uptake and damage and also help to identify variation in O3 sensitivity between species and plant functional groups [45].
These flux metrics rely on being able to assess the time over the day and the growing season when O3 uptake causes most plant damage. This is when stomatal O3 flux exceeds the detoxification capacity of the plant [64,65]. Musselman et al. [66] hypothesised that O3 detoxification capacity varies diurnally (and most likely seasonally) with photosynthesis, which fuels the metabolic synthesis of antioxidants [66]. The existence of this detoxification capacity is also crucially important in determining how plants respond to changing diurnal and seasonal O3 profiles. The current flux-based ERRs [67] estimate this detoxification capacity (‘y’ threshold) via empirical analysis [33,60]. An alternative approach involves cellular level process-based modelling whereby detoxification is determined by apoplastic ascorbate, which is modelled as a function of gsto, mesophyll cell wall thickness and tortuosity, chloroplast volume, apoplast pH and ascorbate to O3 reaction stoichiometry [68]. Neither of these modelling methods consider the ‘costs’ of maintaining such defence mechanisms, which might be expected to require increased respiration rates [23]. Understanding how these detoxification rates might vary diurnally, seasonally and by species and genotype is crucial to our ability to define damage thresholds by O3 metric. The existence and value of such thresholds have a substantial bearing on the effectiveness of emission reduction policy, as discussed in §5a.
Empirical flux-based models have their shortcomings. Firstly, there are uncertainties in estimating gsto, largely due to challenges in the parameterization of gsto models for different species, cultivars and ecotypes. This is further complicated by the fact that O3 can both positively and negatively affect gsto depending upon species and O3 exposure profile [69] and that O3 can modify the stomatal responses of plants to naturally occurring environmental stresses such as drought (e.g. [70–72]). There are only a limited number of flux-based ERRs for yield and biomass effects (table 1). ERRs exist for four crop species (wheat, potato, tomato and rice), five forest tree species/types (beech/birch, poplar, Norway spruce and Mediterranean deciduous oak and Mediterranean evergreen species) and one grassland species (perennial ryegrass) with only three ERRs for species/species types outside of Europe, all of which are Asian species. Interactive effects of co-occurring pollution and environmental stress are not included and hence only the relative risk, rather than absolute damage to O3 under multiple stress conditions, can be assessed [23]. Finally, these models only assess impacts to individual species and therefore are unable to infer the level of O3-induced competition effects on forest and grassland communities evident from experiments. In spite of these limitations, both concentration- and flux-based ERRs have played an important role in enabling national, regional and global assessments of O3 damage.
Table 1.
Characteristics of current flux-based ERRs and associated target level (e.g. critical levels) for species and species groups. N.B. For Europe, only those ERRs that have been established under the CLRTAP are included. PODy is the phytotoxic ozone dose accumulated above a threshold ‘y’, CL is the critical level (see text for further definitions).
| species of vegetation type | global region covered | POD ‘y’ threshold (nmol O3 m−2 PLA s−1) | potential maximum rate of reduction (%) per mmol m−2 PLA of PODy | response variable | potential effect at CL (% reduction) | CL (mmol O3 m−1 PLA) | reference |
|---|---|---|---|---|---|---|---|
| crops | |||||||
| wheat | boreal, temperate & Mediterranean Europea | 6 | 3.85 | grain yield | 5% | 1.3 | [67] |
| 6 | 3.35 | 1000-grain weight | 5% | 1.5 | [67] | ||
| 6 | 2.54 | protein yield | 5% | 2 | [67] | ||
| subtropical China | 12 | 0.082 | grain yield | — | — | [73] | |
| 0–18b | 0.0214 | whole plant biomass | — | — | [74] | ||
| rice | Japand | 10 | 0.487 | grain yield | — | — | [75] |
| potato | boreal, temperate & Mediterranean Europea | 6 | 1.34 | tuber yield | 5% | 3.8 | [67] |
| tomato | boreal, temperate & Mediterranean Europea | 6 | 2.53 | fruit yield | 5% | 2 | [67] |
| 6 | 1.3 | fruit quality | 5% | 3.8 | [67] | ||
| forest trees | |||||||
| beech/birch | boreal & temperate Europea | 1 | 0.93 | whole tree biomass | 4% | 5.2 | [67] |
| poplarc | temperate, semi-humid continental northern China | 7 | 1.2 | whole tree biomass | 5% | 3.8 | [76] |
| continental monsoon China | 7 | 0.56 | whole tree biomass | 5% | 9.8 | [77] | |
| Norway spruce | boreal & temperate Europea | 1 | 0.22 | whole tree biomass | 2% | 9.2 | [67] |
| Deciduous Oak species | Mediterranean Europea | 1 | 0.32 | whole tree biomass | 4% | 14 | [67] |
| 0.45 | root biomass | 4% | 10.3 | [67] | |||
| evergreen tree species | Mediterranean Europea | 1 | 0.09 | above-ground biomass | 4% | 47.3 | [67] |
| semi-natural vegetation | |||||||
| temperate perennial grasslands | boreal, temperate & Mediterranean Europea | 1 | 0.99 | above-ground biomass | 10% | 10.2 | [67] |
| 1 | 0.62 | total biomass | 10% | 16.2 | [67] | ||
| 1 | 1.54 | flower number | 10% | 6.6 | [67] | ||
| Mediterranean annual pasture | boreal, temperate & Mediterranean Europea | 1 | 0.85 | above-ground biomass | 10% | 16.9 | [67] |
| 1 | 1.61 | flower/seed biomass | 10% | 10.8 | [67] | ||
aFurther detail on biogeographical zones is provided in [67].
bVariable threshold defined as a function of gross photosynthesis.
cDifferences between CLs likely due to different number and O3 sensitivity of poplar clones used by [76] (five clones) and [77] (two clones).
dRice plants grown and fumigated in glasshouses.
(b). Risk assessment methodologies
There are three main approaches to using ERRs in geospatial risk assessments to inform air quality management. These methods combine interpolated or modelled O3 concentration fields (and associated meteorological data where additional plant-based modelling is required such as estimation of gsto) with data describing the distribution, both temporally and spatially, of the vegetation type being investigated.
The first approach requires the establishment of target levels for O3 exposure to calculate exceedance and assess likely risk. This approach has been most widely developed and applied for vegetation in Europe largely thanks to the ‘effects-based’ approach coordinated within the UNECEs CLRTAP [67]. For gaseous pollutants such as O3, targets were defined as ‘Critical Levels’ (CLs) - ‘the concentration, cumulative exposure or cumulative stomatal flux of atmospheric pollutants above which direct adverse effects on sensitive vegetation may occur according to present knowledge’ [67]. These CLs are identified through statistical analysis of ERRs that consider how best to combine experimental data from multiple experiments, years and locations and how to allow for the pre-industrial levels of O3 concentration ([67]; table 1). Critical levels are defined for a species- or vegetation-specific percentage effect that is statistically significant (which will vary dependent upon the 95% confidence intervals of the ERR) and have used both AOT40 and flux-based metrics. These CLs have supported the development of EU ‘target values’ for the National Emissions Ceiling Directive (NEC) that represent the legally binding emission reduction targets for all EU member states adopted in 2001 [79]. Similar approaches in the USA are referred to as ‘National Ambient Air Quality Standards' (NAAQS), but these currently only exist to protect human health although a vegetation standard has been proposed [80]. At the global level, the World Health Organisation (WHO) recommends vegetation target values for O3 based on the UNECE CLRTAP CL approach; these are based on the AOT40 metric since the WHO guidelines were established back in 2000. There are currently no official target values for vegetation outside of Europe. Some protection to vegetation may be afforded via human health standards for O3 where these exist (a notable exception is India) although these may be less stringent than the standards needed to protect vegetation.
The second type of assessment uses ERRs to estimate the spatial extent and magnitude of damage. In addition to the O3 concentration fields and vegetation distribution, statistics describing the actual yield or biomass of crop and forest species are required to estimate damage in relative terms. As well as providing regional/global damage assessments (see §4) these risk assessments provide an opportunity to explore the ability of different metrics to estimate damage. For example, flux has been identified as a better predictor of the distribution and scale of damage for bio-monitoring and beech growth across Europe [62,81]. By contrast, neither flux-based nor AOT40 metrics could explain forest defoliation and declines in basal area increment in an Alpine region in northern Italy [82].
The third type of assessment incorporates ERRs into existing process-based modelling frameworks to allow the assessment of direct and indirect impacts of O3 on growth, development and biomass/yield via changes in photosynthesis, C allocation and transpiration [23]. These models have O3 directly or indirectly impacting on photosynthesis (and indirectly on gsto via coupling approaches) using a relationship between canopy conductance and gross primary productivity (GPP) so that GPP is ultimately limited by O3 exposure according to an ERR (e.g. [83,84]). Often this approach uses land-ecosystem models and allows the interactive effects of O3 with other environmental factors to be explored. There are some limitations with this type of modelling. Firstly, some use concentration-based threshold indices (e.g. AOT40) that ignore O3 concentrations below thresholds which we know can contribute to damage. Secondly, the daily time step of some approaches precludes the capture of covarying factors that are known to determine actual levels of O3 uptake. Thirdly, the use of season-long statistical relationships of O3 response in combination with daily or monthly plant processes will miss interactions between O3 uptake, detoxification and prevailing environmental conditions that will influence damage. Finally, these methods require robust and appropriate ERRs such as the response of photosynthesis to O3 concentrations or PODy. This last aspect has been studied in relation to terrestrial ecosystem models for forest trees [85]. Substantial differences were found both in the ERRs used to describe leaf-level photosynthetic responses to O3 and in the methods used to relate these ERRs to reductions in whole tree biomass. ERRs required re-parameterization before they were able to successfully simulate the whole tree biomass reductions found in O3 fumigation field experiments.
4. Key findings from application of risk assessment methods
Even with the uncertainties in these concentration- and flux-based risk assessment methods they still provide useful information on risk and how this might change in light of emission reduction policies or climate change (the latter though only when using flux-based indices and only in relation to how climate variables would influence O3 uptake).
(a). Agriculture
A substantial number of modelling efforts have been conducted to assess the magnitude and extent of O3 impacts on arable crops. The majority of these studies have used concentration-based O3 metrics (M7 and AOT40) and hence should be treated with some caution since they are unable to allow for the modifying influence of environment on O3 sensitivity; more recent studies have used PODy. Studies assessing the exceedance of CLs have been largely limited to Europe and find exceedances of the AOT40 CL by a factor of three common in central and southern Europe; by contrast, the PODy exceedance tends to be more evenly distributed across Europe and only one to two times the CL [86]. A study that applied the AOT40 metric with CLs derived for rice and wheat from an OTC fumigation study conducted in the Yangtze River Delta [87] found exceedances of the CL by factors of approximately two and five across 75% and 83% of the rice and wheat growing areas, respectively [88].
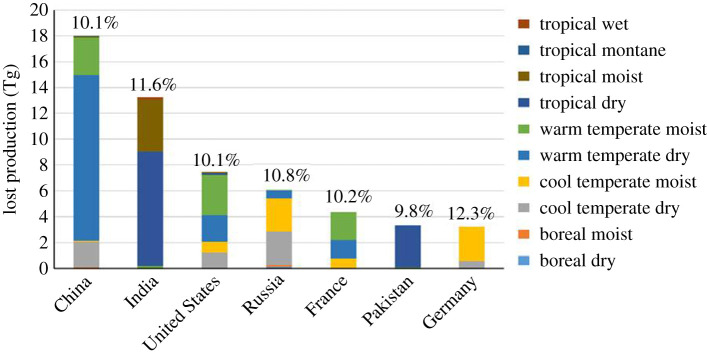
Global-scale assessments of crop damage using ERRs suggest yield reductions for wheat, maize, soya bean and rice of between 3 and 16% which would translate into economic losses of $US 14–26 billion in the year 2000 [89,90]. These losses look set to worsen in the future with additional yield losses for wheat, soya bean and maize of between 0.1 and 11% globally by 2030 based on lower (B1) and upper (A1) IPCC SRES emission projections to 2030 [91]. More recent ERR damage studies for wheat using PODy have importantly allowed an analysis of the biophysical conditions associated with elevated O3 concentrations that will most likely to lead to damage. Relative yield losses for wheat of approximately 6–15% for China and approximately 8–22% for India were found using the flux-based method with the warmer regions of India identified as having particularly high yield losses [92]. Global wheat yield and production losses for 2010–2012 were found to be particularly large in humid rain-fed and irrigated areas of major wheat-producing countries (e.g. USA, France, India, China and Russia) with reductions of approximately 10 and 6% in the NH and SH, respectively [93]. Highest yield losses were found under biophysical conditions that enhanced stomatal uptake of O3, the warm-temperate-moist, tropical-moist and tropical-wet climates of the NH (estimated as having 12–17% mean yield losses) and the tropical-moist and -wet climates of the SH (with 9–11% mean yield losses). Figure 1 shows how these biophysical regions spilt out across the countries most affected by O3-induced yield losses, clearly identifying China and India as having the majority of warm temperate dry and tropical dry conditions which suffer production losses [93]. Findings also showed that O3 could reduce the potential yield benefits of increasing irrigation usage in response to climate change as added irrigation increases O3 uptake and damage. This study clearly shows the opportunities afforded by the flux-based metric in identifying biophysical conditions that might enhance O3 sensitivity.
Figure 1.
Production losses in wheat due to O3 for the seven most affected countries allowing for irrigation. The mean percentage yield loss per country is provided above each bar. For further details see [93]. (Online version in colour.)
Application of the ERRs within process-based models find that the effect of O3 on productivity (often defined as GPP or NPP) is generally greater than that resulting from the change in climate when both are considered over time periods of a number of decades. For example, a larger effect of O3 on NPP (−2.6 to −6.8%) was found for the USA during the late 1980s and early 1990s than that caused by the long-term trends (1950–1995) in climate variability (−1.6%) [95]. Environmental conditions that would support elevated O3 levels are often likely to co-occur with high temperatures and soil water stress. A study of O3-drought interactions in China found that climate variability and O3 together led to an annual mean reduction of crop yield by 10% during 1981–2010 [96]. Crop response to air pollution and climate variables will likely occur under a variety of crop management practices and in combination with regional scale land-use change. Simulations were conducted to explore the interactive effects of elevated CO2 concentration, climate variability, change in land use (with and without N fertilization), and O3 (via direct effects on GPP based on the AOT40 index) on crop productivity across the USA for 1950–1995 [95]. This simulation study found that both O3 and climate change effects were substantially less than the influence of agricultural management (+46.2%) and change in land use (−26.8%) on C sequestration. Similarly, a study of agricultural NPP found increases between 1980 and 2005 that were predominantly due to changes in management practice (application of fertilizer) but that these increases would have been greater in the absence of the combined effect of climate change and O3 [97]. Since O3 stress often coincides with other common stresses impacting agriculture (i.e. heat stress, pests and diseases, aridity and nutrient stress), especially in locations such as China, India and the USA, it is becoming ever more important that we improve our understanding of the impact of these stress combinations on productivity [94].
(b). Forests and grasslands
The complex nature of forest systems with their multi-decadal life span, mix of species within a forest stand, variable age structure and community dynamics, coupled with the fact that ERRs are established for annual changes in whole tree biomass, mean that regional risk assessments using ERRs alone have to date been limited to estimating potential relative risk. A comparison of the spatial extent of risk inferred from application of different metrics found that flux-based metrics reduced both the spatial gradient and relative risk to forests across Europe when compared with concentration-based AOT40 metrics for the year 2000 [86]. Emission reductions according to a current legislation scenario dramatically reduced relative exceedances by 2020 although CLs for forests were still exceeded across most of Europe. A similar study in China found that AOT40 exceeded the CL on average by about five times with higher risks in northern compared with southern tropical and subtropical regions of China [98]. Feng et al. [88] assessed O3 damage to annual forest tree biomass growth across China using an AOT40 metric ERR developed for broadleaf deciduous forests from analysis of fumigation experiments on young European forest trees [33]. The study estimated O3-induced reductions in growth of annual biomass of 11–13% finding a similar O3 risk to evergreen broadleaf forests of (sub-) tropical China and deciduous temperate forests of north–central China. This was due to the longer growing season of the evergreen trees compensating for their exposure to lower O3 concentrations. However, this study assumed evergreen tree response could be simulated using a deciduous tree ERR which is likely to overestimate damage.
Studies using ERRs in combination with process-based models have also been reported for forest trees. The interactive effect of O3, CO2 and N deposition on hardwoods was simulated using the PnET-CN model [99] and found that O3 counteracts the effect of increased CO2 and N deposition on forest growth and C sequestration in the northeastern USA. Similar results were found by a study that explored the relative contributions of changes in temperature, precipitation, CO2 concentration, N deposition and O3 exposure (the latter using the PODy metric) on European forest growth between 1900 and 2050 [100]. Compared with 1990 levels, this study estimated an increase of 41% in average total C sequestration in forests and forest soils between 1950 and 2000, which was predominantly due to increases in CO2 concentration and N deposition. These simulations compared favourably, although were slightly lower than those observed in net annual increment data. From 2000 to 2050, growth is expected to decline with CO2 and temperature playing the greatest role in encouraging growth, helped somewhat by decreases in O3 exposure; decrease in N deposition was the main factor decreasing growth rates. Ideally, forest community dynamics would also be included in simulations of the impacts of O3. A recent study explored the successional dynamics of species composition and structural change of a typical temperate deciduous forest in the southeastern USA [101]. The simulation was conducted for a 500-year period using an individual-based gap model that includes basic physiology as well as species-specific metabolic properties for 10 abundant species and 22 other species. The study found no reduction in forest productivity from elevated O3 due to increases in the biomass of more tolerant species compensating for O3-induced losses in sensitive species.
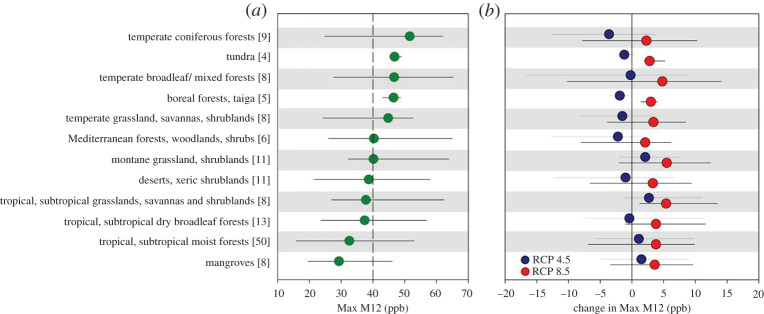
Due to the complexity of grassland ecosystems, studies have only assessed potential risk using concentration-based ERRs. An important global study explored the potential effects of current and future O3 exposures (using the M12 metric) for Global 200 terrestrial ecoregions in 12 major biomes which included both forest and grassland biomes [24]. Figure 2a shows the range of mean M12 values within each major biome for the year 2000 based on the highest of four 3-month M12 means over the whole area of each ecoregion. Approximately 40% of the ecoregions had a mean M12 value above 40 ppb with the highest M12 values found in temperate forests and grasslands, boreal forests and tundra. Figure 2b shows the change in M12 from 2000 to 2050 for a range of emission scenarios (RCP4.5 and 8.5; IPCC [102]). For the more optimistic scenario (RCP4.5) the changes in biome mean M12 are relatively small (−3.6 to +2.7 ppb), and biome mean M12 values decline by 2050 in the temperate and boreal biomes, which had the highest M12 values in 2000, while mean M12 values for tropical, subtropical and montane biomes tend to increase. For the more pessimistic scenario (RCP8.5) increases of over 10 ppb in M12 were found in forests and grasslands, and in the region covering part of India, the Himalayas and western China. These are all regions identified as having high N deposition by 2030 [103], which highlights the need for improved understanding of O3-N interactions in unmanaged systems [24].
Figure 2.
(a) Simulated O3 exposure in 2000 in G200 terrestrial ecoregions (ERs). (b) Change in simulated O3 exposure between 2000 and 2050 under RCP4.5 and RCP8.5. ERs are grouped by major biome, and the number of ERs in each biome is shown within brackets. For further details see [24]. (Online version in colour.)
Importantly, these risk assessments conducted for agriculture, forests and grasslands suggest that policies represented by the more optimistic scenarios do not always lead to a reduction in the spatial extent of O3 risk. In fact, for some regions, particularly in Asia, the extent and magnitude of risks are likely to increase in the future [24,93]. Coupled with these increases in O3 risk are changes in other stress factors such as enhanced N deposition [103], climate change and elevated CO2 [102], which modelling suggests can have substantial impacts on modifying the sensitivity of vegetation to O3 damage.
5. Future ozone trends and implications for vegetation
There are essentially two options available to reduce the damage to vegetation resulting from elevated O3, the first is to reduce the concentrations of O3 pollution in the atmosphere via mitigation, i.e. a reduction in O3 precursor emissions, and the second is to develop adaptation measures that reduce the adverse consequences of O3 pollution.
(a). Mitigation
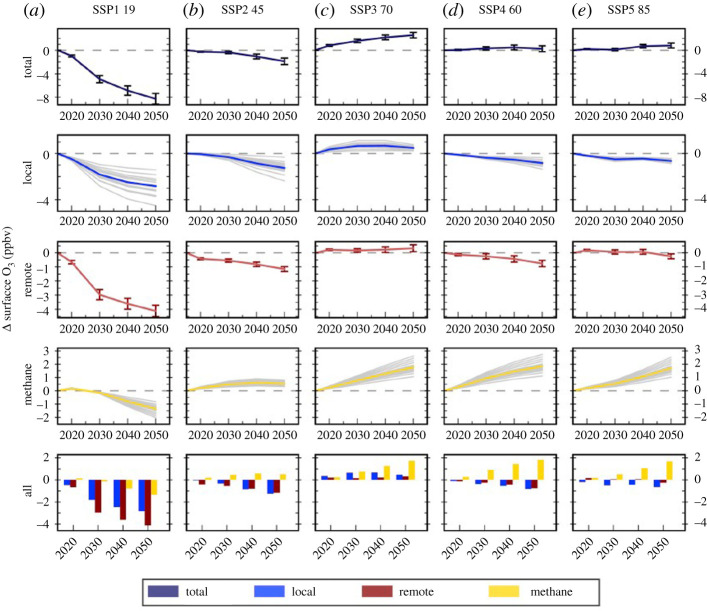
The changes apparent in the global distribution of O3 (see §3) clearly show a shift of O3 precursor emissions towards Asia where the lower latitudes are expected to differentially increase the tropospheric O3 burden due to the greater convection, reaction rates and NOx sensitivity of these regions [56]. It is also important to note that the particular precursor emissions driving O3 formation vary globally depending upon the prevailing atmospheric pollutant composition. In polluted regions, O3 formation is driven by the rapid photochemical oxidation of predominantly short-lived NMVOCs in the presence of NOx; CH4 has little effect on the O3 formed daily in urban plumes as it reacts very slowly (having a lifetime of 8–9 years). However, since CH4 is well-mixed throughout the troposphere and is more abundant than all NMVOCs combined, anthropogenic CH4 is estimated to contribute approximately seven times that of anthropogenic NMVOCs to the total tropospheric O3 burden, being especially important in less polluted environments [104], and will result in an increase in background O3 upon which local-to-regional O3 builds. The interplay between CH4 and O3 is further complicated by the role that O3 precursors play on CH4 chemistry. NOx tends to increase hydroxyl radical (OH) concentrations, which reduce the lifetime of CH4 and hence CH4 concentrations. By contrast, CO, CH4 and VOC emissions tend to reduce OH, increasing CH4 concentrations. Understanding this chemistry, and how it varies geographically, seasonally and between polluted and non-polluted environments, is crucial to developing successful mitigation strategies. A source-attribution study investigated selected socioeconomic pathways (SSPs; [105]) and found that changes in CH4 abundance, predominantly due to emissions from the energy, waste and agricultural sectors were the dominant drivers of changes in global surface O3 (figure 3) [106]. By contrast, the study found that changes in emissions from energy, industry, transport and residential sectors were more important regionally [106]. This analysis highlights that local emission reductions are not always enough to reduce future regional surface O3 concentrations and that hemispheric scale emission controls, particularly for CH4, will be required to reduce long-range sources of O3 and keep the regional surface O3 below present-day concentrations.
Figure 3.
Total annual mean changes in regional surface O3 concentrations over East Asia and the contribution of local (blue), remote (red) and methane (gold) sources between 2015 and 2050 from the parameterization for the CMIP6 emissions under the (a) SSP1 1.9, (b) SSP2 4.5, (c) SSP3 7.0, (d) SSP4 6.0 and (e) SSP5 8.5 scenarios. See [106] for further details. (Online version in colour.)
This raises the question: What are the implications of different emission control options for vegetation damage? This is especially important given that the differential effects of CH4 and NOx emission reductions on global versus regional-to-local O3 concentrations will also affect the diurnal and seasonal O3 concentration profiles. NOx controls will tend to reduce peak O3 concentrations at the local scale due to the shorter lifetime of these chemical species in the atmosphere; such controls are often driven by the countries own self-interest; reductions in CH4 will tend to reduce chronic (or background O3 concentrations) [107]. It is also worth remembering that the longer lifetime of CH4 leads to a slow O3 response to control measures relative to NOx and NMVOC controls that would realize almost immediate effects. The removal of the entire anthropogenic CH4 load by the year 2050 was found to reduce global O3 crop damage (estimated using concentration-based metrics) by 26% leading to a 1% increase in global crop production of the four major staple crops (wheat, maize, rice and soya bean) [107]. These yield benefits correspond to a difference in global economic value for the four crops of US$ 4 to 7 billion, of which 40% is realized in East Asia, 20% in North America and 12% in Europe (based on 2000–2010 average crop production and producer prices). For such assessments, it is crucial that O3 damage metrics are able to capture the influence of changing characteristics of O3 profiles (diurnal, seasonal and spatial distribution). Key issues here are whether the O3 metric has a threshold for damage and, if so, how close is the threshold to any changes in O3 profile resulting from emission reductions. It is also important to know whether acute O3 is more damaging than chronic O3 when delivered as equivalent growing season O3 exposures. It has been found that the AOT40 and PODy flux-based metrics respond quite differently to sets of changing O3 profile over the course of 6 or more years [108]. The AOT40 metric, but not the PODy metric, declined at a location (Harwell, UK) whose O3 concentrations were driven by NOx emission reduction policy. By contrast, at a location more influenced by background O3 concentrations (Auchencorth, UK), the PODy metric increased over time, an effect magnified for species with lower ‘y’ thresholds. The importance of the ‘y’ threshold level in determining the mitigation impact of global simulations of 50% emission reductions across all O3 precursor source sectors has also been explored [109]. Larger benefits were found for C3 cropland, C3 grassland and C4 grassland (which have ‘y’ values of 5 nmol m−2 s−1) compared with deciduous broadleaf, evergreen needleleaf and shrub species with ‘y’ values of approximately 1.6 nmol m−2 s−1. These findings are particularly interesting when compared with the ‘y’ thresholds described in table 1 for the Asian species, which are often far higher (above 10 nmol m−2 s−1); using O3 metrics with such high thresholds may reduce the perceived benefits of CH4 emission reductions.
So which metric is most suitable for predicting damage for a wide variety of O3 profiles? Evidence collected from wheat fumigation experiments found that PODy ERRs established under conditions of enhanced peak O3 concentrations were also valid for higher background and lower peak concentrations, commonly associated with the current European O3 profiles [110]. However, it has also been suggested that plants subjected to repeated exposures at low levels will become more resistant to later exposures [111], possibly due to changes in gene expression. It may also be that the longer the time period between high O3 peaks, the more able the plant is to maintain normal plant growth and development. However, a long-term shift in energy allocation under continuous O3 exposure may be less detrimental than long periods between acute temporary exposures [1]. Further work is required to understand the influence of changing O3 profiles in relation to detoxification thresholds and plant damage.
It is important to be aware that future O3 concentrations will not only be dependent upon emission pathways. They will depend on changes in climate variables (particularly temperature, humidity and solar radiation) that will influence photochemical reaction rates, circulation and mixing of air masses [112]. Climate and land use-induced changes in biogenic VOC emissions as well as feedbacks caused by O3 itself acting as a GHG will also influence O3 production and loss in the atmosphere. As such, ecosystem geography and the meteorological and chemical atmospheric background composition in different global regions will all contribute to regional differences in mitigation impacts [109].
(b). Adaptation
Adaptation options specifically designed to reduce O3 impacts have not been widely investigated, most likely since O3 also causes substantial damage to human health and is a GHG, so there are multiple benefits to be gained from mitigation control options [113]. In addition, adaptation is only really a feasible option for agricultural crops, which would leave forests and grasslands with no protection from prevailing O3 concentrations. However, since evidence suggests that O3 may cause substantial yield losses, especially in food-insecure regions in Asia, it is imperative to consider what options might be available or useful to enhance productivity while mitigation actions are implemented. Two types of adaptation options can be considered, technology options that might focus on crop breeding, for example of physiological traits that reduce O3 sensitivity of different cultivars, and management practice options that would improve crop tolerance and avoidance of elevated O3.
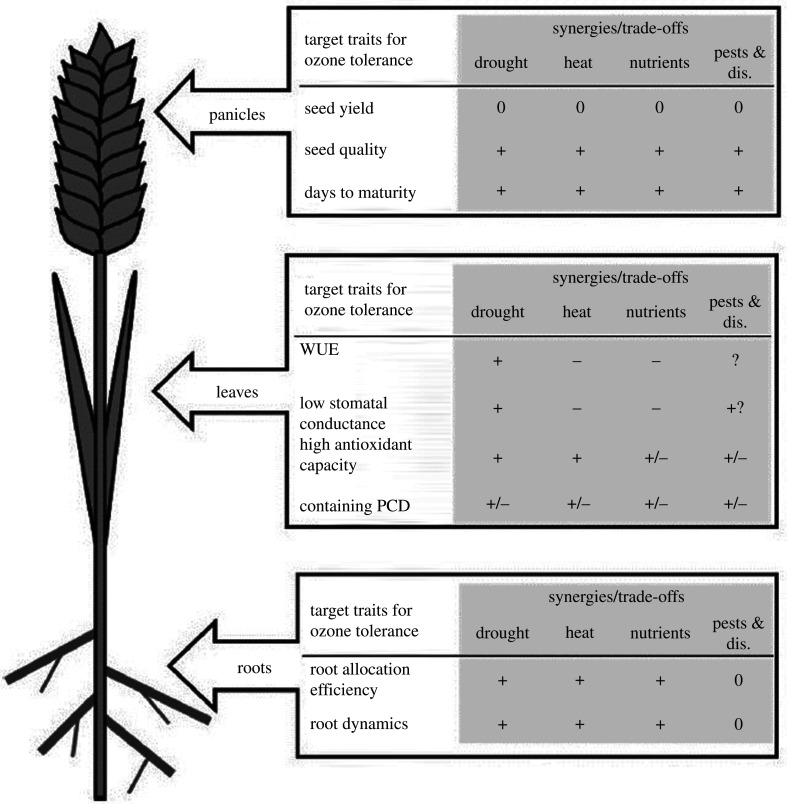
In relation to technology options, ERRs demonstrate the variation in sensitivity of cultivars of the same species to O3 [20,21] and hence the potential to breed O3-tolerant varieties. A recent review identified some challenges with two crop-breeding approaches considered suitable to provide O3 tolerance in rice: marker-assisted selection and transgenic approaches [4]. The challenges identified were: O3 heterogeneity across breeding trial sites, the ‘small effect loci’ genetic architecture of O3 tolerance and the challenges in convincing farmers and breeders of the necessity of breeding for O3 tolerance. However, ultimately the review emphasized that the substantial naturally occurring variability in O3 tolerance of germplasm offered a very real possibility of breeding tolerant varieties. A number of plant traits have been identified that might contribute to improved O3 tolerance, it is important to assess the synergies and trade-offs of such plant traits in relation to other well-known stressors that are likely to occur when O3 levels are high (figure 4; [94]). Identified traits include those that reduce gas exchange (and hence O3 uptake) while maintaining non-limiting levels of photosynthesis (i.e. traits that enhance water use efficiency (WUE)); such traits need to avoid trade-offs of reduced transpirational cooling (especially under co-occurring heat stress) and reduced soil nutrient uptake. Other traits might be those that enhance the detoxification capacity of plants while also minimizing any other gene-related trade-offs. Such traits could include breeding for high levels of antioxidants [114], for increased leaf mass per unit area (to lengthen the pathway and subsequent reaction time with apoplastic antioxidants and resistance to transport of O3 (and its reactive oxygen species) to the cellular sites of damage [115]), and to increase functional redox balance [116], which may also help with defence against other abiotic stresses which cause damage through similar oxidative stress pathways [117]. Breeding plants to reduce the induction of programmed cell death, a key response to oxidative stress, could enhance O3 tolerance [118] but would need to be balanced against unwanted interference with pathogen tolerance [94]. A focus on crop breeding to enhance root physiological traits to overcome the O3 impact on root:shoot ratios supporting continued water and nutrient uptake ensuring high harvest indices and quality of harvestable products (e.g. sugar and protein contents) were also identified as important. Finally, phenology, that is already an important focus of many crop breeding programmes around the world [119], could help plants to avoid harmful O3 exposures. For example, Indian cultivars with different heat stress traits including ‘early maturing’ heat avoidance varieties and ‘late’ or ‘timely’ sown heat-tolerant varieties were found to have different sensitivities to O3 [120]. Such strategies need to balance the benefits of avoiding O3 stress against stresses such as heat and water stress to ensure enough time for a productive grain-filling period.
Figure 4.
An ideotype for an O3-tolerant crop. ‘+’ indicates where there would be a benefit for other stresses of improving O3 tolerance for the trait while ‘−’ indicates a trade-off, and ‘0’ indicates no effect. See [94] for further details.
A number of crop-management practices have been considered as potentially able to confer O3 avoidance including changes to sowing date, irrigation scheduling and chemical protection [94]. For example, a global modelling study found that O3-induced crop yield losses for irrigated crops are usually equal to or greater than for rain-fed crops, especially in India. This was due to irrigation allowing crop calendars to capture optimum radiation and temperature conditions, but also inadvertently causing co-occurrence with seasonal peaks in O3 formation [121]. The study found that shifting crop calendars could reduce regional O3 damage for specific crop-location combinations (e.g. up to 25% for rain-fed soya bean in India), but this had little impact at the global level.
6. Development of improved modelling approaches
A new generation of dynamic process-based models that rely on an understanding of the mechanisms by which O3 causes damage rather than ERRs, are being developed and applied to better understand O3 impacts on vegetation [23]. These models often differentiate between short-term effects of O3 that are simulated as instantaneous effects on photosynthesis, usually modelled as a reduction in the plants maximum carboxylation capacity (Vcmax) [64], and long-term effects of accumulated O3 dose that will cause an earlier and enhanced onset of senescence [122]. These types of modelling approaches have been developed and scaled to estimate consequent effects on C assimilation for both crops (e.g. [122–124] and forests [125,126]). These process-based models rely to some extent on empirical relationships but it is possible to confine these to fundamental plant processes to allow more integrated modelling of O3 impacts. By focusing on leaf-level photosynthesis, and the coupling of photosynthesis to gsto, the assessment of O3 flux can be intrinsically linked to the fundamental processes that determine C assimilation and subsequent allocation that supports plant metabolism, respiration, growth and productivity. However, care should be taken to consider and allow for modification to the coupling (or indeed decoupling) of photosynthesis to gsto that might occur on exposure to O3 [70,126]. A robust mechanistic understanding of how these fundamental plant physiology processes are impacted by O3 should allow the incorporation of the influence of climate variables and plant characteristics (i.e. those associated with both species and genotype as well as environment such as elevation, geographical location, soil textures, etc.) on O3 damage.
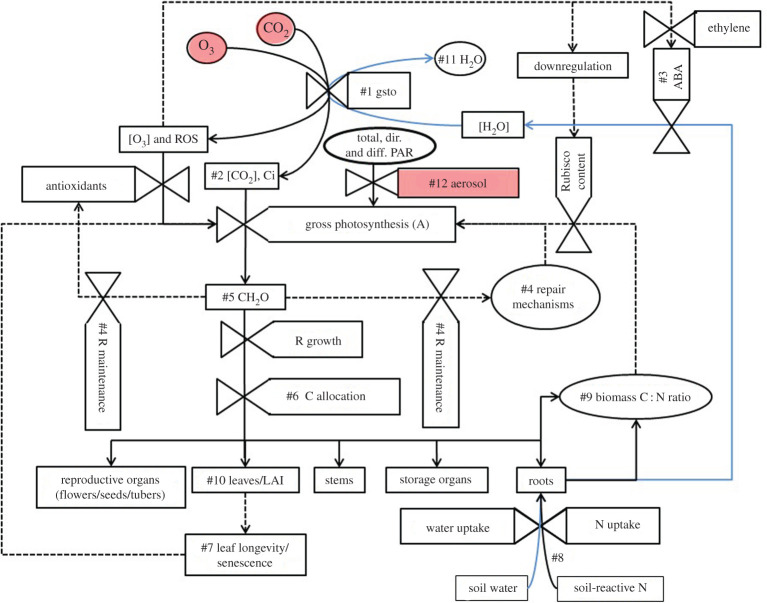
Figure 5 provides a conceptual overview of key mechanisms involved in two key resource use efficiencies—WUE and nitrogen use efficiency (NUE)—and how modelling could incorporate the effect of O3 damage on these processes. The focus on resource-use efficiency provides a useful way of integrating the effect of O3 on a variety of processes that combine to influence an overarching aspect of plant physiology. Ozone pollution will influence WUE through changes in gsto (#1; [69]) and ultimately transpiration [127]. This will occur either by directly impairing stomatal functioning (through damage to guard cells [128]) or indirectly through reductions in photosynthesis (caused by damage to photosynthetic machinery) leading to an increase in sub-stomatal CO2 concentrations (Ci; #2) and closure of stomata [23]. Ozone may also reduce root:shoot biomass ratios [11] leading to reduced soil water uptake by roots (#8) and can accelerate leaf senescence (#7; [129], reducing plant water utilization. These effects will influence transpiration (#11; [130]) and have implications for C assimilation (#5), allocation (#6) and ultimately crop growth and yield [11].
Figure 5.
A conceptual overview of the mechanisms by which pollution (O3 and aerosols) and elevated CO2 (shaded) will influence WUE and NUE. This overview describes the stomatal uptake of O3 pollution and impairment of radiation by aerosol pollution and the consequent effects on C assimilation, allocation and the crop's ability to take up nutrients and water from the soil. Also shown are feedbacks (dashed lines) that will alter the uptake of O3 (e.g. through changes in stomatal conductance (gsto)) and modification of C assimilation (via changes in respiration (R), photosynthetic capacity and leaf longevity). Modified from Emberson et al. [23]. (Online version in colour.)
Ozone can cause changes in N uptake and utilization via the following mechanisms: through early senescence (#7; [129]), which will shorten the crop development period and duration of grain filling leading to reduced N uptake (#8; [22]), and effects on N remobilization altering protein and starch contents of grain yield (#9; [22]); by reduced root:shoot ratio and root activity through altered allocation patterns (#6; [11]) causing reduced N uptake (#8; [22]); by reduced transpiration (#4) due to stomatal closure, which would lead to lower N translocation to the shoots and roots (#9; [27]); and finally by reduced C assimilation (#5) leading to reduced total plant growth (biomass) causing reduced total plant N demand and hence uptake (#9; [22]). Photosynthetic NUE is also important and dependent upon the CO2 saturation of Rubisco (#2, [75]). Understanding the relationship between gross photosynthetic capacity, chlorophyll degradation during leaf senescence (#7) and the shift from N assimilation to N remobilization are all important in the assessment of O3 effects on NUE.
Robust process-based models capable of incorporating O3 damage require specific empirical data (e.g. that describe O3 effects on key physiological processes such as respiration, gsto and photosynthesis and how these vary within canopies and across growing seasons). These data would help define key physiological, developmental, resource-use and growth processes and their parameterization for particular species and genotypes. One way to achieve better-targeted data collection and subsequent analysis is to increase the collaboration between experimentalists and modellers to improve understanding, and hence modelling of O3 effects for a range of environmental stress conditions; such an approach is currently underway in an AgMIP-Ozone initiative [23]. Such collaborative efforts could also inform the development of ecosystem models that are included in land-surface exchange schemes. Currently, some of these land surface models may lack the finer detailed aspects of crop modelling (e.g. developmental stage, C allocation algorithms) and still rely indirectly on empirical ERRs that integrate biomass and yield responses. However, these models do offer the opportunity to simulate a wider range of ecosystems and the exchange of gases (CO2, H2O and potentially O3) at various scales from cellular, to leaf, canopy and ecosystem levels incorporating the influence of multiple global changes including O3. Future innovations of these ecosystem models will also be important for the development of the new generation of earth system models [131] that would eventually enable holistic assessments of the exchange and impacts of pollution within the context of the entire earth system. Here, one of the key challenges has been to identify how best to model the interface between the atmosphere and land surface. The tight coupling between stomatal O3 deposition and O3 effects [132,133] would suggest that efforts to further develop O3 deposition schemes to use coupled photosynthesis-gsto approaches may help to align the air quality community with the land-surface and earth system modelling community. This could provide opportunities to compare and contrast approaches for assessing the risk of pollution to ecosystems and to work together to develop improved and more powerful modelling tools that can support both air quality and climate policy.
7. Conclusion
Current levels of O3 concentration are known to be causing damage to sensitive genotypes, species and species groups in North America, Europe and Asia. Future O3 concentrations, even under optimistic emission scenario pathways, will continue to cause damage to vegetation, particularly across many parts of South and East Asia with additional yield losses for wheat, soya bean and maize of between 0.1 and 11% globally by 2030. Concentration-based O3 risk assessment methods based on empirically derived ERRs for whole plant biomass and yield have limitations since they are unable to incorporate environmental conditions that we know will influence O3 damage. Even where concentration-based ERRs are coupled with process-based models, inconsistencies between the ERR and process-based modelling may produce misleading results in terms of O3 interactions with other stressors and hence risk and damage estimates. The use of flux-based ERRs has identified the global climate variable conditions likely to cause the highest yield losses in wheat as being the warm-temperate-moist, tropical-moist and tropical-wet climates of the NH (with mean yield losses of between 12 and 17%); further work is required to understand impacts on forests and grasslands and how elevated CO2, N and management practices might also influence O3 damage. These flux-based ERRs use a detoxification threshold; it will be crucial to understand how such damage thresholds might influence the efficacy of global versus local to regional emission reduction strategies that will differentially alter the diurnal amplitude and seasonality of O3 profiles. An alternative dynamic process-based modelling approach would be useful to gain a fuller understanding of the interactions between O3 and other stresses related to changes in climate, N deposition and hydrology thereby supporting the development of both mitigation and adaptation options to confer O3 tolerance and avoidance. Such approaches would require the collection of targeted empirical data and improved dialogue between experimentalists and modellers, which should lead to improved scientific knowledge to support air quality, climate change and land-use policymaking.
Data accessibility
This article has no additional data.
Competing interests
I declare I have no competing interests.
Funding
L.E. acknowledges the support of the Research Council of Norway through funding for the CiXPAG project (no. 244551) and the Department for Environment, Food and Rural Affairs (UK) for funding the SUSCAP project. This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 771134. The project SUSCAP was carried out under the ERA-NET Cofund SusCrop (Grant N°771134), being part of the Joint Programming Initiative on Agriculture, Food Security and Climate Change (FACCE-JPI).
References
- 1.Grulke NE, Heath RL. 2020. Ozone effects on plants in natural ecosystems. Plant Biol. 22(S1), 12–37. ( 10.1111/plb.12971) [DOI] [PubMed] [Google Scholar]
- 2.Cooper OR, et al. 2014. Global distribution and trends of tropospheric ozone: an observation-based review. Elementa 2, 1–28. [Google Scholar]
- 3.Mills G, Wagg S, Harmens H. 2013. Ozone pollution: Impacts on ecosystem services and biodiversity. See http://nora.nerc.ac.uk/id/eprint/502675/1/N502675CR.pdf.
- 4.Frei M. 2015. Breeding of ozone resistant rice: relevance, approaches and challenges. Environ. Pollut. 197, 144–155. ( 10.1016/j.envpol.2014.12.011) [DOI] [PubMed] [Google Scholar]
- 5.Heggestad HE, Middleton JT. 1959. Ozone in high concentrations as cause of tobacco leaf injury. Science 129, 208–210. ( 10.1126/science.129.3343.208) [DOI] [PubMed] [Google Scholar]
- 6.Haagen-Smit AJ. 1952. Chemistry and physiology of Los Angeles smog. Ind. Eng. Chem. Res. 44, 1342–1346. ( 10.1021/ie50510a045) [DOI] [Google Scholar]
- 7.Lesser VM, Rawlings JO, Spruill SE, Somerville MC. 1990. Ozone effects on agricultural crops: statistical methodologies and estimated dose-response relationships. Crop Sci. 30, 148–155. ( 10.2135/cropsci1990.0011183X003000010033x) [DOI] [Google Scholar]
- 8.Jäger HJ, Unsworth M, De Temmermann L, Mathy P (eds). 1992. Effects of air pollution on agricultural crops in Europe. CEC Air Pollution Research Report 46. [Google Scholar]
- 9.Fuhrer J. 2009. Ozone risk for crops and pastures in present and future climates. Naturwissenschaften 96, 173–194. ( 10.1007/s00114-008-0468-7) [DOI] [PubMed] [Google Scholar]
- 10.Ainsworth EA. 2017. Understanding and improving global crop response to ozone pollution. Plant J. 90, 886–897. ( 10.1111/tpj.13298) [DOI] [PubMed] [Google Scholar]
- 11.Feng Z, Kobayashi K. 2009. Assessing the impacts of current and future concentrations of surface ozone on crop yield with meta-analysis. Atmos. Environ. 43, 1510–1519. ( 10.1016/j.atmosenv.2008.11.033) [DOI] [Google Scholar]
- 12.Pleijel H, Broberg MC, Uddling J, Mills G. 2018. Current surface ozone concentrations significantly decrease wheat growth, yield and quality. Sci. Total Environ. 613–614, 687–692. ( 10.1016/j.scitotenv.2017.09.111) [DOI] [PubMed] [Google Scholar]
- 13.Broberg MC, Daun S, Pleijel H. 2020. Ozone induced loss of seed protein accumulation is larger in soybean than in wheat and rice. Agronomy 10, 357 ( 10.3390/agronomy10030357) [DOI] [Google Scholar]
- 14.Fishman J, Creilson JK, Parker PA, Ainsworth EA, Vining GG, Szarka J, Booker FL, Xu X. 2010. An investigation of widespread ozone damage to the soybean crop in the upper Midwest determined from ground-based and satellite measurements. Atmos. Environ. 44, 2248–2256. ( 10.1016/j.atmosenv.2010.01.015) [DOI] [Google Scholar]
- 15.Kaliakatsou E, Bell JNB, Thirtle C, Rose D, Power SA. 2010. The impact of tropospheric ozone pollution on trial plot winter wheat yields in Great Britain - an econometric approach. Environ. Pollut. 158, 1948–1954. ( 10.1016/j.envpol.2009.10.033) [DOI] [PubMed] [Google Scholar]
- 16.Hayes F, et al. 2016. Consistent ozone-induced decreases in pasture forage quality across several grassland types and consequences for UK lamb production. Sci. Total Environ. 543, 336–346. ( 10.1016/j.scitotenv.2015.10.128) [DOI] [PubMed] [Google Scholar]
- 17.Hayes F, Jones MLM, Mills G, Ashmore M. 2007. Meta-analysis of the relative sensitivity of semi-natural vegetation species to ozone. Environ. Pollut. 146, 754–762. ( 10.1016/j.envpol.2006.06.011) [DOI] [PubMed] [Google Scholar]
- 18.Sanz J, González-Fernández I, Calvete-Sogo H, Lin JS, Alonso R, Muntifering R, Bermejo V. 2014. Ozone and nitrogen effects on yield and nutritive quality of the annual legume Trifolium cherleri. Atmos. Environ. 94, 765–772. ( 10.1016/j.atmosenv.2014.06.001) [DOI] [Google Scholar]
- 19.Emberson LD, et al. 2009. A comparison of North American and Asian exposure–response data for ozone effects on crop yields. Atmos. Environ. 43, 1945–1953. ( 10.1016/j.atmosenv.2009.01.005) [DOI] [Google Scholar]
- 20.Osborne SA, Mills G, Hayes F, Ainsworth EA, Büker P, Emberson L. 2016. Has the sensitivity of soybean cultivars to ozone pollution increased with time? An analysis of published dose-response data. Global Change Biol. 22, 3097–3111. ( 10.1111/gcb.13318) [DOI] [PubMed] [Google Scholar]
- 21.Biswas DK, et al. 2008. Genotypic differences in leaf biochemical, physiological and growth responses to ozone in 20 winter wheat cultivars released over the past 60 years. Global Change Biol. 14, 46–59. [Google Scholar]
- 22.Broberg MC, Uddling J, Mills G, Pleijel H. 2017. Fertilizer efficiency in wheat is reduced by ozone pollution. Sci. Total Environ. 607–608, 876–880. ( 10.1016/j.scitotenv.2017.07.069) [DOI] [PubMed] [Google Scholar]
- 23.Emberson LD, et al. 2018. Ozone effects on crops and consideration in crop models. Eur. J. Agron. 100, 19–34. ( 10.1016/j.eja.2018.06.002) [DOI] [Google Scholar]
- 24.Fuhrer J, Val Martin M, Mills G, Heald CL, Harmens H, Hayes F, Sharps K, Bender J, Ashmore MR. 2016. Current and future ozone risks to global terrestrial biodiversity and ecosystem processes. Ecol. Evol. 6, 8785–8799. ( 10.1002/ece3.2568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jolivet Y, et al. 2016. Deciphering the ozone-induced changes in cellular processes: a prerequisite for ozone risk assessment at the tree and forest levels. Ann. For. Sci. 73, 923–943. ( 10.1007/s13595-016-0580-3) [DOI] [Google Scholar]
- 26.Chappelka AH, Samuelson LJ. 1998. Ambient ozone effects on forest trees of the eastern United States: a review. New Phytol. 139, 91–108. ( 10.1046/j.1469-8137.1998.00166.x) [DOI] [Google Scholar]
- 27.Skärby L, Ro-Poulsen H, Wellburn FAM, Sheppard LJ. 1998. Impacts of ozone on forests: a European perspective. New Phytol. 139, 109–122. ( 10.1046/j.1469-8137.1998.00184.x) [DOI] [Google Scholar]
- 28.Percy KE, Nosal M, Heilman W, Dann T, Sober J, Legge AH, Karnosky DF. 2007. New exposure-based metric approach for evaluating O3 risk to North American aspen forests. Environ. Pollut. 147, 554–566. ( 10.1016/j.envpol.2006.10.009) [DOI] [PubMed] [Google Scholar]
- 29.Sun G, McLaughlin SB, Porter JH, Uddling J, Mulholland PJ, Adams MB, Pederson N. 2012. Interactive influences of ozone and climate on streamflow of forested watersheds. Global Change Biol. 18, 3395–3409. ( 10.1111/j.1365-2486.2012.02787.x) [DOI] [Google Scholar]
- 30.Super I, De AV-G, Krol J, Maarten C. 2015. Cumulative ozone effect on canopy stomatal resistance and the impact on boundary layer dynamics and CO2 assimilation at the diurnal scale: a case study for grassland in the Netherlands. J. Geophys. Res. Biogeosci. 120, 1348–1365. ( 10.1002/2015JG002996) [DOI] [Google Scholar]
- 31.Wittig VE, Ainsworth EA, Naidu SL, Karnosky DF, Long SP. 2009. Quantifying the impact of current and future tropospheric ozone on tree biomass, growth, physiology and biochemistry: a quantitative meta-analysis. Global Change Biol. 15, 396–424. ( 10.1111/j.1365-2486.2008.01774.x) [DOI] [Google Scholar]
- 32.Karnosky DF, Skelly JM, Percy KE, Chappelka AH. 2007. Perspectives regarding 50 years of research on effects of tropospheric ozone air pollution on US forests. Environ. Pollut. 147, 489–506. ( 10.1016/j.envpol.2006.08.043) [DOI] [PubMed] [Google Scholar]
- 33.Büker P, et al. 2015. New flux based dose-response relationships for ozone for European forest tree species. Environ. Pollut. 206, 163–174. ( 10.1016/j.envpol.2015.06.033) [DOI] [PubMed] [Google Scholar]
- 34.Reich PB. 1987. Quantifying plant response to ozone: a unifying theory. Tree Physiol. 3, 63–91. ( 10.1093/treephys/3.1.63) [DOI] [PubMed] [Google Scholar]
- 35.Dizengremel P, Le Thiec D, Hasenfratz-Sauder MP, Vaultier MN, Bagard M, Jolivet Y. 2009. Metabolic-dependent changes in plant cell redox power after ozone exposure. Plant Biol. 11(Suppl. 1), 35–42. ( 10.1111/j.1438-8677.2009.00261.x) [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Feng Z, Wang X, Niu J. 2012. Responses of native broadleaved woody species to elevated ozone in subtropical China. Environ. Pollut. 163, 149–157. ( 10.1016/j.envpol.2011.12.035) [DOI] [PubMed] [Google Scholar]
- 37.Dickson RE, et al. 2000. Forest Atmosphere Carbon Transfer Storage (FACTS II)—The Aspen Free-air CO2 and O3 Enrichment (FACE) project: an overview. Gen Tech Report, NC-214.
- 38.Isebrands JG, et al. 2003. Growth responses of aspen clones to elevated carbon dioxide and ozone. Dev. Environ. Sci. 3, 411–435. [Google Scholar]
- 39.Percy KE, et al. 2002. Altered performance of forest pests under atmospheres enriched by CO2 and O3. Nature 420, 403–407. ( 10.1038/nature01028) [DOI] [PubMed] [Google Scholar]
- 40.Talhelm AF, et al. 2014. Elevated carbon dioxide and ozone alter productivity and ecosystem carbon content in northern temperate forests. Global Change Biol. 20, 2492–2504. ( 10.1111/gcb.12564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matyssek R, et al. 2010. Enhanced ozone strongly reduces carbon sink strength of adult beech (Fagus sylvatica)—resume from the free-air fumigation study at Kranzberg Forest. Environ. Pollut. 158, 2527–2532. ( 10.1016/j.envpol.2010.05.009) [DOI] [PubMed] [Google Scholar]
- 42.Pretzsch H, Dieler J, Matyssek R, Wipfler P. 2010. Tree and stand growth of mature Norway spruce and European beech under long-term ozone fumigation. Environ. Pollut. 158, 1061–1070. ( 10.1016/j.envpol.2009.07.035) [DOI] [PubMed] [Google Scholar]
- 43.Nunn AJ, et al. 2006. Testing the unifying theory of ozone sensitivity with mature trees of Fagus sylvatica and Picea abies. Tree Physiol. 26, 1391–1403. ( 10.1093/treephys/26.11.1391) [DOI] [PubMed] [Google Scholar]
- 44.Matyssek R, et al. 2012. Forests under climate change and air pollution: gaps in understanding and future directions for research. Environ. Pollut. 160, 57–65. ( 10.1016/j.envpol.2011.07.007) [DOI] [PubMed] [Google Scholar]
- 45.Feng Z, Büker P, Pleijel H, Emberson L, Karlsson PE, Uddling J. 2018. A unifying explanation for variation in ozone sensitivity among woody plants. Global Change Biol. 24, 78–84. ( 10.1111/gcb.13824) [DOI] [PubMed] [Google Scholar]
- 46.Volk M, Bungener P, Contat F, Montani M, Fuhrer J. 2006. Grassland yield declined by a quarter in 5 years of free-air ozone fumigation. Global Change Biol. 12, 74–83. ( 10.1111/j.1365-2486.2005.01083.x) [DOI] [Google Scholar]
- 47.Bassin S, Volk M, Fuhrer J. 2007. Factors affecting the ozone sensitivity of temperate European grasslands: an overview. Environ. Pollut. 146, 678–691. ( 10.1016/j.envpol.2006.06.010) [DOI] [PubMed] [Google Scholar]
- 48.Volk M, Obrist D, Novak K, Giger R, Bassin S, Fuhrer J. 2011. Subalpine grassland carbon dioxide fluxes indicate substantial carbon losses under increased nitrogen deposition, but not at elevated ozone concentration. Global Change Biol. 17, 366–376. ( 10.1111/j.1365-2486.2010.02228.x) [DOI] [Google Scholar]
- 49.Thwaites RH, Ashmore MR, Morton AJ, Pakeman RJ. 2006. The effects of tropospheric ozone on the species dynamics of calcareous grassland. Environ. Pollut. 144, 500–509. ( 10.1016/j.envpol.2006.01.028) [DOI] [PubMed] [Google Scholar]
- 50.Bassin S, Volk M, Suter M, Buchmann N, Fuhrer J. 2007. Nitrogen deposition but not ozone affects productivity and community composition of subalpine grassland after 3 yr of treatment. New Phytol. 175, 523–534. ( 10.1111/j.1469-8137.2007.02140.x) [DOI] [PubMed] [Google Scholar]
- 51.Bassin S, Volk M, Fuhrer J. 2013. Species composition of subalpine grassland is sensitive to nitrogen deposition, but not to ozone, after seven years of treatment. Ecosystems 16, 1105–1117. ( 10.1007/s10021-013-9670-3) [DOI] [Google Scholar]
- 52.Volk M, Wolff V, Bassin S, Ammann C, Fuhrer J. 2014. High tolerance of subalpine grassland to long-term ozone exposure is independent of N input and climatic drivers. Environ. Pollut. 189, 161–168. ( 10.1016/j.envpol.2014.02.032) [DOI] [PubMed] [Google Scholar]
- 53.Scebba F, Canaccini F, Castagna A, Bender J, Weigel HJ, Ranieri A. 2006. Physiological and biochemical stress responses in grassland species are influenced by both early-season ozone exposure and interspecific competition. Environ. Pollut. 142, 540–548. ( 10.1016/j.envpol.2005.10.014) [DOI] [PubMed] [Google Scholar]
- 54.Hayes F, Mills G, Williams P, Harmens H, Büker P. 2006. Impacts of summer ozone exposure on the growth and overwintering of UK upland vegetation. Atmos. Environ. 40, 4088–4097. ( 10.1016/j.atmosenv.2006.03.012) [DOI] [Google Scholar]
- 55.Andersen CP. 2001. Understanding the Role of Ozone Stress in Altering Belowground Processes. In Trends in European forest tree physiology research: cost action E6: EUROSILVA (eds Huttunen S, Heikkilä H, Bucher J, Sundberg B, Jarvis P, Matyssek R), pp. 65–79. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 56.Zhang Y, Cooper OR, Gaudel A, Thompson AM, Nédélec P, Ogino SY, West JJ. 2016. Tropospheric ozone change from 1980 to 2010 dominated by equatorward redistribution of emissions. Nat. Geosci. 9, 875–879. ( 10.1038/ngeo2827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mills G, et al. 2018. Tropospheric ozone assessment report: Present-day tropospheric ozone distribution and trends relevant to vegetation. Elem. Sci. Anth. 6, 47 ( 10.1525/elementa.302) [DOI] [Google Scholar]
- 58.Lu X, et al. 2018. Severe surface ozone pollution in china: a global perspective. Environ. Sci. Technol. Lett. 5, 487–494. ( 10.1021/acs.estlett.8b00366) [DOI] [Google Scholar]
- 59.Karlsson PE, et al. 2007. Risk assessments for forest trees: the performance of the ozone flux versus the AOT concepts. Environ. Pollut. 146, 608–616. ( 10.1016/j.envpol.2006.06.012) [DOI] [PubMed] [Google Scholar]
- 60.Pleijel H, Danielsson H, Emberson L, Ashmore MR, Mills G. 2007. Ozone risk assessment for agricultural crops in Europe: further development of stomatal flux and flux-response relationships for European wheat and potato. Atmos. Environ. 41, 3022–3040. ( 10.1016/j.atmosenv.2006.12.002) [DOI] [Google Scholar]
- 61.Emberson LD, Ashmore MR, Cambridge HM, Simpson D, Tuovinen JP. 2000. Modelling stomatal ozone flux across Europe. Environ. Pollut. 109, 403–413. ( 10.1016/S0269-7491(00)00043-9) [DOI] [PubMed] [Google Scholar]
- 62.Mills G, Hayes F, Simpson D, Emberson L, Norris D, Harmens H, Bãœker P. 2011. Evidence of widespread effects of ozone on crops and (semi-)natural vegetation in Europe (1990–2006) in relation to AOT40- and flux-based risk maps. Global Change Biol. 17, 592–613. ( 10.1111/j.1365-2486.2010.02217.x) [DOI] [Google Scholar]
- 63.Mills G, et al. 2011. New stomatal flux-based critical levels for ozone effects on vegetation. Atmos. Environ. 45, 5064–5068. ( 10.1016/j.atmosenv.2011.06.009) [DOI] [Google Scholar]
- 64.Martin MJ, Farage PK, Humphries SW, Long SP. 2000. Can the stomatal changes caused by acute ozone exposure be predicted by changes occurring in the mesophyll? A simplification for models of vegetation response to the global increase in tropospheric elevated ozone episodes. Aust. J. Plant Physiol. 27, 211–219. [Google Scholar]
- 65.Massman WJ. 2004. Toward an ozone standard to protect vegetation based on effective dose: a review of deposition resistances and a possible metric. Atmos. Environ. 38, 2323–2337. ( 10.1016/j.atmosenv.2003.09.079) [DOI] [Google Scholar]
- 66.Musselman RC, Lefohn AS, Massman WJ, Heath RL. 2006. A critical review and analysis of the use of exposure- and flux-based ozone indices for predicting vegetation effects. Atmos. Environ. 40, 1869–1888. ( 10.1016/j.atmosenv.2005.10.064) [DOI] [Google Scholar]
- 67.CLRTAP. 2017. Mapping Critical Levels for Vegetation, Chapter III. In Manual on methodologies and criteria for modelling and mapping critical loads and levels and air pollution effects, risks and trends (eds Mills et al.), pp. 1–66. Bangor: CEH. [Google Scholar]
- 68.Plöchl M, Lyons T, Ollerenshaw J, Barnes J. 2000. Simulating ozone detoxification in the leaf apoplast through the direct reaction with ascorbate. Planta 210, 454–467. ( 10.1007/PL00008153) [DOI] [PubMed] [Google Scholar]
- 69.Mills G, Harmens H, Wagg S, Sharps K, Hayes F, Fowler D, Sutton M, Davies B. 2016. Ozone impacts on vegetation in a nitrogen enriched and changing climate. Environ. Pollut. 208, 898–908. ( 10.1016/j.envpol.2015.09.038) [DOI] [PubMed] [Google Scholar]
- 70.Mills G, Hayes F, Wilkinson S, Davies WJ. 2009. Chronic exposure to increasing background ozone impairs stomatal functioning in grassland species. Global Change Biol. 15, 1522–1533. ( 10.1111/j.1365-2486.2008.01798.x) [DOI] [Google Scholar]
- 71.Wilkinson S, Davies WJ. 2009. Ozone suppresses soil drying- and abscisic acid (ABA)-induced stomatal closure via an ethylene-dependent mechanism. Plant Cell Environ. 32, 949–959. ( 10.1111/j.1365-3040.2009.01970.x) [DOI] [PubMed] [Google Scholar]
- 72.Wilkinson S, Davies WJ. 2010. Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant Cell Environ. 33, 510–525. ( 10.1111/j.1365-3040.2009.02052.x) [DOI] [PubMed] [Google Scholar]
- 73.Feng Z, Tang H, Uddling J, Pleijel H, Kobayashi K, Zhu J, Oue H, Guo W. 2012. A stomatal ozone flux-response relationship to assess ozone-induced yield loss of winter wheat in subtropical China. Environ. Pollut. 164, 16–23. ( 10.1016/j.envpol.2012.01.014) [DOI] [PubMed] [Google Scholar]
- 74.Wu R, Zheng Y, Hu C. 2016. Evaluation of the chronic effects of ozone on biomass loss of winter wheat based on ozone flux-response relationship with dynamical flux thresholds. Atmos. Environ. 142, 93–103. ( 10.1016/j.atmosenv.2016.07.025) [DOI] [Google Scholar]
- 75.Yamaguchi M, Hoshino D, Inada H, Akhtar N, Sumioka C, Takeda K, Izuta T. 2014. Evaluation of the effects of ozone on yield of Japanese rice (Oryza sativa L.) based on stomatal ozone uptake. Environ. Pollut. 184, 472–480. ( 10.1016/j.envpol.2013.09.024) [DOI] [PubMed] [Google Scholar]
- 76.Hu E, Gao F, Xin Y, Jia H, Li K, Hu J, Feng Z. 2015. Con centration- and flux-based ozone dose-response relationships for five poplar clones grown in North China. Environ. Pollut. 207, 21–30. ( 10.1016/j.envpol.2015.08.034) [DOI] [PubMed] [Google Scholar]
- 77.Shang B, Feng Z, Li P, Yuan X, Xu Y, Calatayud V. 2017. Ozone exposure- and flux-based response relationships with photosynthesis, leaf morphology and biomass in two poplar clones. Sci. Total Environ. 603–604, 185–195. ( 10.1016/j.scitotenv.2017.06.083) [DOI] [PubMed] [Google Scholar]
- 78.Maas R, Grennfelt P (eds). 2016. Towards cleaner air. Scientific Assessment Report, p. 50. Oslo, Norway: EMEP steering body and working group on effects of the convention on long-range transboundary air pollution. [Google Scholar]
- 79.Hettelingh JP, et al. 2013. Assessing interim objectives for acidification, eutrophication and ground-level ozone of the EU National Emission Ceilings Directive with 2001 and 2012 knowledge. Atmos. Environ. 75, 129–140. ( 10.1016/j.atmosenv.2013.03.060) [DOI] [Google Scholar]
- 80.Federal Register. 2017. Air Quality Designations for the 2015 Ozone National Ambient Air Quality Standards (NAAQS).
- 81.Braun S, Schindler C, Rihm B. 2014. Growth losses in Swiss forests caused by ozone: epidemiological data analysis of stem increment of Fagus sylvatica L. and Picea abies Karst. Environ. Pollut. 192, 129–138. ( 10.1016/j.envpol.2014.05.016) [DOI] [PubMed] [Google Scholar]
- 82.Ferretti M, et al. 2018. Scarce evidence of ozone effect on recent health and productivity of alpine forests—a case study in Trentino, N. Italy . Environ. Sci. Pollut. Res. 25, 8217–8232. ( 10.1007/s11356-018-1195-z) [DOI] [PubMed] [Google Scholar]
- 83.Felzer BS, Cronin TW, Melillo JM, Kicklighter DW, Schlosser CA. 2009. Importance of carbon-nitrogen interactions and ozone on ecosystem hydrology during the 21st century. J. Geophys. Res. 114, 1–10. [Google Scholar]
- 84.Ren W, et al. 2007. Effects of tropospheric ozone pollution on net primary productivity and carbon storage in terrestrial ecosystems of China. J. Geophys. Res. 112, D22S09. [Google Scholar]
- 85.Franz M, et al. 2018. Evaluation of simulated ozone effects in forest ecosystems against biomass damage estimates from fumigation experiments. Biogeosciences 15, 6941–6957. ( 10.5194/bg-15-6941-2018) [DOI] [Google Scholar]
- 86.Simpson D, Ashmore MR, Emberson L, Tuovinen J-P. 2007. A comparison of two different approaches for mapping potential ozone damage to vegetation: a model study. Environ. Pollut. 146, 715–725. ( 10.1016/j.envpol.2006.04.013) [DOI] [PubMed] [Google Scholar]
- 87.Wang X, et al. 2012. Effects of elevated O3 concentration on winter wheat and rice yields in the Yangtze River Delta, China. Environ. Pollut. 171, 118–125. ( 10.1016/j.envpol.2012.07.028) [DOI] [PubMed] [Google Scholar]
- 88.Feng Z, et al. 2019. Economic losses due to ozone impacts on human health, forest productivity and crop yield across China. Environ. Int. 131(July), 104966 ( 10.1016/j.envint.2019.104966) [DOI] [PubMed] [Google Scholar]
- 89.Van Dingenen R, Dentener FJ, Raes F, Krol MC, Emberson L, Cofala J. 2009. The global impact of ozone on agricultural crop yields under current and future air quality legislation. Atmos. Environ. 43, 604–618. ( 10.1016/j.atmosenv.2008.10.033) [DOI] [Google Scholar]
- 90.Avnery S, Mauzerall DL, Liu J, Horowitz LW. 2011. Global crop yield reductions due to surface ozone exposure: 1. Year 2000 crop production losses and economic damage. Atmos. Environ. 45, 2284–2296. ( 10.1016/j.atmosenv.2010.11.045) [DOI] [Google Scholar]
- 91.Avnery S, Mauzerall DL, Liu J, Horowitz LW. 2013. Global crop yield reductions due to surface ozone exposure: 2. Year 2030 potential crop production losses and economic. Atmos. Environ. 71, 408– 409 ( 10.1016/j.atmosenv.2011.01.002) [DOI] [Google Scholar]
- 92.Tang H, Takigawa M, Liu G, Zhu J, Kobayashi K. 2013. A projection of ozone-induced wheat production loss in China and India for the years 2000 and 2020 with exposure-based and flux-based approaches. Global Change Biol. 19, 2739–2752. ( 10.1111/gcb.12252) [DOI] [PubMed] [Google Scholar]
- 93.Mills G, et al. 2018. Ozone pollution will compromise efforts to increase global wheat production. Global Change Biol. 24, 3560–3574. ( 10.1111/gcb.14157) [DOI] [PubMed] [Google Scholar]
- 94.Mills G, et al. 2018. Closing the global ozone yield gap: quantification and cobenefits for multistress tolerance. Global Change Biol. 24, 4869–4893. ( 10.1111/gcb.14381) [DOI] [PubMed] [Google Scholar]
- 95.Felzer B, Kicklighter D, Melillo J, Wang C, Zhuang Q, Prinn R. 2004. Effects of ozone on net primary production and carbon sequestration in the conterminous United States using a biogeochemistry model. Tellus. 56B, 230–248. ( 10.3402/tellusb.v56i3.16415) [DOI] [Google Scholar]
- 96.Tian H, et al. 2011. China's terrestrial carbon balance: contributions from multiple global change factors. Global Biogeochem. Cycles. 25, GB1007 ( 10.1029/2010GB003838) [DOI] [Google Scholar]
- 97.Ren W, Tian H, Tao B, Huang Y, Pan S. 2012. China's crop productivity and soil carbon storage as influenced by multifactor global change. Global Change Biol. 18, 2945–2957. ( 10.1111/j.1365-2486.2012.02741.x) [DOI] [PubMed] [Google Scholar]
- 98.Li P, De Marco A, Feng Z, Anav A, Zhou D, Paoletti E. 2018. Nationwide ground-level ozone measurements in China suggest serious risks to forests. Environ. Pollut. 237, 803–813. ( 10.1016/j.envpol.2017.11.002) [DOI] [PubMed] [Google Scholar]
- 99.Ollinger S V, Aber JD, Reich PB, Freuder RJ. 2002. Interactive effects of nitrogen deposition, tropospheric ozone, elevated CO2 and land use history on the carbon dynamics of northern hardwood forests. Global Change Biol. 8, 545–562. ( 10.1046/j.1365-2486.2002.00482.x) [DOI] [Google Scholar]
- 100.de Vries W, Posch M, Simpson D, Reinds GJ. 2017. Modelling long-term impacts of changes in climate, nitrogen deposition and ozone exposure on carbon sequestration of European forest ecosystems. Sci. Total Environ. 605–606, 1097–1116. ( 10.1016/j.scitotenv.2017.06.132) [DOI] [PubMed] [Google Scholar]
- 101.Wang B, Shugart HH, Shuman JK, Lerdau MT. 2016. Forests and ozone: productivity, carbon storage, and feedbacks. Sci. Rep. 6, 22133 ( 10.1038/srep22133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.IPCC. et al. 2013. Climate change 2013: the physical science basis. In Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change (ed Stocker TF.). [Google Scholar]
- 103.Bobbink R, et al. 2010. Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol. Appl. 20, 30–59. ( 10.1890/08-1140.1) [DOI] [PubMed] [Google Scholar]
- 104.Fiore AM, Jacob DJ, Field BD, Streets DG, Fernandes SD, Jang C. 2002. Linking ozone pollution and climate change: the case for controlling methane. Geophys. Res. Lett. 29, 25-1–25-4. ( 10.1029/2002GL015601) [DOI] [Google Scholar]
- 105.Rao S, et al. 2017. Future air pollution in the shared socio-economic pathways. Glob. Environ. Change 42, 346–358. ( 10.1016/j.gloenvcha.2016.05.012) [DOI] [Google Scholar]
- 106.Turnock ST, Wild O, Sellar A, O'Connor FM. 2019. 300 years of tropospheric ozone changes using CMIP6 scenarios with a parameterised approach. Atmos. Environ. 213, 686–698. ( 10.1016/j.atmosenv.2019.07.001) [DOI] [Google Scholar]
- 107.Van Dingenen R, Crippa MJ, Anssens-Maenhout G, Guizzardi D, Dentener F. 2018. Global trends of methane emissions and their impacts on ozone concentrations. Vol. EUR29394EN, JRC Science for Policy Report. 2018. p.1–90.
- 108.Malley CS, Heal MR, Mills G, Braban CF. 2015. Trends and drivers of ozone human health and vegetation impact metrics from UK EMEP supersite measurements (1990-2013). Atmos. Chem. Phys. 15, 4025–4042. ( 10.5194/acp-15-4025-2015) [DOI] [Google Scholar]
- 109.Unger N, Zheng Y, Yue X, Harper KL. 2020. Mitigation of ozone damage to the world's land ecosystems by source sector. Nat. Clim. Change 10, 134–137. ( 10.1038/s41558-019-0678-3) [DOI] [Google Scholar]
- 110.Harmens H, Hayes F, Mills G, Sharps K, Osborne S, Pleijel H. 2018. Wheat yield responses to stomatal uptake of ozone: peak vs rising background ozone conditions. Atmos. Environ. 173, 1–5. ( 10.1016/j.atmosenv.2017.10.059) [DOI] [Google Scholar]
- 111.McCool PM, Musselman RC, Younglove T, Teso RR. 1988. Response of kidney bean to sequential ozone exposures. Environ. Exp. Bot. 28, 307–313. ( 10.1016/0098-8472(88)90054-8) [DOI] [Google Scholar]
- 112.Rasmussen DJ, Hu J, Mahmud A, Kleeman MJ. 2013. The Ozone − Climate Penalty: Past. Present, and Future. [DOI] [PMC free article] [PubMed]
- 113.Shindell D, et al. 2012. Simultaneously mitigating near-term climate change and improving human health and food security. Science 335, 183–189. ( 10.1126/science.1210026) [DOI] [PubMed] [Google Scholar]
- 114.Feng Z, Pang J, Nouchi I, Kobayashi K, Yamakawa T, Zhu J. 2010. Apoplastic ascorbate contributes to the differential ozone sensitivity in two varieties of winter wheat under fully open-air field conditions. Environ. Pollut. 158, 3539–3545. ( 10.1016/j.envpol.2010.08.019) [DOI] [PubMed] [Google Scholar]
- 115.Tingey DT, Taylor GE. 1982. Variation in plant response to ozone: a conceptual model of physiological events. In Effects of gaseous pollution in agriculture and horticulture (eds Unsworth DP, Ormrod MH), pp. 113–138. London, UK: Butterworth Scientific. [Google Scholar]
- 116.Wu LB, Ueda Y, Lai SK, Frei M. 2017. Shoot tolerance mechanisms to iron toxicity in rice (Oryza sativa L). Plant Cell Environ. 40, 570–584. ( 10.1111/pce.12733) [DOI] [PubMed] [Google Scholar]
- 117.Gill SS, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. ( 10.1016/j.plaphy.2010.08.016) [DOI] [PubMed] [Google Scholar]
- 118.Ueda Y, Frimpong F, Qi Y, Matthus E, Wu L, Höller S, Kraska T, Frei M. 2015. Genetic dissection of ozone tolerance in rice (Oryza sativa L.) by a genome-wide association study. J. Exp. Bot. 66, 293–306. ( 10.1093/jxb/eru419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Farooq M, Bramley H, Palta JA, Siddique KHM. 2011. Heat stress in wheat during reproductive and grain-filling phases. CRC Crit. Rev. Plant Sci. 30, 491–507. ( 10.1080/07352689.2011.615687) [DOI] [Google Scholar]
- 120.Jamir C. 2011. Assessing ozone impacts on arable crops in south Asia: identification of suitable risk assessment methods to improve crop biotechnology. York, UK: University of York. [Google Scholar]
- 121.Teixeira E, Fischer G, van Velthuizen H, van Dingenen R, Dentener F, Mills G, Walter C, Ewert F. 2011. Limited potential of crop management for mitigating surface ozone impacts on global food supply. Atmos. Environ. 45, 2569–2576. ( 10.1016/j.atmosenv.2011.02.002) [DOI] [Google Scholar]
- 122.Ewert F, Porter JR. 2000. Ozone effects on wheat in relation to CO2: modelling short-term and long-term responses of leaf photosynthesis and leaf duration. Global Change Biol. 6, 735–750. ( 10.1046/j.1365-2486.2000.00351.x) [DOI] [Google Scholar]
- 123.Tao F, Feng Z, Tang H, Chen Y, Kobayashi K. 2017. Effects of climate change, CO2 and O3 on wheat productivity in Eastern China, singly and in combination. Atmos. Environ. 153, 182–193. ( 10.1016/j.atmosenv.2017.01.032) [DOI] [Google Scholar]
- 124.Schauberger B, Rolinski S, Schaphoff S, Müller C. 2019. Global historical soybean and wheat yield loss estimates from ozone pollution considering water and temperature as modifying effects. Agric. Forest Meteorol. 265, 1–15. ( 10.1016/j.agrformet.2018.11.004) [DOI] [Google Scholar]
- 125.Deckmyn G, de Beeck M O, Löw M, Then C, Verbeeck H, Wipfler P, Ceulemans R. 2007. Modelling ozone effects on adult beech trees through simulation of defence, damage, and repair costs: implementation of the CASIROZ ozone model in the ANAFORE forest model. Plant Biol. 9, 320–330. ( 10.1055/s-2006-924762) [DOI] [PubMed] [Google Scholar]
- 126.Lombardozzi D, Levis S, Bonan G, Sparks JP. 2012. Predicting photosynthesis and transpiration responses to ozone: decoupling modeled photosynthesis and stomatal conductance. Biogeosciences 9, 3113–3130. ( 10.5194/bg-9-3113-2012) [DOI] [Google Scholar]
- 127.Masutomi Y, Kinose Y, Takimoto T, Yonekura T, Oue H, Kobayashi K. 2019. Ozone changes the linear relationship between photosynthesis and stomatal conductance and decreases water use efficiency in rice. Sci. Total Environ. 655, 1009–1016. ( 10.1016/j.scitotenv.2018.11.132) [DOI] [PubMed] [Google Scholar]
- 128.McAinsh MR, Evans NH, Montgomery LT, North KA. 2002. Calcium signalling in stomatal responses to pollutants. New Phytol. 153, 441–447. ( 10.1046/j.0028-646X.2001.00336.x) [DOI] [PubMed] [Google Scholar]
- 129.Osborne S, Pandey D, Mills G, Hayes F, Harmens H, Gillies D, Emberson L. 2019. New insights into leaf physiological responses to ozone for use in crop modelling. Plants 8, 84 ( 10.3390/plants8040084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bernacchi CJ, Kimball BA, Quarles DR, Long SP, Ort DR. 2006. Decreases in stomatal conductance of soybean under open-air elevation of [CO2] are closely coupled with decreases in ecosystem evapotranspiration. Plant Physiol. 143, 134–144. ( 10.1104/pp.106.089557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Flato GM. 2011. Earth system models: an overview. Wiley Interdiscip. Rev. Clim. Change 2, 783–800. ( 10.1002/wcc.148) [DOI] [Google Scholar]
- 132.Emberson LD, Ashmore MR, Simpson D, Tuovinen J-P, Cambridge HM. 2001. Modelling and mapping ozone deposition in Europe. Water Air Soil Pollut. 577–582. ( 10.1023/A:1013851116524) [DOI] [Google Scholar]
- 133.Simpson D, et al. 2012. The EMEP MSC-W chemical transport model – technical description. Atmos. Chem. Phys. 12, 7825–7865. ( 10.5194/acp-12-7825-2012) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.