Abstract
The interaction between polycomb-repressive complexes 1/2 (PRC1/2) and long non-coding RNA (lncRNA), such as the X inactive specific transcript Xist and the HOX transcript antisense RNA (HOTAIR), has been the subject of intense debate. While cross-linking, immuno-precipitation and super-resolution microscopy argue against direct interaction of Polycomb with some lncRNAs, there is increasing evidence supporting the ability of both PRC1 and PRC2 to functionally associate with RNA. Recent data indicate that these interactions are in most cases spurious, but nonetheless crucial for a number of cellular activities. In this review, we suggest that while PRC1/2 recruitment by HOTAIR might be direct, in the case of Xist, it might occur indirectly and, at least in part, through the process of liquid–liquid phase separation. We present recent models of lncRNA-mediated PRC1/2 recruitment to their targets and describe potential RNA-mediated roles in the three-dimensional organization of the nucleus.
Keywords: long non-coding RNAs (lncRNA), Xist RNA, HOTAIR RNA, polycomb-repressive complexes 1/2 (PRC1/2), RNA–protein interaction, RNA secondary structure, phase separation
1. Polycomb-group repressive complexes
Polycomb-repressive complexes 1/2 (PCR 1/2) are repressive proteins, firstly described in Drosophila melanogaster, responsible for the Hox-genes silencing [1], and competing with activating factors such as these from the Thritorax-group proteins [2]. In Drosophila, Polycomb complexes are recruited to target genes by recognition of polycomb response elements, also called PREs [3]. While mammals largely lack canonical PREs, CpG islands seem to pay an equivalent role [4]. Polycomb-mediated silencing is essential for several cellular functions, from pluripotency [5] to lineage specification [6] senescence and cancer [7,8]. In mammals, Polycomb complexes come in two main flavours, polycomb-repressive complex 1 (PRC1) and Polycomb-repressive complex 2 (PRC2). Each of these complexes can be further divided into three [9] or six [10] subcomplexes, respectively, depending on the complex composition and cellular function [11,12]. Polycomb complexes are responsible for placing repressive chemical marks on histone tails, regulating chromatin functions. In particular, PRC2 places methyl groups at the lysine 27 of the histone H3 [13], via Ezh2, its catalytic subunit, while PRC1 places mono-ubiquitin moieties at lysine 119/120 of histone H2A [14], via the Ring1A/B catalytic subunits. Polycomb marks on the chromatin, are, in turn, read by Polycomb complexes subunits [15,16] (positive reinforcing loops) and other complexes (readers) to stabilize gene silencing [17]. Both Polycomb complexes are capable of binding RNA, and this function of Polycomb complexes is crucial to ensure correct gene expression [18–20]. While PRC1/2 complexes are thought to have non-catalytic roles in genome architecture (e.g. by organizing the genome in three-dimensional) [21], the catalytic activity of these complexes is critical for polycomb-mediated silencing [22–24]. As the role of these marks has been discussed elsewhere, we refer the reader to other excellent reviews [25,26]. In our review, we focus on the role of RNA and in particular of long-coding RNAs, in the recruitment of these complexes to the chromatin, using the two most studies lncRNAs, Xist and HOTAIR, as models.
2. Direct versus indirect binding of polycomb-repressive complexes 1/2 components to Xist and HOTAIR
Long non-coding RNAs (lncRNAs) are RNA molecules longer than 200 bases that lack protein-coding potential [27,28]. They represent a significant portion of the cell transcriptome [29] and work as activators or repressors of gene transcription acting on different regulatory mechanisms [30–32]. lncRNAs can act as scaffolds for protein recruitment [33–40] and behave as guides and/or sponges for titrating RNAs and proteins, influencing transcription at regulatory regions or triggering transcriptional interference [41–43]. In the large spectrum of activities, the RNA structure plays a central role and dictates precise functionalities by creating spatial patterns and alternative conformations and binding sites for proteins [44,45]. In this review, we will focus on the two best-studied lncRNAs, Xist and HOTAIR, to critically discuss what we know about the interaction of PRC1/2 complexes with RNA.
Xist is a long non-coding RNA and the master-regulator of X chromosome inactivation (XCI) [46–49]. Xist works as a scaffold for the recruitment of repressive complexes on the inactive X chromosome (Xi) [46,50]. As for its structure, six conserved repetitive regions (Rep), named A to F, have been reported to be essential for its function [30,44]. The interaction between Xist and PRC1/2 has been studied in detail. In particular, PRC1 has been reported to interact with Xist B-repeats and PRC2 with Xist A-repeats (see below) (figure 1a). In the case of PRC1-Xist B repeats, a study from the Heard laboratory showed that a region encompassing the Xist B/C-repeat is necessary for PRC1 recruitment [52]. The Brockdorff laboratory mapped this interaction to the B repeat mostly, and proved that HNRNPK, which physically interacts with PRC1, is directly involved in RNA binding (figure 1a) [54]. For the PRC2-Xist interaction with the A-repeats, there is not agreement in literature. A seminal study from the Lee laboratory has shown that Xist A-repeats directly recruits EZH2 via direct interaction with its stem and loops [51]. However, different lines of evidence stemming from developmental studies suggest that Xist expression and PRC2 recruitment can be decoupled. In particular, in developing female embryos, Xist RNA clouds seems to precede H3K37me3 domains, making a direct interaction unlikely [55,56]. In agreement with these observations, super-resolution microscopy [57] and genetics analysis [58] point towards a non-direct interaction. In particular, Almeida et al. suggest that Xist attracts PRC2 to the chromatin via the recognition of the chromatin mark placed by PRC1 (i.e. H2AK119ub), in agreement with other models of PRC1/2 recruitment [59,60] (discussed in more details below).
Figure 1.
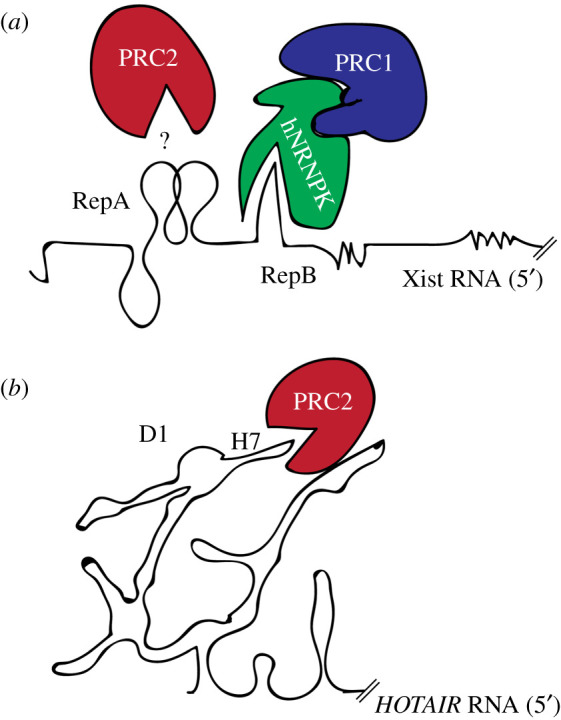
Xist and HOTAIR interactions with Polycomb proteins. (a) Schematic representation of possible PRC1/2 interactions via the Xist A- [51] and B- [52] repeats (black lines). The question marks indicates the debated interaction of PRC2 with the A-repeat (b) Schematic representation of HOTAIR interaction at domain 1 (D1), helix 7 (H7) [53] with PRC2 (black lines).
HOTAIR [61] is another well-known lncRNA regulating the expression of the HOX genes during development [61]. HOTAIR works as a scaffold for the recruitment of the PRC2 members EZH2, SUZ12, and it is also able to act in trans to allow the establishment of a repressed chromatin state at the HOX clusters [62,63]. How HOTAIR interacts with PRC2 in vivo is still debated, an in vitro study indicates a direct interaction between HOTAIR and EZH2 at its 5′ [63,64]. In particular, HOTAIR interaction with PRC2, mapped at the HOTAIR repeat D1 helix 7 (H7) [53], appears to be direct (in the range of 200 nM) [63,64]. HOTAIR-PRC2 interactions might be very different from those of Xist-PRC1/2 (figure 1b). The interaction between HOTAIR and PRC2 is likely sustained by the repetitive Guanine stretches (G-tracts) found in the D1 helix [64]. This interpretation is in line with data from Somarowth et al. showing equal affinity of the PRC2 complex to natively purified or refolded HOTAIR 5′/3′ using in vitro assays [53]. Noticeably, the putative Xist-PRC2 interaction region (A-repeats) is missing the key RNA recognition sequences needed for specific interactions (discussed below) [65].
3. Xist and HOTAIR show different modes of interactions with polycomb-repressive complexes 1/2 components
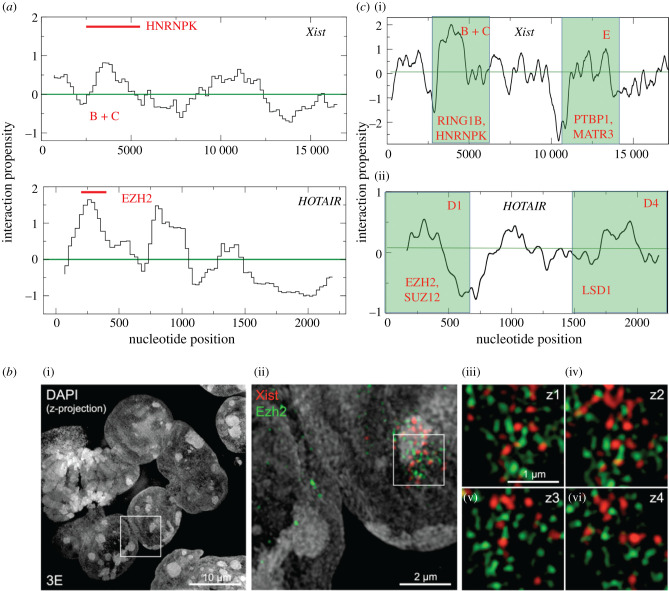
We analysed our previously published data on Xist and HOTAIR [35,66,67] binding abilities to PRC1/2 components. In our studies, we employed the catRAPID [35,68] method to estimates the binding potential of proteins to RNA molecules through van der Waals, hydrogen bonding and secondary structure propensities of both protein and RNA sequences. This allows the identification of binding partners with high confidence [69]. In agreement with experimental evidence [54], catRAPID identified a direct interaction between Xist 5′-end and HNRNPK [35] (Global Score = 0.99 on a scale ranging from 0 to 1, where 0 indicates no RNA-binding ability and 1 strong affinity; figure 2a; by contrast, the negative control Dyskerin Pseudouridine Synthase 1 DKC1 has a score of 0.01). To identify interactions of long non-coding RNAs such as Xist, catRAPID exploits a special pipeline that is based on the division of the transcript into fragments and calculation of their individual binding propensities (Z-normalized to 0 mean and standard deviation of 1), which is useful to spot the binding sites (figure 2a) [35]. PRC1 catalytic subunits Ring1A/B showed a high catRAPID score (Global Score = 0.98) [35], in accordance with what reported by Chu et al. [39] using Xist complementary oligo probes in pull-down experiments. Yet, it should be mentioned that Chu et al. [39] used formaldehyde fixation conditions to identify Xist binders, which indicates that non-direct interactions can be detected in their experiments. Other PRC1 components and PRC2 subunits did not rank high in our catRAPID analysis [35]. This is in agreement with the observation that PRC2 elements are under-represented in proteomic [36,37,39] and genetic screens [33,34] designed to reveal Xist interactomes. As for other PRC1 and PRC2 elements, we predicted low interaction propensities. For example, SUZ12, EZH1 and EZH2 have Global Score values of 0.01, 0.22 and 0.35, respectively. This is in line with the results of the previous analysis [70]. In brief, using randomized Xist A-repeats as a control, Ezh2 has been predicted to bind Xist with low affinity (EZH2-A-repeats interaction propensity is approximately 1, using a scale where positive interactions have scores greater than 10). These findings are in good agreement with three-dimensional-SIM data (figure 2b), showing the poor overlap between Xist and PRC2 [57], suggesting that this interaction might be sustained by intermediary proteins or via an indirect cascade (i.e. through PRC1-mediated H2A119 ubiquitination, see below).
Figure 2.
Xist and HOTAIR RNA predicted structure and interaction propensity and super-resolution microscopy. (a). Xist interaction propensity profile (Z-normalized binding propensities of RNA regions) calculated with catRAPID indicates that the binding of HNRNPK is in the region comprising the Xist Rep B and the Xist Rep C [35], in agreement with experimental evidence [54]. (b) Xist and PRC2 do not directly interact. Representative image of Xist and PRC2 catalytic subunit Ezh2 from Cerase et al. [57]. Reproduced with the permission of the editor, PNAS February 11, 2014 111 (6) 2235–2240. (c) HOTAIR interaction propensity calculated with catRAPID indicates the binding of EZH2 in the D1 domain [66], in agreement with experimental evidence [57,58].
On the other hand, catRAPID predictions indicate that HOTAIR and EZH2 might directly interact (Global Score = 0.99; figure 2a; by contrast, the negative control, the keratin-associated protein KRTAP21 has a score of 0.01, which is in agreement with previous biochemical evidence [63,64]). In both Xist and HOTAIR analyses, protein interactions strictly occur in highly structured regions of the transcripts (figure 2c) that contain G-rich stretches. These findings are in line with recent studies revealing that double-stranded regions in RNA molecules provide the scaffold for protein complexes [71,72]. Indeed, since RNA transcripts are highly flexible, an increase in secondary structure makes the protein partners bind tightly [72], favouring their accumulation on the scaffold, which can induce the formation of phase-separated assemblies (discussed below) [71].
In regards to the RNA structure, the CROSS (Computational Recognition of Secondary Structure) algorithm predicts the propensity of a nucleotide to be double-stranded given the neighbour nucleotides and the crowded cellular environment [73]. CROSS has been previously employed to compute the structural properties of Xist and HOTAIR [73,74]. In accordance with dimethyl sulfate (DMS)-sensitivity experiments [75], CROSS [73] analysis predicts that, Xist B and C Repeats (nucleotides approximately 2000–5500) as well as Xist A repeats (nucleotides approximately 1–400) and E (nucleotides approximately 10 000–12 000) of Xist are highly structured. Among Xist-interacting proteins binding to RepE, there are the splicing regulators polypyrimidine Tract Binding Protein 1 (PTBP1), MATRIN-3 (MATR3), CUG-Binding Protein 1 (CELF1) and TAR-DNA Binding Protein (TDP-43) [35–37,39].
In the case of HOTAIR, CROSS [73] identifies specific regions in the D1 region (nucleotides 1–500) as the most structured, together with the adjacent D2 region (nucleotides 500–1000 and nucleotides 1500–2200), which agrees with DMS experiments [53] (figure 2c, bottom). In addition to EZH2 [76], HOTAIR was shown to interact with the histone demethylases LSD1 (lysine-specific demethylase 1A). LSD1 is a flavin-dependent monoamine oxidase that demethylates lysines, specifically lysine 4 on histone H3. LSD1 is known to form a multi-protein complex with REST (RE1-Silencing Transcription factor) and CoREST that are critical players in gene silencing [63,64].
4. Polycomb-repressive complexes 1/2–long non-coding RNA interactions and phase separation
Phase separation is defined as the process by which a homogeneous solution divides in two or more separated phases. Paraspeckles are a classic example of phase-separated cellular entities, nucleoli and stress granules [19,44–49], which are membrane-less assemblies composed of RNA and proteins. Formation of cytoplasmic stress granules is an evolutionary conserved mechanism. For example, stress granules are formed in response to environmental changes (i.e. heat shock) and favour the confinement of enzymes and nucleic acids in discrete regions of the nucleus or cytoplasm [77]. Structurally disordered and nucleic acid binding domains promote protein–protein and protein–RNA interactions in large ‘higher-order’ assemblies [78,79]. Intrinsically disordered proteins, which are enriched in polar and non-polar amino acids such as arginine and phenylalanine, have been shown to promote phase transitions in the cell [45].
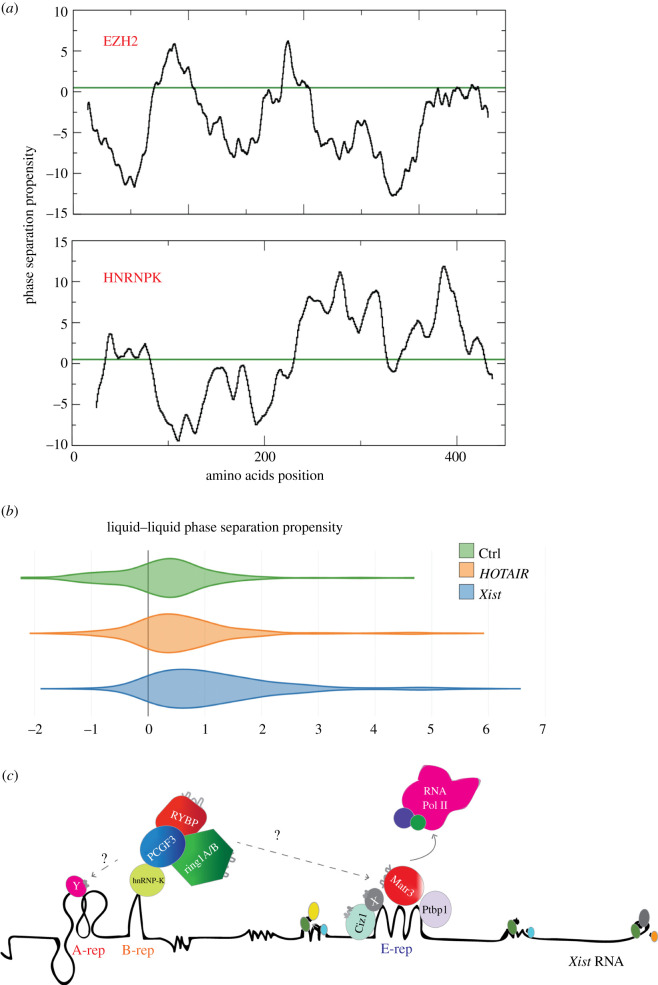
In a recent publication [67], we reasoned that Xist exerts its functions—at least in part—through the formation of silencing granules by phase separation, in which PRC1 and PRC2 are also recruited. More precisely, we suggested that non-canonical recruitment of repressive PRC1 complexes is promoted or reinforced by the formation of higher order assemblies. In this scenario, the primary de novo recruitment of PRC1/2 would happen through the Xist B repeats [54] direct interaction and involve proteins with a strong propensity to phase separate. As predicted by the catGRANULE algorithm [45] that estimates the ability of proteins to form liquid-like assemblies containing protein and RNA molecules [67], both EZH2 and HNRNPK are prone to phase-separate (figure 3a and table 1). Yet, HNRNPK shows a much higher granulation score than EZH2 (1.60 versus 0.71; note that the score is z-normalized and 0 correspond to the average protein propensity), which suggests enhanced ability to form large ribonucleoprotein complexes. In agreement with this observation, experimental [57,81] and computational studies [67] have indicated that Xist could phase separate with its associated proteins, but no evidence has been proposed so far on HOTAIR ability to form such assemblies. This finding is in line with the fact that PRC2 components might directly binding to HOTAIR, while most of Xist-Polycomb associations [51,82] are largely indirect [54] (figures 1 and 2). Indeed, analysing the whole protein interactomes of both Xist [36,39] and HOTAIR [83], we found that Xist binding partners are highly prone to phase separation, while HOTAIR interactions show lower propensity to phase separate, which is in accordance with the observation that indirect protein–protein interactions may mediate associations through structurally disordered domains (figure 3b) [67]. We note that HOTAIR binding partners have a non-negligible propensity to phase separate with respect to a similar length negative control (antisense of 3′ UTR of Alpha Synuclein; around 2500 nucleotides; figure 3b) [80], which suggests that HOTAIR might form medium-size assemblies [84].
Figure 3.
Xist and HOTAIR phase separation propensity and phase separation by Xist RNA through recruitment of phase-separating proteins. (a) Phase separation propensities profiles reveal that structurally disordered regions in EZH2 (https://pfam.xfam.org/protein/Q15910) and HNRNPK (https://pfam.xfam.org/protein/P61979) promote the formation of high-order assemblies. HNRNPK shows higher phase separation propensity than EZH2. (b) Comparison between Xist and HOTAIR interactomes indicates that Xist interactions are enriched in elements prone to phase separation (***p-value < 0.001; Kolmogorov–Smirnov test, table 1). Comparison with control RNA (antisense of the 3′ UTR of Alpha Synuclein) [80] indicates that HOTAIR has non-negligible propensity to associate with phase-separating proteins (***p-value < 0.001; Kolmogorov–Smirnov test). (c) The most-likely Xist-mediated PRC2 recruitment pathway involves PRC1 recruitment via repeat B interaction through HNRNPK direct interaction (light green). H2A ubiquitination by PRC1 may induce PRC2 recruitment on the Xi as previously shown (see main text). We suggest that Xist might also recruit PRC1/2 complexes by phase separation through mediation of structurally disordered proteins the Xist binding repeat E. Phase-separated PRC1/2 recruitment could occur through a direct interaction with repeat Xist E. We suggest that the PRC1/2 oligomerization can further recruit repressive proteins and/or disordered proteins, contributing to the eviction of Pol II and basic transcription factors, recruiting more structurally disordered proteins and in turn, inducing further granule formation, heterochromatinization and gene repression. Xist repeats are shown; A repeat (pink), B repeat (orange); E repeat (blue). Proteins are shown by name. Waved grey profiles on proteins, indicate intrinsically disordered regions; Xist RNA (black line).
Table 1.
Liquid–liquid Phase Separation (LLPS) propensity of PRC1 and PRC2 components. The score is Z-normalized and values >0 indicate that the protein is prone to phase-separate.
| gene | LLPS |
|---|---|
| HNRNPK | 1.601 |
| RING1 | 1.499 |
| JARD2 | 1.339 |
| SUZ12 | 1.226 |
| CBX2 | 1.175 |
| CBX4 | 1.169 |
| CBX8 | 1.058 |
| EZH2 | 0.711 |
| CBX6 | 0.592 |
| PHC1 | 0.556 |
| EED | 0.509 |
| RBBP4 | 0.466 |
| PHC2 | 0.401 |
| RING2 | 0.107 |
| BMI1 | −0.059 |
| PCGF2 | −0.096 |
| PHC3 | −0.438 |
| CBX7 | −0.439 |
In the Xist case, PRC1 positive feedback recruitment may be reinforced by liquid-like interactions in which specific elements such as CBX2 [85] (liquid–liquid phase separation propensities of 1.17 [45]) as well as SAM-domain multimerization [86] or intrinsically disordered domains could be involved. Based on their phase separation scores, we speculate that other proteins such as HNRNPU (phase separation propensity of 2.5) and MATR3 (liquid–liquid phase separation propensity of 1.5) might contribute towards the recruitment of polycomb proteins to the Xist body (table 1). These interactions might also be mediated by intrinsically disordered proteins yet to be discovered binding the Xist A-, D-3′end repeats. This protein multimerization driven by phase separation and the RNA–protein interactions might be playing a critical role in this process [67] and, in turn, trigger RNA Polymerase II (Pol-II) and basic transcription factors eviction, inducing gene silencing and heterochromatinization (figure 3c).
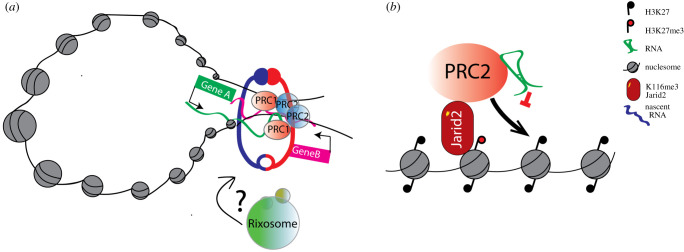
5. Non-catalytic functions of polycomb-repressive complex in shaping the three-dimensional genome might be mediated by RNA interactions
Work from different laboratories has shown that PRC1/2 complexes are essential regulators of cellular three-dimensional structure (recently reviewed by Illingworth RS [85] and Cheutin and Cavalli [87]). Very recent work from the Cavalli lab has elegantly shown how PRC1 can exert different and apparently opposing functions such as gene repression, three-dimensional organization of the genome and gene activation [88]. In brief, Loubiere and colleagues showed, using PRC1 mutants at the duchsund locus in Drosophila, that genes are positively and negatively regulated by PRC1. In particular, they suggest that while in the absence of activating transcription factors (TFs), PRC1 is mostly involved in gene silencing, in the presence of TF, PRC1 might be able to regulate gene expression by making PRC1-dependent promoter enhancer contacts [88]. As PRC1 has also been shown to have a role in regulating occupancy, elongation and phosphorylation of RNA polymerase II (Pol-II) [89,90], it is tempting to speculate that these functions of PRC1 might be, in part, mediated by its ability to bind to RNA via RING1A/B or CBX7 [91] proteins (figure 4a). In support of this idea/interpretation, a paper from the Moazed laboratory [92] has shown that the Rixosome, a conserved RNA degradation machinery, interacts with PRC1/2, and it is recruited at Polycomb sites for efficient gene silencing. Similarly, Garland et al. [93] showed a link between the RNA degradation pathways and Polycomb silencing. In particular, they showed that KO of Zcfh31, a component of the poly(A) RNA exosome targeting (PAXT) complex, increases the cellular level of poly-adenylated RNA, triggering the destabilization of the PRC2 complex, impaired chromatin binding and reduction of gene silencing [93]. Furthermore, work from several laboratories has shown that Polycomb can interact with RNAs [94,95], nascent transcripts [96] or with R-loops at Polycomb-repressed targets [94,97]. These lines of evidence support the idea that the interaction of Polycomb proteins with RNA might be spurious, yet it is critical for numerous cellular functions, from nuclear three-dimensional organization [85,87,98–100], repression of target genes [94,101,102], spreading on PRC1/2 [103], cellular differentiation and lineage commitment.
Figure 4.
RNA sustains Polycomb complexes functions. RNA can facilitate PRC1/2 complex and sustain three-dimensional contacts and loops (also mediated by the cohesin complex; red/blue ring) to coordinate gene expression by brining co-regulated genes together (gene A, green; Gene B, purple; green/blue ribbons represent nascent RNA from gene A/B). Rixosome could also be participating to these interactions. (B) RNA inhibits PRC2 catalytic activity. RNA (green) can inhibit PRC2 catalytic activity. Its activity can be relieved by H3K27me3 tails (red lollipop) or methylated Jarid2 proteins.
6. Conclusion
Elegant biochemistry work from several laboratories showed that PRC1 [19] and specific PRC2 subcomplexes [20,104] (i.e. PRC2.1, PRC2.2 depending on the accessory subunits present in the complex, reviewed in Van Mierlo and colleagues [9]) bind to RNA with different affinities and specificities. Recent work suggests that the interaction of PRC1/2 components to RNA is promiscuous [18,105], and in part mediated by protein–protein interactions [65]. It has also been shown that EZH2–RNA interactions can catalytically inactivate or expel EZH2 [101,104,106–108], suggesting that RNA binding is essential for the modulation of polycomb catalytic activities [50] (figure 4b). However, allosteric RNA inhibition can be relieved both by H3K27me3 and methylated JARID protein interactions (the latter also in agreement with Cifuentes-Rojas and colleagues [106,109]). These lines of evidence suggest a new model of PRC2 recruitment that can explain both de novo polycomb recruitment (RNA binding) and spreading (using established polycomb domains) [20].
Taking into account previous experimental and computational work, we suggest that the ‘canonical’, direct lncRNA-mediated PRC2 recruitment has to be revisited [105]. As for Xist, the de novo recruitment of PRC1 and PRC2 is highly unlikely to occur through a mechanism of recruitment to the chromatin associated with catalytically inactivated complexes (i.e. allosteric inhibition). Although the recruitment of Xist to pre-existing CpG islands might partially alleviate its catalytic inhibition (104). Alternatively, these interactions occur indirectly (no complex inhibition), through intermediate proteins or by means of liquid–liquid phase separation (figure 3a–c). For example, Xist A-repeats, the putative Xist-PRC2 interaction region, are missing the key RNA recognition sequences needed for specific interactions [65], which suggests that these interactions, although critical, might also be spurious [18,65,105] (binding many RNAs with low affinity) or indirect. As for the HOTAIR-mediated de novo Polycomb recruitment (possibly mediated by direct interactions), it is possible that residual H3K27me3 at the HOX locus might alleviate allosteric inhibition [110]. For PRC1/2 recruitment on the inactive X chromosome (Xi) at the onset of XCI, it is likely that de novo accumulation largely depends on PRC1-mediated mark on the chromatin, such as H2A-119ub (figure 1a) [23,54,58,104]. In this regard, work from the Pasini and Klose laboratory elegantly proved that H2A119 ubiquitination is essential for PRC1/2 silencing and PRC2 de novo recruitment [22,23,59]. We believe that more work has to be done in order to have a final model of lncRNA and Polycomb recruitment, capable of reconciling all this evidence.
7. Material and methods
7.1. RNA–protein interaction predictions and granule propensity
To compute protein-RNA interactions, we used the catRAPID approach that evaluates the interaction propensities of polypeptide and nucleotide chains based on their physico-chemical properties predicted from primary structure [35,66]. Structural disorder, nucleic acid-binding propensity and amino acid patterns such as arginine-glycine and phenylalanine-glycine are key features of proteins coalescing in granules [45]. These features were combined in a computational approach, catGRANULE, that we employed to identify RBPs assembling into granules (scores >0 indicate granule propensity). We predicted the secondary structure of transcripts using CROSS [73,74]. The algorithm predicts the structural profile (single- and double-stranded state) at single-nucleotide resolution using sequence information only and without sequence length restrictions (scores > 0 indicate double stranded regions).
HOTAIR repeats annotation: D1 (nucleotides 1–530) consists of 12 helices, 8 terminal loops and 4 junctions (three 3-way junctions and one 4-way junction). D2 (nucleotides 531–1040) consists of 15 helices, 11 terminal loops and 4 junctions (three 5-way junctions and one 3-way junction). D3 (nucleotides 1041–1513) is the smallest of all the four domains and consists of 9 helices, 6 terminal loops and 3 junctions (two 4-way junctions and one 3-way junction). Finally, D4 (nucleotides 1514–2148) is the largest among the four domains and consists of 20 helices, 13 terminal loops and 7 junctions (one 6-way, two 4-way and four 3-way junctions).
Supplementary Material
Acknowledgements
We thank all members of the Tartaglia and Cerase's laboratories. We also thank Roberto Bonasio and Chen Davidovich, Giacomo Cavalli and John Rinn for helpful discussions. We thank Luciano Di Croce and Chen Davidovich and Neil Blackledge for critical reading.
Data accessibility
Relevant data are available at 1) http://crg-webservice.s3.amazonaws.com/submissions/2020-03/251545/output/index.html?unlock=073199e0ac (Ezh2-Hotair); http://crg-webservice.s3.amazonaws.com/submissions/2020-03/251800/output/index.html?unlock=6cbd243faa (HnrnpK-Xist); http://crg-webservice.s3.amazonaws.com/submissions/2020-03/251574/output/index.html?unlock=c891ca7c43 (Xist); http://crg-webservice.s3.amazonaws.com/submissions/2020-03/251583/output/index.html?unlock=0c097f6326 (Hotair).
Funding
A.C. had been funded by a Rett Syndrome Research Trust (RSRT) and a BARTS Charity grants and by QMUL intramural support. The research leading to these results has been supported by European Research Council (RIBOMYLOME 309545 and ASTRA 855923), the Spanish Ministry of Economy and Competitiveness (BFU2017-86970-P) and H2020 projects (IASIS 727658 and INFORE 825080).
References
- 1.Beuchle D, Struhl G, Muller J. 2001. Polycomb group proteins and heritable silencing of Drosophila Hox genes. Development 128, 993–1004. [DOI] [PubMed] [Google Scholar]
- 2.Geisler SJ, Paro R. 2015. Trithorax and Polycomb group-dependent regulation: a tale of opposing activities. Development 142, 2876–2887. ( 10.1242/dev.120030) [DOI] [PubMed] [Google Scholar]
- 3.Dorafshan E, Kahn TG, Schwartz YB. 2017. Hierarchical recruitment of polycomb complexes revisited. Nucleus 8, 496–505. ( 10.1080/19491034.2017.1363136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer M, Trupke J, Ringrose L. 2016. The quest for mammalian polycomb response elements: are we there yet? Chromosoma 125, 471–496. ( 10.1007/s00412-015-0539-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aloia L, Di Stefano B, Di Croce L. 2013. Polycomb complexes in stem cells and embryonic development. Development 140, 2525–2534. ( 10.1242/dev.091553) [DOI] [PubMed] [Google Scholar]
- 6.Illingworth RS, Holzenspies JJ, Roske FV, Bickmore WA, Brickman JM. 2016. Polycomb enables primitive endoderm lineage priming in embryonic stem cells. eLife 5, e14926 ( 10.7554/eLife.14926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan HL, et al. 2018. Polycomb complexes associate with enhancers and promote oncogenic transcriptional programs in cancer through multiple mechanisms. Nat. Commun. 9, 3377 ( 10.1038/s41467-018-05728-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito T, Teo YV, Evans SA, Neretti N, Sedivy JM. 2018. Regulation of cellular senescence by polycomb chromatin modifiers through distinct DNA damage- and histone methylation-dependent pathways. Cell reports 22, 3480–3492. ( 10.1016/j.celrep.2018.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Mierlo G, Veenstra GJC, Vermeulen M, Marks H. 2019. The complexity of PRC2 subcomplexes. Trends Cell Biol. 29, 660–671. ( 10.1016/j.tcb.2019.05.004) [DOI] [PubMed] [Google Scholar]
- 10.Aranda S, Mas G, Di Croce L. 2015. Regulation of gene transcription by polycomb proteins. Sci. Adv. 1, e1500737 ( 10.1126/sciadv.1500737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidal M, Starowicz K. 2017. Polycomb complexes PRC1 and their function in hematopoiesis. Exp. Hematol. 48, 12–31. ( 10.1016/j.exphem.2016.12.006) [DOI] [PubMed] [Google Scholar]
- 12.Cao Q, et al. 2016. BCOR regulates myeloid cell proliferation and differentiation. Leukemia 30, 1155–1165. ( 10.1038/leu.2016.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plath K, et al. 2003. Role of histone H3 lysine 27 methylation in X inactivation. Science 300, 131–135. ( 10.1126/science.1084274) [DOI] [PubMed] [Google Scholar]
- 14.de Napoles M, et al. 2004. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev. Cell 7, 663–676. ( 10.1016/j.devcel.2004.10.005) [DOI] [PubMed] [Google Scholar]
- 15.Margueron R, et al. 2009. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature 461, 762–767. ( 10.1038/nature08398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper S, et al. 2014. Targeting polycomb to pericentric heterochromatin in embryonic stem cells reveals a role for H2AK119u1 in PRC2 recruitment. Cell Reports 7, 1456–1470. ( 10.1016/j.celrep.2014.04.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasini D, Hansen KH, Christensen J, Agger K, Cloos PAC, Helin K. 2008. Coordinated regulation of transcriptional repression by the RBP2 H3K4 demethylase and polycomb-repressive complex 2. Genes Dev. 22, 1345–1355. ( 10.1101/gad.470008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Goodrich KJ, Gooding AR, Naeem H, Archer S, Paucek RD, Youmans DT, Cech TR, Davidovich C. 2017. Targeting of polycomb repressive complex 2 to RNA by short repeats of consecutive guanines. Mol. Cell 65, 1056–1067. ( 10.1016/j.molcel.2017.02.003) [DOI] [PubMed] [Google Scholar]
- 19.Bonasio R, Lecona E, Narendra V, Voigt P, Parisi F, Kluger Y, Reinberg D. 2014. Interactions with RNA direct the Polycomb group protein SCML2 to chromatin where it represses target genes. eLife 3, e02637 ( 10.7554/eLife.02637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, et al. 2019. RNA exploits an exposed regulatory site to inhibit the enzymatic activity of PRC2. Nat. Struct. Mol. Biol. 26, 237–247. ( 10.1038/s41594-019-0197-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon JA, Kingston RE. 2013. Occupying chromatin: polycomb mechanisms for getting to genomic targets, stopping transcriptional traffic, and staying put. Mol. Cell 49, 808–824. ( 10.1016/j.molcel.2013.02.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackledge NP, Fursova NA, Kelley JR, Huseyin MK, Feldmann A, Klose RJ. 2020. PRC1 catalytic activity is central to polycomb system function. Mol. Cell 77, 857–874. ( 10.1016/j.molcel.2019.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamburri S, Lavarone E, Fernández-Pérez D, Conway E, Zanotti M, Manganaro D, Pasini D. 2019. Histone H2AK119 Mono-ubiquitination is essential for polycomb-mediated transcriptional repression. Mol. Cell 77, 840–856. ( 10.1016/j.molcel.2019.11.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laugesen A, Hojfeldt JW, Helin K. 2016. Role of the polycomb repressive complex 2 (PRC2) in transcriptional regulation and cancer. Cold Spring Harb. Perspect. Med. 6, a026575 ( 10.1101/cshperspect.a026575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loubiere V, Martinez AM, Cavalli G. 2019. Cell fate and developmental regulation dynamics by polycomb proteins and 3D genome architecture. Bioessays 41, e1800222 ( 10.1002/bies.201800222) [DOI] [PubMed] [Google Scholar]
- 26.Blanco E, Gonzalez-Ramirez M, Alcaine-Colet A, Aranda S, Di Croce L. 2020. The bivalent genome: characterization, structure, and regulation. Trends in genetics : TIG 36, 118–131. ( 10.1016/j.tig.2019.11.004) [DOI] [PubMed] [Google Scholar]
- 27.Ransohoff JD, Wei Y, Khavari PA. 2018. The functions and unique features of long intergenic non-coding RNA. Nature reviews. Mol. Cell Biol. 19, 143–157. ( 10.1038/nrm.2017.104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engreitz JM, Ollikainen N, Guttman M. 2016. Long non-coding RNAs: spatial amplifiers that control nuclear structure and gene expression. Nat. rev. Mol. Cell Biol. 17, 756–770. ( 10.1038/nrm.2016.126) [DOI] [PubMed] [Google Scholar]
- 29.Iyer MK, et al. 2015. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genetics 47, 199–208. ( 10.1038/ng.3192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerase A, Pintacuda G, Tattermusch A, Avner P. 2015. Xist localization and function: new insights from multiple levels. Genome Biol. 16, 166 ( 10.1186/s13059-015-0733-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pintacuda G, Cerase A. 2015. X inactivation lessons from differentiating mouse embryonic stem cells. Stem Cell Rev. 11, 699–705. ( 10.1007/s12015-015-9597-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long Y, Wang X, Youmans DT, Cech TR. 2017. How do lncRNAs regulate transcription? Sci. Adv. 3, eaao2110 ( 10.1126/sciadv.aao2110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moindrot B, Cerase A, Coker H, Masui O, Grijzenhout A, Pintacuda G, Schermelleh L, Nesterova TB, Brockdorff N. 2015. A pooled shRNA screen identifies Rbm15, Spen, and Wtap as factors required for Xist RNA-mediated silencing. Cell Rep. 12, 562–572. ( 10.1016/j.celrep.2015.06.053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monfort A, Di Minin G, Postlmayr A, Freimann R, Arieti F, Thore S, Wutz A. et al. 2015. Identification of spen as a crucial factor for Xist function through forward genetic screening in haploid embryonic stem cells. Cell Rep. 12, 554–561. ( 10.1016/j.celrep.2015.06.067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cirillo D, Blanco M, Armaos A, Buness A, Avner P, Guttman M, Cerase A, Tartaglia GG. 2016. Quantitative predictions of protein interactions with long noncoding RNAs. Nat. Methods 14, 5–6. ( 10.1038/nmeth.4100) [DOI] [PubMed] [Google Scholar]
- 36.Minajigi A, et al. 2015. Chromosomes. A comprehensive Xist interactome reveals cohesin repulsion and an RNA-directed chromosome conformation. Science 349, aab2276 ( 10.1126/science.aab2276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McHugh CA, et al. 2015. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 521, 232–236. ( 10.1038/nature14443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CK, et al. 2016. Xist recruits the X chromosome to the nuclear lamina to enable chromosome-wide silencing. Science 354, 468–472. ( 10.1126/science.aae0047) [DOI] [PubMed] [Google Scholar]
- 39.Chu C, Zhang QC, Da Rocha ST, Flynn RA, Bharadwaj M, Calabrese JM, Magnuson T, Heard E, Chang HY. 2015. Systematic discovery of Xist RNA binding proteins. Cell 161, 404–416. ( 10.1016/j.cell.2015.03.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinter SF. 2016. A Tale of Two Cities: How Xist and its partners localize to and silence the bicompartmental X. Seminars in Cell Dev. Biol. 56, 19–34. ( 10.1016/j.semcdb.2016.03.023) [DOI] [PubMed] [Google Scholar]
- 41.Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. 2011. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147, 358–369. ( 10.1016/j.cell.2011.09.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirose T, et al. 2014. NEAT1 long noncoding RNA regulates transcription via protein sequestration within subnuclear bodies. Mol. Biol. cell 25, 169–183. ( 10.1091/mbc.E13-09-0558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu XS, et al. 2017. LncRNA-PAGBC acts as a microRNA sponge and promotes gallbladder tumorigenesis. EMBO Rep. 18, 1837–1853. ( 10.15252/embr.201744147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pintacuda G, Young AN, Cerase A. 2017. Function by Structure: Spotlights on Xist Long Non-coding RNA. Front. Mol. Biosci. 4, 90 ( 10.3389/fmolb.2017.00090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bolognesi B, Lorenzo Gotor N, Dhar R, Cirillo D, Baldrighi M, Tartaglia GG, Lehner B. 2016. A concentration-dependent liquid phase separation can cause toxicity upon increased protein expression. Cell Rep. 16, 222–231. ( 10.1016/j.celrep.2016.05.076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mira-Bontenbal H, Gribnau J. 2016. New Xist-interacting proteins in X-chromosome inactivation. Curr. Biol. 26, R338–R342. ( 10.1016/j.cub.2016.03.022) [DOI] [PubMed] [Google Scholar]
- 47.Robert Finestra T, & Gribnau J. 2017. X chromosome inactivation: silencing, topology and reactivation. Curr. Opin. Cell Biol. 46, 54–61. ( 10.1016/j.ceb.2017.01.007) [DOI] [PubMed] [Google Scholar]
- 48.van Bemmel JG, Mira-Bontenbal H, Gribnau J. 2016. Cis- and trans-regulation in X inactivation. Chromosoma 125, 41–50. ( 10.1007/s00412-015-0525-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brockdorff N, Bowness JS, Wei G. 2020. Progress toward understanding chromosome silencing by Xist RNA. Genes Dev. 34, 733–744. ( 10.1101/gad.337196.120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Almeida M, Bowness JS, Brockdorff N. 2020. The many faces of Polycomb regulation by RNA. Curr. Opin. Genetics Dev. 61, 53–61. ( 10.1016/j.gde.2020.02.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. 2008. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322, 750–756. ( 10.1126/science.1163045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.da Rocha ST, et al. 2014. Jarid2 Is Implicated in the Initial Xist-induced targeting of PRC2 to the inactive X chromosome. Mol. cell 53, 301–316. ( 10.1016/j.molcel.2014.01.002) [DOI] [PubMed] [Google Scholar]
- 53.Somarowthu S, Legiewicz M, Chillón I, Marcia M, Liu F, Pyle AM. 2015. HOTAIR forms an intricate and modular secondary structure. Mol. cell 58, 353–361. ( 10.1016/j.molcel.2015.03.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pintacuda G, et al. 2017. hnRNPK Recruits PCGF3/5-PRC1 to the Xist RNA B-repeat to establish polycomb-mediated chromosomal silencing. Mol. cell 68, 955–969. ( 10.1016/j.molcel.2017.11.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mak W, Nesterova TB, de Napoles M, Appanah R, Yamanaka S, Otte AP. 2004. Reactivation of the paternal X chromosome in early mouse embryos. Science 303, 666–669. ( 10.1126/science.1092674) [DOI] [PubMed] [Google Scholar]
- 56.Okamoto I, Otte AP, Allis CD, Reinberg D, Heard E. 2004. Epigenetic dynamics of imprinted X inactivation during early mouse development. Science 303, 644–649. ( 10.1126/science.1092727) [DOI] [PubMed] [Google Scholar]
- 57.Cerase A, et al. 2014. Spatial separation of Xist RNA and polycomb proteins revealed by superresolution microscopy. Proc. Natl Acad. Sci. USA 111, 2235–2240. ( 10.1073/pnas.1312951111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Almeida M, et al. 2017. PCGF3/5-PRC1 initiates polycomb recruitment in X chromosome inactivation. Science 356, 1081–1084. ( 10.1126/science.aal2512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blackledge NP, et al. 2014. Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell 157, 1445–1459. ( 10.1016/j.cell.2014.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Comet I, Helin K. 2014. Revolution in the polycomb hierarchy. Nat. Struct. Mol. Biol. 21, 573–575. ( 10.1038/nsmb.2848) [DOI] [PubMed] [Google Scholar]
- 61.Rinn JL, et al. 2007. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129, 1311–1323. ( 10.1016/j.cell.2007.05.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta RA, et al. 2010. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464, 1071–1076. ( 10.1038/nature08975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsai MC, et al. 2010. Long noncoding RNA as modular scaffold of histone modification complexes. Science 329, 689–693. ( 10.1126/science.1192002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu L, Murat P, Matak-Vinkovic D, Murrell A, Balasubramanian S. 2013. Binding interactions between long noncoding RNA HOTAIR and PRC2 proteins. Biochemistry 52, 9519–9527. ( 10.1021/bi401085h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Long Y, et al. 2017. Conserved RNA-binding specificity of polycomb repressive complex 2 is achieved by dispersed amino acid patches in EZH2. eLife 6, e31558 ( 10.7554/eLife.31558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bellucci M, Agostini F, Masin M, Tartaglia GG. 2011. Predicting protein associations with long noncoding RNAs. Nat. Methods 8, 444–445. ( 10.1038/nmeth.1611) [DOI] [PubMed] [Google Scholar]
- 67.Cerase A, Armaos A, Neumayer C, Avner P, Guttman M, Tartaglia GG. 2019. Phase separation drives X-chromosome inactivation: a hypothesis. Nat. Struct. Mol. Biol. 26, 331–334. ( 10.1038/s41594-019-0223-0) [DOI] [PubMed] [Google Scholar]
- 68.Agostini F, Zanzoni A, Klus P, Marchese D, Cirillo D, Tartaglia GG. 2013. catRAPID omics: a web server for large-scale prediction of protein-RNA interactions. Bioinformatics 29, 2928–2930. ( 10.1093/bioinformatics/btt495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lang B, Armaos A, Tartaglia GG. 2019. RNAct: Protein-RNA interaction predictions for model organisms with supporting experimental data. Nucleic acids Res. 47, D601–D606. ( 10.1093/nar/gky967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Agostini F, Cirillo D, Bolognesi B, Tartaglia GG. 2013. X-inactivation: quantitative predictions of protein interactions in the Xist network. Nucleic acids Res. 41, e31 ( 10.1093/nar/gks968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cid-Samper F, et al. 2018. An integrative study of protein-RNA condensates identifies scaffolding RNAs and reveals players in Fragile X-associated tremor/ataxia syndrome. Cell Rep. 25, 3422–3434. ( 10.1016/j.celrep.2018.11.076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanchez de Groot N, Armaos A, Graña-Montes R, Alriquet M, Calloni G, Vabulas RM, Tartaglia GG. 2019. RNA structure drives interaction with proteins. Nat. Commun. 10, 3246 ( 10.1038/s41467-019-10923-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ponti RD, Armaos A, Vandelli A, Tartaglia GG. 2020. CROSSalive: a web server for predicting the in vivo structure of RNA molecules. Bioinformatics 36, 940–941. ( 10.1093/bioinformatics/btz666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Delli Ponti R, Armaos A, Marti S, & Tartaglia GG. 2018. A method for RNA structure prediction shows evidence for structure in lncRNAs. Front. Mol. Biosci. 5, 111 ( 10.3389/fmolb.2018.00111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fang R, Moss WN, Rutenberg-Schoenberg M, Simon MD. 2015. Probing Xist RNA Structure in Cells Using Targeted Structure-Seq. PLoS genetics 11, e1005668 ( 10.1371/journal.pgen.1005668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhan A, Mandal SS. 2015. LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer. Biochim. Biophys. Acta 1856, 151–164. ( 10.1016/j.bbcan.2015.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hyman AA, Weber CA, Julicher F. 2014. Liquid-liquid phase separation in biology. Annu. rev. Cell Dev. Biol. 30, 39–58. ( 10.1146/annurev-cellbio-100913-013325) [DOI] [PubMed] [Google Scholar]
- 78.Wu H, Fuxreiter M. 2016. The structure and dynamics of higher-order assemblies: amyloids, signalosomes, and granules. Cell 165, 1055–1066. ( 10.1016/j.cell.2016.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bergeron-Sandoval LP, Safaee N, Michnick SW. 2016. Mechanisms and consequences of macromolecular phase separation. Cell 165, 1067–1079. ( 10.1016/j.cell.2016.05.026) [DOI] [PubMed] [Google Scholar]
- 80.Marchese D, et al. 2017. Discovering the 3' UTR-mediated regulation of alpha-synuclein. Nucleic Acids Res. 45, 12 888–12 903. ( 10.1093/nar/gkx1048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pandya-Jones A, et al. 2020. An Xist-dependent protein assembly mediates Xist localization and gene silencing. bioRxiv, 2020.2003.2009.979369 ( 10.1101/2020.03.09.979369) [DOI]
- 82.Maenner S, et al. 2010. 2-D structure of the A region of Xist RNA and its implication for PRC2 association. PLoS Biol. 8, e1000276 ( 10.1371/journal.pbio.1000276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zheng P, Xiong Q, Wu Y, Chen Y, Chen Z, Fleming J, Gao D, Bi L, Ge F. 2015. Quantitative proteomics analysis reveals novel insights into mechanisms of action of long noncoding RNA Hox transcript antisense intergenic RNA (HOTAIR) in HeLa Cells. Mol. Cell Proteomics 14, 1447–1463. ( 10.1074/mcp.M114.043984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoon JH, et al. 2013. Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nat. Commun. 4, 2939 ( 10.1038/ncomms3939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Illingworth RS. 2019. Chromatin folding and nuclear architecture: PRC1 function in 3D. Curr. Opin. Genetics Dev. 55, 82–90. ( 10.1016/j.gde.2019.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Isono K, et al. 2013. SAM domain polymerization links subnuclear clustering of PRC1 to gene silencing. Dev. Cell 26, 565–577. ( 10.1016/j.devcel.2013.08.016) [DOI] [PubMed] [Google Scholar]
- 87.Cheutin T, Cavalli G. 2019. The multiscale effects of polycomb mechanisms on 3D chromatin folding. Crit. Rev. Biochem. Mol. Biol. 54, 399–417. ( 10.1080/10409238.2019.1679082) [DOI] [PubMed] [Google Scholar]
- 88.Loubiere V, Papadopoulos GL, Szabo Q, Martinez A-M, Cavalli G. 2020. Widespread activation of developmental gene expression characterized by PRC1-dependent chromatin looping. Sci. Adv. 6, eaax4001 ( 10.1126/sciadv.aax4001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pherson M, Misulovin Z, Gause M, Mihindukulasuriya K, Swain A, Dorsett D. 2017. Polycomb repressive complex 1 modifies transcription of active genes. Sci. Adv. 3, e1700944 ( 10.1126/sciadv.1700944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stock JK, Giadrossi S, Casanova M, Brookes E, Vidal M, Koseki H, Brockdorff N, Fisher AG, Pombo A. 2007. Ring1-mediated ubiquitination of H2A restrains poised RNA polymerase II at bivalent genes in mouse ES cells. Nat. Cell Biol. 9, 1428–1435. ( 10.1038/ncb1663) [DOI] [PubMed] [Google Scholar]
- 91.Yap KL, Li S, Muñoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, Gil J, Walsh MJ, Zhou MM. 2010. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol. Cell 38, 662–674. ( 10.1016/j.molcel.2010.03.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou H, et al. 2019. An RNA degradation complex required for silencing of Polycomb target genes. bioRxiv, 2019.2012.2023.887547 ( 10.1101/2019.12.23.887547) [DOI]
- 93.Garland W, et al. 2019. A functional link between nuclear RNA decay and transcriptional control mediated by the polycomb repressive complex 2. Cell rep. 29, 1800–1811. ( 10.1016/j.celrep.2019.10.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kanhere A, et al. 2010. Short RNAs are transcribed from repressed polycomb target genes and interact with polycomb repressive complex-2. Mol. Cell 38, 675–688. ( 10.1016/j.molcel.2010.03.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Long Y, Hwang T, Gooding AR, Goodrich KJ, Rinn JL, Cech TR. 2020. RNA is essential for PRC2 chromatin occupancy and function in human pluripotent stem cells. Nat. Genetics. ( 10.1038/s41588-020-0662-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wei C, et al. 2016. RBFox2 binds nascent RNA to globally regulate polycomb complex 2 targeting in mammalian genomes. Mol. Cell 62, 982 ( 10.1016/j.molcel.2016.06.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Skourti-Stathaki K, Torlai Triglia E, Warburton M, Voigt P, Bird A, Pombo A. 2019. R-loops enhance polycomb repression at a subset of developmental regulator genes. Mol. Cell 73, 930–945. ( 10.1016/j.molcel.2018.12.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pachano T, Crispatzu G, Rada-Iglesias A. 2019. Polycomb proteins as organizers of 3D genome architecture in embryonic stem cells. Brief Funct. Genomics 18, 358–366. ( 10.1093/bfgp/elz022) [DOI] [PubMed] [Google Scholar]
- 99.Bonev B, et al. 2017. Multiscale 3D genome rewiring during mouse neural development. Cell 171, 557–572. ( 10.1016/j.cell.2017.09.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hacisuleyman E, et al. 2014. Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat.struct. mol. biol. 21, 198–206. ( 10.1038/nsmb.2764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beltran M, et al. 2019. G-tract RNA removes Polycomb repressive complex 2 from genes. Nat. Struct. Mol. Biol. 26, 899–909. ( 10.1038/s41594-019-0293-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Surface LE, Thornton SR, Boyer LA. 2010. Polycomb group proteins set the stage for early lineage commitment. Cell Stem Cell 7, 288–298. ( 10.1016/j.stem.2010.08.004) [DOI] [PubMed] [Google Scholar]
- 103.Schertzer MD, et al. 2019. lncRNA-induced spread of polycomb controlled by genome architecture, RNA abundance, and CpG Island DNA. Mol. cell 75, 523–537. ( 10.1016/j.molcel.2019.05.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang X, Paucek RD, Gooding AR, Brown ZZ, Ge EJ, Muir TW, Cech TR. 2017. Molecular analysis of PRC2 recruitment to DNA in chromatin and its inhibition by RNA. Nat. Struct. Mol. Biol. 24, 1028–1038. ( 10.1038/nsmb.3487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davidovich C, Zheng L, Goodrich KJ, Cech TR. 2013. Promiscuous RNA binding by Polycomb repressive complex 2. Nat. Struct. Mol. Biol. 20, 1250–1257. ( 10.1038/nsmb.2679) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cifuentes-Rojas C, Hernandez AJ, Sarma K, Lee JT. 2014. Regulatory interactions between RNA and polycomb repressive complex 2. Mol. Cell 55, 171–185. ( 10.1016/j.molcel.2014.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.West JA, et al. 2016. Structural, super-resolution microscopy analysis of paraspeckle nuclear body organization. J. Cell Biol. 214, 817–830. ( 10.1083/jcb.201601071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beltran M, et al. 2016. The interaction of PRC2 with RNA or chromatin is mutually antagonistic. Genome Res. 26, 896–907. ( 10.1101/gr.197632.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Botta-Ofilia T, et al. 2018. Phase separation driven by RNA scaffolds and protein sequestration in FXTAS. BioRxiv , April 13 th , 2018. ( 10.1101/298943) [DOI]
- 110.Coleman RT, Struhl G. 2017. Causal role for inheritance of H3K27me3 in maintaining the OFF state of a Drosophila HOX gene. Science 356 ( 10.1126/science.aai8236) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Relevant data are available at 1) http://crg-webservice.s3.amazonaws.com/submissions/2020-03/251545/output/index.html?unlock=073199e0ac (Ezh2-Hotair); http://crg-webservice.s3.amazonaws.com/submissions/2020-03/251800/output/index.html?unlock=6cbd243faa (HnrnpK-Xist); http://crg-webservice.s3.amazonaws.com/submissions/2020-03/251574/output/index.html?unlock=c891ca7c43 (Xist); http://crg-webservice.s3.amazonaws.com/submissions/2020-03/251583/output/index.html?unlock=0c097f6326 (Hotair).