Abstract
Trichomoniasis is the third most common sexually transmitted infection in humans and is caused by the protozoan parasite, Trichomonas vaginalis (Tv). Pathogenic outcomes are more common in women and generally include mild vaginitis or cervicitis. However, more serious effects associated with trichomoniasis include adverse reproductive outcomes. Like other infectious agents, pathogenesis from Tv infection is predicted to be the result of both parasite and host factors. At the site of infection, neutrophils are the most abundant immune cells present and probably play key roles in both parasite clearance and inflammatory pathology. Here, we discuss the evidence that neutrophils home to the site of Tv infection, kill the parasite, and that in some circumstances, parasites possibly evade neutrophil-directed killing. In vitro, the parasite is killed by neutrophils using a novel antimicrobial mechanism called trogocytosis, which probably involves both innate and adaptive immunity. While mechanisms of evasion are mostly conjecture at present, the persistence of Tv infections in patients argues strongly for their existence. Additionally, many strains of Tv harbour microbial symbionts Mycoplasma hominis or Trichomonasvirus, which are both predicted to impact neutrophil responses against the parasite. Novel research tools, especially animal models, will help to reveal the true outcomes of many factors involved in neutrophil-Tv interactions during trichomoniasis.
Keywords: neutrophil, Trichomonas vaginalis, trogocytosis, Mycoplasma hominis, inflammation, sexually transmitted infection
1. Introduction
Trichomonas vaginalis (Tv) is a human-specific extracellular, flagellated protozoan parasite responsible for the third most common sexually transmitted infection (STI) in the United States (US) and worldwide, called trichomoniasis [1–3]. Worldwide, trichomoniasis case numbers approach 400 million, making it the most common non-viral sexually transmitted infection [4]. Despite the high prevalence of Tv infection, trichomoniasis is classified as a neglected infectious disease in the US owing to its high prevalence and the relative lack of research regarding the infection [3]. It is commonly treated with 5-nitroimidazole drugs such as metronidazole or tinidazole. Unfortunately, antibiotic-resistant Tv strains are on the rise, making treatment of some infections difficult [1]. However, no other treatment options are currently approved to treat or prevent trichomoniasis [1].
Tv attacks the host by attaching to, and often subsequently killing cells in the urogenital tract, such as cervicovaginal and prostate epithelial cells [5,6]. The process of Tv attachment to host cells is called cytoadherence [6,7]. During cytoadherence, the parasite alters its usually pear-shaped morphology to adopt an amoeboid form, increasing host cell surface area coverage [5,8], which is postulated to aid in the parasite's retention within the host [5,8]. It is also posited that the parasite obtains nutrients through the destruction of host epithelial cells [5]. The degradation of cervicovaginal epithelial cells is thought to be the source of vaginitis and colpitis macularis (commonly referred to as ‘strawberry cervix') [9]. Other adverse effects may include pelvic inflammatory disease and infertility [10–12]. Infection during pregnancy is associated with pre-term delivery, causing low birth weight infants, putatively owing to early rupture of the uterine membrane [10,11,13,14]. While Tv infection is overwhelmingly asymptomatic in men, some patients may experience penile discharge, discomfort during urination or irritation in the urethra [15]. Trichomoniasis also contributes to the spread of human immunodeficiency virus (HIV), as incidences of HIV have been found to be higher in Tv+ populations [11,16,17]. Epithelial cell damage caused by Tv may also allow for increased malignancy of cervical neoplasms, as later grades of cervical cancers were found to be increased in human papillomavirus+ patients that are co-infected with Tv [18–20]. One major factor in the broad spectrum of disease severity associated with trichomoniasis is likely to be the strain of parasite, because clinical isolates vary broadly in their ability to kill cervicovaginal and prostate epithelial cells in vitro [6]. However, host factors such as the individualized microbiome [21,22], and immune response most likely also play a role [7]. In particular, many of the aforementioned symptoms are linked to inflammation [10].
Innate immune cells called neutrophils are considered to be the major player in Tv-associated inflammation, as they are the most inflammatory cells in the immune system and are abundantly recruited to the vagina during Tv infection [23]. Neutrophils are also the most abundant immune cell type in the blood [24] and are the first cells recruited to the site of most infections, as they extravasate from the blood in large numbers, responding to local inflammatory cues [24–26]. Once in the infected tissue, their effector functions serve to quickly and efficiently kill pathogens to reduce their dissemination [24,26]. A white-frothy discharge rich in neutrophils has long been a clinical hallmark of trichomoniasis [9]. In trichomoniasis patients, neutrophils are abundant in wet mount smears from vaginal discharges and penile urethral samples [23,27,28]. Furthermore, the quest to establish a mouse model to study trichomoniasis has been stymied by the large influx of neutrophils to the vagina following inoculation with Tv, arguing for both the recruitment of neutrophils to the infection and also their ability to kill the parasite [29]. However, while this evidence supports that neutrophils play important roles in clearing Tv, neutrophils are also well-known to cause myriad inflammatory pathologies [30] and could therefore also drive many symptoms and sequelae of trichomoniasis. Therefore, whether the cumulative impact of neutrophil activity during trichomoniasis is beneficial or detrimental to the host is unknown, and most likely depends on many variables that occur during natural infection. Undoubtedly, however, a better understanding of the actions of neutrophils during trichomoniasis is important for understanding the pathogenesis of and immunity to Tv.
2. Neutrophil homing to Tv infection
Neutrophils probably home to the site of Tv infection following cues from both the parasite itself and host-produced factors (figure 1). The first cells to encounter the parasite during the initial stage of infection are the urogenital epithelial cells that the parasite attaches to (described above), as well as tissue-resident macrophages and dendritic cells [31]. Responding to cues from cells at the infection site, neutrophils rapidly leave the blood to infiltrate infected tissues, in a process known as extravasation [25]. Therefore, it is useful to consider neutrophil homing to Tv in two phases: (i) extravasation from the blood into the infected tissue, and (ii) homing to individual parasites once in the infected tissue. Importantly, both of these processes are predicted to be influenced by the presence of microbial symbionts within Tv.
Figure 1.
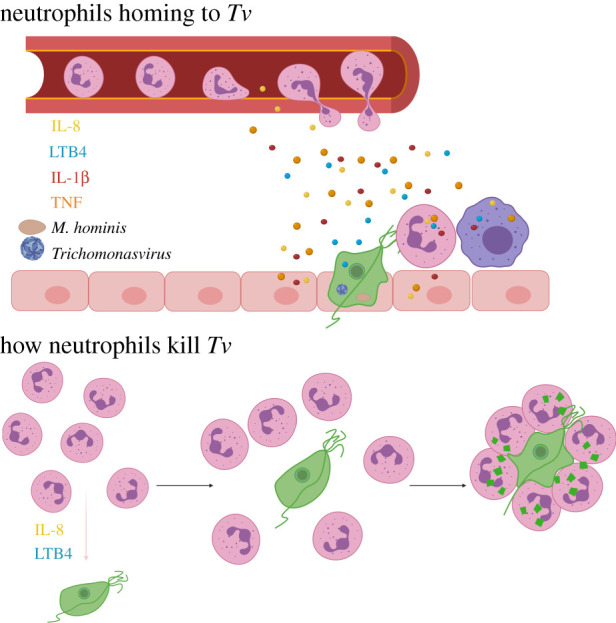
Neutrophils flow rapidly through blood, but activation of the endothelium by inflammatory mediators near an infection site can begin the process of neutrophil extravasation through diapedesis. Inflammatory mediators are secreted in response to Tv (green) from epithelial cells (pink), resident macrophages (purple) and neutrophils that arrive first (pink cells with multi-lobed nuclei). The greatest amount of inflammatory cytokines is triggered by strains of Tv that harbour symbionts Mycoplasma hominis or Trichomonasvirus. Once out of the blood, neutrophils follow IL-8 and LTB4 gradients to home to parasites. Upon encountering Tv, neutrophils swarm and trogocytose the parasite.
2.1. Extravasation and homing to individual parasites
The process of extravasation is mediated by adhesive molecules on both the neutrophil and vascular endothelial cells. First, selectins are upregulated on vascular endothelial cells in response to local inflammatory cytokines such as interleukin (IL)-1β and tumour necrosis factor (TNF) [32]. Neutrophils flow rapidly through the blood, however, when they pass through capillaries where endothelial cells have upregulated selectins, the neutrophils decelerate and roll along capillary walls, owing to the neutrophil's constitutively expressed low-affinity selectin ligands [25]. Following this rolling, neutrophil activation occurs, both by signalling through the selectin ligand, and by inflammatory cytokines in the area such as IL-8 [25]. Neutrophil activation results in the expression of the active-confirmation integrin that binds intercellular adhesion molecules (ICAMs) on the surface of the endothelial cells with high affinity, causing a halt [25]. The neutrophil then passes through the endothelium at the cell–cell boundaries and also penetrates the basement membrane, entering the tissue: a process known as diapedesis [25]. Leukotriene chemical mediators have also been shown to activate endothelium and increase vascular permeability to promote extravasation and diapedesis [33]. Therefore, inflammatory cytokines, such as IL-1β, TNF and IL-8, and leukotrienes secreted at the site of Tv infection are hypothesized to be important in neutrophil extravasation from the blood into the tissue.
Once in the tissues, neutrophils continue to follow chemotactic cues to home directly to pathogens. Neutrophils also exhibit ‘swarming motility,' attacking pathogens in aggregate [25]. In the case of Tv, visualization of vaginal smears shows neutrophils following the parasite in swarms [34], an observation that we also made with axenically grown trichomonads co-cultured with neutrophils isolated from peripheral blood [35].
Early work showed that Tv produces a chemotactic factor that attracts neutrophils [36]. This factor was found to be leukotriene-B4 (LTB4), an eicosanoid lipid mediator that plays roles in promoting extravasation [33,36]. In addition to parasite-produced LTB4, neutrophils themselves make LTB4, perpetuating a positive feedback loop once the first neutrophil has encountered a trichomonad, signalling for more recruitment to the infected area [37]. LTB4 has also been detected at the site of Tv infection in vaginal discharges, supporting its involvement during natural infection [38,39]. Analyses also showed that higher levels of LTB4 at the infection site were correlated with symptomaticity [39], indicating the role of LTB4 in modulating inflammation. Patients with higher LTB4 levels also had more neutrophils at the infection site, supporting a role for neutrophils in pathogenesis [39]. As neutrophils greatly outnumber trichomonads in vaginal discharges (greater than 100 : 1) [23], the major source of LTB4 during infection is likely to be neutrophils. It is not clear, however, whether LTB4 production and secretion by Tv itself confer any advantage to the parasite in the host, but its conservation, despite attracting neutrophils, points to a potentially redundant function for this eicosanoid in an essential function in Tv biology.
Tv has also long been known to induce IL-8 secretion from host cells [40–42] and has been demonstrated to stimulate IL-8 from a variety of cell types that are present at the site of initial infection such as epithelial cells and macrophages [41,43–46]. IL-1β and TNF have also been shown to be induced from host cells by the parasite [43–46], supporting a model that local cytokine responses from epithelial cells and resident macrophages during initial Tv infection can activate both the local endothelium and neutrophils within the blood to promote extravasation of neutrophils into the infected tissue. Once in the tissue, IL-8 is thought to be the most important chemokine for neutrophil homing to the parasite, as IL-8 is the most potent neutrophil-recruitment chemokine [47] and is consistently found to be abundantly secreted from host cells following Tv challenge [43–45]. Interestingly, patients infected with Tv that displayed symptoms had a higher neutrophil presence and IL-8 concentrations, compared to those who were asymptomatic [41], again supporting the notion that neutrophils may play a role in pathogenesis.
Furthermore, the protein C5a, which is generated when the complement system (described below) is activated, also aids in diapedesis and serves as a chemoattractant for neutrophils [25,48]. As Tv is known to activate complement [49], C5a may also contribute to neutrophil homing to Tv during infection.
2.2. The role of microbial symbionts in Tv immune activation
Many Tv strains harbour microbial symbionts that are likely to strongly promote neutrophil infiltration and attraction, by triggering increased inflammatory cytokine responses. IL-8, IL-1β and TNF were all markedly increased in instances where the parasite was harbouring either of its two microbial endosymbionts, Mycoplasma hominis or Trichomonasvirus. Human M. hominis is an obligate parasitic bacterium with a minimal genome and limited biosynthetic abilities [50–52]. While it can be detected in the vaginal microflora of healthy women [53], it is more commonly found in women with bacterial vaginosis (BV) [53,54] and is associated with pre-term birth [55,56]. While M. hominis can invade host epithelial cells [57,58], intriguingly, the bacteria can also reside within Tv as a symbiont [50]. Trichomonasvirus is a double-stranded RNA (dsRNA) virus in the totiviridae family [59,60]. Both symbionts are highly prevalent in clinical isolates of Tv, although their prevalence among strains varies by geographical region. The prevalence and other aspects of the symbionts' biology and contribution to pathogenesis are nicely reviewed elsewhere [51,61]. For the purposes of this review, however, it is important to note that the presence of the symbionts greatly impacts which pattern-recognition receptors (PRRs) are stimulated on host cells, since Tv, M. hominis, and Trichomonasvirus all have distinct pathogen-associated molecular patterns (PAMPs) [62]. PRR engagement is a strong determinant of the quantity and quality of cytokine and chemokine secretion [62].
The major PAMP on Tv is currently thought to be the dominant surface lipoglycan (LG) (also referred to as the lipophosphoglycan (LPG)), an abundant Tv glycoconjugate that coats the surface of the parasite [63,64], and binds to host cell galectins 1 and 3, resulting in cytokine production [65]. Trichomonasvirus may conceivably trigger either of the known PRRs that recognize dsRNA, Toll-like receptor 3 (TLR3), and/or the RIG-I-like receptors RIG-I and MDA5 [66]. Data currently supports that Trichomonasvirus engages TLR3 when Tv strains containing Trichomonasvirus are used to stimulate host cells [45]. Mycoplasma hominis is thought to activate host cells through TLR2, as is common for mycoplasmas [67,68]. Therefore, the presence of the symbionts expands the PRR- triggering capacity of the parasite. Lysis of the parasite, which occurs from antibiotic treatment, was shown to promote increased cytokine production from host cells, presumably owing to increased release of intracellular symbionts into the extracellular space, where they can more easily access host cell PRRs [45]. Therefore, the symbionts may have an even greater effect on host cell cytokine secretion in vivo when the parasite is under assault by neutrophils, potentially promoting the increased release of the symbionts from lysed parasites.
In studies where we compared strains harbouring or cleared of M. hominis, IL-1β was completely absent and IL-8 was severely (greater than fivefold) reduced, when monocytes were stimulated by M. hominis-free strains [43], showing that M. hominis is probably responsible for a majority of inflammatory cytokine secretion induced by the parasite. In similar experiments using a myeloid cell line, Fiori et al. [44] also observed substantial increases in IL-1β and TNF when M. hominis was added to Tv, and in fact, TNF was completely absent when strains without the addition of M. hominis were used. Fichorova et al. [45] compared cytokine responses from epithelial cells in Trichomonasvirus+ versus Trichomonasvirus- strains and similarly observed increases in IL-1β and IL-8 induced from strains that harbour the virus compared to those that do not. Collectively, these data support that a major factor in activating host cells to produce neutrophil-recruitment factors may actually be ligands from Tv's symbionts rather than the parasite itself. Therefore, whether the infecting strain of the parasite harbours either or both of these symbionts probably has major impacts on pathogenesis.
3. Mechanism of neutrophil killing of Tv
Once neutrophils home to Tv, it is conceivable that they could either succeed or fail at killing the parasite, and clearing infection. In either scenario, neutrophil activity could result in collateral damage to host tissues and therefore contribute to pathogenesis. However, if neutrophils succeed at killing Tv, this may outweigh or limit the duration of any collateral damage. In this section, we will discuss supporting evidence that neutrophils kill Tv, and we discuss potential evasion mechanisms further on in this review. However, we acknowledge that during a natural infection, multiple variables exist that may contribute to the outcome, including the strain, whether symbionts are present, host immune variability, and also the cervicovaginal microbiome (CVM).
3.1. How neutrophils kill
Neutrophils have three main killing mechanisms used against pathogens: phagocytosis, extracellular degranulation and NETosis [69]. Phagocytosis is the engulfment of whole pathogens and is followed by subsequent digestion of the pathogen when the phagosome fuses either with a lysosome, or with neutrophil toxic granules. Neutrophil toxic granules are organelles that contain antimicrobial contents such as pore-forming toxins, proteases and reactive chemical species. Degranulation is the exocytosis of these toxic granules from neutrophils, where they may intoxicate pathogens in the extracellular space. NETosis, short for Neutrophil Extracellular Traps, is the ejection of DNA, histones and toxic granules from neutrophils into the extracellular space, resulting in pathogen ensnarement in ‘NETs' of unravelled DNA. The immobilized pathogens are then subject to toxic granules and/or nearby phagocytic cells [69]. Importantly, while phagocytosis and extracellular degranulation ensue rapidly after pathogen encounter, NETs generally take 2–4 h to deploy [69]. It is not fully clear whether neutrophils receive a signal to determine which mechanism to employ following sensing of the pathogen, or whether they attempt phagocytosis and degranulation first, and NETosis ensues later if the earlier mechanisms are not productive. However, intriguing work using Candida albicans and Mycobacterium bovis showed that neutrophils phagocytosed small yeast and individual bacteria while they NETosed hyphae and large bacterial aggregates and showed that signalling downstream of successful phagocytosis downregulated the NETosis machinery, pointing to a size-sensing mechanism that neutrophils may broadly use to regulate the employment of discrete killing mechanisms [70]. It is also possible that the three mechanisms are not mutually exclusive, but that neutrophils eventually use a combination of the three during a particular infection. This is a logical hypothesis, as many pathogens may be morphologically heterogenous in vivo, with some pathogens existing as individuals, and others in biofilm form. Furthermore, polymicrobial infections may require multiple strategies to be simultaneously employed [71]. In any case, neutrophils cause inflammation of host tissues at the infection site, owing to their aggressive effector functions, such as the release of NETs, toxic granules, reactive oxygen species (discussed below) and proinflammatory cytokines into the extracellular space [40].
3.2. Neutrophils kill Tv using trogocytosis
We recently developed an in vitro co-culture system to assess neutrophil killing of Tv, using neutrophils purified from peripheral blood of healthy donors, and found that neutrophils of all 20+ donors tested rapidly and efficiently killed Tv [35]. We then investigated the mechanism(s) that neutrophils used to kill Tv in this system and took a process-of-elimination approach in which we inhibited each of the three mechanisms described above and assessed the ability of the neutrophils to kill Tv in their absence. To block the effects of NETosis, we used DNase; to determine whether extracellular degranulation was involved, we stimulated neutrophils and Tv in separate chambers of a trans-well plate, allowing the granule components of stimulated neutrophils to diffuse across the wells, but precluding cell–cell contact; to inhibit phagocytosis, we used the classic engulfment inhibitors cytochalasin D and wortmannin to impair actin polymerization and PI3 K signalling, respectively. We were surprised to find that while our data did not support a role for NETosis or extracellular degranulation in this rapid killing in vitro, our data pointed to phagocytosis as the mechanism, because killing was inhibited by the engulfment inhibitors [35]. However, the size of Tv would appear to preclude the use of phagocytosis, as Tv (10–15 µm diameter) [72] are larger than neutrophils (average 8.85 µm diameter) [72].
On closer examination using live imaging techniques, we found that neutrophils use a fourth, previously uncharacterized antimicrobial mechanism called trogocytosis (trogo = to nibble) to kill Tv [35]. In trogocytosis, neutrophils surround and ‘nibble' a target, often until the membrane is breached [73]. We used three-dimensional live confocal and four-dimensional super-resolution live confocal microscopy to observe neutrophils swarming individual trichomonads and ingesting small pieces of their membranes. In these experiments, we used propidium iodide, a membrane-impermeable nucleic acid sensor to track Tv viability in real-time during interaction with neutrophils and found that Tv were viable until multiple fragments of Tv membrane accumulated in the neutrophils. On average, parasites could survive 7 min after trogocytosis commenced and until 3–8 ‘bites' were taken, although we did observe variability. Furthermore, this was a process that neutrophils performed in aggregate, with an average of 3–6 neutrophils being present in the swarm during the killing, often nibbling from different angles [7,35]. We therefore hypothesize that Tv death occurs when a sufficient amount of membrane has been removed from the parasite's surface, or when the number/rate of ‘bites' overwhelms membrane repair machinery.
It was reassuring to find that our observations, made using modern imaging technology, corroborated observations from decades ago. Rein et al. [23] observed multiple neutrophils swarming one trichomonad and ingesting fragments of the parasite, although the term ‘trogocytosis' was not yet coined and technologies were limited to strengthen the author's confidence that this was indeed a novel process. The authors observed that ‘trichomonads usually escaped from single [neutrophils],' [23, p. 577] similar to our observations that multiple neutrophils attack a single trichomonad at different angles, presumably to trap the motile parasite from swimming away.
Matlung et al. [73] also recently reported neutrophils performing cytotoxic trogocytosis against cancer cells, further supporting that neutrophils have a trogocytic mechanism in their arsenal against targets. In this study, researchers found that trogocytic killing was independent of neutrophil degranulation, as neutrophils from human donors with a genetic degranulation deficiency were still competent to kill cancer cells [73]. We ruled out contact-independent extracellular degranulation as a mechanism used by neutrophils to kill Tv because of the inability of stimulated neutrophils to inflict damage to Tv across a trans-well membrane insert [35]. However, it is not yet known whether the granules being released directly at the junction between neutrophils and Tv during trogocytosis could be aiding in the degradation of the parasite membrane into ‘bites.' Interestingly, we found that serine proteases are required for trogocytosis and killing of Tv [35]. While serine proteases are present at the neutrophil plasma membrane, they are also a component of neutrophil toxic granules, pointing to a potential role for granule exocytosis in neutrophil trogocytic killing. Future experiments will determine if neutrophil toxic granules have a role in neutrophil trogocytic killing of Tv.
Additionally, Matlung et al. [73] used electron microscopy and intravital imaging to generate very high-resolution images of trogocytosis in action and demonstrating that neutrophil trogocytic killing of cancer cells occurs in vivo. Therefore, this study provides evidence that neutrophil trogocytic killing of targets occurs in vivo. While, we are currently not able to test whether trogocytosis of Tv occurs in vivo owing to severe limitations of Tv mouse models, the Matlung study increases our confidence that the observation of trogocytosis is not an in vitro artefact. Still, neutrophil killing of Tv during trichomoniasis infection in vivo may be more complicated than in vitro systems can capture. While NETs did not play a role in our in vitro model of rapid killing (10 min – 2 h), it is possible that they could be employed at a later stage in some challenging infections where parasites are in microcolonies or enmeshed in biofilms with other organisms [74]. Mouse models that can recapitulate trichomonas-associated pathology and neutrophil recruitment, especially in the context of a humanized CVM, would be powerful tools in illuminating a more complete picture of neutrophil killing of Tv during infection. Still, the efficiency with which trogocytic killing occurs in vitro points to an important role for this process in infection control.
The term trogocytosis was coined in 1990, to describe the ‘nibbling' of rat neuronal cells by the brain-eating amoeba Naegleria fowleri, which was distinct from phagocytosis (phago; devour), in that only small fragments of the target cells were ingested, rather than being eaten whole [75]. In 2003, the term was again used to describe membrane and protein exchange that occurs during antigen presentation at the immunological synapse from an antigen-presenting cell to a T-cell [76,77], although in this case the trogocytosis did not result in the death of the target cell. Cellular nibbling or gnawing of one cell on an adjacent cell has now been observed across animals and amoebozoas, and during many different biological scenarios such as development, neural remodelling, infection, and mounting and executing immune responses [78]. Importantly, trogocytosis does not always lead to the death of the trogocytosed cell, so it may be helpful to begin to classify trogocytic processes into cytotoxic trogocytosis and non-cytotoxic trogocytosis. Matlung et al. [73] have proposed the term ‘trogoptosis' to describe the death of a target cell following trogocytosis. The first demonstration of cytotoxic trogocytosis was the observation that the parasite Entamoeba histolytica kills host cells using trogocytosis [79]. Furthermore, while membrane transfer always occurs during trogocytosis, it is not clear if cytosol is also exchanged in each case, as methods to detect small amounts of cytosol uptake with confidence do not exist. Matlung et al. [73] observed a reduction in cytosolic signal in the target cell following trogocytosis, indicating cytosolic transfer, and Ralston et al. [79] observed cytosolic transfer in 90% of trogocytic instances using human cells and E. histolytica. However, while we did observe some ‘bites' of Tv membrane containing Tv cytosol in neutrophils following trogocytosis, not all ‘bites' were observed to contain it, and we were not able to confirm that our detection method was sensitive enough to say with confidence that not all bites contain cytosol (F. Mercer, P. J. Johnson 2018, unpublished observation). Therefore, it is currently unknown whether cytosol transfer generally occurs during cytotoxic trogocytosis.
Another interesting observation about trogocytosis is that it appears to occur only on live cell targets, while the same targets would otherwise be phagocytosed if they were already dead. Amoebic trogocytosis was demonstrated to occur exclusively on live cell targets, whereas amoeba phagocytosed pre-killed, intact cells [79]. We similarly found that dead-intact trichomonads were engulfed whole, via phagocytosis [35], and work from others showed that despite the parasite being slightly larger than neutrophils, phagocytosis is possible under conditions in which it is forced, such as using centrifugation of neutrophils and parasites together [23,80]. In the case of neutrophil trogocytosis of Tv, one hypothesis is that the parasite's motile nature contributes to the evasion of phagocytosis, necessitating trogocytosis. However, in the case of E. histolytica trogocytosis of human cells, the targets were not motile. Therefore, it appears that amoeba, and potentially also neutrophils, may have sensing mechanisms by which they can assess whether a target should be phagocytosed or trogocytosed. However, currently the mechanism by which neutrophils are activated to specifically undergo trogocytosis is not characterized. Serum opsonins appear to play a role in initiating the process, as we will discuss below, however, serum opsonins also play similar roles in phagocytosis, so it is not yet known what differentially regulates the two processes.
3.3. A potential role for reactive oxygen species
When neutrophils become activated by a pathogen, reactive oxygen species (ROS) are produced and released from the cell and into the phagosome, in a process known as oxidative burst [81]. ROS in the phagosome helps to damage ingested pathogens, and ROS in the extracellular space can damage extracellular pathogens, but can collaterally damage host cells as well [82,83]. Activation of the ROS pathway is also involved in NET release [84]. Therefore, neutrophil ROS participate in the other neutrophil killing mechanisms; however, it is not known whether they play a role in trogocytic killing of Tv. Matlung et al. [73] found that neutrophil trogocytic killing of cancer cells was not affected in the absence of ROS generation, as neutrophils from patients with genetic deficiencies in ROS production were still able to kill cancer cells via trogocytosis, and as killing proceeded in the presence of diphenyleneiodonium (DPI), a chemical inhibitor of ROS production, suggesting that neutrophil trogocytic killing does not require ROS. However, studies report contradicting results on the role of neutrophil ROS in the killing of Tv. Rein et al. [23] concluded that neutrophil ROS production played a role in killing trichomonads in vitro, because the killing of Tv by neutrophils was reduced in the presence of catalase or superoxide dismutase, enzymes that break down intermediates in the ROS pathway, and because ROS could be detected at the trichomonad-neutrophil interface using biochemical methods. However, in the same study, the researchers also found that neutrophils isolated from patients unable to synthesize ROS were able to kill Tv, confounding their results [23].
We observed no reduction in Tv killing by neutrophils in the presence of catalase [35], supporting that ROS does not play a role in trogocytic killing. These inconsistent results may be attributed to the strains of Tv used in the experiments, as Tv has been reported to produce anti-oxidants to guard against ROS [85]. Oxygen concentrations during the assays could also contribute to discrepancies, as we performed our assays in a standard 5% CO2 incubator, while Rein et al. [23] performed their catalase inhibition experiments in open air [35]. Hypoxia (low oxygen) has been found to be associated with lower levels of neutrophil activity [86]; however, anaerobic conditions may be more physiologically relevant, particularly during infection [87]. Vaginal oxygen levels are highly variable, further confounding decisions about what an appropriate level would be to conduct these experiments [88]. Therefore, the contribution of ROS to killing may also best be assessed in an animal model.
4. The role of opsonins in Tv-neutrophil interaction
While we observed that neutrophils kill Tv using trogocytosis, the subcellular and molecular players in the trogocytic process are under-characterized. However, one of the clearest results observed by numerous groups studying Tv interaction with neutrophils is that in the absence of human serum, killing was completely abolished [35,73,80], pointing to an important role for serum opsonins in neutrophil trogocytic killing of Tv. Opsonins are serum proteins that coat pathogens, allowing cross-linking of a cell bearing an opsonin receptor to the ‘opsonized’ pathogen [23,35]. Neutrophils express opsonin receptors [89–91], thus opsonins can enhance contact between neutrophils and pathogens and act as tags for phagocytosis and trogocytosis [73]. Opsonins include antibodies and complement proteins [62].
4.1. The role of antibodies in neutrophil killing of Tv
Antibodies are produced in response to specific pathogens, and bind those pathogens or their components with high specificity. Antibodies also contain an Fc domain that can bind to Fc receptors on neutrophils or other immune cells, thus mediating opsonization [24,62,91]. While long-term immunity against Tv seems tenuous [92,93], evidence indicates that antibodies against the parasite and its components are formed during infection with Tv. First, serum derived from trichomoniasis patients had bright reactivity against Tv [49]. Furthermore, immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies were detected in Tv-infected patients in the vagina and endocervix, indicating that Tv elicits antibody production at the infection site [94,95]. Animal models of intravaginal trichomonad infection also show IgM, IgG and IgA responses [96,97].
Many of the early researchers studying the interaction of neutrophils with Tv emphasized that antibodies were not necessary for trichomonacidal activity because the killing observed was unaffected by adsorption of serum to Tv, to deplete any antibodies that may have bound to the parasite [23,80]. However, these studies used serum from individual healthy donors with no history of STI's [23], or no history of sexual activity [80]. Therefore, antibodies were unlikely to contribute to the killing measured in these studies, and therefore no change would be expected when antibodies are blocked.
Our studies are in agreement that neutrophils can kill Tv in the absence of antibodies; however, our data support that killing is enhanced by up to twofold if specific antibodies are present. We found that opsonization mediated by antibodies had a role in neutrophil killing of Tv, as trogocytic activity and parasite killing was reduced by about half when Fc receptors were blocked [35]. In these experiments, we used human serum from a commercial source, which was pooled from hundreds of male donors, of which several had probably encountered Tv, owing to the high prevalence of Tv in the general population. Furthermore, we did indeed confirm that antibodies which could bind to Tv were present in our commercial serum batch, using flow cytometry [35]. A role for antibodies in neutrophil trogocytic targets was also confirmed for neutrophil killing of cancer cells. When Fc receptors were blocked, Matlung et al. [73] observed a reduced trogocytic activity against cancer cells. Therefore, antibodies probably play a role in neutrophil trogocytic killing of Tv; however, the specific Fc receptors involved, and the full contribution of antibody to killing Tv in vivo remains to be determined.
4.2. The role of complement in neutrophil killing of Tv
The complement system is a group of reactive proteins found constitutively in human serum that bind to pathogens and mediate various downstream effector functions in pathogen clearance [62]. One major function of complement is to opsonize pathogens with fragments of complement proteins, which can cross-link to complement receptors on neutrophils and other immune cells. The major complement protein involved in opsonization is called iC3b [62].
Complement proteins have long been known to bind Tv, as other downstream effects of complement, such as direct parasite lysis have been observed in some circumstances [23,49,98]. Furthermore, several groups have demonstrated an essential role for the complement system in neutrophil killing of Tv because heat-inactivation of serum (which inactivates complement proteins), reduced killing [23,80,98]. While several groups have shown that complement can be activated spontaneously on the parasite surface [49,80,98], using a pathway called the alternative pathway of complement activation, one group showed that the presence of anti-Tv antibodies can also enable complement to be activated through an antibody-enhanced process called the classical pathway of complement activation [80]. These data further support that while antibodies are not necessary for anti-trichomonal host responses, they can enhance them. We have shown that iC3b coats the surface of Tv that have been incubated with human serum, pointing to complement opsonization as a method by which neutrophils are activated to trogocytose the parasite [35].
To mediate opsonization, iC3b can bind to receptors CR1, CR3, CR4 and C1qR [90]. Matlung et al. [73] showed that CR3 is required for neutrophil trogocytic killing of cancer cells; however, the specific receptors involved in killing Tv are not yet defined. Discerning the roles of antibody and complement in opsonizing Tv for trogocytic killing are important, as complement is a function of the innate immune system, while antibody develops after a first encounter with the pathogen [62]. Therefore, identifying which players are required and which specific roles they play in neutrophil trogocytosis will help to define when Tv can be cleared upon initial infection and when stronger recall responses are necessary.
5. Tv evasion from neutrophils
Importantly, clinical observation shows that many patients do not clear Tv on their own, but require antibiotic therapy, pointing to an inability of neutrophils to effectively clear the parasite during infection, and implicating that Tv employs neutrophil evasion strategies. In the subsections below, we present evidence that several neutrophil evasion strategies may exist. However, as the data is collected from reductive in vitro models, the effectiveness of each strategy remains to be tested in conditions that more closely resemble natural infection.
5.1. ‘Running or hiding’
In live imaging studies that we performed on neutrophils and Tv, we always vortexed parasites prior to adding them to the imaging platform to break up clumps, which would otherwise make it very difficult to visualize trichomonads interacting with neutrophils in a steady plane-of-view [35]. In these experiments, we always saw several neutrophils swarm around individual parasites, often attacking from all sides, and on average, 3-6 neutrophils were present in a swarm around a parasite [7,35]. However, many strains of Tv grow in clumps, and during infection, this clumping behaviour may facilitate the formation of microcolonies [21]. In fact, some strains that demonstrate higher pathogenic behaviours in vitro tend to clump more [99,100], pointing to clumping as a virulence trait of the parasite. As clumps of Tv would make it very difficult for multiple neutrophils to surround individual trichomonads, it is probable that clumping behaviour facilitates evasion from trogocytosis. Furthermore, if parasites on the outside of the clump are trogocytosed and killed first, it may take neutrophils longer to reach the parasites on the inside, giving those trichomonads longer to employ some other potential evasion strategies described below.
In addition to protective clumping behaviour, Tv may also repel away from neutrophils during infection. One study shows evidence that in vitro, trichomonads avoided travelling towards neutrophils by repelling away from ROS products produced during neutrophil oxidative bursts. In a chemotaxis assay using a plate in which trichomonads were separated from neutrophils by a filter, fewer trichomonads migrated into the filter when neutrophils were stimulated compared to when they were not [27]. One alternative interpretation of these results could be that conditions in which neutrophils were activated resulted in lower parasite viability, and thus fewer parasites able to chemotax at all. However, our results using trans-well assays with neutrophils and Tv separated by a filter demonstrate that Tv cannot be killed by soluble factors from activated neutrophils [35]. Therefore, we support the interpretation that Tv were repelled from activated neutrophils in these assays. Chemorepulsion by Tv depended on the dose and the type of stimulus on the neutrophils. To determine which antimicrobial molecule the stimulated neutrophils were producing to induce chemorepulsion, the stimulated neutrophils were treated with catalase or superoxide dismutase to break down oxygen metabolites. The results demonstrated chemorepulsion by Tv was induced by neutrophil ROS products [27]. However, as these assays demonstrated chemorepulsion within 45 min, it is not clear whether chemorepulsion would aid in trichomonad escape of neutrophil trogocytosis, which is a rapid process often complete within 15 min. However, it is conceivable that in tissues with large numbers of activated neutrophils, trichomonads may use chemorepulsion to avoid areas where neutrophils have recently cast NETs that could possibly ensnare them. While neutrophil oxidative burst may enhance Tv repulsion, other studies demonstrated that it may also induce apoptosis in neutrophils.
5.2. Inducing neutrophil apoptosis
Tv has demonstrated the ability to induce apoptosis in neutrophils by activating ROS. While neutrophils are short-lived cells, apoptosis occurred significantly more in neutrophils incubated with Tv than neutrophils alone, when incubated for 12 h [101,102]. To confirm that the apoptotic pathway was induced by Tv, neutrophils, trichomonads and a caspase-3 inhibitor were cultured together [102]. Caspases are proteases that play a role in signalling programmed cell death; of the broad class of caspases, caspase-3 and caspase-8 facilitate apoptosis [103]. Addition of a caspase-3 inhibitor to the co-culture resulted in a reduction in apoptosis [102]. These results suggested that caspase-3 in neutrophils was induced by Tv, which led to premature apoptosis. Elevated levels of ROS can trigger apoptosis in neutrophils [104,105] and the addition of ROS inhibitor DPI reduced apoptosis in neutrophils triggered by Tv [101]; therefore, Tv induction of ROS from neutrophils is thought to be the mechanism of Tv-induced neutrophil apoptosis. However, as trogocytic killing of Tv is rapid (approx. 15 min), it is improbable that individual parasites are able to evade killing by inducing this neutrophil suicide, which takes up to 12 h to ensue. However, at a population level, this mechanism may impede neutrophil killing of parasites that take longer to approach because they are on the inside of a microcolony or within a polymicrobial biofilm [21]. However, as neutrophils are continually replaced from the blood during persistent infections, it is not clear what the impact of neutrophil apoptosis is in vivo.
5.3. Suppression of neutrophil recruitment
As described above, mediators from the parasite itself, as well as inflammatory products that Tv induces from epithelial cells, monocytes and other neutrophils, serve as chemo-attractants for neutrophils to home to the site of infection. The major neutrophil-recruitment factor is likely to be the cytokine IL-8, as it is released rapidly and abundantly from multiple cellular sources and facilitates both extravasation and homing to microenvironments within tissues. Interestingly, Tv was recently shown to produce and secrete exosomes, small extracellular vesicles that contain RNA and protein and can be uptaken by host cells [46,106]. In addition to increasing host cell susceptibility to parasite attachment, Tv exosomes also seem to have the ability to suppress neutrophil recruitment, as host epithelial cells that were pretreated with Tv exosomes showed a reduction in IL-8 response upon subsequent parasite encounter [46]. These data support a model in which parasites secrete exosomes at the infection site to prime more distal areas of the vagina for colonization, by promoting epithelial cell adherence, and decreasing the number of neutrophils homing to the area. Furthermore, in preliminary mouse models, the application of parasite exosomes to mouse vaginas 48 h prior to infection reduced gross inflammation and reduced the Th17 response, which is a type of immune response associated with neutrophils [107]. Therefore, Tv exosomes appear to have immunosuppressive properties in vivo.
5.4. Evading antibody-mediated trogocytosis
As described above, neutrophil trogocytic killing of Tv was shown to be partially mediated by antibodies [35]. However, Tv secretion products were shown to contain cysteine proteases that have the ability to degrade antibodies in vitro [108,109], pointing to a mechanism by which the parasite could degrade antibody and escape trogocytosis. Furthermore, we found Tv to be capable of killing lymphocytes, and to preferentially target B-cells [43], the producers of antibodies. The B-cell cytotoxic effects of Tv were mediated by both contact-dependent and soluble factors [43]. As B-cells can be detected in the cervicovaginal mucosa during infection [110,111], it is also conceivable that Tv could be impeding the formation of antibody responses in the local infection site.
5.5. Escape from neutrophil extracellular traps
In addition to behaviours that point to mechanisms of trogocytosis evasion, it is also possible that Tv could evade NETosis, a late-stage, final effort neutrophil killing strategy that could conceivably be a relevant killing mechanism in the context of Tv microcolonies or biofilms [21,112]. Interestingly, Tv's symbiont M. hominis contains a virulence factor that may play a role in degrading NETs released by neutrophils [112]. An in vitro assay of activated neutrophils and M. hominis confirmed that NETs were released from neutrophils, but that they were degraded in the presence of M. hominis, and specifically owing to M. hominis gene MHOM_0730, which encodes for a surface lipoprotein with a nuclease domain [112]. Control studies demonstrated MHOM_0730's ability to degrade linear double stranded DNA (dsDNA), circular dsDNA and single stranded DNA [112]; therefore, one property of strains containing the M. hominis symbiont could be their increased resistance to neutrophil attack. However, as these strains are also more immuno-stimulatory [43,44] and likely to elicit increased neutrophil recruitment, the ultimate result of M. hominis presence on infection persistence is unclear. Nonetheless, as neutrophils are associated with inflammation regardless of whether they succeed in killing the parasite, we hypothesize that M. hominis + strains are more pathogenic. Furthermore, proteomic analysis of Tv secreted products revealed the presence of a DNase in the Tv genome [113]. While it is unclear whether this DNase is expressed during infection or whether it is competent to degrade NETs, this represents another exiting possible avenue by which Tv could evade neutrophil killing in some circumstances.
6. Conclusion and open questions
The interaction between neutrophils and Tv has been studied for over four decades since it was first noted that neutrophils are present in high numbers in vaginal discharge of trichomoniasis patients [23]. Expanding on the current knowledge of how the host handles Tv infection is important for the development of novel prevention and treatment options, especially in light of increased antibiotic-resistant Tv strains [1,114,115]. Neutrophils probably extravasate and home to the site of Tv infection following gradients of LTB4 secreted by both the parasite and the host, and IL-8 secreted by the host. Once at the infection site, neutrophils may kill the parasites; however, many of the effector functions of neutrophils can also cause inflammation in the host [35,40,98,101]. Both degranulation and NETosis release toxic granules into the extracellular space, potentially damaging the surrounding host tissue [69]. While phagocytosis and trogocytosis do not appear to involve the release of toxins into the environment, neutrophils still die en masse following the attack of pathogens using these mechanisms, and the resultant dead cell products could still elicit tissue inflammation. Furthermore, neutrophil activation to perform any of these mechanisms is also accompanied by the secretion of inflammatory cytokines such as IL-8, IL-6, IL-1β and TNF [26], which recruit other immune cells, and have broad inflammatory effects such as vasodilation. So far, the only killing mechanism that has been shown to be effective against Tv is trogocytosis, which requires cell–cell contact. Both complement proteins and antibodies seem to facilitate this contact, as blocking Fc receptors hinders trogocytic killing, and using complement-deficient serum failed to eliminate Tv [35,49]. Tv has also plausibly evolved to evade neutrophils in order to survive and strategies such as inhibiting neutrophil recruitment, repelling away from neutrophils, hiding in aggregates or biofilms, inducing neutrophil death, and thwarting various neutrophil effector functions may all contribute to Tv evasion of neutrophils. Certainly, neutrophil-Tv interaction is very dynamic. However, much remains to be determined about the subcellular and molecular particulars of neutrophil-Tv interactions, what the downstream immunological consequences are, how all of the discoveries made in vitro contribute to actual outcomes in vivo, and what contributions the vaginal microbiota and the parasite's own symbionts make to ultimate outcomes of the neutrophil-trichomonad struggle.
6.1. Molecular mechanisms of neutrophil trogocytosis of Tv
The recently discovered mechanism of neutrophil trogocytosis of Tv remains to be further studied. Thus far, it is known that trogocytosis is a contact-dependent process, though the determination of specific neutrophil receptors remains to be elucidated.
While antibodies and complement factors are implemented in cross-linking the neutrophil to Tv to initiate trogocytosis, whether any other adhesion factors are involved, and what specific molecules and organelles carry out the acquisition of Tv material by neutrophils is unclear. Our data showing that trogocytosis and parasite killing is reduced in the presence of a serine protease inhibitor indicates that neutrophil serine proteases play a role in the trogocytic process. However, which specific serine protease, which subcellular location it acts from, and which targets on the parasite that it attacks are not known. It is also possible that the serine protease acts on a host target, which is more directly involved in mediating the nibbling phenotype. Cysteine proteases are effectors of trogocytosis in E. histolytica [116], but the effect of proteases of neutrophil trogocytosis of cancer cells has not been tested and is instead thought to be purely mechanical, as a result of actin-myosin contraction [73]. As mentioned above, for several instances of trogocytosis, including neutrophil trogocytosis of Tv, the effector cell trogocytoses live cell targets, but phagocytoses the same cellular targets if they are dead, pointing to a signalling mechanism downstream of sensing live versus dead cells [35,79]. However, it is not known whether there are any trogocytosis-specific players function in neutrophil trogocytosis of Tv.
6.2. Downstream immunological consequences of trogocytosis
As neutrophils are usually the first immune cells that respond to an infection, their actions can have formative effects on how the subsequent immune response proceeds. Neutrophils can shape the tissue environment by cytokine secretion and tissue damage. However, how adaptive immune responses are formed following trogocytic killing of pathogens is unknown. While Tv material is detected in neutrophils following trogocytosis, the fate of these ‘bites' in unknown. In E. histolytica, trogocytosed bites of host cells fuse with lysosomes, and lysosomal degradation is required for sustained trogocytosis [117]. However, it is not known whether ‘trogosomes' containing Tv material become degraded by lysosomes or toxic granules, or whether the Tv material is subsequently loaded onto major histocompatibility complex class I or II, which would have implications for the formation of T-cell responses.
Furthermore, it is not known whether Tv material enters the cytosol of neutrophils, which would have implications for both antigen presentation as well as neutrophil cell death.
Macrophages undergo a process called pyroptosis, a highly inflammatory programmed cell death that is initiated by inflammasomes. Inflammasomes are complexes formed by cytosolic nod-like receptors (NLRs) and activate caspase enzymes [118]. Although it has been shown that macrophages are able to undergo pyroptosis when challenged with Tv, it is unknown if neutrophils do the same [119]. For this to occur, Tv material would have to enter the cytosol in order to activate the cytosolic NLRs. Alternatively, the material of either of Tv's symbionts could potentially activate inflammasomes if it enters the cytosol. It is not known whether ‘trogosomes’ contain Tv material only, whether M. hominis and Trichomonasvirus are contained within ‘bites' as well, and whether any of this material can subsequently enter the cytosol.
6.3. Neutrophil heterogeneity
Recently, the primitive implication of neutrophils as dirty-bombs, sent to die at the site of infection, has been challenged. Intravital microscopy has recently revealed neutrophils responding to sterile injury and playing roles in tissue repair [120] and also travelling back out of inflamed tissues, in a process termed ‘reverse migration' [120,121]. Therefore, some neutrophils may have longer lasting and more restorative roles than once thought. Similar to the paradigm of the ‘classical' versus ‘alternatively activated' macrophage (clunkily termed M1 and M2), different subsets of neutrophils, N1 and N2, have also been proposed, and are nicely reviewed elsewhere [122]. N2 neutrophils and neutrophils that may actually have suppressive function, termed Granulocytic–Myeloid-derived suppressor cells (G-MDSC), are thought to play immunosuppressive roles in tumour microenvironments and may have more tolerogenic roles in regeneration and wound healing, and resolution of inflammation. These tolerogenic neutrophils may even have functions in antigen presentation [123]. However, it is not known whether N2 neutrophils play a role in responding to Tv, or whether both types of neutrophils can perform trogocytosis.
6.4. Contributions by Tv's symbionts
Some important roles for symbionts M. hominis and Trichomonasvirus in stimulating immune cells have been recently revealed [61], but many previous studies that claimed to axenically prepare their Tv parasites might not have checked for these intracellular ‘hangers-on', particularly before 1986 in the case of Trichomonasvirus [60] and 1998 in the case of M. hominis [124]. Furthermore, while old literature often used strains collected from symptomatic patients, defined strains of Tv now exist as community resources on American Type Culture Collection (ATCC). It would therefore be potentially beneficial to the field if some experiments were repeated with more defined parameters, such as consistent strains, and comparing the conditions +/− either symbiont. With these variables under control, it may be possible to better understand neutrophil activation and response among different strains and from different microbial stimuli. In particular, it will be interesting to determine if there are differences in activating ROS, trogocytosis and NETs from neutrophils if strains harbour these symbionts that have been demonstrated to activate discrete PRRs. Researchers may then be able to make better predictions about outcomes during infection resulting from pathogenic strains of the parasite and whether the microbial symbionts contribute to pathogenesis in vivo.
6.5. Effect of the microbiome
Another exciting future direction will be to assess the effect of neutrophils during Tv infection depending on changes in the CVM. The human CVM is generally rich in lactobacillus species during the steady-state, but can become overgrown with pathobionts such as Gardnerella vaginalis, in a state known as BV [21]. The BV state is strongly correlated with Tv infection, although it is not yet known whether BV predisposes to Tv or vice versa. However, while lactobacilli decrease Tv colonization of epithelial monolayers [125,126], biofilms formed by the pathobionts increase adherence and killing of epithelial cells by the parasite [21], which presumably increases inflammation in the tissue. Therefore, we would predict increased neutrophil recruitment to tissues infected with Tv and in a state of BV dysbiosis. We also hypothesize that the BV biofilms may stymie neutrophil efforts to trogocytose parasites, thus the overall effect of this additional neutrophil recruitment may be more pathogenic than productive. However, these hypotheses remain to be tested. Importantly, mouse models that can re-capitulate good levels of parasite colonization, and ideally a humanized CVM would be powerful tools to test how neutrophil-Tv-microbiome dynamics ultimately play out during infection.
A wealth of in vitro data has characterized interactions between this highly prevalent human parasite and its most abundant host cell adversary, giving clues about how attacks are mounted in time and space, and the impacts of the parasite, the host and the microbial partners of each, on the disease. However, many open questions remain about the specific molecular players that participate, and the ultimate outcomes of these interactions. Delving further into these questions will aid in a better understanding of trichomoniasis that can inspire improvements in future prevention and treatment strategies.
Acknowledgements
We would like to acknowledge our colleagues in the Department of Biological Sciences at Cal Poly Pomona, and support from the administration within the College of Science. We also thank Patricia Johnson at UCLA for supporting F.M.s work on neutrophils and Tv, and thank colleagues Victor Torres, Natalia de Miguel, Cheryl Okumura, Angelica Riestra, and Daniele Dessi for productive discussions about the experiments and interpretation.
Data accessibility
This article has no additional data.
Authors' contributions
S.B.B. and J.A.M. performed literature search and wrote the first drafts of the manuscript. S.B.B. wrote sections on abstract, introduction, killing and evasion. J.A.M. wrote sections on introduction, homing, opsonization and conclusions/ open questions. J.A.M. generated the figure. F.M. edited the manuscript.
Competing interests
We declare we have no competing interests.
Funding
F.M. is funded by NIH grant no. 1SC3GM135046-0, Cal State University Program for Education and Research in Biotechnology (CSUPERB) and the Cal State University Agricultural Research Institute (ARI) grant no. 21-04-112. Work described in this review was supported by NIH grant no. F32 AI122643 to F.M. S.B.B. is funded by The Cal Poly Pomona President's Scholars and Project Hatchery Programs. J.A.M. is funded by the NIH Research Training Initiative for Student Enhancement (RISE), and Project Hatchery programs.
References
- 1.Leitsch D. 2016. Recent advances in the Trichomonas vaginalis field. F1000Res 5 ( 10.12688/f1000research.7594.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirt RP, Sherrard J. 2015. Trichomonas vaginalis origins, molecular pathobiology and clinical considerations. Curr. Opin. Infect Dis. 28, 72–79. ( 10.1097/QCO.0000000000000128) [DOI] [PubMed] [Google Scholar]
- 3.Secor WE, Meites E, Starr MC, Workowski KA. 2014. Neglected parasitic infections in the United States: trichomoniasis. Am. J. Trop. Med. Hyg. 90, 800–804. ( 10.4269/ajtmh.13-0723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organization WH. 2018. Report on global sexually-transmitted infection surveillance.
- 5.Midlej V, Benchimol M. 2010. Trichomonas vaginalis kills and eats–evidence for phagocytic activity as a cytopathic effect. Parasitology 137, 65–76. ( 10.1017/S0031182009991041) [DOI] [PubMed] [Google Scholar]
- 6.Lustig G, Ryan CM, Secor WE, Johnson PJ. 2013. Trichomonas vaginalis contact-dependent cytolysis of epithelial cells. Infect Immun. 81, 1411–1419. ( 10.1128/IAI.01244-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercer F, Johnson PJ. 2018. Trichomonas vaginalis: pathogenesis, symbiont interactions, and host cell immune responses. Trends Parasitol. 34, 683–693. ( 10.1016/j.pt.2018.05.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arroyo R, Gonzalez-Robles A, Martinez-Palomo A, Alderete JF. 1993. Signalling of Trichomonas vaginalis for amoeboid transformation and adhesion synthesis follows cytoadherence. Mol. Microbiol. 7, 299–309. ( 10.1111/j.1365-2958.1993.tb01121.x) [DOI] [PubMed] [Google Scholar]
- 9.Schwebke JR, Burgess D. 2004. Trichomoniasis. Clin. Microbiol. Rev. 17, 794–803. table of contents ( 10.1128/CMR.17.4.794-803.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fichorova RN. 2009. Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J. Reprod. Immunol. 83, 185–189. ( 10.1016/j.jri.2009.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Gerwen OT, Muzny CA. 2019. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res 8 ( 10.12688/f1000research.19972.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiringa AE, Ness RB, Darville T, Beigi RH, Haggerty CL. 2019. Trichomonas vaginalis, endometritis and sequelae among women with clinically suspected pelvic inflammatory disease. Sex Transm Infect. 96, 436–438. [DOI] [PubMed] [Google Scholar]
- 13.Mielczarek E, Blaszkowska J. 2016. Trichomonas vaginalis: pathogenicity and potential role in human reproductive failure. Infection. 44, 447–458. ( 10.1007/s15010-015-0860-0) [DOI] [PubMed] [Google Scholar]
- 14.Nakubulwa S, Kaye DK, Bwanga F, Tumwesigye NM, Mirembe FM. 2015. Genital infections and risk of premature rupture of membranes in Mulago Hospital, Uganda: a case control study. BMC Res. Notes 8, 573 ( 10.1186/s13104-015-1545-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prevention CfDCa. 2020. STD facts. See https://wwwcdcgov/std/trichomonas/stdfact-trichomoniasishtm.
- 16.Fastring DR, et al. 2014. Co-occurrence of Trichomonas vaginalis and bacterial vaginosis and vaginal shedding of HIV-1 RNA. Sex. Transm. Dis. 41, 173–179. ( 10.1097/OLQ.0000000000000089) [DOI] [PubMed] [Google Scholar]
- 17.McClelland RS, Sangare L, Hassan WM, Lavreys L, Mandaliya K, Kiarie J, Ndinya-Achola J, Jaoko W, Baeten JM. 2007. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J. Infect. Dis. 195, 698–702. ( 10.1086/511278) [DOI] [PubMed] [Google Scholar]
- 18.Tao L, et al. 2014. Prevalence and risk factors for cervical neoplasia: a cervical cancer screening program in Beijing. BMC Public Health 14, 1185 ( 10.1186/1471-2458-14-1185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh I, Muwonge R, Mittal S, Banerjee D, Kundu P, Mandal R, Biswas J, Basu P. 2017. Association between high risk human papillomavirus infection and co-infection with Candida spp. and Trichomonas vaginalis in women with cervical premalignant and malignant lesions. J. Clin. Virol. 87, 43–48. ( 10.1016/j.jcv.2016.12.007) [DOI] [PubMed] [Google Scholar]
- 20.Yang S, Zhao W, Wang H, Wang Y, Li J, Wu X. 2018. Trichomonas vaginalis infection-associated risk of cervical cancer: a meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 228, 166–173. ( 10.1016/j.ejogrb.2018.06.031) [DOI] [PubMed] [Google Scholar]
- 21.Hinderfeld AS, Simoes-Barbosa A. 2019. Vaginal dysbiotic bacteria act as pathobionts of the protozoal pathogen Trichomonas vaginalis. Microbio. Pathog. 138, 103820 ( 10.1016/j.micpath.2019.103820) [DOI] [PubMed] [Google Scholar]
- 22.Hinderfeld AS, Phukan N, Bar AK, Roberton AM, Simoes-Barbosa A. 2019. Cooperative Interactions between Trichomonas vaginalis and associated bacteria enhance paracellular permeability of the cervicovaginal epithelium by dysregulating tight junctions. Infect. Immun. 87 ( 10.1128/IAI.00141-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rein MF, Sullivan JA, Mandell GL. 1980. Trichomonacidal activity of human polymorphonuclear neutrophils: killing by disruption and fragmentation. J. Infect. Dis. 142, 575–585. ( 10.1093/infdis/142.4.575) [DOI] [PubMed] [Google Scholar]
- 24.Rosales C. 2018. Neutrophil: a cell with many roles in inflammation or several cell types? Front. Physiol. 9, 113 ( 10.3389/fphys.2018.00113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ley K, Hoffman HM, Kubes P, Cassatella MA, Zychlinsky A, Hedrick CC, Catz SD. 2018. Neutrophils: new insights and open questions. Sci. Immunol. 3. [DOI] [PubMed] [Google Scholar]
- 26.Kolaczkowska E, Kubes P. 2013. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175. ( 10.1038/nri3399) [DOI] [PubMed] [Google Scholar]
- 27.Styrt B, Sugarman B, Mummaw N, White JC. 1991. Chemorepulsion of trichomonads by products of neutrophil oxidative metabolism. J. Infect. Dis. 163, 176–179. ( 10.1093/infdis/163.1.176) [DOI] [PubMed] [Google Scholar]
- 28.Vesic S, Vukicevic J, Dakovic Z, Tomovic M, Dobrosavljevic D, Medenica L, Pavlović MD. 2007. Male urethritis with and without discharge: relation to microbiological findings and polymorphonuclear counts. Acta Dermatovenerol Alp Pannonica Adriat. 16, 53–57. [PubMed] [Google Scholar]
- 29.Cobo ER, Eckmann L, Corbeil LB. 2011. Murine models of vaginal trichomonad infections. Am. J. Trop. Med. Hyg. 85, 667–673. ( 10.4269/ajtmh.2011.11-0123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright HL, Moots RJ, Bucknall RC, Edwards SW. 2010. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 49, 1618–1631. ( 10.1093/rheumatology/keq045) [DOI] [PubMed] [Google Scholar]
- 31.Iijima N, Thompson JM, Iwasaki A. 2008. Dendritic cells and macrophages in the genitourinary tract. Mucosal Immunol. 1, 451–459. ( 10.1038/mi.2008.57) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pober JS, Sessa WC. 2007. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 7, 803–815. ( 10.1038/nri2171) [DOI] [PubMed] [Google Scholar]
- 33.Nohgawa M, Sasada M, Maeda A, Asagoe K, Harakawa N, Takano K, Yamamoto K, Okuma M. 1997. Leukotriene B4-activated human endothelial cells promote transendothelial neutrophil migration. J. Leukoc. Biol. 62, 203–209. ( 10.1002/jlb.62.2.203) [DOI] [PubMed] [Google Scholar]
- 34.Demirezen S, Safi Z, Beksac S. 2000. The interaction of Trichomonas vaginalis with epithelial cells, polymorphonuclear leucocytes and erythrocytes on vaginal smears: light microscopic observation. Cytopathology 11, 326–332. ( 10.1046/j.1365-2303.2000.00237.x) [DOI] [PubMed] [Google Scholar]
- 35.Mercer F, Ng SH, Brown TM, Boatman G, Johnson PJ. 2018. Neutrophils kill the parasite Trichomonas vaginalis using trogocytosis. PLoS Biol. 16, e2003885 ( 10.1371/journal.pbio.2003885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaio MF, Lin PR, Lee CS, Hou SC, Tang P, Yang KD. 1992. A novel neutrophil-activating factor released by Trichomonas vaginalis. Infect. Immun. 60, 4475–4482. ( 10.1128/IAI.60.11.4475-4482.1992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaio MF, Lin PR. 1995. Influence of humoral immunity on leukotriene B4 production by neutrophils in response to Trichomonas vaginalis stimulation. Parasite Immunol. 17, 127–133. ( 10.1111/j.1365-3024.1995.tb01014.x) [DOI] [PubMed] [Google Scholar]
- 38.Ryu JS, Min DY. 2006. Trichomonas vaginalis and trichomoniasis in the Republic of Korea. Korean J. Parasitol. 44, 101–116. ( 10.3347/kjp.2006.44.2.101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaio MF, Lin PR. 1995. Leucotriene B4 levels in the vaginal discharges from cases of trichomoniasis. Ann. Trop. Med. Parasitol. 89, 85–88. ( 10.1080/00034983.1995.11812934) [DOI] [PubMed] [Google Scholar]
- 40.Ryu JS, Kang JH, Jung SY, Shin MH, Kim JM, Park H, Min D-Y. 2004. Production of interleukin-8 by human neutrophils stimulated with Trichomonas vaginalis. Infect. Immun. 72, 1326–1332. ( 10.1128/IAI.72.3.1326-1332.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaio MF, Lin PR, Liu JY, Tang KD. 1994. Monocyte-derived interleukin-8 involved in the recruitment of neutrophils induced by Trichomonas vaginalis infection. J. Infect. Dis. 170, 1638–1640. ( 10.1093/infdis/170.6.1638) [DOI] [PubMed] [Google Scholar]
- 42.Shaio MF, Lin PR, Liu JY, Yang KD. 1995. Generation of interleukin-8 from human monocytes in response to Trichomonas vaginalis stimulation. Infect. Immun. 63, 3864–3870. ( 10.1128/IAI.63.10.3864-3870.1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mercer F, Diala FG, Chen YP, Molgora BM, Ng SH, Johnson PJ. 2016. Leukocyte lysis and cytokine induction by the human sexually transmitted parasite Trichomonas vaginalis. PLoS Negl. Trop. Dis. 10, e0004913 ( 10.1371/journal.pntd.0004913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiori PL, Diaz N, Cocco AR, Rappelli P, Dessi D. 2013. Association of Trichomonas vaginalis with its symbiont Mycoplasma hominis synergistically upregulates the in vitro proinflammatory response of human monocytes. Sex. Transm. Infect. 89, 449–454. ( 10.1136/sextrans-2012-051006) [DOI] [PubMed] [Google Scholar]
- 45.Fichorova RN, et al. 2012. Endobiont viruses sensed by the human host: beyond conventional antiparasitic therapy. PLoS ONE 7, e48418 ( 10.1371/journal.pone.0048418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Twu O, de Miguel N, Lustig G, Stevens GC, Vashisht AA, Wohlschlegel JA, Johnson PJ. 2013. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate hostratioparasite interactions. PLoS Pathog. 9, e1003482 ( 10.1371/journal.ppat.1003482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim D, Haynes CL. 2012. Neutrophil chemotaxis within a competing gradient of chemoattractants. Anal. Chem. 84, 6070–6078. ( 10.1021/ac3009548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akk A, et al. 2019. Complement activation on neutrophils initiates endothelial adhesion and extravasation. Mol. Immunol. 114, 629–642. ( 10.1016/j.molimm.2019.09.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gillin FD, Sher A. 1981. Activation of the alternative complement pathway by Trichomonas vaginalis. Infect. Immun. 34, 268–273. ( 10.1128/IAI.34.1.268-273.1981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dessi D, Delogu G, Emonte E, Catania MR, Fiori PL, Rappelli P. 2005. Long-term survival and intracellular replication of Mycoplasma hominis in Trichomonas vaginalis cells: potential role of the protozoon in transmitting bacterial infection. Infect. Immun. 73, 1180–1186. ( 10.1128/IAI.73.2.1180-1186.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dessi D, Margarita V, Cocco AR, Marongiu A, Fiori PL, Rappelli P. 2019. Trichomonas vaginalis and Mycoplasma hominis: new tales of two old friends. Parasitology. 146, 1150–1155. ( 10.1017/S0031182018002135) [DOI] [PubMed] [Google Scholar]
- 52.Margarita V, Rappelli P, Dessi D, Pintus G, Hirt RP, Fiori PL. 2016. Symbiotic association with Mycoplasma hominis can influence growth rate, atp production, cytolysis and inflammatory response of Trichomonas vaginalis. Front. Microbiol. 7 ( 10.3389/fmicb.2016.00953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Margarita V, Fiori PL, Rappelli P. 2020. Impact of symbiosis between Trichomonas vaginalis and Mycoplasma hominis on vaginal dysbiosis: a mini review. Front Cell Infect Microbiol. 10, 179 ( 10.3389/fcimb.2020.00179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Onderdonk AB, Delaney ML, Fichorova RN. 2016. The human microbiome during bacterial vaginosis. Clin. Microbiol. Rev. 29, 223–238. ( 10.1128/CMR.00075-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murtha AP, Edwards JM. 2014. The role of Mycoplasma and Ureaplasma in adverse pregnancy outcomes. Obstet. Gynecol. Clin. North Am. 41, 615–627. ( 10.1016/j.ogc.2014.08.010) [DOI] [PubMed] [Google Scholar]
- 56.Thi Trung Thu T, Margarita V, Cocco AR, Marongiu A, Dessi D, Rappelli P, Fiori PL. 2018. Trichomonas vaginalis transports virulent Mycoplasma hominis and transmits the infection to human cells after metronidazole treatment: a potential role in bacterial invasion of fetal membranes and amniotic fluid. J. Pregnancy 2018, 5037181 ( 10.1155/2018/5037181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hopfe M, Deenen R, Degrandi D, Kohrer K, Henrich B. 2013. Host cell responses to persistent mycoplasmas–different stages in infection of HeLa cells with Mycoplasma hominis. PLoS ONE 8, e54219 ( 10.1371/journal.pone.0054219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, Cassell GH, Waites KB. 2009. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod. Sci. 16, 56–70. ( 10.1177/1933719108325508) [DOI] [PubMed] [Google Scholar]
- 59.Parent KN, et al. 2013. Structure of a protozoan virus from the human genitourinary parasite Trichomonas vaginalis. mBio 4 ( 10.1128/mBio.00056-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang AL, Wang CC. 1986. The double-stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc. Natl Acad. Sci. USA 83, 7956–7960. ( 10.1073/pnas.83.20.7956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fichorova R, Fraga J, Rappelli P, Fiori PL. 2017. Trichomonas vaginalis infection in symbiosis with Trichomonasvirus and Mycoplasma. Res. Microbiol. 168, 882–891. ( 10.1016/j.resmic.2017.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chaplin DD. 2010. Overview of the immune response. J. Allergy Clin. Immunol. 125(2 Suppl. 2), S3–23. ( 10.1016/j.jaci.2009.12.980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryan CM, Mehlert A, Richardson JM, Ferguson MA, Johnson PJ. 2011. Chemical structure of Trichomonas vaginalis surface lipoglycan: a role for short galactose (beta1-4/3) N-acetylglucosamine repeats in host cell interaction. J. Biol. Chem. 286, 40 494–40 508. ( 10.1074/jbc.M111.280578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fichorova RN, Trifonova RT, Gilbert RO, Costello CE, Hayes GR, Lucas JJ, Singh BN. 2006. Trichomonas vaginalis lipophosphoglycan triggers a selective upregulation of cytokines by human female reproductive tract epithelial cells. Infect. Immun. 74, 5773–5779. ( 10.1128/IAI.00631-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fichorova RN, et al. 2016. Trichomonas vaginalis lipophosphoglycan exploits binding to galectin-1 and -3 to modulate epithelial immunity. J. Biol. Chem. 291, 998–1013. ( 10.1074/jbc.M115.651497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jensen S, Thomsen AR. 2012. Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. J Virol. 86, 2900–2910. ( 10.1128/JVI.05738-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Love W, Dobbs N, Tabor L, Simecka JW. 2010. Toll-like receptor 2 (TLR2) plays a major role in innate resistance in the lung against murine Mycoplasma. PLoS ONE 5, e10739 ( 10.1371/journal.pone.0010739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goret J, Beven L, Faustin B, Contin-Bordes C, Le Roy C, Claverol S, Renaudin H, Bébéar C, Pereyre S. 2017. Interaction of Mycoplasma hominis PG21 with human dendritic cells: interleukin-23-inducing mycoplasmal lipoproteins and inflammasome activation of the cell. J. Bacteriol. 199 ( 10.1128/JB.00213-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liew PX, Kubes P. 2019. The neutrophil's role during health and disease. Physiol Rev. 99, 1223–1248. ( 10.1152/physrev.00012.2018) [DOI] [PubMed] [Google Scholar]
- 70.Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V. 2014. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 15, 1017–1025. ( 10.1038/ni.2987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peters BM, Jabra-Rizk MA, O'May GA, Costerton JW, Shirtliff ME. 2012. Polymicrobial interactions: impact on pathogenesis and human disease. Clin. Microbiol. rev. 25, 193–213. ( 10.1128/CMR.00013-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheon SH, Kim SR, Song HO, Ahn MH, Ryu JS. 2013. The dimension of Trichomonas vaginalis as measured by scanning electron microscopy. Korean J. Parasitol. 51, 243–246. ( 10.3347/kjp.2013.51.2.243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matlung HL, et al. 2018. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 23, 3946–3959. e6 ( 10.1016/j.celrep.2018.05.082) [DOI] [PubMed] [Google Scholar]
- 74.Aquino MFK, Hinderfeld AS, Simoes-Barbosa A. 2020. Trichomonas vaginalis Trends Parasitol. 36, 646–647. ( 10.1016/j.pt.2020.01.010) [DOI] [PubMed] [Google Scholar]
- 75.Marciano-Cabral F, Zoghby KL, Bradley SG. 1990. Cytopathic action of Naegleria fowleri amoebae on rat neuroblastoma target cells. J. Protozool. 37, 138–144. ( 10.1111/j.1550-7408.1990.tb05884.x) [DOI] [PubMed] [Google Scholar]
- 76.Joly E, Hudrisier D. 2003. What is trogocytosis and what is its purpose? Nat. Immunol. 4, 815 ( 10.1038/ni0903-815) [DOI] [PubMed] [Google Scholar]
- 77.Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, Jackson MR, Sprent J, Cai Z. et al. 1999. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science 286, 952–954. ( 10.1126/science.286.5441.952) [DOI] [PubMed] [Google Scholar]
- 78.Bettadapur A, Miller HW, Ralston KS. 2020. Biting off what can be chewed: trogocytosis in health, infection, and disease. Infect Immun. 88 ( 10.1128/IAI.00930-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ralston KS, Solga MD, Mackey-Lawrence NM, Somlata, Bhattacharya A, Petri WA Jr. 2014. Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature 508, 526–530. ( 10.1038/nature13242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shaio MF, Chang FY, Hou SC, Lee CS, Lin PR. 1991. The role of immunoglobulin and complement in enhancing the respiratory burst of neutrophils against Trichomonas vaginalis. Parasite Immunol. 13, 241–250. ( 10.1111/j.1365-3024.1991.tb00279.x) [DOI] [PubMed] [Google Scholar]
- 81.Winterbourn CC, Kettle AJ, Hampton MB. 2016. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 85, 765–792. ( 10.1146/annurev-biochem-060815-014442) [DOI] [PubMed] [Google Scholar]
- 82.Ward RA, Nakamura M, McLeish KR. 2000. Priming of the neutrophil respiratory burst involves p38 mitogen-activated protein kinase-dependent exocytosis of flavocytochrome b558-containing granules. J. Biol. Chem. 275, 36 713–36 719. ( 10.1074/jbc.M003017200) [DOI] [PubMed] [Google Scholar]
- 83.Clark RA. 1999. Activation of the neutrophil respiratory burst oxidase. J. Infect. Dis. 179(Suppl. 2), S309–S317. ( 10.1086/513849) [DOI] [PubMed] [Google Scholar]
- 84.Rohm M, Grimm MJ, D'Auria AC, Almyroudis NG, Segal BH, Urban CF. 2014. NADPH oxidase promotes neutrophil extracellular trap formation in pulmonary aspergillosis. Infect. Immun. 82, 1766–1777. ( 10.1128/IAI.00096-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ellis JE, Yarlett N, Cole D, Humphreys MJ, Lloyd D. 1994. Antioxidant defences in the microaerophilic protozoan Trichomonas vaginalis: comparison of metronidazole-resistant and sensitive strains. Microbiology 140, 2489–2494. ( 10.1099/13500872-140-9-2489) [DOI] [PubMed] [Google Scholar]
- 86.McGovern NN, et al. 2011. Hypoxia selectively inhibits respiratory burst activity and killing of Staphylococcus aureus in human neutrophils. J. Immunol. 186, 453–463. ( 10.4049/jimmunol.1002213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eltzschig HK, Carmeliet P. 2011. Hypoxia and inflammation. N. Engl. J. Med. 364, 656–665. ( 10.1056/NEJMra0910283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hill DR, Brunner ME, Schmitz DC, Davis CC, Flood JA, Schlievert PM, Wang-Weigand SZ, Osborn TW. 2005. In vivo assessment of human vaginal oxygen and carbon dioxide levels during and post menses. J. Appl. Physiol. (1985) 99, 1582–1591. ( 10.1152/japplphysiol.01422.2004) [DOI] [PubMed] [Google Scholar]
- 89.Whitehouse J, et al. 2016. Population variation in anti-S. aureus IgG isotypes influences surface protein A mediated immune subversion. Vaccine 34, 1792–1799. ( 10.1016/j.vaccine.2016.02.034) [DOI] [PubMed] [Google Scholar]
- 90.Sengelov H. 1995. Complement receptors in neutrophils. Crit. Rev. Immunol. 15, 107–131. ( 10.1615/CritRevImmunol.v15.i2.10) [DOI] [PubMed] [Google Scholar]
- 91.Rosales C. 2017. Fcgamma receptor heterogeneity in leukocyte functional responses. Front. Immunol. 8, 280 ( 10.3389/fimmu.2017.00280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nu PA T, Rappelli P, Dessi D, Nguyen VQ, Fiori PL. 2015. Kinetics of circulating antibody response to Trichomonas vaginalis: clinical and diagnostic implications. Sex. Transm. Infect. 91, 561–563. ( 10.1136/sextrans-2014-051839) [DOI] [PubMed] [Google Scholar]
- 93.Forna F, Gulmezoglu AM. 2003. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev. 2019, issue 5, CD000218 ( 10.1002/14651858.CD000218) [DOI] [PubMed] [Google Scholar]
- 94.Bastida-Corcuera FD, Singh BN, Gray GC, Stamper PD, Davuluri M, Schlangen K, Corbeil RR, Corbeil LB. 2013. Antibodies to Trichomonas vaginalis surface glycolipid. Sex. Transm. Infect. 89, 467–472. ( 10.1136/sextrans-2012-051013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chipperfield EJ, Evans BA. 1972. The influence of local infection on immunoglobulin formation in the human endocervix. Clin. Exp. Immunol. 11, 219–233. [PMC free article] [PubMed] [Google Scholar]
- 96.Kaur S, Khurana S, Bagga R, Wanchu A, Malla N. 2008. Antitrichomonas IgG, IgM, IgA, and IgG subclass responses in human intravaginal trichomoniasis. Parasitol. Res. 103, 305–312. ( 10.1007/s00436-008-0971-y) [DOI] [PubMed] [Google Scholar]
- 97.Paintlia MK, Kaur S, Gupta I, Ganguly NK, Mahajan RC, Malla N. 2002. Specific IgA response, T-cell subtype and cytokine profile in experimental intravaginal trichomoniasis. Parasitol. Res. 88, 338–343. ( 10.1007/s004360100396) [DOI] [PubMed] [Google Scholar]
- 98.Holbrook TW, Boackle RJ, Vesely J, Parker BW. 1982. Trichomonas vaginalis: alternative pathway activation of complement. Trans. R. Soc. Trop. Med. Hyg. 76, 473–475. ( 10.1016/0035-9203(82)90140-7) [DOI] [PubMed] [Google Scholar]
- 99.Coceres VM, Alonso AM, Nievas YR, Midlej V, Frontera L, Benchimol M, Johnson PJ, de Migue N. 2015. The C-terminal tail of tetraspanin proteins regulates their intracellular distribution in the parasite Trichomonas vaginalis. Cell. Microbiol. 17, 1217–1229. ( 10.1111/cmi.12431) [DOI] [PubMed] [Google Scholar]
- 100.Pachano T, Nievas YR, Lizarraga A, Johnson PJ, Strobl-Mazzulla PH, de Miguel N. 2017. Epigenetics regulates transcription and pathogenesis in the parasite Trichomonas vaginalis. Cell. Microbiol. 19 ( 10.1111/cmi.12716) [DOI] [PubMed] [Google Scholar]
- 101.Song HO, Shin MH, Ahn MH, Min DY, Kim YS, Ryu JS. 2008. Trichomonas vaginalis: reactive oxygen species mediates caspase-3 dependent apoptosis of human neutrophils. Exp. Parasitol. 118, 59–65. ( 10.1016/j.exppara.2007.06.010) [DOI] [PubMed] [Google Scholar]
- 102.Kang JH, Song HO, Ryu JS, Shin MH, Kim JM, Cho YS, Alderete JF, Ahn MH, Min DY. 2006. Trichomonas vaginalis promotes apoptosis of human neutrophils by activating caspase-3 and reducing Mcl-1 expression. Parasite Immunol. 28, 439–446. ( 10.1111/j.1365-3024.2006.00884.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li J, Yuan J. 2008. Caspases in apoptosis and beyond. Oncogene 27, 6194–6206. ( 10.1038/onc.2008.297) [DOI] [PubMed] [Google Scholar]
- 104.Scheel-Toellner D, et al. 2004. Reactive oxygen species limit neutrophil life span by activating death receptor signaling. Blood 104, 2557–2564. ( 10.1182/blood-2004-01-0191) [DOI] [PubMed] [Google Scholar]
- 105.Gardai S, Whitlock BB, Helgason C, Ambruso D, Fadok V, Bratton D, Henson PM. 2002. Activation of SHIP by NADPH oxidase-stimulated Lyn leads to enhanced apoptosis in neutrophils. J. Biol. Chem. 277, 5236–5246. ( 10.1074/jbc.M110005200) [DOI] [PubMed] [Google Scholar]
- 106.Rai AK, Johnson PJ. 2019. Trichomonas vaginalis extracellular vesicles are internalized by host cells using proteoglycans and caveolin-dependent endocytosis. Proc. Natl Acad. Sci. USA 116, 21 354–21 360. ( 10.1073/pnas.1912356116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Olmos-Ortiz LM, Barajas-Mendiola MA, Barrios-Rodiles M, Castellano LE, Arias-Negrete S, Avila EE, Cuéllar-Mata P. 2017. Trichomonas vaginalis exosome-like vesicles modify the cytokine profile and reduce inflammation in parasite-infected mice. Parasite Immunol. 39. [DOI] [PubMed] [Google Scholar]
- 108.Provenzano D, Alderete JF. 1995. Analysis of human immunoglobulin-degrading cysteine proteinases of Trichomonas vaginalis. Infect. Immun. 63, 3388–3395. ( 10.1128/IAI.63.9.3388-3395.1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hernandez HM, Marcet R, Sarracent J. 2014. Biological roles of cysteine proteinases in the pathogenesis of Trichomonas vaginalis. Parasite. 21, 54 ( 10.1051/parasite/2014054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anjuere F, Bekri S, Bihl F, Braud VM, Cuburu N, Czerkinsky C, Hervouet C, Luci C. 2012. B cell and T cell immunity in the female genital tract: potential of distinct mucosal routes of vaccination and role of tissue-associated dendritic cells and natural killer cells. Clin. Microbiol. Infect. 18(Suppl. 5), 117–122. ( 10.1111/j.1469-0691.2012.03995.x) [DOI] [PubMed] [Google Scholar]
- 111.Oh JE, Iijima N, Song E, Lu P, Klein J, Jiang R, Kleinstein SH, Iwasaki A. 2019. Migrant memory B cells secrete luminal antibody in the vagina. Nature 571, 122–126. ( 10.1038/s41586-019-1285-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cacciotto C, et al. 2019. MHO_0730 as a surface-exposed calcium-dependent nuclease of Mycoplasma hominis promoting neutrophil extracellular trap formation and escape. J. Infect. Dis. 220, 1999–2008. ( 10.1093/infdis/jiz406) [DOI] [PubMed] [Google Scholar]
- 113.Stafkova J, et al. 2018. Dynamic secretome of Trichomonas vaginalis: case study of beta-amylases. Mol. Cell Proteomics. 17, 304–320. ( 10.1074/mcp.RA117.000434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leitsch D. 2017. A review on metronidazole: an old warhorse in antimicrobial chemotherapy. Parasitology 146, 1–12. [DOI] [PubMed] [Google Scholar]
- 115.Leitsch D. 2017. Drug susceptibility testing in microaerophilic parasites: cysteine strongly affects the effectivities of metronidazole and auranofin, a novel and promising antimicrobial. Int. J. Parasitol. Drugs Drug Resist. 7, 321–327. ( 10.1016/j.ijpddr.2017.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gilmartin AA, Ralston KS, Petri WA. 2020. Inhibition of amebic cysteine proteases blocks amebic trogocytosis but not phagocytosis. J. Infect. Dis. 221, 1734–1739. ( 10.1093/infdis/jiz671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gilmartin AA, Ralston KS, Petri WA Jr. 2017. Inhibition of amebic lysosomal acidification blocks amebic trogocytosis and cell killing. mBio 8 ( 10.1128/mBio.01187-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu L, Sun B. 2019. Neutrophil pyroptosis: new perspectives on sepsis. Cell Mol. Life Sci. 76, 2031–2042. ( 10.1007/s00018-019-03060-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Riestra AM, Valderrama JA, Patras KA, Booth SD, Quek XY, Tsai CM, Nizet V. 2019. Trichomonas vaginalis induces NLRP3 inflammasome activation and pyroptotic cell death in human macrophages. J. Innate Immun. 11, 86–98. ( 10.1159/000493585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes P. 2017. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 358, 111–116. ( 10.1126/science.aam9690) [DOI] [PubMed] [Google Scholar]
- 121.de Oliveira S, Rosowski EE, Huttenlocher A. 2016. Neutrophil migration in infection and wound repair: going forward in reverse. Nat. Rev. Immunol. 16, 378–391. ( 10.1038/nri.2016.49) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Deniset JF, Kubes P. 2018. Neutrophil heterogeneity: Bona fide subsets or polarization states? J. Leukoc. Biol. 103, 829–838. ( 10.1002/JLB.3RI0917-361R) [DOI] [PubMed] [Google Scholar]
- 123.Singhal S, et al. 2016. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell. 30, 120–135. ( 10.1016/j.ccell.2016.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rappelli P, Addis MF, Carta F, Fiori PL. 1998. Mycoplasma hominis parasitism of Trichomonas vaginalis. Lancet 352, 1286 ( 10.1016/S0140-6736(05)61372-4) [DOI] [PubMed] [Google Scholar]
- 125.Phukan N, Brooks AES, Simoes-Barbosa A. 2018. A cell surface aggregation-promoting factor from Lactobacillus gasseri contributes to inhibition of Trichomonas vaginalis adhesion to human vaginal ectocervical cells. Infect. Immun. 86 ( 10.1128/IAI.00907-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Phukan N, Parsamand T, Brooks AE, Nguyen TN, Simoes-Barbosa A. 2013. The adherence of Trichomonas vaginalis to host ectocervical cells is influenced by lactobacilli. Sex. Transm. Infect. 89, 455–459. ( 10.1136/sextrans-2013-051039) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.


