Abstract
RNA m6A methylation is a post-transcriptional modification that occurs at the nitrogen-6 position of adenine. This dynamically reversible modification is installed, removed and recognized by methyltransferases, demethylases and readers, respectively. This modification has been found in most eukaryotic mRNA, tRNA, rRNA and other non-coding RNA. Recent studies have revealed important regulatory functions of the m6A including effects on gene expression regulation, organism development and cancer development. In this review, we summarize the discovery and features of m6A, and briefly introduce the mammalian m6A writers, erasers and readers. Finally, we discuss progress in identifying additional functions of m6A and the outstanding questions about the regulatory effect of this widespread modification.
Keywords: m6A methylation, writers, erasers, readers, functions
1. Introduction
There has been extensive study of gene expression regulation. Chemical modification in DNA and RNA can regulate gene expression, which has evolved to ensure that the right genes are properly expressed for the conditions of a particular environment and at the necessary time. There has been awareness that the epigenetic modification of DNA can regulate gene expression and chromatin organization. This recently coined an additional regulatory layer termed ‘epitranscriptomics’ that depends on biochemical modifications to the RNA [1]. One of the most common RNA modifications is m6A methylation, or N6-methyladenosine, which refers to methylation of the adenosine base at the nitrogen-6 position. This methylation is a dynamically reversible modification that is installed, removed and recognized by methyltransferases, demethylases and readers, respectively [2]. This modification has been found in many eukaryotes, from plant to mammals, and even in viruses [3–6]. The m6A methylation is widely distributed in various RNA, with an average of three m6A sites per mRNA [7]. The m6A modification was first identified in the 1970s, but research on its potential function was initially limited owing to a lack of technologies for global detection of the m6A modification. In 2011, the obesity-associated protein (FTO) was found to effectively remove m6A modification on RNA [8], suggesting that m6A modification might serve a regulatory role. The development of next generation sequencing methods has facilitated further functional study of m6A modification.
In this review, we summarize the discovery and the main features of m6A modification, and briefly introduce the mammalian m6A writers, erasers and readers that interact with m6A sites to mediate the fate of mRNA (figure 1). We next describe the emerging knowledge of the functions of m6A in post-transcriptional gene expression regulation, animal development and cancer development. Finally, we discuss the emerging challenge and outstanding questions of this field, which should advance our understanding of m6A.
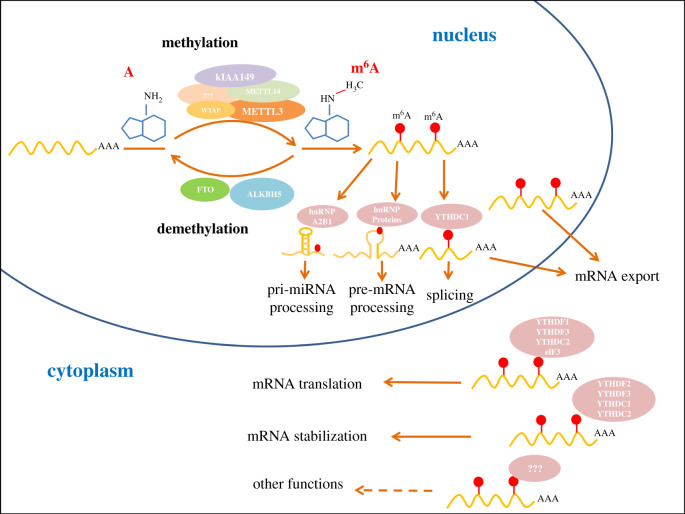
Figure 1.
The patterns and functions of m6A methylation. The m6A methylation, occurs at the sixth N atom of RNA adenine, is installed by methyltransferase and erased by demethylase in the nucleus. The m6A readers that preferentially recognize m6A-containing RNA can impact the fate of the methylated RNA and give diverse regulatory function. In the nucleus, combination of m6A with hnRNP proteins or YTHDC1 can affect splicing of pre-mRNAs and combination with YTHDC1 mediates the export of methylated mRNA. In addition, combination with hnRNPA2B1 facilitates the processing of methylated pri-miRNA. In cytoplasm, YTHDF1, YTHDC2 and eIF3 bind to the methylated mRNAs to promote translation. YTHDF2, YTHDC1 and YTHDC2 bind to the methylated mRNAs to accelerate decay. Furthermore YTHDF3 combining with the YTHDF1 can promote targeted mRNA translation and combining with YTHDF2 can accelerate degradation. More m6A readers and other functions need to identify in m6A-modified mRNA.
2. Discovery and features of m6A
In the 1970s, several groups characterizing mRNA 5ʹ structures in mammalian cells serendipitously discovered that polyadenylate RNA was rich in m6A modifications [9,10]. However, concerns about contamination from small amounts of known m6A sources, such as rRNA and small nucleolar RNAs [11–13], prevented confirmation of m6A as a ubiquitous modification in mRNA that is related to biogenesis [14]. In 2012, two groups of researchers firstly identified m6A peaks corresponding to 5678 mRNA transcripts and 6990 mRNA transcripts in mouse and human cells, and observed strong conservation of these m6A peaks in humans and mice [4,5]. The applied method was MeRIP-Seq or m6A-seq, which relies on the use of highly specific m6A antibodies to immunoprecipitate methylated mRNAs and then uses next generation sequencing to map methylated transcripts [4,5]. However this method lacks high sensitivity and resolution. The development of m6A-miCLIP and PA-m6A-seq methods have allowed more subtle mapping of m6A modification [15,16]. The technologies for detection and analysis of m6A sites continue to advance, providing more insight into the importance of this modification and its function in gene regulation.
Two mechanisms for regulating m6A deposition have been described to date. First, histone H3 trimethylation at Lys36 (H3K36me3) can globally regulate m6A deposition. Approximately 70% of m6A peaks are enriched near H3K36me3 sites. The depletion of H3K36me3 led to a reduction of the m6A level, because H3K36me3 is coupled with METTL14, which recruits the m6A methyltransferase complex to newly synthesized RNAs and with RNA polymerase II mediates the co-transcriptional deposition of m6A [17]. Second, transcription factors can mediate the dynamic level of m6A methylation. For example, Zfp217 can reduce the level of m6A by activating the demethylase FTO and SMAD2/D3 can recruit the m6A methyltransferase complex to newly synthesized RNA to facilitate m6A deposition [18,19].
There are several features of m6A modification: (i) mapping of m6A sites revealed that they preferentially map near stop codons, in the 3′ untranslated regions (UTRs), followed by the coding sequences (CDS) and the 5′ UTR regions [4,5]; (ii) the m6A motif was originally identified as (G/A) (m6A) C [20,21]. Recently, this motif has been more fully described as G [G/A] (m6A) CU, with almost 90% of the m6A peaks containing these motifs [4,5,22]; (iii) this modification is widely distributed among species including human, mammals, yeast, Arabidopsis and even viruses [3–6,23]; (iv) in addition to being found in mRNA, the m6A modification has been observed in tRNA, rRNA and other abundant non-coding RNA [24]. Signals for m6A have also been found in several classes of lncRNAs, including the well-known XIST and MALAT1 [4,5,25,26]. The m6A modification can also alter the expression of mature miRNA by affecting the production of pri-miRNA [27]. Recent studies have shown that intracellular m6A methylation can regulate the translation, destabilization, export and biogenesis of circRNAs [28–31]; and (v) in mammals, m6A modification is widely present in multiple tissues, with highest levels in liver, kidney and brain [5]. Recent work has shown that m6A and m6Am are highly specific to the brain, and some tissue-specific m6A signals may distinguish different human and mouse tissue types [32]. Overall, m6A modification is universal and exhibits organizational preference.
3. Cellular system of m6A methylation
3.1. m6A writers
The m6A modification is performed by a methyltransferase complex, or ‘writer’. This complex consists of two subunit complexes: an m6A-METTL complex (MAC) and an m6A-METTL-associated complex (MACOM), which can transfer the methyl group from S-adenosylmethionine (SAM) to the N6-amine of adenosine [33]. The m6A-METTL complex includes methyltransferase 3 (METTL3) and methyltransferase 14 (METTL14), which form a stable heterodimer. In 1997, a 70 kDa protein called MTA-70 (or METTL3) was successfully isolated and found to contain a classic SAM-binding methyltransferase domain (SAM) [34]. METTL3 is the catalytic subunit and binds to SAM [34] and METTL14 acts to stabilize the conformation and promote binding to RNA [35,36]. The lack of METTL3 could promote the apoptosis of HeLa cells and causes a decrease of m6A level [37]. METTL3 is highly conserved in eukaryotes and its homologues have been found in yeast, plants and flies [38–40]. Notably, the absence of METTL3 can block development in yeast and flies, and can lead to death in Arabidopsis and mice [39–41]. An early study revealed METTL14 is highly similar to METTL3 [42], and further research confirmed that METTL14 is also a methyltransferase [43]. METTL14 can synergistically increase METTL3 methyltransferase activities [43,44]. Interestingly, the knockdown of METTL14 resulted in a greater reduction in m6A levels than the knockdown of METTL3 in HeLa and 293T cells [43]. The METTL3/14 complex can selectively methylate RRACH sequences [43].
Subsequent efforts focused on m6A-METTL-associated complexes and how these complexes promote methyltransferase activities. The Wilms tumour-associated protein (WTAP) can interact with the METTL3/14 complex to promote mRNA methylation [43]. Although WTAP lacks methyltransferase activity in vitro, it promotes the localization of the METTL3/14 complex to nuclear speckles and facilitates mRNAs methylation [45]. Interfering with WTAP significantly reduces the level of m6A and prevents METTL3/14 complex localization to nuclear speckles [45]. KIAA1429 (also VIRMA) is a newly discovered component of the methyltransferase complex. Proteomic studies revealed important interactions with KIAA1429 and WTAP, and the absence of KIAA1429 substantially reduces the level of m6A modification [46,47]. Recent studies showed KIAA1429 is critical for the specific installation of m6A to 3′ UTR sites [48]. The RNA-binding protein 15/15B (RBM15/15B) preferentially binds to U-rich regions to recruit the m6A complex and may promote the methylation of specific RNA [25]. Another methyltransferase, METTL16, can install m6A on U6 snRNA and other highly structured ncRNAs and pre-mRNAs [49–51]. METTL16 may act a splicing enhancer to produce stable mature MAT2A mRNA encoding SAM synthetase during low-SAM conditions [49]. Recent study revealed the role of METTL16 in promoting early mouse embryonic development through regulation of SAM availability [52]. In Arabidopsis, HAKAI was identified as a new element by interaction with WTAP and was found necessary for m6A methylation [53,54]. The CCCH-type 13 zinc finger protein (ZC3H13) and its homologous protein FLacc in Drosophila are also involved in m6A installation by promoting the localization of WTAP and the deposition of m6A [55,56]. Most recently, ZCCHC4, a new m6A methyltransferase, was reported to methylate human 28S rRNA within the AAC motif [57]. The known m6A methyltransferases and their functions are listed in table 1.
Table 1.
Functions of m6A writers and erasers.
| molecule | effect on m6A modification | other functions | references | |
|---|---|---|---|---|
| m6A writers | METTL3 | catalytic core of methyltransferase | enhances translation | [34,58,59] |
| METTL14 | stabilize METTL3/14 complex and promote the binding to RNA | [35,36] | ||
| WTAP | promote the localization of METTL3/14 complex | [45] | ||
| KIAA149(VIRMA) | interactions with WTAP and installation of m6A to the 3′ UTR | [48] | ||
| RBM15/15B | binding to U-rich regions to recruit the methyltransferase complex | [25] | ||
| METTL16 | promote methylation of U6 snRNA, ncRNAs and pre-mRNAs | facilitate splicing of specific mRNA | [49–52] | |
| HAKAI | necessary for the m6A methylation in Arabidopsis | [53,54] | ||
| ZC3H13 | promote the WTAP localization and m6A deposition | [55,56] | ||
| ZCCHC4 | methylate human 28S rRNA | [57] | ||
| m6A erasers | FTO | remove m6A and m6Am | regulate pre-mRNA alternative splicing | [8,60,61] |
| ALKBH5 | remove m6A | regulate mRNA processing, metabolism and export | [62–64] |
3.2. m6A erasers
Until endogenous enzymes capable of demethylation of m6A were found, m6A modification was regarded as a static modification. An important recent study identified FTO and ALKBH5 as m6A demethylases that can remove m6A methylation. These ‘erasers’ belong to the AlkB family and require the involvement of ferrous ion, α-ketoglutarate and oxygen [65,66]. FTO is associated with weight gain and obesity in humans [67]. Initial studies demonstrated that FTO could demethylate 3-methylthymidine (3mT) in single-stranded DNA and 3-methyluracil (3mU) in single-stranded RNA [65,68]. In 2011, FTO was shown to effectively remove m6A methylation of mRNA in vitro and inside cells [8]. Subsequent study revealed that FTO can produce two intermediates in removing m6A : N6-hydroxymethyladenosine (hm6A) and N6-formyladenosine (f6A), which is unrecognized by m6A ‘readers’ [69]. Knockdown of FTO in HeLa cells can increase the level of m6A and overexpression can reduce the level of m6A in mRNA [8]. More recently, FTO has been found to preferentially target intronic regions in pre-mRNAs rather than mRNAs, so can regulate pre-mRNA alternative splicing and 3′ UTR processing [60]. In addition to m6A, FTO can also effectively remove m1A from specific tRNAs and cap-m6Am from mRNAs and some snRNAs [61]. FTO has higher demethylation activity for m6Am and can stabilize the 5′ cap in mRNA, making an effect on mRNA stability likely [61]. Most recently, FTO was shown to remove m6Am methylation in snRNAs, suggesting that methylation information in snRNA may influence mRNA splicing [70].
Recently, ALKBH5 was identified as a second mammalian m6A demethylase [62]. ALKBH5 is enriched in the nucleus, unlike FTO, which is detected in the cytosol and nucleus [61,62]. Based on its localization, ALKBH5 may target nuclear RNAs and also interact with mRNA processing factors to regulate mRNA processing, metabolism and export [62,63]. The m6A demethylation process catalysed by ALKBH5 does not produce any intermediates. A lack of ALKBH5 in HeLa cells increased the m6A level by 9%, while overexpression of ALKBH5 decreased m6A level by 29% in total mRNA [62]. ALKBH5 was found to be highly expressed in the testicles of mice, and knockout of ALKBH5 inhibited spermatogenesis and decreased male fertility [62]. ALKBH5 can also modulate correct splicing and promote the production of longer 3′ UTR mRNAs in the nuclei of spermatocytes and round spermatids [64]. The known m6A demethylases and their functions are listed in table 1.
3.3. m6A readers
Although methyltransferase and demethylase endow the structural characteristics of RNA, m6A readers preferentially recognize m6A-containing mRNA, and impact the fate of target mRNA to give diverse regulatory functions. Recent studies have confirmed that m6A readers have a YTH domain that enables them to selectively target m6A-containing mRNA [71,72]. Proteins with a YTH domain for recognition of m6A-containing mRNA include: YTHDC1, YTHDC2, YTHDF1, YTHDF2 and YTHDF3. YTHDF2, which has the highest affinity to m6A, can selectively bind the m6A motif to regulate mRNA degradation [73]. Studies have found that mRNA bound to YTHDF2 can be transferred to an RNA degradation site using an N-terminal, such as the processor (p-body), and YTHDF2 can also directly recruit the CCR4-NOT deadenylase complex to accelerate degradation [73,74]. Several studies suggested that the IDR domain plays an effector function, where the IDR of YTHDF2 bound to mRNA allows targeting of P-bodies and also interaction with CCR4-NOT and endoribonuclease RNase P/MRP [29,73,74]. Importantly, YTHDF2 can block demethylation of 5′ UTR by FTO to stabilize methylation levels in cells [75]. A ratio of total mRNA by 21%, suggesting that the YTHDF2 destabilizes m6A-modified mRNA [73]. Related proteins YTHDF1 and YTHDF3 can promote translation by recruiting translation initiation factors in HeLa cells [76,77]. Knockout of YTHDF1 does not affect overall mRNA stability, but the overall translation efficiency is significantly reduced owing to interaction of YTHDF1 with eIF3 and other translation initiation factors [76]. Interestingly, YTHDF3 was proposed to complex with both YTHDF1 and YTHDF2 to promote mRNA translation and degradation upon binding its targets [78]. However, the mechanisms by which binding affects translation and degradation have not been fully described. YTHDC1, also known as YT521-B, has a variety of regulatory functions, including regulation of mRNA splicing [79], accelerating mRNA export [80], silencing the X chromosome [25] and promoting the decay of specific transcripts [81]. Recent studies have shown that YTHDC2 can increase the translation efficiency of its targets as well as decrease their mRNA abundance and is also involved in the regulation of meiosis and spermatogenesis [82,83].
In addition to the YTH domain family, eukaryotic initiation factor 3 (eIF3), a component of the 43S translation initiation complex, directly binds to the 5′ UTR of m6A mRNA and affects translation initiation [84]. Member of the heterogeneous nuclear ribonucleoprotein family, hnRNPC, hnRNPG, and hnRNPA2B1, were identified as m6A readers that regulate alternative splicing events [85–88]. The hnRNPC protein is a nuclear RNA-binding protein that is involved in the processing of pre-mRNA [89,90]. The m6A region of mRNA often lacks secondary structure which promotes hnRNPC binding to RNA, allowing it to regulate the abundance and alternative splicing of target genes [87,91]. Another member of the hnRNP family, hnRNPA2B1, was identified as an m6A binding protein that affects m6A-dependent alternative splicing and microRNA maturity [27,85]. The hnRNPG protein selectively binds m6A-modified RNA using Arg-Gly-Gly (RGG) motifs and interacts with RNA polymerase II (RNAPII) to regulate exon splicing [86,88]. In another class of m6A readers, insulin-like growth factor 2 binding protein 1-3 (IGF2BP1-3) and Prrc2a stabilize m6A-containing mRNA [92,93]. The known m6A readers and their functions are listed in table 2.
Table 2.
Functions of m6A readers.
| molecule | functions | references | |
|---|---|---|---|
| YTH domain family | YTHDF1 | promote m6A-modified RNA translation | [77] |
| YTHDF2 | regulate m6A-modified RNA degradation | [29,73,74] | |
| YTHDF3 | promote m6A-modified RNA translation and degradation | [77,78] | |
| YTHDC1 | regulate m6A-modified RNA splicing, export and degradation | [79–81] | |
| YTHDC2 | promote m6A-modified RNA translation and degradation | [82,83] | |
| hnRNP family | hnRNPC | regulate the abundance and alternative splicing of target genes | [87] |
| hnRNPG | regulate the alternative splicing of target genes | [86,88] | |
| hnRNPA2B1 | regulate the alternative splicing of target genes and microRNA maturity | [27,85] | |
| others | eIF3 | promote m6A-modified RNA translation | [84] |
| IGF2BP1–3 | stabilize m6A-modified mRNA | [92] | |
| Prrc2a | stabilize m6A-modified mRNA | [93] |
4. Biological function of m6A
With the improvement of m6A sequencing and detecting technology, many regulatory functions and mechanisms of m6A have been revealed in a variety of biological processes. Several studies have examined the biological function of m6A in gene expression regulation [94], organism development [95] and cancer development [96].
4.1. The regulation of gene expression
Modification by m6A regulates gene expression by affecting the splicing, translation, stability and localization of mRNA.
4.1.1. mRNA splicing
The function of m6A was initially proposed to be the regulation of mRNA splicing because characterized m6A residues were observed in the nucleus and in introns of pre-mRNA, and because intron splicing can reduce the m6A level of total RNA [97,98]. Knockout of WTAP or METTL3 causes variable mRNA splicing isoforms [45]. Several m6A reader proteins can promote splicing events, including YTHDC1, which regulates splicing via recruiting other splicing-related proteins [79], as well as hnRNPC and hnRNPA2B1 that regulate splicing via binding to m6A-dependent structural switches [87,88]. Additionally, hnRNPG as splicing factors can interact with both nascent RNA and the carboxy-terminal domain (CTD) of RNAPII to regulate alternative splicing of m6A-modified RNA by hnRNPG binding and RNAPII occupancy [86]. In addition, ALKBH5 has been shown to affect splicing rates [62]. Further studies revealed that the deletion of METTL3 in mouse embryonic stem cells (mESCs) can reduce 0.5% of alternative splicing events [41,99]. Overall these results support a model in which m6A regulates mRNA splicing.
4.1.2. mRNA translation
Early studies found significantly enrichment of ribosome-related components in m6A-containing mRNA that was not observed in mRNA without m6A [100]. The m6A reader YTHDF1 increases the translation efficiency of m6A-modified mRNA through direct interaction with translation initiation factors and ribosomal subunits [76,78]. Another YTH domain protein, YTHDF3, interacts with YTHDF1 in HeLa cells to promote translation, but a clear mechanism by which the combination of these two factors affect translation has not been described [78]. Notably, increased 5′ UTR methylation in the form of m6A can promote translation initiation independent of a 5′-end N7-methylguanosine cap [75]. Separately from catalytic activity, METTL3 enhances translation of bound RNA by directly recruiting translation initiation factors in an RNA-independent manner [58]. Most recently, a study showed that METTL3 can interact with the eIF3 h subunit at the 5′-end of mRNA bound to specific sites near the translation stop codon to facilitate circularization of the mRNA for ribosome recycling and translational control [59].
4.1.3. mRNA stability
Knockdown of METTL3 and METTL14 led to a modest increase in stability of methylated transcripts, suggesting that m6A can influence mRNA stability [44]. Studies on the half-life of target mRNAs revealed a significant increase in stability when YTHDF2 was not present, indicating that YTHDF2 accelerates mRNA degradation. Importantly, YTHDF2 localized to P-bodies, a subset of cellular processing bodies [73]. Consistent with this view, a study found that YTHDF2 recruits CCR4-NOT through direct interaction with CNOT1 to promote degradation of methylated transcripts [74]. Another m6A reader involved in RNA degradation is YTHDC2. The researchers observed that a slight increase in the expression of m6A-modified transcripts in YTHDC2-knockout testes [83]. Unlike the m6A reader, m6A may regulate RNA stability by affecting its secondary structure. The RNA-binding protein HuR, which binds to the U-rich region of the 3′ UTR in mRNA, blocks binding of the miRNA and thus prevents degradation [101]. Studies have shown that m6A interferes with HuR binding in miRNA target genes, therefore promoting the degradation of mRNA. At the same time, knockout of METTL3 inhibits Ago2 binding to target mRNA and increases its stability [44]. A new mechanism of m6A-modified RNAs degradation was reported recently, in which HRSP12 acts as an adaptor to connect YTHDF2 and RNase P/MRP (endoribonucleases) resulting in endoribonucleolytic cleavage of YTHDF2-bound RNAs [29].
4.1.4. mRNA export
Knockdown of METTL3 can prevent the nuclear export of circadian clock genes Per2 and Arntl, resulting in a prolonged circadian period [102]. ALKBH5 is mainly localized in nuclear speckles and depletion of ALKBH5 can accelerate the nuclear export of target RNAs [62]. Combined depletion of WTAP and KIAA1429 led to a nuclear accumulation of specific m6A-modified transcripts [103]. The m6A reader YTHDC1 mediates the export of methylated mRNA from the nucleus to the cytoplasm in human cells. YTHDC1 can interact with SRSF3 and SRSF3 interacts with the nuclear export receptor NXF1 to mediate the export of mRNA. The knockdown of YTHDC1 does not affect the overall level of m6A, but does result in nuclear accumulation of mRNA [80]. The nuclear export of mRNA is controlled by the TREX complex and the heterodimeric nuclear export receptor NXF1-P15 [104]. The m6A writer complex can recruit TREX to m6A-modified mRNAs and TREX can stimulate recruitment of YTHDC1 and NXF1, resulting in the export of mRNA [103]. Both Zika virus and HIV-1 have a high level of m6A methylation, with accelerated nuclear export, and other processing steps dependent on m6A during replication, suggesting that methylation may be necessary for nuclear export of mRNA [105].
4.2. Organism development
Growing research indicates that m6A modification is necessary for early embryo development. Early studies showed a lack of Ime4 in Drosophila, the METTL3 homologous protein, has a semi-lethal effect on development, and the fertility of adult individuals is reduced owing to impaired NOTCH signalling [40]. Recent study indicates that depletion of Ime4 in Drosophila does not really cause prominent lethality in adults. The study showed that m6A methyltransferase plays a critical regulator in controlling neuronal functions and sex determination by its nuclear reader YT521-B [106]. However, depletion of METTL3 in mice has a lethal effect on embryonic development [41]. Furthermore, in Arabidopsis, the absence of the orthologue of the yeast and human mRNA adenosine methylase (MTA) can affect embryonic development and in yeast, Ime4 plays an important role in cell meiosis [23,107]. During the maternal to zygomatic transition (MZT) in zebrafish, maternal mRNAs with m6A modification were rapidly cleared by YTHDF2. Knockout of YTHDF2 increased the stability of maternal mRNAs and prevented the egg transforming into the fertilized state, ultimately slowing the embryo from entering the MZT and delaying development of the offspring zebrafish [108]. These studies indicate that m6A modification is required for early embryo development in animals.
Recent studies have shown that m6A is involved in various physiological processes, such as stem cell self-renewal and differentiation, lipid metabolism, glucose metabolism, DNA damage repair, control of heat shock response, and circadian rhythm. The lack of YTHDF1 can impair hippocampal-dependent neurological functions in mice such as spatial learning and memory, but overexpression of YTHDF1 in the hippocampus can restore this damage. It was showed that binding of YTHDF1 to methylated transcripts can promote the function of synaptic transmission and long-term potentiation genes [109]. After an organism was subjected to heat shock, METTL3 rapidly bound to heat shock genes and YTHDF2 can compete with the FTO to prevent 5′ UTR demethylation, thus enhancing translation [75]. Similarly, after DNA ultraviolet damage, transcripts methylated by METTL3 are rapidly localized at the site of injury, and then recruit DNA polymerase κ (Pol κ) to promote damage repair [110]. The process of m6A methylation also plays a vital physiological role in the circadian rhythm cycle. Reduced m6A can prevent the nuclear export of circadian clock genes Per2 and Arntl [102]. There are many reported roles of m6A in lipid metabolism. FTO-dependent demethylation led to lipid accumulation and triglyceride deposition in skeletal muscle cells and hepatocytes [111,112]. FTO can also affect glucose metabolism by reducing the m6A level of FOXO1, an important transcription factor that regulates hepatic gluconeogenesis [113].
Many studies have emphasized a role of m6A in the regulation of stem cell differentiation. Earlier research showed that knockdown of METTL3 or METTL14 reduced the level of m6A and self-renewal in mESCs [44]. However, conflicting results indicated that knockout of METTL3 in mESCs increased self-renewal and impaired differentiation towards cardiomyocytes and neurons by enhancing the level of regulator Nanog necessary for self-renewal [114]. The knockout of METTL3 in early mouse embryos failed to transform naive mESCs into the primed state, resulting in post-implantation embryo death. However the knockdown of METTL3 at a primed pluripotency state promoted differentiation [41]. This result suggests that m6A may serve as a switch to regulate the expression of multiple pluripotency genes and developmental regulators in early embryos. Similarly in mouse embryonic fibroblasts, knockdown of METTL3 resulted in a decrease in m6A abundance and improved reprogramming efficiency [115]. In addition, in haematopoietic stem cells (HSCs) the knockout of YTHDF2 can maintain the function of HSCs by regulating the stability of multiple mRNAs critical for HSC self-renewal [116]. Taken together, these results suggested that m6A is required for maintaining pluripotency and stem cell differentiation.
4.3. Cancer development
The process of m6A methylation has been related to the development of human diseases, especially cancer proliferation, apoptosis and metastasis. The deletion of WTAP in a human acute myeloid leukaemia (AML) cell line reduced proliferation and increased differentiation and apoptosis [117]. Consistent with that effect of WTAP deletion, deletion of METTL3 in the AML cell line promoted cell differentiation and apoptosis by reducing translation of METTL3-binding genes, including MYC, BCL2 and PTEN [118]. Meanwhile, METTL14 play an oncogenic role in the AML cell line by regulating m6A methylation of tumour genes MYB and MYC [119]. However, the reduction of m6A plays an oncogenic role in some AMLs. In the t (11q23), t (15; 17), and FLT3-ITD type AML cell lines, FTO is highly expressed, which can promote leukemogenesis. FTO can also suppress AML cell differentiation induced by all-trans-retinoic acid (ATRA) treatment [120].
In breast cancer (BC), HBXIP can upregulate METTL3 by suppressing miRNA let-7 g, and METTL3 promotes HBXIP expression through increased m6A modification, leading to an accelerated proliferation of BC cells [121]. In human pancreatic cancer (PC), YTHDF2 is upregulated to promote cancer cell proliferation and the epithelial-mesenchymal transition (EMT) [122]. The overall m6A level was significantly enriched in PC cells and overexpression of ALKBH5 can inhibit cancer cell migration and invasion by demethylating lncRNA KCNK15-AS1 [123]. In human hepatoma cells (HCC), decreased m6A and METTL14 was detected and overexpression of METTL14 can interact with DGCR8 to regulate the maturation of pri-miRNA126 in an m6A-dependent manner, reducing the metastasis of hepatoma cells [124]. However, METTL3 is upregulated in HCC and knockdown of METTL3 significantly inhibits the proliferation, migration and metastasis of cancer cells. The results indicated that METTL3 can increase m6A abundance in SOCS2 mRNA, with degradation that is dependent on YTHDF2, which ultimately promoted liver cancer [125]. The conflicting results demonstrate that more work remains to explore the function of m6A methylation.
Glioblastoma stem cells (GSCs) possess self-renewal and differentiation capabilities in malignant tumours. The knockdown of METTL3 and METTL14 in GSCs reduced the m6A level, thereby enhancing the expression of oncogenes including ADAM19, EPHA3 and KLF4, which promoted cell growth and self-renewal. However, overexpression of METTL3 or knockdown of FTO inhibited GSC growth and self-renewal, resulting in inhibition of tumorigenesis [126]. The latest research has shown that m6A modification affects tumour antigen-specific T cell immune responses by regulating the translation efficiency of lysosomal cathepsin in dendritic cells [127]. Knockout of YTHDF1 in mice enhances the response of tumour antigen-specific CD8+ T cells. Further study showed that the mRNAs of multiple lysosomal cathepsins are m6A modified and methylated transcripts can be recognized by YTHDF1, resulting in increased translation [127].
Recent studies provide evidence that m6A methylation may be used as a potential prognostic biomarker of the tumour. In gastric cancer, reduced m6A modification can promote gastric cancer malignancy by activating oncogenic signalling, and FTO acting as an oncogene can promote tumour growth [128,129]. Similarly, hnRNPC was identified as an independent prognostic biomarker in oral squamous cell carcinoma (OSCC) and the overexpression of hnRNPC facilitated the development of OSCC cells in vitro [130]. MALDI-TOF-MS revealed that the methylation level of miRNA methylation was significantly higher in PC compared with normal tissues, so evaluating miRNA methylation is a promising diagnostic strategy [131]. However, whether m6A methylation can serve as a molecular tool to regulate gene expression for treatment of human diseases is a key question to be addressed.
5. Conclusion
Similar to DNA methylation, RNA m6A methylation is a dynamic reversible modification catalysed by methyltransferase and demethylase, where the proteins that recognize this modification alter the function of the target mRNA. With improved technology to detect and analyse m6A, recent years have witnessed a rapid advance in studies on m6A methylation. The m6A methylation is widely found in various RNAs in both prokaryotes and eukaryotes, and m6A methylation can regulate RNA stabilization, transport, splicing and translation. In addition, m6A methylation can alter RNA structures to affect the interaction of mRNA binding proteins [88]. Additionally, m6A is closely related to embryonic development, cancer metastasis, immune response, stem cell self-renewal differentiation, lipid metabolism, glucose metabolism, DNA damage repair, heat shock response control and circadian rhythm control.
Several challenging questions about m6A methylation remain to be addressed. In mammals a consensus sequence of m6A: G [G/A] (m6A) CU has been defined. However, although this consensus motif is ubiquitous in the transcriptome, only a fraction of these sites are methylated in vivo. Thus, we still need to elucidate the mechanisms for selective specificity in the m6A-modified transcripts. The result of this selection may be related to different requirements for development and environmental stimuli. Functions of m6A may vary in different environmental stimuli or cellular type. For example, in heat stress, the level of m6A increases in the 5′ UTR and promotes the expression of HSF by initiating independent-cap translation, thus promoting the response of the cell heat shock pathway [75]. This dynamical change probably leads to different fates of the methylated RNAs for various environmental stimuli or cellular types. Additionally, the diversity in the binding classes of m6A readers also can change the fate of methylated transcripts. YTHDF3 has two different functions, acting to promote targeted mRNA translation with YTHDF1 and acting to accelerate degradation with YTHDF2 [78]. The mechanism by which YTHDF3 combines with YTHDF1 or YTHDF2 remains unclear. This complexity means that simply exploring the m6A functions in single environmental systems may not adequately reveal the comprehensive roles of m6A methylation in multiple biological processes. YTHDC1 possesses a variety of regulatory functions, but how YTHDC1 selects different sets of m6A-modified RNA is unclear [79–81]. Indeed, there is limited understanding of how m6A readers identify and select their target transcripts to modulate the fate of modified RNAs. In addition, METTL3 and ALKBH5 have regulatory functions for modified RNAs that are independent of both catalytic activity and m6A readers [62,59]. Thus, we should continue to investigate the function and molecular mechanisms of m6A methylase to better understand this complex process. Collectively, m6A methylation needs us to uncover the occurrence and function from different layers.
Data accessibility
This article has no additional data.
Authors' contributions
W.R. and H.C. conceived and designed the review; W.R. wrote the manuscript; X.Z., B.Y., A.Q., X.S., Y.H., X.L. and C.L. contributed to revision and finalization of the manuscript.
Competing interests
The authors declare no competing interests.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 31772574).
References
- 1.Saletore Y, Meyer K, Korlach J, Vilfan ID, Jaffrey S, Mason CE. 2012. The birth of the Epitranscriptome: deciphering the function of RNA modifications. Genome Biol. 13, 175 ( 10.1186/gb-2012-13-10-175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer KD, Jaffrey SR. 2014. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev. Mol. Cell Biol. 15, 313–326. ( 10.1038/nrm3785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krug RM, Morgan MA, Shatkin AJ. 1976. Influenza viral mRNA contains internal N6-methyladenosine and 5′-terminal 7-methylguanosine in cap structures. J. Virol. 20, 45–53. ( 10.1128/JVI.20.1.45-53.1976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominissini D, et al. 2012. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206. ( 10.1038/nature11112) [DOI] [PubMed] [Google Scholar]
- 5.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. 2012. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149, 1635–1646. ( 10.1016/j.cell.2012.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo GZ, et al. 2014. Unique features of the m6A methylome in Arabidopsis thaliana. Nat. Commun. 5, 5630 ( 10.1038/ncomms6630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yue Y, Liu J, He C. 2015. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 29, 1343–1355. ( 10.1101/gad.262766.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia G, et al. 2011. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 7, 885 ( 10.1038/nchembio.687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry R, Kelley D. 1974. Existence of methylated messenger RNA in mouse L cells. Cell 1, 37–42. ( 10.1016/0092-8674(74)90153-6) [DOI] [Google Scholar]
- 10.Desrosiers R, Friderici K, Rottman F. 1974. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc. Natl Acad. Sci. USA 71, 3971–3975. ( 10.1073/pnas.71.10.3971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi YC, Busch H. 1978. Modified nucleotides in T1 RNase oligonucleotides of 18S ribosomal RNA of the Novikoff hepatoma. Biochemistry 17, 2551–2560. ( 10.1021/bi00606a015) [DOI] [PubMed] [Google Scholar]
- 12.Bringmann P, Lührmann R. 1987. Antibodies specific for N6-methyladenosine react with intact snRNPs U2 and U4/U6. FEBS Lett. 213, 309–315. ( 10.1016/0014-5793(87)81512-0) [DOI] [PubMed] [Google Scholar]
- 13.Shimba S, Bokar JA, Rottman F, Reddy R. 1995. Accurate and efficient N-6-adenosine methylation in spliceosomal U6 small nucelar RNA by HeLa cell extract in vitro. Nucleic Acids Res. 23, 2421–2426. ( 10.1093/nar/23.13.2421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao G, Li HB, Yin Z, Flavell RA. 2016. Recent advances in dynamic m6A RNA modification. Open Biol. 6, 160003 ( 10.1098/rsob.160003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. 2015. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat. Methods 12, 767–772. ( 10.1038/nmeth.3453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen K, et al. 2015. High-resolution N6-methyladenosine (m6A) map using photo-crosslinking-assisted m6A sequencing. Angew. Chem. Int. Ed. 54, 1587–1590. ( 10.1002/anie.201410647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang H, et al. 2019. Histone H3 trimethylation at lysine 36 guides m6A RNA modification co-transcriptionally. Nature 567, 414–419. ( 10.1038/s41586-019-1016-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song T, et al. 2019. Zfp217 mediates m6A mRNA methylation to orchestrate transcriptional and post-transcriptional regulation to promote adipogenic differentiation. Nucleic Acids Res. 47, 6130–6144. ( 10.1093/nar/gkz312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertero A, et al. 2018. The SMAD2/3 interactome reveals that TGFβ controls m6A mRNA methylation in pluripotency. Nature 555, 256–259. ( 10.1038/nature25784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schibler U, Kelley DE, Perry RP. 1977. Comparison of methylated sequences in messenger RNA and heterogeneous nuclear RNA from mouse L cells. J. Mol. Biol. 115, 695–714. ( 10.1016/0022-2836(77)90110-3) [DOI] [PubMed] [Google Scholar]
- 21.Wei CM, Moss B. 1977. Nucleotide sequences at the N6-methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry 16, 1672–1676. ( 10.1021/bi00627a023) [DOI] [PubMed] [Google Scholar]
- 22.Meyer KD, Jaffrey SR. 2014. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev. Mol. Cell Biol. 15, 313–326. ( 10.1038/nrm3785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwala SD, Blitzblau HG, Hochwagen A, Fink GR. 2012. RNA methylation by the MIS complex regulates a cell fate decision in yeast. PLoS Genet. 8, e1002732 ( 10.1371/journal.pgen.1002732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi H, Wei J, He C. 2019. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol. Cell 74, 640–650. ( 10.1016/j.molcel.2019.04.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, Jaffrey SR. 2016. m6A RNA methylation promotes XIST-mediated transcriptional repression. Nature 537, 369–373. ( 10.1038/nature19342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu N, Parisien M, Dai Q, Zheng G, He C, Pan T. 2013. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA 19, 1848–1856. ( 10.1261/rna.041178.113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alarcón CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. 2015. N6-methyladenosine marks primary microRNAs for processing. Nature 519, 482–485. ( 10.1038/nature14281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, et al. 2017. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 27, 626–641. ( 10.1038/cr.2017.31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park OH, Ha H, Lee Y, Boo SH, Kwon DH, Song HK, Kim YK. 2019. Endoribonucleolytic cleavage of m6A-containing RNAs by RNase P/MRP complex. Mol. Cell 74, 494–507. e498. ( 10.1016/j.molcel.2019.02.034) [DOI] [PubMed] [Google Scholar]
- 30.Chen RX, et al. 2019. N6-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat. Commun. 10, 1–15. ( 10.1038/s41467-019-12651-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang C, et al. 2020. m6A-dependent biogenesis of circular RNAs in male germ cells. Cell Res. 30, 211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li K, et al. 2020. Landscape and regulation of m6A and m6Am methylome across human and mouse tissues. Mol. Cell 77, 426–440. e426. ( 10.1016/j.molcel.2019.09.032) [DOI] [PubMed] [Google Scholar]
- 33.Lence T, Paolantoni C, Worpenberg L, Roignant JY. 2019. Mechanistic insights into m6A RNA enzymes. Biochim. Biophys. Acta Gene Regul. Mech. 1862, 222–229. ( 10.1016/j.bbagrm.2018.10.014) [DOI] [PubMed] [Google Scholar]
- 34.Bokar J, Shambaugh M, Polayes D, Matera A, Rottman F. 1997. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA 3, 1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, et al. 2016. Structural basis of N6-adenosine methylation by the METTL3–METTL14 complex. Nature 534, 575–578. ( 10.1038/nature18298) [DOI] [PubMed] [Google Scholar]
- 36.Wang P, Doxtader KA, Nam Y. 2016. Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol. Cell 63, 306–317. ( 10.1016/j.molcel.2016.05.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bokar JA. 2005. The biosynthesis and functional roles of methylated nucleosides in eukaryotic mRNA. In Fine-tuning of RNA functions by modification and editing (ed. Grosjean H.), pp. 141–177. Berlin, Germany: Springer. [Google Scholar]
- 38.Clancy MJ, Shambaugh ME, Timpte CS, Bokar JA. 2002. Induction of sporulation in Saccharomyces cerevisiae leads to the formation of N6-methyladenosine in mRNA: a potential mechanism for the activity of the IME4 gene. Nucleic Acids Res. 30, 4509–4518. ( 10.1093/nar/gkf573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong S, Li H, Bodi Z, Button J, Vespa L, Herzog M, Fray RG. 2008. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell 20, 1278–1288. ( 10.1105/tpc.108.058883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hongay CF, Orr-Weaver TL. 2011. Drosophila Inducer of MEiosis 4 (IME4) is required for Notch signaling during oogenesis. Proc. Natl Acad. Sci. USA 108, 14 855–14 860. ( 10.1073/pnas.1111577108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geula S, et al. 2015. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science 347, 1002–1006. ( 10.1126/science.1261417) [DOI] [PubMed] [Google Scholar]
- 42.Bujnicki JM, Feder M, Radlinska M, Blumenthal RM. 2002. Structure prediction and phylogenetic analysis of a functionally diverse family of proteins homologous to the MT-A70 subunit of the human mRNA: m6A methyltransferase. J. Mol. Evol. 55, 431–444. ( 10.1007/s00239-002-2339-8) [DOI] [PubMed] [Google Scholar]
- 43.Liu J, et al. 2014. A METTL3–METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 10, 93–95. ( 10.1038/nchembio.1432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. 2014. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat. Cell Biol. 16, 191–198. ( 10.1038/ncb2902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ping XL, et al. 2014. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 24, 177–189. ( 10.1038/cr.2014.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horiuchi K, Kawamura T, Iwanari H, Ohashi R, Naito M, Kodama T, Hamakubo T. 2013. Identification of Wilms' tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J. Biol. Chem. 288, 33 292–33 302. ( 10.1074/jbc.M113.500397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz S, et al. 2014. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep. 8, 284–296. ( 10.1016/j.celrep.2014.05.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yue Y, et al. 2018. VIRMA mediates preferential m6A mRNA methylation in 3′ UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 4, 10 ( 10.1038/s41421-018-0019-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, Conrad NK. 2017. The U6 snRNA m6A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell 169, 824–835. e814. ( 10.1016/j.cell.2017.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warda AS, Kretschmer J, Hackert P, Lenz C, Urlaub H, Höbartner C, Sloan KE, Bohnsack MT. 2017. Human METTL16 is a N6-methyladenosine (m6A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 18, 2004–2014. ( 10.15252/embr.201744940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown JA, Kinzig CG, DeGregorio SJ, Steitz JA. 2016. Methyltransferase-like protein 16 binds the 3′-terminal triple helix of MALAT1 long noncoding RNA. Proc. Natl Acad. Sci. USA 113, 14 013–14 018. ( 10.1073/pnas.1614759113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mendel M, Chen KM, Homolka D, Gos P, Pandey RR, McCarthy AA, Pillai RS. 2018. Methylation of structured RNA by the m6A writer METTL16 is essential for mouse embryonic development. Mol. Cell 71, 986–1000. e1011. ( 10.1016/j.molcel.2018.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horiuchi K, Kawamura T, Iwanari H, Ohashi R, Naito M, Kodama T, Hamakubo T. 2013. Identification of Wilms’ tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J. Biol. Chem. 288, 33 292–33 302. ( 10.1074/jbc.M113.500397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Růžička K, et al. 2017. Identification of factors required for m6A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol. 215, 157–172. ( 10.1111/nph.14586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wen J, et al. 2018. Zc3h13 regulates nuclear RNA m6A methylation and mouse embryonic stem cell self-renewal. Mol. Cell 69, 1028–1038. e1026. ( 10.1016/j.molcel.2018.02.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knuckles P, et al. 2018. Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA-binding factor Rbm15/Spenito to the m6A machinery component Wtap/Fl(2)d. Genes Dev. 32, 415–429. ( 10.1101/gad.309146.117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma H, et al. 2019. N6-Methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat. Chem. Biol. 15, 88–94. ( 10.1038/s41589-018-0184-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin S, Choe J, Du P, Triboulet R, Gregory RI. 2016. The m6A methyltransferase METTL3 promotes translation in human cancer cells. Mol. Cell 62, 335–345. ( 10.1016/j.molcel.2016.03.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choe J, et al. 2018. mRNA circularization by METTL3–eIF3 h enhances translation and promotes oncogenesis. Nature 561, 556–560. ( 10.1038/s41586-018-0538-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartosovic M, Molares HC, Gregorova P, Hrossova D, Kudla G, Vanacova S. 2017. N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3′-end processing. Nucleic Acids Res. 45, 11 356–11 370. ( 10.1093/nar/gkx778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei J, et al. 2018. Differential m6A, m6Am, and m1A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol. Cell 71, 973–985. e975. ( 10.1016/j.molcel.2018.08.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng G, et al. 2013. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 49, 18–29. ( 10.1016/j.molcel.2012.10.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aik W, Scotti JS, Choi H, Gong L, Demetriades M, Schofield CJ, McDonough MA. 2014. Structure of human RNA N6-methyladenine demethylase ALKBH5 provides insights into its mechanisms of nucleic acid recognition and demethylation. Nucleic Acids Res. 42, 4741–4754. ( 10.1093/nar/gku085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang C, Klukovich R, Peng H, Wang Z, Yu T, Zhang Y, Zheng H, Klungland A, Yan W. 2018. ALKBH5-dependent m6A demethylation controls splicing and stability of long 3′-UTR mRNAs in male germ cells. Proc. Natl Acad. Sci. USA 115, E325–E333. ( 10.1073/pnas.1717794115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gerken T, et al. 2007. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 318, 1469–1472. ( 10.1126/science.1151710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Falnes PØ, Johansen RF, Seeberg E. 2002. AlkB-mediated oxidative demethylation reverses DNA damage in Escherichia coli. Nature 419, 178–182. ( 10.1038/nature01048) [DOI] [PubMed] [Google Scholar]
- 67.Dina C, et al. 2007. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 39, 724–726. ( 10.1038/ng2048) [DOI] [PubMed] [Google Scholar]
- 68.Jia G, Yang CG, Yang S, Jian X, Yi C, Zhou Z, He C. 2008. Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Lett. 582, 3313–3319. ( 10.1016/j.febslet.2008.08.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fu Y, et al. 2013. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat. Commun. 4, 1798 ( 10.1038/ncomms2822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mauer J, et al. 2019. FTO controls reversible m6Am RNA methylation during snRNA biogenesis. Nat. Chem. Biol. 15, 340–347. ( 10.1038/s41589-019-0231-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stoilov P, Rafalska I, Stamm S. 2002. YTH: a new domain in nuclear proteins. Trends Biochem. Sci. 27, 495–497. ( 10.1016/S0968-0004(02)02189-8) [DOI] [PubMed] [Google Scholar]
- 72.Theler D, Dominguez C, Blatter M, Boudet J, Allain FHT. 2014. Solution structure of the YTH domain in complex with N6-methyladenosine RNA: a reader of methylated RNA. Nucleic Acids Res. 42, 13 911–13 919. ( 10.1093/nar/gku1116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X, et al. 2014. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120. ( 10.1038/nature12730) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, Ma J, Wu L. 2016. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4–NOT deadenylase complex. Nat. Commun. 7, 12626 ( 10.1038/ncomms12626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB. 2015. Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature 526, 591–594. ( 10.1038/nature15377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang X, et al. 2015. N6-methyladenosine modulates messenger RNA translation efficiency. Cell 161, 1388–1399. ( 10.1016/j.cell.2015.05.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li A, et al. 2017. Cytoplasmic m6A reader YTHDF3 promotes mRNA translation. Cell Res. 27, 444–447. ( 10.1038/cr.2017.10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, Liu C, He C. 2017. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 27, 315–328. ( 10.1038/cr.2017.15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiao W, et al. 2016. Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol. Cell 61, 507–519. ( 10.1016/j.molcel.2016.01.012) [DOI] [PubMed] [Google Scholar]
- 80.Roundtree IA, et al. 2017. YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. Elife 6, e31311 ( 10.7554/eLife.31311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shima H, et al. 2017. S-Adenosylmethionine synthesis is regulated by selective N6-adenosine methylation and mRNA degradation involving METTL16 and YTHDC1. Cell Rep. 21, 3354–3363. ( 10.1016/j.celrep.2017.11.092) [DOI] [PubMed] [Google Scholar]
- 82.Tanabe A, et al. 2016. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF-1α mRNA is translated. Cancer Lett. 376, 34–42. ( 10.1016/j.canlet.2016.02.022) [DOI] [PubMed] [Google Scholar]
- 83.Hsu PJ, et al. 2017. Ythdc2 is an N6-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 27, 1115–1127. ( 10.1038/cr.2017.99) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB, Jaffrey SR. 2015. 5′ UTR m6A promotes cap-independent translation. Cell 163, 999–1010. ( 10.1016/j.cell.2015.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. 2015. HNRNPA2B1 is a mediator of m6A-dependent nuclear RNA processing events. Cell 162, 1299–1308. ( 10.1016/j.cell.2015.08.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou KI, Shi H, Lyu R, Wylder AC, Matuszek Ż, Pan JN, He C, Parisien M, Pan T. 2019. Regulation of co-transcriptional pre-mRNA splicing by m6A through the low-complexity protein hnRNPG. Mol. Cell 76, 70–81. e79. ( 10.1016/j.molcel.2019.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. 2015. N6-methyladenosine-dependent RNA structural switches regulate RNA–protein interactions. Nature 518, 560–564. ( 10.1038/nature14234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, Pan T. 2017. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 45, 6051–6063. ( 10.1093/nar/gkx141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.König J, Zarnack K, Rot G, Curk T, Kayikci M, Zupan B, Turner DJ, Luscombe NM, Ule J. 2010. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat. Struct. Mol. Biol. 17, 909–915. ( 10.1038/nsmb.1838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cienikova Z, Damberger FF, Hall J, Allain FHT, Maris C. 2014. Structural and mechanistic insights into poly(uridine) tract recognition by the hnRNP C RNA recognition motif. J. Am. Chem. Soc. 136, 14 536–14 544. ( 10.1021/ja507690d) [DOI] [PubMed] [Google Scholar]
- 91.Wan Y, et al. 2014. Landscape and variation of RNA secondary structure across the human transcriptome. Nature 505, 706–709. ( 10.1038/nature12946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang H, et al. 2018. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 20, 285–295. ( 10.1038/s41556-018-0045-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu R, et al. 2019. A novel m6A reader Prrc2a controls oligodendroglial specification and myelination. Cell Res. 29, 23–41. ( 10.1038/s41422-018-0113-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roundtree IA, Evans ME, Pan T, He C. 2017. Dynamic RNA modifications in gene expression regulation. Cell 169, 1187–1200. ( 10.1016/j.cell.2017.05.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frye M, Harada BT, Behm M, He C. 2018. RNA modifications modulate gene expression during development. Science 361, 1346–1349. ( 10.1126/science.aau1646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pan Y, Ma P, Liu Y, Li W, Shu Y. 2018. Multiple functions of m6A RNA methylation in cancer. J. Hematol. Oncol. 11, 48 ( 10.1186/s13045-018-0590-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carroll S, Narayan P, Rottman F. 1990. N6-methyladenosine residues in an intron-specific region of prolactin pre-mRNA. Mol. Cell. Biol. 10, 4456–4465. ( 10.1128/MCB.10.9.4456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salditt-Georgieff M, Jelinek W, Darnell JE, Furuichi Y, Morgan M, Shatkin A. 1976. Methyl labeling of HeLa cell hnRNA: a comparison with mRNA. Cell 7, 227–237. ( 10.1016/0092-8674(76)90022-2) [DOI] [PubMed] [Google Scholar]
- 99.Ke S, et al. 2017. m6A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev. 31, 990–1006. ( 10.1101/gad.301036.117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bodi Z, Bottley A, Archer N, May ST, Fray RG. 2015. Yeast m6A methylated mRNAs are enriched on translating ribosomes during meiosis, and under rapamycin treatment. PLoS ONE 10, e0132090 ( 10.1371/journal.pone.0132090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kundu P, Fabian MR, Sonenberg N, Bhattacharyya SN, Filipowicz W. 2012. HuR protein attenuates miRNA-mediated repression by promoting miRISC dissociation from the target RNA. Nucleic Acids Res. 40, 5088–5100. ( 10.1093/nar/gks148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fustin JM, et al. 2013. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell 155, 793–806. ( 10.1016/j.cell.2013.10.026) [DOI] [PubMed] [Google Scholar]
- 103.Lesbirel S, Viphakone N, Parker M, Parker J, Heath C, Sudbery I, Wilson SA. 2018. The m6A-methylase complex recruits TREX and regulates mRNA export. Sci. Rep. 8, 1–12. ( 10.1038/s41598-018-32310-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lesbirel S, Wilson SA. 2019. The m6A-methylase complex and mRNA export. Biochim. Biophys. Acta Gene Regul. Mech. 1862, 319–328. ( 10.1016/j.bbagrm.2018.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lichinchi G, Zhao BS, Wu Y, Lu Z, Qin Y, He C, Rana TM. 2016. Dynamics of human and viral RNA methylation during Zika virus infection. Cell Host Microbe 20, 666–673. ( 10.1016/j.chom.2016.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lence T, et al. 2016. m6A modulates neuronal functions and sex determination in Drosophila. Nature 540, 242–247. ( 10.1038/nature20568) [DOI] [PubMed] [Google Scholar]
- 107.Bodi Z, Zhong S, Mehra S, Song J, Li H, Graham N, May S, Fray RG. 2012. Adenosine methylation in Arabidopsis mRNA is associated with the 3′ end and reduced levels cause developmental defects. Front. Plant Sci. 3, 48 ( 10.3389/fpls.2012.00048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao BS, Wang X, Beadell AV, Lu Z, Shi H, Kuuspalu A, Ho RK, He C. 2017. m6A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature 542, 475–478. ( 10.1038/nature21355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shi H, et al. 2018. m 6 A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature 563, 249–253. ( 10.1038/s41586-018-0666-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xiang Y, et al. 2017. RNA m6A methylation regulates the ultraviolet-induced DNA damage response. Nature 543, 573–576. ( 10.1038/nature21671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu W, Feng J, Jiang D, Zhou X, Jiang Q, Cai M, Wang X, Shan T, Wang Y. 2017. AMPK regulates lipid accumulation in skeletal muscle cells through FTO-dependent demethylation of N6-methyladenosine. Sci. Rep. 7, 41606 ( 10.1038/srep41606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kang H, Zhang Z, Yu L, Li Y, Liang M, Zhou L. 2018. FTO reduces mitochondria and promotes hepatic fat accumulation through RNA demethylation. J. Cell. Biochem. 119, 5676–5685. ( 10.1002/jcb.26746) [DOI] [PubMed] [Google Scholar]
- 113.Peng S, et al. 2019. Identification of entacapone as a chemical inhibitor of FTO mediating metabolic regulation through FOXO1. Sci. Transl. Med. 11, eaau7116 ( 10.1126/scitranslmed.aau7116) [DOI] [PubMed] [Google Scholar]
- 114.Batista PJ, et al. 2014. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell 15, 707–719. ( 10.1016/j.stem.2014.09.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen T, et al. 2015. m6A RNA methylation is regulated by microRNAs and promotes reprogramming to pluripotency. Cell Stem Cell 16, 289–301. ( 10.1016/j.stem.2015.01.016) [DOI] [PubMed] [Google Scholar]
- 116.Li Z, et al. 2018. Suppression of m6A reader Ythdf2 promotes hematopoietic stem cell expansion. Cell Res. 28, 904–917. ( 10.1038/s41422-018-0072-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bansal H, et al. 2014. WTAP is a novel oncogenic protein in acute myeloid leukemia. Leukemia 28, 1171–1174. ( 10.1038/leu.2014.16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vu LP, et al. 2017. The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat. Med. 23, 1369–1376. ( 10.1038/nm.4416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Weng H, et al. 2018. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m6A modification. Cell Stem Cell 22, 191–205. e199. ( 10.1016/j.stem.2017.11.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Z, et al. 2017. FTO plays an oncogenic role in acute myeloid leukemia as a N6-methyladenosine RNA demethylase. Cancer Cell. 31, 127–141. ( 10.1016/j.ccell.2016.11.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cai X, et al. 2018. HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7 g. Cancer Lett. 415, 11–19. ( 10.1016/j.canlet.2017.11.018) [DOI] [PubMed] [Google Scholar]
- 122.Chen J, et al. 2017. YTH domain family 2 orchestrates epithelial-mesenchymal transition/proliferation dichotomy in pancreatic cancer cells. Cell Cycle 16, 2259–2271. ( 10.1080/15384101.2017.1380125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.He Y, et al. 2018. ALKBH5 inhibits pancreatic cancer motility by decreasing long non-coding RNA KCNK15-AS1 methylation. Cell. Physiol. Biochem. 48, 838–846. ( 10.1159/000491915) [DOI] [PubMed] [Google Scholar]
- 124.Jz M, et al. 2017. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N6-methyladenosine-dependent primary MicroRNA processing. Hepatology 65, 529–543. ( 10.1002/hep.28885) [DOI] [PubMed] [Google Scholar]
- 125.Chen M, et al. 2018. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology 67, 2254–2270. ( 10.1002/hep.29683) [DOI] [PubMed] [Google Scholar]
- 126.Cui Q, et al. 2017. m6A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 18, 2622–2634. ( 10.1016/j.celrep.2017.02.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Han D, et al. 2019. Anti-tumour immunity controlled through mRNA m6A methylation and YTHDF1 in dendritic cells. Nature 566, 270–274. ( 10.1038/s41586-019-0916-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang C, Zhang M, Ge S, Lin X, Huang W, Gan Y, Gao J, Shen L. 2019. Reduced m6A modification predicts malignant phenotypes and augmented tumorigenic signaling in gastric cancer. Cancer Med. 8, 4766–4781. ( 10.1002/cam4.2360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shimura T, Kandimalla R, Toiyama Y, Okugawa Y, Kusunoki M, Goel A. 2019. Novel evidence for m6A methylation regulators as prognostic biomarkers and potential therapeutic targets in gastric cancer. In Proc. AACR Ann. Meeting, 29 March–3 April 2019, Atlanta, GA. [DOI] [PMC free article] [PubMed]
- 130.Huang G, Wu QQ, Zheng ZN, Shao TR, Chen YC, Zeng WS. 2020. Comprehensive bioinformatics analysis reveals that N6-methyladenosine (m6A) methylation reader HNRNPC facilitates progression of OSCC through promoting epithelial-mesenchymal transition (EMT). Aging 12, 11667–11684. ( 10.18632/aging.103333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Konno M, et al. 2019. Distinct methylation levels of mature microRNAs in gastrointestinal cancers. Nat. Commun. 10, 1–7. ( 10.1038/s41467-019-11826-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.