Abstract
Background
Pneumonia is the most frequent complication of COVID-19, due to an aberrant host immune response that is associated with an acute respiratory distress syndrome, and, in most critical patients, with a “cytokine storm”. IL-6 might play a key role in the cytokine storm and might be a potential target to treat severe and critical COVID-19. Tocilizumab is a recombinant humanized monoclonal antibody, directed against IL-6 receptor.
Methods
This multicentre study project includes a single-arm phase 2 study and a further parallel cohort, enrolling hospitalized patients with COVID-19 pneumonia and oxygen saturation at rest in ambient air ≤93% or requiring respiratory support. Patients receive tocilizumab 8 mg/kg (up to 800 mg) as one intravenous administration. A second administration (same dose) after 12 h is optional. Two-week and one-month lethality rates are the co-primary endpoints. Sample size planned for the phase 2 study is 330 patients. The parallel cohort will include patients who cannot enter the phase 2 study because being intubated from more than 24 h, or having already received tocilizumab, or the phase 2 study has reached sample size. Primary analysis will include patients enrolled in the phase 2 study. Results of the primary analysis will be validated in the prospective cohort of patients consecutively registered after phase 2 closure from March 20 to March 24, who were potentially eligible for the phase 2 study.
Conclusion
This trial aims to verify the safety and efficacy of tocilizumab in the Italian population with COVID-19 pneumonia and respiratory impairment. EudraCT Number: 2020–001110-38; Clinicaltrials.gov ID NCT04317092
Keywords: COVID-19 pneumonia, Tocilizumab, Phase 2 study
List of abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| COVID | CoronaVirus Disease |
| CRP | C-reactive protein |
| CRS | cytokine release syndrome |
| CTCAE | Common Terminology Criteria for Adverse Events |
| FiO2 | inspired fraction of oxygen |
| IDMC | Independent Data Monitoring Committee |
| IL-6 | interleukin 6 |
| ISS | Italian National Institute of Health |
| ITT | intention-to-treatment |
| mITT | modified intention-to-treatment |
| PaO2 | partial pressure of oxygen |
| PCR | Polymerase Chain Reaction |
| SARS-CoV-2 | Severe acute respiratory syndrome CoronaVirus 2 |
| SOFA | Sequential Organ Failure Assessment. |
1. Background
Pneumonia is the most frequent and serious complication of COVID-19, a disease that results from SARS-CoV-2 infection. In particular, SARS-CoV-2 infection induces an excessive and aberrant host immune response that is associated with an acute respiratory distress syndrome, with typical radiological findings and, in most critical patients, with a so-called “cytokine storm”, characterized by the plasma increase of many cytokines that produce long-term damage and fibrosis of lung tissue [[1], [2], [3], [4], [5]].
Interleukin 6 (IL-6) is a pleiotropic proinflammatory multifunctional cytokine produced by a variety of cell types. IL-6 is involved in various physiological processes such as activation of T-cells, induction of acute phase proteins, stimulation of growth and differentiation of hematopoietic precursor cells, hepatic, cutaneous and neural cell proliferation, metabolism bone, lipid metabolism, and tissue fibrosis. Elevated tissue and serum levels of IL-6 are implicated in the pathogenesis of various inflammatory and autoimmune disorders including many forms of rheumatic diseases; they are also implicated in the cytokine release syndrome (CRS) [6].
Tocilizumab is a recombinant humanized monoclonal antibody, of the IgG1 class, directed against both the soluble IL-6 receptor (sIL-6R) and the receptor bound to the membrane (mIL-6R). Tocilizumab is indicated for the treatment of severe rheumatoid arthritis, systemic juvenile idiopathic arthritis, juvenile idiopathic polyarthritis and for the treatment of the severe or life-threatening cytokine release syndrome induced by the chimeric antigen receptor T-cell (CAR-T) in adults and pediatric patients 2 years of age or older [[7], [8], [9], [10]].
In an experience disclosed by Chinese researchers, 21 patients with severe or critical COVID-19 pneumonia were treated with tocilizumab 400 mg iv (i.e. the expected dose for the treatment of CRS) with reduction of oxygen requirement (15/20), resolution of CT lesions (19/21), normalization of lymphocyte count (10/19), reduction of C-reactive protein levels (16/19), hospital discharge (19/21) with an average hospitalization duration of 13.5 days [11]. These results are considered by the Chinese authors to be very positive and gave rise to the design of a randomized trial (tocilizumab vs control) which will include approximately 190 patients and is expected to reach the planned accrual by mid-May 2020.
2. Study rationale
IL-6 might play a key role in the cytokine storm induced bySARS-CoV-2 and interfering of IL-6 might be a potentially therapeutic strategy for severe and critical COVID-19. Encouraging preliminary data in Chinese patients prompted a randomized trial. This trial aims to verify the safety and efficacy of tocilizumab in the Italian population with COVID-19 pneumonia and respiratory impairment.
3. Methods
3.1. Overall design
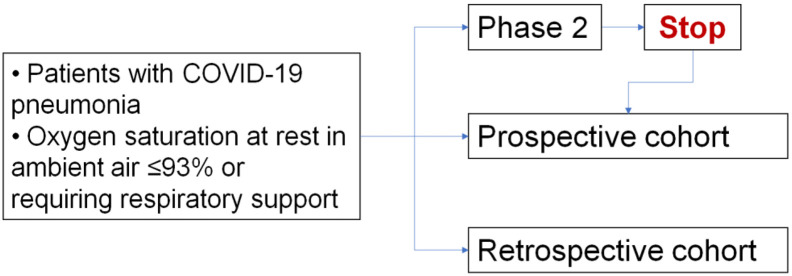
This study project includes a single-arm phase 2 study, a further parallel prospective cohort, and a retrospective cohort, enrolling patients with COVID-19 pneumonia (Fig. 1 ).
Fig. 1.
Study design flowchart.
The phase 2 study is a multicenter, single-arm, single-stage study. All the patients enrolled are treated with tocilizumab. Two-week (14 days) and one-month (30 days) lethality rates are the co-primary endpoints. Based on the data of the Italian National Institute of Health referring to mortality in the Veneto region, as for April 15 (personal communication of partial data subsequently published in ref. number [12]), two-week and 1-month lethality rates for the population defined by the selection criteria were assumed to be 20% and 35%, respectively (P0).
To verify the hypothesis that the experimental drug may produce a 10% reduction of the lethality (from 20% to 10% at two weeks and from 35% to 25% at one month from registration in the study, P1), 330 patients will provide 99% and 95% power, respectively, with a 2.5%bilateral alpha error for each test. The phase 2 population will be defined according to the intent-to-treat strategy. At least 400 patients will be considered to allow for possible ineligibility found after registration.
The two further cohorts will include patients who are treated with tocilizumab and cannot enter the phase 2 study because:
-
(a)
emergency conditions or infrastructural or operational limits prevented registration before the administration of the experimental drug (retrospective cohort) or.
-
(b)
they had been intubated more than 24 h before registration or.
-
(c)
the phase 2 study has been closed due to reached sample size.
This means that, after closure of the phase 2 enrolment, patients who might be eligible for the phase 2 study will be included in a validation prospective cohort study.
The same information planned for the phase 2 cohort is in principle required also for the prospective cohort study, whose sample size is not defined a priori, and that will close at the end of the overall project.
3.2. Study setting
This is a non-profit investigator initiated trial. In this trial, the experimental drug tocilizumab is be provided at no cost by the manufacturer (Roche).
Study protocol, patient information, and informed consent at beginning and at each required amendment are submitted to the National Ethical Committee for approval. After being approved, on March 19, 2020, the study started at each Italian centre requiring to participate. More than 300 centres are recruiting participants.
3.3. Outcome measures
Primary objective of the study is to evaluate the efficacy of tocilizumab by describing:
-
•
Lethality rate two weeks after registration in the phase 2 ITT population
-
•
Lethality rate one month after registration in the phase 2 ITT population
Secondary objectives of the study are:
-
•
To evaluate lethality rate at two weeks and one month according to delay of treatment (received early after registration, received late, not received at all)
-
•
To evaluate lethality rate at two weeks and one month in the subgroup of phase 2 patients who did actually receive the experimental drug (modified ITT)
-
•
To evaluate lethality rate at two weeks and one month in the prospective ITT cohort, with the same eligibility criteria of phase 2, enrolled consecutively after phase 2 closure up to March 24, 2020.
-
•To evaluate in the two cohorts:
-
oTime to death
-
oRespiratory function in terms of:
-
▪time to invasive mechanical ventilation (if not previously initiated)
-
▪time to definitive extubation (if previously intubated)
-
▪time to independence from non-invasive mechanical ventilation
-
▪time to independence from oxygen therapy
-
▪
-
oWhether IL-6 and CRP levels are predictive of treatment efficacy
-
oTrend of the PaO2/FiO2 ratio
-
oTrend of body temperature and lymphocyte count
-
oChange of the “Sequential Organ Failure Assessment” (SOFA)
-
oDuration of hospitalization
-
oRadiological response
-
o
-
•
To describe the toxicity of tocilizumab
-
•
To describe in the prospective and retrospective population all the endpoints proposed for the phase 2 study
3.4. Characteristics of participants
Participants are eligible to be included in the study if the following criteria apply:
-
1.
Any gender
-
2.
No age limit
-
3.
Informed consent for participation in the study (consent can be oral if a written consent cannot be expressed. If the subject is incapable of giving an informed consent and an authorized representative is not available without a delay that would, in the opinion of the Investigator, compromise the potential life-saving effect of the treatment this can be administered without consent. Consent to remain in the research should be sought as soon the conditions of the patient will allow it)
-
4.
Virological diagnosis of SARS-CoV-2 infection (real-time PCR)
-
5.
Hospitalized due to clinical/instrumental diagnosis of pneumonia
-
6.
Oxygen saturation at rest in ambient air ≤93%or requiring oxygen therapy or mechanical ventilation either non invasive or invasive (intubated). Patients intubated more than 24 h before registration are not eligible for the phase 2 study, but are allowed in the parallel cohort.
-
7.
Patients with criteria #4 and #5 and #6 who have been already treated with tocilizumab before registration are eligible for the retrospective part of the parallel cohort.
Participants are excluded from the study if any of the following criteria apply:
-
1.
Known hypersensitivity to tocilizumab or its excipients
-
2.
Known active infections or other clinical condition that contraindicate tocilizumab and cannot be treated or solved according to the judgement of the clinician
-
3.
ALT / AST > 5 times the upper limit of the normality
-
4.
Neutrophils <500 / mmc
-
5.
Platelets <50.000 / mmc
-
6.
Bowel diverticulitis or perforation
3.5. Intervention and procedures
All the enrolled patients (in both phase 2 and parallel cohort) are treated with tocilizumab 8 mg/kg (up to a maximum of 800 mg) as one intravenous administration. A second administration (same dose) can be given after 12 h if respiratory function has not recovered, at discretion of the Investigator.
There is no contraindication to concomitant treatment (including antiviral drugs) that can be defined in advance given the severity of the disease and the availability of very few data on pharmacological interactions of the tocilizumab schedule planned in this study. In case of suspected or demonstrated concomitant infections that can be successfully treated with antimicrobials in order to make the patient eligible, such treatments are allowed. Any medication that the participant is receiving at the time of enrollment or receives during the study is recorded.
Screening evaluations must be completed and reviewed before registration of patients to confirm that potential participants meet eligibility criteria. Daily assessments for evaluating the respiratory function and other clinical and laboratory parameters are planned during the study and are summarized in Table 1 .
Table 1.
Schedule of assessments.
| Procedure | Baseline before first tocilizumab administration | Treatment and hospitalization period |
Discharge | Follow-up |
|
|---|---|---|---|---|---|
| Before the eventual second administration of tocilizumab | Every day while hospitalized | On day 30 | |||
| Informed consent | X | ||||
| Inclusion and exclusion criteria | X | ||||
| Demography | X | ||||
| Full physical examination including height and weight | X | ||||
| Medical history (includes past and current medical conditions, and substance usage) | X | ||||
| Arterial Blood Gas (ABG) Analysisa | X | X | X | X | |
| Respiratory assistance assessment | X | X | X | X | |
| Laboratory assessmentsb | X | X | X | X | |
| IL-6 (recommended but not mandatory)and CRP levels | X | X | X | X | |
| 12‑lead ECG | X | X | X | X | |
| Vital signs | X | X | X | X | |
| SOFA scorec | X | X | X | X | |
| Thoracic CT scan or Chest XRd | X | X | |||
| AE review | X | X | X | X | X |
| Concomitant medication review | X | X | X | X | |
| Survival follow-up | X | X | |||
Twice in a day.
At least blood count, bilirubin, AST, ALT, creatinine, PT, PTT, LDH, D-dimer.
SOFA score is calculated considering PaO2/FiO2, Glasgow coma scale, mean arterial pressure, and bilirubin, platelet and creatinine levels.
Radiological evaluation is optional. If baseline evaluation (CT or XR) is available a re-evaluation is planned on day 7 and subsequently if clinically indicated.
3.6. Statistical considerations
For purposes of analysis, the following populations are defined:
-
•
Enrolled - All participants who sign the informed consent and are registered (including prospective and retrospective cohorts)
-
•
Phase 2 – ITT - All patients enrolled in the phase 2 cohort. The ITT population will be the primary analysis set and will provide an estimate of the effect of treatment offer. Because of the limited availability of the study drug immediately after the start of the study it is expected that several patients could have received the drug some days after registration or could not have get it at all owing to death or discharge.
-
•
Phase 2 – mITT - The subgroup of patients in the phase 2 ITT population who have received at least one dose of study drug. The mITT population will be the efficacy secondary analysis set and will provide an estimate of the effect of treatment in treated patients.
-
•
Validation – ITT - All patients consecutively and prospectively registered after phase 2 closure from March 20 to March 24who were potentially eligible for the phase 2 study but could not be enrolled because of the completion of the phase 2 cohort.
-
•
Validation – mITT - The subgroup of patients in the Validation ITT population who have received at least one dose of study drug.
-
•
Safety - All patients in the phase 2 and validation cohorts who began the infusion of the first dose of study treatment.
-
•
Prospective - All patients who have been registered in the parallel cohort before receiving the study drug.
-
•
Retrospective - All patients who have been registered in the parallel cohort after having received the study drug.
Primary and secondary analyses will be stratified by age categories, gender and eventually other clinically relevant factors (comorbidities, smoke habits etc.).
For the purpose of primary efficacy analyses the co-primary endpoint are defined as follows:
-
•
2-week lethality is defined as the ratio of the number of subjects dead within 14 days from study start out of phase 2 patients with baseline information. Point estimate will be complemented by exact 97.5% confidence interval.
-
•
1-month lethality is defined as the ratio of the number subjects dead within 30 days from study start out of phase 2 patients with baseline information. Point estimate will be complemented by exact 97.5% confidence interval.
In addition, lethality rates will be described separately by age group, and other baseline characteristics of patients.
Sample size for the phase 2 study was initially calculated using 1-month lethality rate as the primary endpoint and 1-month lethality for the eligible population was estimated around 15% based on March 10th daily report of the Italian National Institute of Health on Italian breakout (http://www.salute.gov.it/imgs/C_17_pagineAree_5351_4_file.pdf accessed Sep 30th, 2020). Subsequently, biweekly report of the Italian National Institute of Health (ISS) on deceased COVID-19 patients described median hospitalization times equal to 10 days and 5 days from onset of symptoms and death, and hospitalization and death, respectively. Accordingly, it appeared that death estimate at 14 days might be very informative, being also less prone to possible loss of information than 30-day estimate. Therefore, 14-days death rate was added as co-primary endpoint together with the 1-month death rate. As a consequence, the alpha error was splitted as 0.025 for each end-point. With the availability of more Italian data, it was also evident that the initial null hypothesis (30-day death rate 15%) was seriously underestimated. However, a main concern was the accessibility of data referring to patients hospitalized with severe or critical COVID-19 pneumonia. Thanks to a personal communication of data of the Italian National Institute of Health (ISS) we accessed to data referring to Veneto region, as for April 15, that showed death rates of 15.6% (day 14) and 28.2% (day 30) calculated by the Kaplan-Meier product limit method; such data have been subsequently reported combined with other data by Giorgi Rossi et al. [12]. These estimates were not simply applicable to the whole Italian population, because Veneto was among the regions with lower death rate. Therefore, assuming that case mix of our sample was similar to case mix of the whole Italian population, we amended the null hypotheses of the phase 2 study (P0) setting the new values of death rates to 20% and 35% at 14 and 30 days, respectively; such rates were in fact conservatively lower than the result of calculation that brought to 23% and 39% (details on the calculation are available in the Statistical Analysis Plan of the study) [13].
All secondary efficacy analyses will be considered as being supportive of the primary results.
Safety analyses will be performed in the Safety Population. For each patient and for each type of toxicity described according to CTCAE, the worst degree ever suffered during treatment will be used for descriptive analyses.
Details of statistical analysis are reported in the Statistical Analysis Plan of the study, separately reported [13].
3.7. Ethics
The procedures set out in this study protocol are designed to ensure that the promoter and the Investigators abide by the principles of the Good Clinical Practice guidelines of the International Conference on Harmonization (ICH) and the Declaration of Helsinki in the conduct, evaluation and documentation of this study. The study has carried out adhering to local legal requirements and the applicable national law, whichever represents the greater protection for the individual.
The Ethical Committee of the National Institute of the Infectious Diseases Lazzaro Spallanzani, as National Ethical Committee for COVID-19 trials according to the Italian Decree nr.18 of March17, 2020, approved the study.
The physicians treating the hospitalized patient are responsible for information of the patient and obtaining of the Informed Consent. The consent can be oral if a written consent cannot be expressed. If the subject is incapable of giving an informed consent and an authorized representative is not available without a delay that would, in the opinion of the Investigator, compromise the potential life-saving effect of the treatment this can be administered without consent. Consent to remain in the research should be sought as soon the conditions of the patient will allow it.
3.8. Data monitoring committee
An Independent Data Monitoring committee (IDMC) has been nominated to warrant the quality of the study management and analysis. The IDMC is made of 5 members, selected among statisticians, trialists and experts in Internal Medicine and Resuscitation.
The IDMC is responsible for:
-
•
reviewing efficacy and safety data through progress reports produced by the promoter and recommending, for example, modifications in case of unexpected or unexpectedly severe toxicities for study treatment, or in case of preliminary data suggesting inactivity or surprisingly positive efficacy in specific subgroups of patients. These corrections may be modifications of the treatment, the inclusion criteria or conditions for retreatment, or the sample size, or the study procedures or early study termination.
-
•
evaluating the effect on the study of possible changes in scientific evidence, such as results of other studies, and recommending modifications as above on the basis of such external data.
Considering the setting of the present study, which apply to a health emergency situation, progress report are produced at least bi-weekly and the IDMC examine all the reports produced, in collaboration with the steering committee and/or within closed meetings, and can suggest possible modifications as described above.
3.9. Data collection procedures
Patient registration and data collection are centralized at the Clinical Trials Unit of the National Cancer Institute of Naples and are web-based (http://www.usc-intnapoli.net).
Data monitoring activities during pandemic can be primarily or exclusively performed without peripheral visits. Remote monitoring is performed through periodic, comprehensive connections through the web or the telephone with all participating centres by promoter personnel or representatives.
4. Discussion
This project was written at the time of the coronavirus pandemic and while in Italy the number of people who get infected or was hospitalized for respiratory complication was dramatically increasing. Therefore, the clinical and operational scenario was extremely variable and it was expected that it will remain so for an unforeseeable time. In addition, very few solid evidence was available on the course of the disease and on the significance of intermediate end-points, before the use of the experimental drug. Therefore, it was accepted in advance that the study protocol might need repeated amendments to comply with evolving knowledge on the pandemic, the rate of complications, and the therapeutic scenario for patients who develop pneumonia. A high degree of adaptivity was therefore planned, that have been strictly discussed with the Independent Data Monitoring Committee.
Likewise, the health emergency with dramatically increasing numbers of infected and critical patients, combined with a large use of off-label tocilizumab ongoing at that time, prompted by some positive case-reports in Italy, affected the choice of the study design. Also being aware that a not randomized trial wouldn't have provided definitive results of the efficacy of the drug, we considered this design unfeasible in the setting we were at the moment of study design for several reasons. First of all, a randomized trial would have needed a longer time to be organized and would have caused a highest operative stress on participating physicians and a more complex informed consent process for the patients. Moreover, we anticipated that rate of cross-over violations while having the drug available for the experimental arm and critical patients in the control arm, might have been very high.
4.1. Trial status
The study was authorized on March 18; 2020 and opened on the dedicated web platform at 14:00 on March 19, 2020. The sample size of 330 patients enrolled in the phase 2 was reached in less than 24 h. As a consequence, the coordinating centre operatively decided to conservatively inflate the recruitment sample size for phase 2 up to at least 400 to allow for possible ineligibility found after registration. All the drug that was available in Italy was distributed by the manufacturer (Roche) in the first 48 h of the study. Further drug was to be distributed by the manufacturer during the subsequent days according to drug availability. As a consequence, (a) patients registered in the study cohorts received the drug at a variable time distance from the date of registration, due to drug unavailability, and (b) some registered patients might not receive the drug at all, due to hospital discharge or death before being treated.
This issues prompted the first amendment of the study, to clarify how the analysis populations are to be defined to pursue the primary study end-point. The version 2 of the protocol was approved on March 24, 2020.
In April, the availability of new and more detailed information on the outcome of the disease in Italy and on the need of dealing with the rate of missing data in the study, prompted the second amendment of the study, also discussed and recommended by the IDMC. This amendment introduced the 14-day lethality rate as co-primary endpoint and revised the expected lethality rates in the eligible population for the formulation of null hypothesis.
The fact that registration in the study was instrumental to obtain availability of the experimental drug in a very critical moment of the spread of the disease, and that there were many difficulties in data collection within the emergency setting, we anticipated a relevant phenomenon of missing data, whit a lowered power of the primary ITT analysis of the phase 2 study. Therefore, to validate the findings of the phase 2 study, in the amendment 2 we defined to perform the same analyses in the cohort of patients, consecutively and prospectively registered from March 20 to March 24, 2020 who shared the same eligibility criteria of the phase 2 cohort, but could not be enrolled because they exceeded the planned phase 2 study size.
The version 3 of the protocol was approved on April 28, 2020. As of May 4, the enrolment in the parallel cohort is still ongoing and more than 5.000 patients have been registered.
5. Conclusions
The TOCIVID-19 trial aims to verify the safety and efficacy of tocilizumab in the Italian population with COVID-19 pneumonia and respiratory impairment. EudraCT Number: 2020–001110-38; Clinicaltrials.gov ID NCT04317092
Funding
The trial sponsor is the National Cancer Institute of Naples. No specific funding was available for this study. Tocilizumab was provided by the pharmaceutical company (Roche) free of charge.
Authorship statement
FP (Chief Investigator), PA, AMM, RP, PP, CS and MCP conceived the study and led the protocol development; LA, MCa, MCo, GD, NCF, FF, MM, VM, CM, EAN, GB contributed to the devolpment of the study protocol. CG (lead trial statistician), PC and LA developed the study desing and statistical analysis plan. All authors provided feedback on drafts of this paper and read and approved the final manuscript.
Declaration of Competing Interest
AMM, RP, PP, LA, MCa, MCo, GD, NCF, FF, MM, VM, CM, EAN, PC, and CG have no competing interests. PA has received fee for advisory/consultant role and research funds from Roche. CS has received consulting fees (less than $10,000) and research support from Roche. FP and MCP coordinate three academic clinical trials in oncology, promoted by the Istituto Nazionale Tumori di Napoli, that are supported by Roche (clilnicaltrials.gov id: NCT01706120, NCT01802749, NCT02633189).
Acknowledgements
The authors thanks the members of the IDMC, Aldo Maggioni (Chair), Paolo Bruzzi, Antonio Pesenti, Valter Torri and Giuseppe Traversa for their valuable advices and recommendations, and all the staff at Clinical Trial Unit of the National Cancer Institute of Naples for their untimed efforts in the development of the protocol.
References
- 1.Hui D.S.C., Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect. Dis. Clin. N. Am. 2019;33:869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azhar E.I., Hui D.S.C., Memish Z.A., Drosten C., Zumla A. The Middle East respiratory syndrome (MERS) Infect. Dis. Clin. N. Am. 2019;33:891–905. doi: 10.1016/j.idc.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y. China medical treatment expert Group for Covid-19 clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G., Fan Y., Lai Y. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. SeminImmunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schirmer M., Muratore F., Salvarani C. Tocilizumab for the treatment of giant cell arteritis. Expert. Rev. ClinImmunol. 2018;14:339–349. doi: 10.1080/1744666X.2018.1468251. [DOI] [PubMed] [Google Scholar]
- 8.Stone J.H., Tuckwell K., Dimonaco S., Klearman M., Aringer M., Blockmans D., Brouwer E., Cid M.C., Dasgupta B., Rech J., Salvarani C., Schett G., Schulze-Koops H., Spiera R., Unizony S.H., Collinson N. Trial of Tocilizumab in Giant-cell arteritis. N. Engl. J. Med. 2017;377:317–328. doi: 10.1056/NEJMoa1613849. [DOI] [PubMed] [Google Scholar]
- 9.Manfredi A., Cassone G., Furini F., Gremese E., Venerito V., Atzeni F., Arrigoni E., Della Casa G., Cerri S., Govoni M., Petricca L., Iannone F., Salvarani C., Sebastiani M. Tocilizumab therapy in rheumatoid arthritis with interstitial lung disease: a multi center retrospective study. Intern. Med. J. 2019 Oct 29 doi: 10.1111/imj.14670. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim Y.F., Moussa R.A., Bayoumi A.M.A., Ahmed A.F. Tocilizumab attenuates acute lung and kidney injuries and improves survival in a rat model of sepsis via down-regulation of NF-κB/JNK: a possible role of P-glycoprotein. Inflammopharmacology. 2020;28:215–230. doi: 10.1007/s10787-019-00628-y. [DOI] [PubMed] [Google Scholar]
- 11.Xu Xiaoling, Han Mingfeng, Li Tiantian. 2020. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. ChinaXiv: 202003.00026v1, Now Published in Proc Natl Acad Sci U S A. (Apr 29. pii: 202005615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giorgi Rossi P., Ferroni E., SpilaAlegiani S., Pitter G., Leoni O., Cereda D., Marino M., Pellizzari M., Sultana J., Trifirò G., Massari M., the ITA-COVID19 working group Survival of hospitalized COVID-19 patients in Northern Italy: a population-based color study by the ITA-COVID19 Network. MedrXiv. 2020 doi: 10.2147/CLEP.S271763. (2020.05.15.20103119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiodini P., Arenare L., Piccirillo M.C., Perrone F., Gallo C. 2020. A phase 2, Open Label, Multicenter, Single Arm Study of Tocilizumabon the Efficacy and Tolerability of Tocilizumab in the Treatment of Patients with COVID-19 Pneumonia (TOCIVID-19 trial): Statistical Analysis Plan. Epidemiologia & Prevenzione. (repo.epiprev.it/1610) [DOI] [PMC free article] [PubMed] [Google Scholar]