Abstract
In this review, we present the environmental perspectives of the viruses and antiviral drugs related to SARS-CoV-2. The present review paper discusses occurrence, fate, transport, susceptibility, and inactivation mechanisms of viruses in the environment as well as environmental occurrence and fate of antiviral drugs, and prospects (prevalence and occurrence) of antiviral drug resistance (both antiviral drug resistant viruses and antiviral resistance in the human). During winter, the number of viral disease cases and environmental occurrence of antiviral drug surge due to various biotic and abiotic factors such as transmission pathways, human behaviour, susceptibility, and immunity as well as cold climatic conditions. Adsorption and persistence critically determine the fate and transport of viruses in the environment. Inactivation and disinfection of virus include UV, alcohol, and other chemical-base methods but the susceptibility of virus against these methods varies. Wastewater treatment plants (WWTPs) are major reserviors of antiviral drugs and their metabolites and transformation products. Ecotoxicity of antiviral drug residues against aquatic organisms have been reported, however more threatening is the development of antiviral resistance, both in humans and in wild animal reservoirs. In particular, emergence of antiviral drug-resistant viruses via exposure of wild animals to high loads of antiviral residues during the current pandemic needs further evaluation.
Keywords: Coronavirs, COVID-19, Water, Antiviral drugs, resistance, Virus, Persistance, Ecotoxicity
Graphical Abstract
1. Introduction
With dreadful global lockdown and search for effective medicine against the novel coronavirus (SARS-CoV-2), efforts to understand the perspectives of coronavirus disease 2019 (COVID-19) is still ongoing. Many countries are implementing different plan of actions to deal with the COVID-19 pandemic and understanding their impact on the society (van der Voorn et al., 2020). Also, the prevalence of SARS-CoV-2 in the aquatic environemnt as a result of this global pandemic is of great concern (Kumar et al., 2020f). In this effort, our team has recently published a review on epidemiology, prognosis, diagnosis, transmission and treatment of COVID-19 (Kumar et al., 2020e). The detection of SARS-CoV-2 RNA are reported in feces of patients in the US and China (Wang et al., 2020a, Liang et al., 2020). Furthermore, reports have suggested that SARS-CoV-2 are present in patients’ stool as well as in wastewater (Kitajima et al., 2020). This is alarming for possible transmission to humans via contaminated water. At the same time, this has provided an opportunity to carry out wastewater-based epidemiological studies for community-wide estimation of COVID-19 prevalence (Kitajima et al., 2020, Kumar et al., 2020d).
Further, transport and infectivity of SARS-CoV-2 in wastewater/environmental waters are highly dependent on the physical and environmental susceptibility of SARS-CoV-2 as well as inactivation. As per the inactivation mechanism, environmental stressors become critical for the disruptions of proteins and lipids of viral envelope leading to their seasonal variations (Kumar et al., 2020b). Along with host immunity, climatic factors would also influence human respiratory air passage defence. Since such information is barely available related to SARS-CoV-2, there is a dire need to understand similarities and dissimilarities of respiratory coronavirus (positive-stranded RNA viruses with nucleocapsid) with various viruses including enteric viruses (Van Der Hoek et al., 2004). Such information will be of immense help to understand structural, and environmental susceptibility and inactivation mechanisms for SARS-CoV-2.
With surge in COVID-19 patients (> 20 million people) as of the second week of August, 2020, large number of antiviral drugs (remdesivir, ivermectine chloroquine and hydroxychloroquine) have been clinically tested. However, there is no data available on the quantum of antiviral drugs being used to treat such extraordinary number of COVID-19 patients. So far, a large number of antiviral drugs have been discovered till date ( Fig. 1), but the quest of finding viral cure seems to be never ending due to insufficient effectiveness of such treatments and high viral mutation rates which lead to emergence of resistant strains (Irwin et al., 2016, Race et al., 2020, Sanjuán et al., 2010, Duffy, 2018). Furthermore, studies reported that the administered drugs are not fully metabolised in the human body, thus generating residues and metabolites. The drug residue and metabolite are discharged into the environment through sewage leading to spikes of antiviral drugs in wastewater and ambient waters (Race et al., 2020, Funke et al., 2016, Khetan and Collins, 2007). In addition, studies confirmed incomplete removal of antiviral drugs in wastewater treatment plants (WWTPs) (Nannou et al., 2020, Mlunguza et al., 2020, Ngumba et al., 2020). The unprecedented use of antiviral drug during pandemic events, has led to the development of antiviral-drug resistant viruses within wild animal reservoir and may compromise the treatment of COVID-19 patients (Ahmed et al., 2020, Musharrafieh et al., 2020). Therefore, the occurrence and fate of antiviral drugs in the environment during this unprecedented pandemic need a special attention.
Fig. 1.
Historical timeline of antiviral drugs approved since 1963 to 2020 (Modified from De Clercq and Li, 2016).
Under the light of above discussions, we hereby present a comprehensive review on physical and environmental susceptibility, seasonal variations, inactivation mechanism, discovery and transport of antiviral drugs, transport and fate of viruses in the Anthroposphere. The review also discusses the role of vital factors like carrier prevalence, treatment efficacy of wastewater burden (virus source), transport among environmental compartments of viruses and their inactivation mechanisms, and the current unprecedented use of antiviral drugs. Special emphasis is given on understanding the transport of both viruses and antiviral drugs, alongside treatments and governing mechanisms of SARS-CoV-2 inactivation. We then finally present the current global status of antiviral drug resistance, and future scenarios of antiviral drug resistance both in pandemic viruses and infected humans. Overall, we intended to prepare an insightful ready reference that can not only help the readers identifying critical variables governing COVID-19, but also raise awareness of some likely aftermaths of the current pandemic.
2. Seasonality of viruses and viral infections
Transmission of viruses and the manifestation of infection depend on transmission pathways, human behaviour, susceptibility and immunity. Environmental factors, such as climatic conditions and behaviour of virus hosts and vectors, also play a formidable role that translates into several distinct seasonal trends of viral infections. Wuhib et al. (1994) investigated the seasonal pervasiveness of Microsporidiosis and Cryptosporidium parvum in immuno-compromised HIV positive (+ve) patients, and reported a profuse infection in the rainy seasons. However, the reported course of seasonal prevalence of these parasites was not statistically significant. Their prevalence depends on the HIV infection and hence, is mainly related to the fecal-oral route, zoonotic emanation, sexual transmission, and renaissance of passive condition observed for HIV.
Howbeit, in the case of viruses, especially influenza viruses, the seasonal pattern is indisputable. Chris et al. (1998) studied the seasonal variation of respiratory infectious virus and observed that the key factors determining the prevalence of the virus were change in temperature and humidity (Christophers et al., 1998). The reported incidences of influenza and other respiratory epidemics are significantly higher in winter season than summer and spring seasons (Mourtzoukou and Falagas, 2007, Peci et al., 2019). In addition, in line with the various epidemiological research, the majority of the respiratory virus outbreaks in temperate zones exhibit seasonal fluctuations. In particular, human coronavirus (types: HKU1, 229E, OC43, and NL63), influenza virus and respiratory syncytial virus culminate in the winter season and are commonly known as winter viruses (Monto, 2002, Landes et al., 2013, Morikawa et al., 2015, Midgley et al., 2017, Killerby et al., 2018).
Incongruence in the replication of these viruses leads to a non-overlapping prevalence with respect to each other. It has been found that respiratory viruses such as respiratory syncytial and influenza viruses, even being pervasive in winter, do not occur simultaneously (Anestad, 1982). Antithetically, human bocavirus, rhinovirus, adenovirus, and human metapneumovirus are identified all along the year and are called as all-year viruses (Bastien et al., 2006). However, Lee et al. (2012) reported that rhinovirus infection surfaced during fall and spring, but the disease ferocity was highest in winter. On the other hand, the rate of recurrence of several enteroviruses escalates during summer implying the significance of seasonality of viral infections (Haynes et al., 2016, Abedi et al., 2018). Further, the transmission efficiency of viral infections via all possible pathways depends on outdoor and indoor climatic factors. Kudo et al. (2019) illustrated that lower humidity could induce a decrease of Mx1 congenic mice weight, increase in mortality rate, pulmonary viral load, and infection with influenza virus.
Viral epidemics such as SARS (2002–2003), MERS (2012–2015) and the current COVID-19 pandemic emerged in winter before their subsequent worldwide spread (Kuiken et al., 2003, Peiris et al., 2003, Paules et al., 2020), these respiratory viruses (coronaviruses: SARS-CoV, MERS-CoV, and SARS-CoV-2) aggrandizes in the winter season. Seasonality also governs the host propensity by moderating human pulmonary or nasopharyngeal innate defense mechanisms, and thus, regulates respiratory virus effectiveness/viability and dissemination (Moriyama et al., 2020). Therefore, the key determining characteristics of respiratory viral outbreak is pathogenicity which is severely affected by seasonality i.e. sunlight, temperature (winter), absolute humidity (dry season), host susceptibility due to cold weather, and seasonal changes in immunity (Dowell, 2001, Shoji et al., 2011, Sloan et al., 2011, Tamerius et al., 2011, Azziz Baumgartner et al., 2012, Fisman, 2012).
3. Transport of viruses
Adsorption and persistence are the two major elements that affect the fate and transport of viruses in the environment. Survival of viruses is typically evaluated by reduction time: T90, T99, or T99.99, which are the time required for viruses to reduce by 90%, 99%, or 99.99%, respectively, under certain environment. The reduction of various viruses in environmental waters are summarized in Table 1. In general, viruses with high persistence have a high capability of causing infection within the environment. Although literature around transport of SARS-CoV-2 in the surface and subsurface medium is not available, several factors, including soil-specific, virus-specific, and environmental factors might influence the transport of the virus in the environment (Aw and Gin, 2011) (Supplementary Table 1). Governing features for the migration of viruses in surface and subsurface systems include temperature, rate of adsorption, virus aggregation, moisture content, pH, the concentration of salts, properties of soil, organic matter, strains of the virus, and hydraulic condition (Bosch et al., 2006).
Table 1.
Survival of viruses in water, wastewater and groundwater (Johnson et al., 1997, Skraber et al., 2009, Carratala et al., 2013, Enriquez-Enriquez, 1994, Ogorzaly et al., 2010, Bae and Schwab, 2008, Sidhu et al., 2015, John and Rose, 2005, Casanova et al., 2009, Gundy et al., 2009, Ye et al., 2016, Casanova and Weaver, 2015, Wang et al., 2005).
| Categories |
Enveloped Viruses |
Non-Enveloped Viruses |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Types of water | Reduction Time | Corona | MHV | TGEV | PP | Entero | Rota/HAV | Noro/Calici | Adeno |
| Surface Water | T90 | 3 d (Casanova and Weaver, 2015) (SARS-CoV, tap) | – | – | – | 10.8 h (Surface water, L) (Bosch et al., 2006) | – | – | – |
| T99 | 6.5–8 d (Casanova et al., 2009) (HCoV, FCoV), | – | – | 3.1 d (Casanova and Weaver, 2015) (River) | 25.7 h (Surface water, L) (Bosch et al., 2006) | – | – | – | |
| Wastewater | T90 | 3 d (Casanova and Weaver, 2015) (SARS-CoV) (Hospital Sewage) | – | 9 d (John and Rose, 2005) (Sewage) | 7 h (Ye et al., 2016) | 672 h (Effluent, D) (Carratala et al., 2013) | – | 540 h (Effluent, D) (Johnson et al., 1997) | 3.12 h (Influent, D) (Skraber et al., 2009) |
| T99 | 1.85–2.36 d (HCoV), 1.62–1.71 d (FCoV) (Casanova and Weaver, 2015) | 49 d (John and Rose, 2005) (Sewage) | – | 53 h (Ye et al., 2016) | – | – | – | 7.44 h (Influent, D) (Skraber et al., 2009) | |
| T99.99 | 2–4 d10(surrogate) (Casanova et al., 2009) | – | – | – | – | – | – | 15.84 h (Influent, D) (Skraber et al., 2009) | |
| Groundwater | T90 | – | 13–19 h (Gundy et al., 2009) | – | 7 h (Ye et al., 2016) | – | 34–200 d (Bae and Schwab, 2008) (Rota), 33.3 d (Sidhu et al., 2015) (HAV) | 5–1266 d, 5.6–11.1 d (Ogorzaly et al., 2010) (Calici) | 278d (20 °C) (Enriquez-Enriquez, 1994) |
TGEV: Transmissible gastroenteritis virus; MHV: Mouse hepatitis virus; SARS-CoV: Severe acute respiratory syndrome coronavirus; PP: Pseudomonas Phage.
3.1. Adsorption
Being amphoteric in nature, coronaviruses are expected to be attracted and trapped by both positively and negatively charged soil colloids and humus species. Major adsorption is expected to occur in soil having a high concentration of clay. Bacteriophages that are considered to be surrogates for enteric viruses and also used as a process control in the detection of SARS-CoV-2 have shown greater adsorption potential to polyelectrolytes (Dang and Tarabara, 2019, Park et al., 2017) and minerals (Walshe et al., 2010). However, soil is a mixture of minerals, organic compounds, and living microorganisms thereby constituting a tridimensional structure with many particularities affecting retention potential where diffusive and convective fluxes are crucial. Thus, it would be difficult to predict the adsorption of SARS-CoV-2 to soil without field data. According to the Derjaguin–Landau–Verwey–Overbeek (DVLO) theory, at higher pH, viruses are weakly adsorbed to soil particles due to an increase in electrostatic repulsion (Armanious et al., 2016). Experiments conducted for adsorption study of human enteroviruses and bacteriophages onto different soils illustrated that decrease in pH increased the adsorption of the most viruses (Goyal and Gerba, 1979, Walshe et al., 2010). The presence of envelope (lipoproteinaceous membrane) and spike proteins, makes SARS-CoV-2 surface property significantly different from non-enveloped viruses (Walls et al., 2020). Presence of various functional groups along with characteristics spike proteins are expected to govern the adsorption, fate and transport of the novel virus in the environment. Despite the lipid layer is significantly vulnerable to several impurities present in the soil, enveloped viruses, such as mouse hepatitis virus (MHV- a murine coronavirus) and Pseudomonas phase (ϕ6) have shown high adsorption potential onto wastewater solid fractions. Such a difference in adsorptive behaviour of enveloped viruses was possibly due to the dominance of both hydrophobic effects and electrostatic force amidst sorbent surface and capsid protein (Armanious et al., 2016, Ye et al., 2016). Similar force of attraction would play a vital role in the transport of amphoteric SARS-CoV-2 in the aqueous medium. Howbeit, saturated soils filled with water during monsoon season and subsequently increased flow rate reported to increase the virus transport due to less interaction time with soil particles (Funderburg et al., 1981, Jin et al., 2000, Williamson et al., 2005, Betancourt et al., 2019, Yates et al., 1987). Similarly, smaller-sized viruses migrate at a faster rate due to lesser entrapment in the soil pore. Virus transport in fine-grained soil is expected to be slower due to its higher probability in virus retention than in coarse-grained soil. A typical diameter of a SARS-CoV-2 virion is 100 nm (Bar-On et al., 2020), which is larger than many of non-enveloped viruses ( Table 2). Therefore, lesser mobility in soil is expected for SARS-CoV-2 owing to its size than that of enveloped viruses which is yet to be demonstrated.
Table 2.
Probable inactivation mechanism for enveloped and non-enveloped viruses (Meister et al., 2018, Duizer et al., 2004, He et al., 2004, Darnell et al., 2004, Rabenau et al., 2005, Kingsley et al., 2002, Croughan and Behbehani, 1988, Wilde et al., 2016, Jeong et al., 2010, Shin and Sobsey, 2008, Tree et al., 2003, Kratzel et al., 2020, Kampf et al., 2020, Hu et al., 2020, Glass and O’Brien, 1980, Kapuscinski and Mitchell, 1980, Xing et al., 2020, Vettori et al., 2000, Babich and Stotzky, 1979, Azadpour-Keeley and Ward, 2005, Schijven and Hassanizadeh, 2000, Yates and Jury, 1995, Park et al., 2011, Šim\uunek et al., 2016, Weber and Stilianakis, 2008, Andersen, 2019).
| Category | Virus |
Morphology |
Inactivation Mechanisms |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Diameter (Average) | Genome Type | Genome Size | UV Light/ozone | Chloride/Iodide/Salts Compounds | Heat (Temperature Dependent) | Acid/Alcohol | Other | ||
| Enveloped Viruses | Coronavirus | 80–120 nm | +ss RNA | 26,000–32,000 bases | UV-C254 (4,016 μW/cm2), UV-A365 (2,133 μW/cm2), ϒ (3–15 k rad.)60Co (Glass and O’Brien, 1980) | Benzalkonium chloride & Laurylamine (RF > 3.8, 60 min) (Kapuscinski and Mitchell, 1980), didecyldimonium chloride (RF > 3.8, 60 min) (Kapuscinski and Mitchell, 1980), Hypochlorite (0.1–0.5%, 2–4 log10) (Šimunek et al., 2016) | Heat Treatment (> 65 °C) (Glass and O’Brien, 1980) Heat Treatment (60 °C, 30 min) (Kapuscinski and Mitchell, 1980), Heat on serum (56 °C, 30 min, 90% inactivation,7–14 min) (Weber and Stilianakis, 2008) | Sterillium (propanol, ethanol) (RF > 4.3, 30 s), (Ethanol, biphenylol)(Kapuscinski and Mitchell, 1980), Ethanol (80%v/v) (3.8 log10) and 2-propanol (75%v/v) (30 S, > 3.8 log10) (Park et al., 2011) | Catalytic Oxidation (Ag, 5 min & Cu, 20 min) (Hu et al., 2020), Formaldehyde (Glass and O’Brien, 1980) & Glutaraldehyde (Glass and O’Brien, 1980), (Kapuscinski and Mitchell, 1980), Incidin Plus, Wine Vinegar (Šimunek et al., 2016), Glutardialdehyde(2%,2–4 log10) (Šimunek et al., 2016) |
| Influenzavirus | 80–120 nm | ss RNA | 13,500 bases | UV-C Irradiation6 | Heat Treatment (70,80,90 °C, 5,2.5,1 min) (Azadpour-Keeley and Ward, 2005) | 70% Ethanol, 1-propanol (1 min) (Azadpour-Keeley and Ward, 2005) | 0.1 mol/LNaOH (Azadpour-Keeley and Ward, 2005), Ethylene Oxide surface treatment (Azadpour-Keeley and Ward, 2005) | ||
| Flavivirus | 50 nm | +ss RNA | 11,000 bases | – | 500 ppm Cl, 50 s (> 4 log10) (Babich and Stotzky, 1979) | Dry Heat Treatment (56–60 °C) (Babich and Stotzky, 1979) | Ammonium/Alcohol Product (> 3.5 log10), 70% isopropyl-alcohol (> 5 log10) (Babich and Stotzky, 1979) | – | |
| Simplexvirus | 150–245 nm | ds DNA | 152,000 | – | 50 mM Zinc Gluconate (2 hrs) (100% Reduction) (Xing et al., 2020), Alcide Disinfectant (Vettori et al., 2000) | Temp. of 56 °C in 30 min (Vettori et al., 2000) | Lysol, Listerine, Alcohol (Vettori et al., 2000) | – | |
| African Swine fever Virus | 170–190 nm | ds DNA | 189,000 bases | Nacl and Phosphate Salt (4, 12, 20, 25 °C) (Croughan and Behbehani, 1988) | |||||
| Non-Enveloped virus | Enetrovirus | 25–30 nm | +ss RNA | 7,200–8,500 bases | Ozone (Duizer et al., 2004) | Chlorine Dioxide (4 log reduction) (Meister et al., 2018), Monochloroamine (Wilde et al., 2016) | Heat (55 °C) (Jeong et al., 2010), Heat (70oC, 30 min) (Shin and Sobsey, 2008) | Methanol & Ethanol (90%) (Tree et al., 2003) | Marine bacteria (Pseudomonas & Vibrio) (He et al., 2004) |
| UV-C Irradiation (Rabenau et al., 2005) | |||||||||
| UV254 (Jeong et al., 2010), Sunlight (Jeong et al., 2010) | 2–4 log10 reduction | Activated Sludge Treatment (Andersen, 2019) | |||||||
| ϒ rays (1.0 Mrad) (Shin and Sobsey, 2008), UV (62.50 mW s cm−2,4 log10) (Yates and Jury, 1995) | Free Cl (Jeong et al., 2010), ClO2 (Jeong et al., 2010) | Anaerobic digestion under thermophilic condition (Shin and Sobsey, 2008) | |||||||
| HEV/HAV | 27–34 nm | +ss RNA | 7,200 bases | Irradiated with 0.6 J/cm2 after dilution with DMEM (Darnell et al., 2004) | Povidone Iodide (Darnell et al., 2004) | 80 °C for 5 min, after dilution with DMEM (Darnell et al., 2004) | Triton, Ethanol, Propanol (Darnell et al., 2004) | Hydrostatic Pressure Processing (300–450 MPa, 5 min) (Xing et al., 2020) | |
| Norovirus | 23–40 nm | +ss RNA | 7,500 bases | UV254 (25–100 mJ/cm2) 4 log10 reduction (Kratzel et al., 2020) | Nacl concn. (0.3, 1.3, 3.3, 6.3% [wt/vol])(Kingsley et al., 2002), Monochloroamine (Wilde et al., 2016), free Cl (1 & 5 mg/L)(Schijven and Hassanizadeh, 2000) | 24–85 °C (20 days) (Kingsley et al., 2002) | – | – | |
| 4 log reduction (60 °C, 10 min) | |||||||||
| Inactivated (85 °C, < 1 min) (Kingsley et al., 2002) | |||||||||
| Calicivirus | 27–40 nm | +ss RNA | 7,500–8,500 bases | UV254 (25–100 mJ/cm2) 4 log10 reduction (Kratzel et al., 2020), UV-B (0–150 mJ/cm2)(Kampf et al., 2020), UV (62.50 mW s cm-2,4 log10)(Yates and Jury, 1995) | Sodium Hypochlorite (300 ppm) (Kampf et al., 2020), Free Cl (30 mg/L, 5 min, 4 log10)(Yates and Jury, 1995) | 3 log reduction at 71.3 °C (Kingsley et al., 2002) | 70% Ethanol (30 min) 3 log10 reduction (Kampf et al., 2020) | – | |
| Astrovirus | 28–35 nm | +ss RNA | 6,800–7,900 bases | – | – | – | Methanol & Ethanol (90%) (Tree et al., 2003) | – | |
| Rotavirus | 76.5 nm | ds RNA | 37,100 bases | propan-1-ol, propan-2-ol, butan-2-ol (40%) (104x drop) (Tree et al., 2003) | |||||
| Adenovirus | 90–100 nm | ds DNA | 26,000–48,000 bases | Monochloroamine (Wilde et al., 2016) | |||||
DMEM-Dulbecco Modified Eagle Medium; ss-single strand, ds-double strand.
3.2. Persistence
While there is a debate about whether virus inactivation follows first-order or time-dependent processes, several factors including temperature, conductivity, and water quality parameters are reported to inactivate the virus by disrupting the protein coat and nucleic acid. Again, the extent of inactivation of enveloped viruses is found to be high compared to the non-enveloped viruses in surface water, groundwater, and wastewater (Table 1). With an increase in temperature, the rate of enveloped virus inactivation reported to increase compared to non-enveloped viruses. Surrogate coronaviruses are known to retain their infectivity in water from days to several weeks depending upon the surrounding temperature. At low temperature viruses are expected to survive for longer duration and subsequently migrate to a greater distance (Casanova et al., 2009). However, an association of viruses with the colloidal and particulate materials can protect the virus from inactivation and such phenomena were observed for poliovirus Type 1 and bacteriophages (Kapuscinski and Mitchell, 1980, Xing et al., 2020). Similarly, clay minerals are reported to protect adsorbed bacteriophages from UV radiation (Vettori et al., 2000). Several authors also highlighted the importance of nutrients such as phosphorus and metals towards the inactivation of viruses (Babich and Stotzky, 1979). Organometallic complexes are reported to alleviate the toxicity of heavy metals towards virus inactivation.
In many cases, septic tank effluents, leachate from sludge disposal sites, and direct land application of wastewater effluents considerably contribute to the groundwater contamination. However, the transportation of viruses in subsurface systems is a function of their retention time, nature, and geology of aquifer. Both efficacy and transport of virus are controlled by soil water content, temperature, sorption and desorption, pH, salt content, type of virus, and hydraulic stresses (Azadpour-Keeley and Ward, 2005). As discussed, both adsorption and inactivation collectively govern viruses transport during soil passage. While advection and dispersion control the virus spread, attenuation of virus concentrations. Irreversible reaction assumes no detachment of virus, and reversible reactions involve equilibrium and kinetics of adsorption. Thus in a subsurface system, adsorption and desorption are rapid in reference to flow velocity, favouring faster equilibrium. There might be another scenario where adsorption is kinetically limited in reference to the flow velocity, with a fixed adsorption and desorption rate coefficients. Hence the corresponding kinetic equations of solute transport must take into account dispersion, advection, and inactivation while modeling virus transport in a three-dimensional saturated flow condition (Schijven and Hassanizadeh, 2000).
Various models were proposed to study the transport phenomena of viruses in environmental matrices. Some of them were VIRAL, working on the principle of irreversible sorption phenomena (Yates and Jury, 1995), VIRULO based on Monte Carlo simulation to predict attenuation of virus in the unsaturated zone (Park et al., 2010, Park et al., 2011) and HYDRUS-2D working based on virus kinetics of deposition, surface chemistry of the sorption sites (Šimunek et al., 2016). While enveloped viruses like SARS-CoV-2 may have notable mobility in the environment, further studies are needed to appraise its persistence and transport. Those above-developed models for non-enveloped viruses may be used to have a conservative estimation of enveloped virus transport. However, additional research is required to model SARS-CoV-2 transport in the soil, which can be used to model the transport of other enveloped viruses.
4. Inactivation mechanism
Viruses that are small and elliptical in shape are considered to be highly infectious. Even a small amount of viral load is enough to cause gastroenteritis. Compared to non-enveloped norovirus, a significant number of enveloped viruses were found in vomit and excreta of infected persons. Infections by most of the viruses are transmitted through contact, fecal-oral route, droplets, or aerosols (Weber and Stilianakis, 2008). Viral particles are also transmitted via contaminated surfaces, clothes, food, water, and fomites (Andersen, 2019). It is reported that SARS-CoV can survive on the surface for several days and could retain its infectivity up to 9 days (Rabenau et al., 2005). Similarly, Otter et al. (2016) revealed that other enveloped viruses, such as MERS and SARS viruses, could survive up to several months. Various factors that govern the survival of viruses are i) type of strain ii) load of titer iii) type of surface and v) environmental condition (Otter et al., 2016).
Disinfection plays an important role in controlling microbial infection in medical settings, such as, health care facilities, and nursing homes (Pfaender et al., 2015). Also, it helps in controlling microbial infection in water treatment plants and WWTPs by destroying or deactivating pathogenic microorganisms (Macinga et al., 2008). Disinfection is one of the most fundamental ways of disrupting virus spread by reducing or inactivating their infectious nature (Hijnen et al., 2006). In this section, different modes of virus inactivation such as, UV, alcohol, heat treatment, and other conventional and advanced methods, are discussed. Possible inactivation mechanism for SARS-CoV-2 has also been presented by referring to the available literature on other envelope viruses and non-enveloped viruses, as shown in Table 2 and Fig. 2.
Fig. 2.
Schematic of SARS-CoV-2 disinfection for an enveloped virus (Modified from Pfaender et al. 2015).
4.1. UV-based inactivation
UV-based disinfection technique is profoundly used in the medical sectors to sterilize medical equipment, and PPEs (Vaidya et al., 2018). UV(C) rays were proven to be more efficient than the UV(A) and UV(B) in inactivating viruses, as they possess high energy and are most absorbed by the DNA and RNA (Ravanat et al., 2001). Darnell et al. (2004) compared the efficiencies of UV(A) and UV(C) to inactivate the viruses within a limited period. UV(C) could increase the rate of virus inactivation to 400 times within 6 min, while UV(A) showed no effects when the experiment was extended up to 15 min. The UV-based disinfection involves the absorption of UV rays by DNA/RNA bases followed by fusion with pyrimidines into covalently linked dimmers, which later becomes non-pairing bases (Perdiz et al., 2000). DNA can absorb UV(C) in the range of 245–285 in the most propitious way and thus proves to be suitable to disinfect microbes (Pratelli, 2008).
4.2. Alcohol
Disinfection by means of alcohol is considered an important measure for the inactivation of viruses. Alcohol-based sanitizers (a mixture of polyquaternium and organic acid) are used to inactivate non-enveloped viruses such as human rotavirus, poliovirus type 1, human norovirus, and murine norovirus that are inactivated by a factor of 103 times within 30 s (Macinga et al., 2008). Ethanol and propanol were also found to inactivate feline calicivirus (norovirus’s surrogate) by 4 log units within 30 s (Gehrke et al., 2004). A notable study by Kampf et al. (2020) reported an efficient method to disinfect SARS-CoV-2 in less than 60 s using ethanol [C2H5OH (65%)], hydrogen peroxide [H2O2 (0.5%)] and sodium hypochlorite [NaClO (0.1%)] (Kampf et al., 2020). Hulkower et al. (2011) reported the reduction of infectivity by > 3 log units with ethanol. Generally, inactivation of viruses by alcohol-based disinfectants involves disruption of the virus envelope within the capsid, without targeting the RNA (Pfaender et al., 2015). Kratzel et al. (2020) evaluated two of the alcohol-based disinfectants based on WHO formulation for the inactivation of SARS-CoV-2, namely, ethanol and 2-propanol, along with glycerol and H2O2. Both the formulations resulted in the reduction of the viral titers in 30 s.
4.3. Chemical-based disinfection
Chemical-based inactivation is also an important tool for disinfecting viruses. Jelsma et al. (2019) studied the effect of sodium chloride (NaCl) and phosphate-based (P-salt) salts on enveloped swine fever viruses present in the porcine intestines. The reduction values were determined at four different temperatures, namely, 4, 12, 20, and 25 °C. Compared to sodium-based salt, phosphate-based (P-salt) salts were found to be more effective across all the studied temperatures. Virus titers showed a reduction of 99% from its initial concentration. One of the enveloped viruses, HSV (Herpes Simplex Virus) was inactivated using zinc gluconate and zinc lactate salts in vitro. The former salt showed inactivation for 80% of the virus particles with more than 98% reduction, while the later inactivated 90% of the samples with more than 97% reduction. The reduction was found to be proportional to the concentration of salt used (Arens and Travis, 2000). Kampf et al. (2020) studied the effect of several biocidal agents on the inactivation of SARS-CoV-2. Ethanol (62–71%), sodium hypochlorite (0.1–0.5%), glutardialdehyde (2%) were found to reduce the titers by 2–4 log10 while on contrary, benzalkonium chloride (0.04%) and ortho-phthalaldehyde (0.55%) were less effective in inactivating SARS-CoV-2.
4.4. Chlorination
Inactivation of many viruses in the drinking water treatment plants is usually done by chlorination. Cromeans et al. (2010) studied the effect of monochloramine on human adenoviruses, enteroviruses, and murine norovirus. Agolini et al. (1999) studied the effect of chlorine disinfectant on HCV (hepatitis C virus) inactivation. Ogata and Shibata (2008) studied the effect of chlorine dioxide (ClO2) gas against Influenza A virus, an enveloped virus. The mechanism involves denaturing of viral envelope proteins, which are responsible for the infectious nature of the virus by ClO2. SARS-CoV-2 has already been detected in many of the untreated wastewater samples at Australia (positive in 22% samples), Netherland (58%), USA(71%), France (100%), and USA (100%) (Ahmed et al., 2020, Medema et al., 2020, Wu et al., 2020, Wurtzer et al., 2020, Nemudryi et al., 2020) Thus, it becomes ever important to disinfect the viruses before they contaminate surface water bodies and enter into urban water cycle. Chlorination is the most common wastewater disinfection method. A2.8 log-unit inactivation by chlorination was reported for poliovirus (Tree et al., 2003). Although information regarding the portion of infectious SARS-CoV virus in feces is still emerging, reports of RNA of SARS-CoV-2 in the feces nof infected patients raises the importance of being extra vigiliant about management of undiluted hospital wastewater (Wang et al., 2020b). Liquid chlorine, chlorine dioxide, and sodium hypochlorite are some of the mostly used disinfectants at health facilities (Lizasoain et al., 2018, Yu et al., 2014). All these chlorine based disinfectants offer several advantages over UV/Ozone and other inactivation processes like i) low power consumption ii) low toxicity iii) simple equipment iv) need no skilled labor v) low set-up and operational cost (Fan et al., 2017). The effective product which inactivates the pathogen during chlorine-based disinfection is HClO (Wang et al., 2020b). Also, due to 80% similarity between SARS-CoV-2 and SARS-CoV-1, the disinfection process of the latter could be well approximated to the former one. Chen et al. (2006) studied the complete inactivation of SARS viruses by adding free Cl (0.5 mg/L) or ClO 2 (2.9 mg/L) after a heat treatment of 30 °C for half an hour. Therefore, it may be an effective wastewater disinfection method for the COVID-19, too; however, further studies are required to ascertain the dose response relationships (Chen et al., 2006).
4.5. Thermal inactivation
Heat treatment for virus inactivation has been in use for decades. Seo et al. (2012) studied the effect of temperature on the virus inactivation. A 102 times reduction was noticed at 24 °C within 12 days; 4 log unit reduction at 70 °C within 2.5 min in human/murine norovirus and a 3 log reduction at 71 °C in feline calicivirus within 2 days. Inactivation involves denaturing viral capsid proteins, which in turn creates gaps in the viral particles making it more vulnerable to proteinase K and RNase treatment (Seo et al., 2012). Recently, Hu et al. (2020) studied the thermal inactivation of SARS-CoV-2 by heating human serum contaminated with the virus at 56 °C for 30 min. In another approach, 90% inactivation of the virus was achieved within 7 and 14 min when experiments were conducted in simulated saliva and culture media, respectively (Ratnesar-Shumate et al., 2020).
4.6. Other inactivation mechanisms
Several studies have reported instances of using microorganisms as an important tool for inactivating enterovirus (Toranzo et al., 1983). Toranzo and Metricic (1982) reported the antiviral properties of isolated marine bacteria. Photocatalysis is an alternative to chemical disinfection. He et al. (2004) studied the efficiencies of Ag/Al2O3 and Cu/Al2O3 on SARS-CoV inactivation and observed complete inactivation in 5 min and 20 min (He et al., 2004). Kingsley et al. (2002) studied the effect of hydrostatic pressure on the inactivation of hepatitis A virus (HAV) virus and observed successful inactivation at high pressure (> 450 MPa) within 5 min. Similarly, poliovirus was inactivated at a pressure of 600 MPa within 5 min (Kingsley et al., 2002). Hydrostatic pressure might have damaged the capsid proteins, which led to virus inactivation. Breidablik et al. (2019) devised ozonized water as an alternative for the alcohol-based disinfectant. After SARS outbreak, several chemical disinfectants were used as potential inactivators (He et al., 2004).
5. Antiviral drugs: environmental occurrence and potential ecotoxicity
Several research groups around the globe are working to develop the vaccine for COVID-19, with an expected 12–18 months of timeline for production and delivery. In the meantime, researchers are also exploring potential antiviral drugs to minimize the mortality rate caused by SARS-COV-2. Fig. 3 shows repurposed antiviral drug targets for SARS-CoV-2 infection. Development of drugs starts with the identification of interactions between a target virus and host receptor (Yang et al., 2017), as polymerase inhibition, budding inhibition, target sites for the virus, human-drug interaction, and a better strategy (Zumla et al., 2016). Drug repurposing is one of the most crucial steps in finding the drug from the already existing drugs, which saves both time and cost during drug development (Zhou et al., 2020). Table 3 summarizes various human viruses, including SARS-CoV-2, and their effective antiviral drugs.
Fig. 3.
Repurposed antiviral drug targets for SARS-CoV-2 infection.
Table 3.
Various human viruses with their ecotoxicological effects and side effects on human (Caly et al., 2020, Enoki et al., 2012, Clancy, 2008, Laine et al., 2015, Vorou, 2016, Straub, 2009, Mitjà and Clotet, 2020, McChesney, 1983, Jensen and Scott-Fordsmand, 2012).
G: Gastroenteritis symptoms (Pain, diarrhea, nausea, vomiting, fever, headache, chills, sweating); F: Fatigue; L: Liver problems; K: Kidney problem; V: Visionary problems; T: Throat problems; A: Antiangiogenic effects; C: Cytotoxic effects; N: Neutropenia; P: Pancytopenia; LA: Loss of appetite; S: Sleep disorder (Insomnia, strange dreams, mood change); B: Behavioral changes (feeling irritable, drowsiness, anxiety, mood change).
Lin et al. (2018) studied the effect of disulfiram drug for the inhibition of SARS and MERS coronaviruses (Lin et al., 2018). It behaves as an allosteric inhibitor for MERS and a competitive inhibitor for SARS by inhibiting the papain-like proteases. Zhou et al. (2020) studied the probability of 16 potential drugs for the SARS-CoV-2 after observing a similarity index of 89.6% and 96% in the nucleocapsid proteins and envelop, respectively, with SARS-CoV. The drugs were mesalazine, toremifene, eplerenone, paroxetine, sirolimus, dactinomycin, irbesartan, mercaptopurine, melatonin, quinacrine, carvedilol, colchicine, camphor, equaline, oxymetholone, and emodin. Additionally, a combination of sirolimus and dactinomycin, toremifene and emodin, and mercaptopurine and melatonin were found to be potential drug combinations against SARS-CoV-2 (Zhou et al., 2020). Some of the old drugs (such as ribavirin, interferon, lopinavir, and ritonavir), formerly used for SARS and MERS, are currently under trial repurposing against SARS-CoV-2 (Zumla et al., 2016).
Hydroxychloroquine (HCQ) was reported to be effective against COVID-19 (Liu et al., 2020). The production of cytokine was often observed in severely-ill COVID-19 patients (Sun et al., 2020) and HCQ was found to be successful in inhibiting the production of cytokine (Liu et al., 2020). Wang et al. (2020a) compared the several drugs for the in-vitro treatment of the virus and found out the remdesivir and chloroquine to be highly efficient and safe drugs. Choy et al. (2020) also observed the combination of remdesivir and emetine to be the most suitable combination for the inhibition of SARS-CoV-2 after trying a combination of 5 drugs. Ko et al. (2020) listed the favourable instances of making remdesivir as an inhibitor for COVID-19, namely, reduction of viral load in SARS-CoV-2, safety i.e., nontoxic effects on infected patients. Similarly, Caly et al. (2020) studied the application of FDA-approved Ivermectin drug in reducing the viral RNA by 5,000 times within 48 h.
Immediately after consumption, antiviral drugs undergo series of biotransformation such as glucuronidation, sulfoxidation, dimethylamine N-demethylation, and sulfate conjugation and finally excreted from the human body to the greater extent as metabolites, including active ones, mostly in urine (Nannou et al., 2020). Therefore, the drug residues and metabolites can be continuously transported through hospital and household wastewater to WWTPs and may pose additional challenges in their detection in complex environmental matrices (Mohapatra et al., 2018). Since WWTPs are not specifically designed to remove such drug residues, WWTPs become a major source of antivirals and their metabolites in the surface water bodies, especially during the pandemics (Nannou et al., 2020). In this section, fate, transport, and occurrence of antiviral drugs with their seasonal variation, and ecotoxicological effects in the environmental waters are discussed.
5.1. Fate and transport of antiviral drugs
In general, pharmaceuticals are expected to be attenuated via hydrolysis, sorption photodegradation, and biodegradation in the natural environment (Mohapatra et al., 2020). Similar mechanisms are expected for attenuation of antiviral drugs in the environment. Pharmaceuticals have a large range of Log Kow (octanol-water partition coefficient) from − 4–8, indicating various absorptive nature in the environment ( Fig. 4); antiviral drugs have relatively small Log Kow of − 0.3 ± 0.3 (mean ± standard error), suggesting a hydrophilic nature. Azuma et al. (2017) evaluated the transport of a set of antiviral drugs by studying their photodegradation, biodegradation, and sorption behaviours in a river for a period of 9 months. Except for favipiravir, laninamivir, and laninamivir octanoate, most of the studied antivirals were found to be persistent and could travel from upstream to downstream in chemically unchanged form. While the majority of antivirals are resistant to photodegradation, favipiravir, which is under clinical trial against SARS-CoV-2, is reported to be removed through photodegradation (Azuma et al., 2017). Most of these antiviral drugs were weakly adsorbed to sediment and sludge samples, indicating removal via sorption is not the primary removal mechanism. Except for laninamivir and oseltamivir, no other antivirals were found to be removed through biodegradation.
Fig. 4.
Mobility curve depiction through partitioning coefficients (Kow) of various pharmaceuticals, including antiviral and antimalarial drugs. (Modified from Sanderson et al., 2004).
While there is no extensive study reported on the transport of antiviral drugs in the natural environment, available data on other drugs suggests that river’s hydrogeological conditions, dissolved organic matter, and physical conditions prevailing in an aquatic environment may control the attenuation of antivirals (Yang et al., 2017). However, it would be difficult to predict the actual attenuation of such drugs in surface water receiving active antiviral drugs from the manufacturing industries along the river course as reported elsewhere (Prasse et al., 2010).
5.2. Occurrence and seasonal variation of antiviral drugs in the environment
The occurrence of antiviral drugs in environmental waters is largely affected by seasonality of diseases, corresponding consumption of antiviral drugs, and environmental factors. The common physical and biological parameters such as water flow rate in the river, penetration depth of sunlight, depth of water column, ambient temperature, biological activity, turbulence, mixing, and rate of exfiltration and infiltration can significantly vary depending on seasons. Thus, seasonality influences the fate and transport of antiviral in the aquatic environment. To study the seasonal variation in the concentration of antiviral drugs such as ganciclovir, ribavirin, acyclovir, stavudine, oseltamivir, zidovudine and oseltamivir carboxylate, Peng et al. (2014) analysed the influent and effluent wastewater samples of a WWTP, landfill leachate, groundwater, reservoir water, and surface water from river Pearl and its tributaries in China. The concentrations of anti-influenza drugs were highest in the winter season (54–113 ng L−1) due to high consumption of antiviral drugs in that season. Kolpin et al. (2004) and Yu et al. (2011) studied the effect of river flow/discharge on several antiviral drugs. The drug acyclovir was detected at the highest concentration in winter (17 ng L−1) than in spring (14 ng L−1), summer (11 ng L−1), and fall (7 ng L−1). Its concentration in the effluent did not show statistically significant seasonal variation over one year of sampling. An anti-influenza drug oseltamivir, and its metabolite oseltamivir carboxylate were detected in a river during the flu epidemic in Japan (Azuma et al., 2012). The maximum concentration of oseltamivir carboxylate was found to be 288 ng L−1, and the concentration trend corresponded with the number of influenza patients obtained from sentinel surveillance (Azuma et al., 2012).
In the case of Nairobi River in Kenya, the highest concentration for zidovudine (9 µg L−1) was detected due to high consumption of this drug during HIV-AIDS outbreak (K’oreje et al., 2012). Unlike the Pearl River, acyclovir was not found in the Nairobi River, indicating the importance of drug usage patterns, consumer habits, and prescription patterns, which varies significantly across the globe. The seasonal variation of pharmaceuticals has been well studied, but similar study for antiviral drugs is still lacking (Vieno et al., 2005). Kumar et al. (2020a) studied the seasonal trend in the occurrence of fluoroquinolone drugs viz., levofloxacin, norfloxacin and ciprofloxacin in Kelani and Brahmaputra rivers from Sri Lanka and India, respectively. It was found that concentration of drugs declined significantly in summer compared to the winter season. Similarly, the occurrence of naproxen, diclofenac, ibuprofen, bezafibrate, and ketoprofen was studied in the influent and effluent streams of a WWTP and nearby drinking water treatment plant (DWTP), as well as at downstream of a river in summer, spring and winter seasons (Vieno et al., 2005). Poor removal for the pharmaceuticals was noticed during the winter season (~25%) attributed to slow microbial activity compared to summer and spring seasons. The mean concentration of the studied drugs was found to increase by 3–5 times in the winter season compared to other seasons. These results suggest that cold/winter seasons adversely elevate the probability of pharmaceutical contamination in environmental water and escalate the chances of their occurrence in drinking water (Vieno et al., 2005).
Collectively, in winter, human immunity tends to be low, especially in colder countries, thus viral infection cases and consumption of antiviral drugs increases. Together with the low flow rate in winter, antiviral concentration levels in environmental waters are typically high in winter. Additional studies may be conducted as a priority basis, especially during this pandemic, to understand occurrence, fate, persistence, transport, and seasonal variation of antivirals, including the ones considered for the application to COVID-19 patients, in the environment.
5.3. Ecotoxicity of antiviral drugs
Like several other pharmaceuticals, antiviral drugs are prognosticated to be one of the most perilous among the therapeutic group, with the help of quantitative structure activity relationship (QSAR) modelling, in terms of their toxicity with regard to fishes, crustaceans, common water fleas and algae (Sanderson et al., 2004, Menon et al., 2020). The ecotoxicological effects of various antiviral drugs, together with their side effects on human are summarized in Table 3.
Prasse et al. (2012) reported the toxicity of the oxidation product of the drug acyclovir, N‐(4–carbamoyl‐2–imino‐5–oxoimidazolidin) formamido‐N‐methoxyacetic acid (COFA) on Aliivibrio fischeri bacteria. Diverse reports are available on the ecotoxic nature of antiviral drugs, nonetheless, antiviral drugs for the treatment of influenza are of major concern (Straub, 2009, Hutchinson et al., 2009, Singer et al., 2011). Tamiflu or oseltamivir ethyl ester was reported to be even more toxic than its hydrolysis metabolite oseltamivir acid, towards algae (Desmodesmus subspicatus, Chlorella vulgaris and Pseudokirchneriella subcapitata), bacteria (Vibrio fischeri), water flea (Daphnia magna) and fish (Cyprinus carpio, Pimephales promelas and Danio rerio) (Chen et al., 2014); Oseltamivir and oseltamivir carboxylate was studied by Mestankova et al. (2012), and reported that for daphnia, algae and fish the no observed effect concentration (NOEC) were higher than 1 mg/L. It has been asserted that the influenza drug amantadine does not pose any harm even in serious influenza pandemic situations, however an unaccustomed ecotoxicity was observed even at concentrations 0.1 mM. Inhibition of D. magna, S. capricornutum and P. phosphoreum was reported between 15 min and 72 h of exposure to amantadine and rimantadine (An et al., 2015, Azuma et al., 2015). In another study, major freshwater bacteria viz., Planctomycetes, α, β and γ-Proteobacteria, and Cytophaga-Flavobacterium-Bacteroides, was observed with Fluorescence In-Situ Hybridization (FISH) technique for the comparative toxic effect of Tamiflu and its metabolite oseltamivir carboxylate and reported lower survival of bacteria in the presence of the parent compound (Caracciolo et al., 2010).
Antiretroviral drugs like abacavir have shown prodigious toxic effects on green algae, crustaceans and diatoms, with half maximal effective concentration (EC50) values ranging from 50 to 100 mg/L (Minguez et al., 2016). Green algae containing chlorophyll, are considered to be the primitive first degree producers in an aquatic ecosystem and hence, toxicity towards it, is of pivotal concern (Ncube et al., 2018). Furthermore, subjection to efavirenz (20.6 ng L−1) elicited hepatocyte cell damage and even caused death in O. mossambicus ( Robson et al., 2017 ). Ecotoxicity assay for acyclovir revealed that toxicity on V. fischeri, Raphidocelis subcapitata, D. magna can be reduced upon treatment with UV254 (1.62 × 10−3 mol ein−1)/H2O2 (30–150 mg/L) (Russo et al., 2017). Emtricitabine and Nevirapine are reported by Ngumba et al. (2016), to show toxicity for water flea, algae and fish. Similarly, Zidovudine also exhibited an ecotoxicological effect on algae. Apart from single compounds, the synergistic effect of parent drugs and their metabolites may exert even more serious ecotoxicological consequences. Researchers have communicated that antiviral drugs viz., Ivermectin, HCQ and chloroquine, regarded as potential drug therapies to treat SARS-CoV-2 infection, are carcinogenic, toxic and teratogenic in nature. Some of these drugs discovered previously for other viral diseases and now under trial to treat the novel coronavirus showed acute toxicity to microalgae: Pseudokirchneriella subcapitata, crustacean: Daphnia magna, zebrafish: Danio rerio (McChesney, 1983, Jensen and Scott-Fordsmand, 2012). Thus, these drugs may prove to be a perilous menace to the aquatic ecosystems, and their toxicity and actual environmental conditions needs to be studied in depth.
6. Antiviral resistance
6.1. Occurrence of antiviral resistance
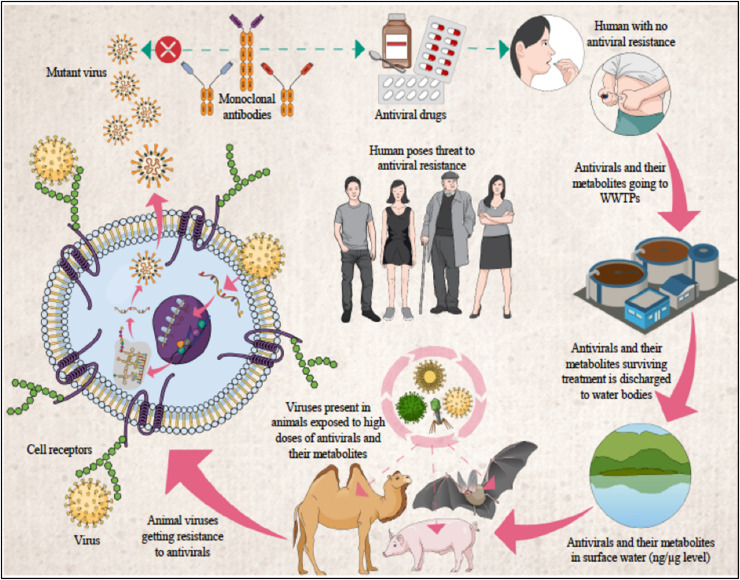
Fig. 5 portrays the global occurrence and prevalence of antiviral resistance in the patients infected by Influenza, Human immunodeficiency virus (HIV), Hepatitis B virus (HBV), Hepatitis C virus (HCV), Herpes simplex virus (HSV), and Human Cytomegalovirus (HCMV). Antiviral drugs released to the environment are of substantial concern, owing to potential transformation in the ecosystem as well as plausible threat of viral resistance development (Singer et al., 2007). In general, WWTPs become major source of the antiviral drugs in the surface water bodies especially during the pandemics. As mentioned above, conventional wastewater treatment at WWTPs doesn’t lead to the complete mineralization of the parent antiviral drugs, but results in residues and formation of metabolites and oxidation/transformation products; those compounds can be as biologically active as their parent compounds. This gives rise to the major concern of the development of antiviral drug-resistant viruses within wild animals, such as bats, pigs, camels, etc., which are natural reservoirs of the viruses (Kumar et al., 2020c). The potential pathways and origins of antiviral drug-resistant viruses through environmental waters are depicted in Fig. 6.
Fig. 5.
Global Occurrence and prevalence of antiviral resistance in the viruses (Influenza, Human immunodeficiency virus: HIV, Hepatitis B virus: HBV, Hepatitis C virus: HCV, Herpes simplex virus: HSV, and Human Cytomegalovirus: HCMV) and virally infected patients.
Fig. 6.
Probable antiviral drug resistant virus mutation in animal reservoirs.
In the case of influenza virus, water fowls are known animal reservoirs (Wallensten et al., 2007). As for SARS-CoV-2, bats and pangolins are considered as wild animal reservoirs (Kumar et al., 2020c, Zhang et al., 2020). Recently, the novel coronavirus has also been detected in eight lions and tigers at New York's Bronx Zoo (Naquin, 2020). Development of antiviral drug-resistant viruses are concerned when those animals with viruses ingest the contaminated environmental waters, then the viruses are continuously exposed to the high loads of antiviral drug residues and their metabolites/transformation products and, thus gain resistance through mutations. This potential development of antiviral drug-resistant viruses within animal reservoirs and their subsequent transmission to humans will compromise the treatment of the viral disease (Kumar et al., 2020c). This all will cause more severity to human being for the current and future pandemic control.
6.2. Clinical implications, management and preventive/control measures against antiviral resistance
When the antiviral resistance is suspected, proper management in terms of clinical treatments, therapies, and laboratory testing can minimise the risk of severe consequences (Strasfeld and Chou, 2010). To guide the therapeutic decisions, an accessible and authorised database about the mutations of drug resistant viruses should be developed (Strasfeld and Chou, 2010). Less toxic and potent antiviral drugs should be developed which can reduce the risk of cross-resistance and simultaneously target the different aspects of viral replications. Understanding the kinetics of emergence of antiviral resistance in the host population (from minor to predominant) is now possible due to the contemporary next-generation sequencing (van der Vries and Ison, 2017). Administration of genetic screening for pre-existing resistant mutants with respect to the particular drugs before the onset of treatments proves to be beneficial as observed in the case of HIV and HCV (Irwin et al., 2016). Such genomic data obtained for screening helps to scan the already known mutants and to detect new resistant mutations. Moreover, for management of antiviral resistant viruses, following actions can be summarised: 1) Discontinuation of ongoing monotherapies: If the ongoing drug therapy is stopped, then generally the resistant virus returns to its wild-type, which can be then easily treated with new drugs or vaccination. 2) Switching to a new antiviral agent or therapy: This will help when the virus is resistant against some particular drug. 3) Adding another drug to the current treatment: New drugs can be more effective against the known resistant mutants than repurposed drugs. 4) Using combination chemotherapies: patient with advanced critical disease/infections can be treated with proper combinations of antiviral agents (Bartholomeusz and Locarnini, 2006).
Management is mainly associated with the already developed resistant mutants or viruses. But following preventive measures can be effective against the development of antiviral resistance as well as for the remediation of environmental pollution by anti-viral drugs:
-
▪
Prevention from the infection and transmission: Most of the viral infections can be prevented and their transmission can be reduced by using simple, inexpensive and effective measures such as hand washing, vaccination, unnecessary injections, use of bed nets, and contraceptives (Drexler, 2014). The general public should be made aware and educated through media and campaigns which clearly emphasize the message regarding the dangers caused by overuse of antiviral or anti-infective drugs.
-
▪
Authorised guidelines: The national (such as https://www.mygov.in/covid-19/) and local authorities should develop and provide the clinical guidelines for different diseases, recommended drug dosage and duration for certain infection treatments. Also, guidelines for the selection of combination therapies and proper antiviral agents should be recommended by the respective authorities and must be communicated effectively to the healthcare personals. Nevertheless, people should be educated with the help of authorised guidelines for proper disposal of the unused and expired drugs.
-
▪
Block interspecies transmission of resistant viruses: Viruses in their natural hosts (animals) can be exposed to antiviral drugs via water that contains residues and metabolites of antiviral drugs, and can subsequently develop antiviral resistance. Although this mechanism is hypothesized and is debatable, natural recombination of genetic materials of different viral strains and drug exposure-induced resistant viral strains can reach human population through direct contact with such natural hosts (reservoirs) or via the intermediate hosts. Thus, avoiding the direct contact with the natural hosts and to some extent intermediate host species, the transmission of resistant viruses can be blocked. For example, in the case of coronaviruses, bats, rats, camels, and pangolins are known natural hosts (Zhang et al., 2020). Thus, identification of their intermediate host species and avoiding contact with them can block the transmission of such coronaviruses to humans.
-
▪
Effective wastewater treatment facilities: A systematic study on the requirement of barriers additional to the conventional wastewater disinfection techniques is recommended. Potential technologies for further assesment includeadvanced treatment technologies, such as advanced oxidation processes, adsorption, membrane separation processes, and/or hybrid treatment systems, (Nannou et al., 2020, Dhangar and Kumar, 2020, Jain et al., 2013, Thakur et al., 2020), which may achieve the required level of SARS-CoV disinfection and also remove the antiviral drug residues and their harmful metabolites/transformation products from treated effluent.
7. Conclusions
While vaccine development against SARS-CoV-2 is underway, the number of confirmed cases has globally crossed 20 million, as of the second week of August, 2020. In such an unprecedented scenario where several countries have already experienced the second wave, the use of alcohol, and chlorine-based disinfectants has been found to be promising to inactivate the virus from various surfaces. Compared to UV-based disinfection, chlorination seems to be a more suitable means for the developing countries due to the low cost and ease of handling. Currently, there is no publication related to persistence and transport of SARS-CoV-2 in land surface and subsurface medium (soil and ground water). However, it is essential to research these aspects of SARS-CoV-2, which particularly critical for the developing countries where lack of sanitation and inadequate management of biomedical wastes are prevelent. It is also imperative to take initiatives to find the answer of questions such as: What will be the different scenarios in case of such a high use of antivirals during this ongoing pandemic of SARS-CoV-2? Can we deny the possibility of the development of stronger viruses, causing severe pandemics than COVID-19 in the near future? What will be the occurrence scenarios of environmental pollution by antiviral drugs and antiviral resistance in different parts of the world? Will there be any correlation of drug residue and antiviral resistance occurence in the ambient water and the number of infected person reported from that region or it will be governed by the capabilities of WWTP infrastructure present in that country?.Finally, how to minimise the pollution of natural waterbodies by antiviral drugs and viruses, especially these antiviral drug concentrations to go the ambient waters like river especially in developing countries where WWTPs are scarce and not even integrated in the hospital effluent?.
CRediT authorship contribution statement
Manish Kumar: Conceptualization, Visualization, Writing - original draft, Revision, and Response. Payal Mazumder: ADR first draft and related illustrations. Sanjeeb Mohapatra: Inactivation Part. Alok Kumar Thakur: Transport portion first draft. Kiran Dhangar: Spread and Seasonality first draft. Kaling Taki: Illustrations and Revision. Santanu Mukherjee: Revision and Response. Arbind Kumar Patel: Environmental Fate. Prosun Bhattacharya: Revision and Improvement. Pranab Mohapatra: Transport portion. Jörg Rinklebe: ADR and related revisions. Masaaki Kitajima: Revision. Faisal I Hai: Inactivation part and revision. Anwar Khursheed: Writing - review & editing. Hiroaki Furumai: Overall read, revision and response. Christian Sonne: Expressions and Revision. Keisuke Kuroda: Visualization, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the fund and support received from the UK-India Education and Research Initiative (UKIERI).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jhazmat.2020.124043.
Appendix A. Supplementary material
Supplementary material
.
References
- Abedi G.R., Watson J.T., Nix W.A., Oberste M.S., Gerber S.I. Enterovirus and parechovirus surveillance—United States, 2014–2016. Morb. Mortal. Wkly. Rep. 2018;67:515–518. doi: 10.15585/mmwr.mm6718a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agolini G., Russo A., Clementi M. Effect of phenolic and chlorine disinfectants on hepatitis C virus binding and infectivity. Am. J. Infect. Control. 1999;27:236–239. doi: 10.1053/ic.1999.v27.a90911. [DOI] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen B.M. Prevention and Control of Infections in Hospitals. Springer; 2019. [Google Scholar]
- Anestad, G. (1982). Interference between outbreaks of respiratory syncytial virus and influenza virus infection.1. [DOI] [PubMed]
- An J., Li G., An T., Song W., Feng H., Lu Y. Photocatalytic degradation of three amantadine antiviral drugs as well as their eco-toxicity evolution. Catal. Today. 2015;258:602–609. [Google Scholar]
- Arens M., Travis S. Zinc salts inactivate clinical isolates of herpes simplex virus in vitro. J. Clin. Microbiol. 2000;38:1758–1762. doi: 10.1128/jcm.38.5.1758-1762.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanious A., Aeppli M., Jacak R., Refardt D., Sigstam T., Kohn T., Sander M. Viruses at solid-water interfaces: a systematic assessment of interactions driving adsorption. Environ. Sci. Technol. 2016;50:732–743. doi: 10.1021/acs.est.5b04644. [DOI] [PubMed] [Google Scholar]
- Aw T.G., Gin K.-H. Prevalence and genetic diversity of waterborne pathogenic viruses in surface waters of tropical urban catchments. J. Appl. Microbiol. 2011;110:903–914. doi: 10.1111/j.1365-2672.2011.04947.x. [DOI] [PubMed] [Google Scholar]
- Azadpour-Keeley A., Ward C.H. Transport and survival of viruses in the subsurface—processes, experiments, and simulation models. Remediat. J. J. Environ. Cleanup Costs Technol. Tech. 2005;15:23–49. [Google Scholar]
- Azuma T., Ishida M., Hisamatsu K., Yunoki A., Otomo K., Kunitou M., Shimizu M., Hosomaru K., Mikata S., Mino Y. Fate of new three anti-influenza drugs and one prodrug in the water environment. Chemosphere. 2017;169:550–557. doi: 10.1016/j.chemosphere.2016.11.102. [DOI] [PubMed] [Google Scholar]
- Azuma T., Nakada N., Yamashita N., Tanaka H. Synchronous dynamics of observed and predicted values of anti-influenza drugs in environmental waters during a seasonal influenza outbreak. Environ. Sci. Technol. 2012;46:12873–12881. doi: 10.1021/es303203c. [DOI] [PubMed] [Google Scholar]
- Azuma T., Nakada N., Yamashita N., Tanaka H. Prediction, risk and control of anti-influenza drugs in the Yodo River Basin, Japan during seasonal and pandemic influenza using the transmission model for infectious disease. Sci. Total Environ. 2015;521:68–74. doi: 10.1016/j.scitotenv.2015.03.069. [DOI] [PubMed] [Google Scholar]
- Azziz Baumgartner E., Dao C.N., Nasreen S., Bhuiyan M.U., Mah-E-Muneer S., Mamun A.A., Sharker M.Y., Zaman R.U., Cheng P.-Y., Klimov A.I. Seasonality, timing, and climate drivers of influenza activity worldwide. J. Infect. Dis. 2012;206:838–846. doi: 10.1093/infdis/jis467. [DOI] [PubMed] [Google Scholar]
- Babich H., Stotzky G. Differential toxicities of mercury to bacteria and bacteriophages in sea and in lake water. Can. J. Microbiol. 1979;25:1252–1257. doi: 10.1139/m79-197. [DOI] [PubMed] [Google Scholar]
- Bae J., Schwab K.J. Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Appl. Environ. Microbiol. 2008;74:477–484. doi: 10.1128/AEM.02095-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On Y.M., Flamholz A., Phillips R., Milo R. Science forum: SARS-CoV-2 (COVID-19) by the numbers. Elife. 2020;9 doi: 10.7554/eLife.57309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomeusz A., Locarnini S.A. Seminars in Liver Disease. Thieme Medical Publishers, Inc.; 333 Seventh Avenue, New York: 2006. Antiviral drug resistance: clinical consequencesand molecular aspects; pp. 162–170. [DOI] [PubMed] [Google Scholar]
- Bastien N., Brandt K., Dust K., Ward D., Li Y. Human bocavirus infection, Canada. Emerg. Infect. Dis. 2006;12:848–850. doi: 10.3201/eid1205.051424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt W.Q., Schijven J., Regnery J., Wing A., Morrison C.M., Drewes J.E., Gerba C.P. Variable non-linear removal of viruses during transport through a saturated soil column. J. Contam. Hydrol. 2019;223 doi: 10.1016/j.jconhyd.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Bosch A., Pintó R.M., Abad F.X. Viruses in Foods. Springer; 2006. Survival and transport of enteric viruses in the environment; pp. 151–187. [Google Scholar]
- Breidablik H.J., Lysebo D.E., Johannessen L., AAse Skare J.R., Andersen O.T. Kleiven, Ozonized water as an alternative to alcohol-based hand disinfection. J. Hosp. Infect. 2019;102:419–424. doi: 10.1016/j.jhin.2019.01.026. [DOI] [PubMed] [Google Scholar]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracciolo A.B., Grenni P., Saccà M.L. Effect of the antiviral drug Oseltamivir (Tamiflu) on the bacterial community structure of a surface water ecosystem analyzed using fluorescence in situ hybridization. Bull. Environ. Contam. Toxicol. 2010;85:443–446. doi: 10.1007/s00128-010-0114-x. [DOI] [PubMed] [Google Scholar]
- Carratala A., Rusinol M., Rodriguez-Manzano J., Guerrero-Latorre L., Sommer R., Girones R. Environmental effectors on the inactivation of human adenoviruses in water. Food Environ. Virol. 2013;5:203–214. doi: 10.1007/s12560-013-9123-3. [DOI] [PubMed] [Google Scholar]
- Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43:1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L.M., Weaver S.R. Inactivation of an enveloped surrogate virus in human sewage. Environ. Sci. Technol. Lett. 2015;2:76–78. [Google Scholar]
- Chen W.-Y., Lin C.-J., Liao C.-M. Assessing exposure risks for aquatic organisms posed by Tamiflu use under seasonal influenza and pandemic conditions. Environ. Pollut. 2014;184:377–384. doi: 10.1016/j.envpol.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Chen C., Zhang X.-J., Wang Y., Zhu L.-X., Liu J. Waste water disinfection during SARS epidemic for microbiological and toxicological control. Biomed. Environ. Sci. BES. 2006;19:173–178. [PubMed] [Google Scholar]
- Choy K.-T., Wong A.Y.-L., Kaewpreedee P., Sia S.-F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P.-H., Huang X. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chris N., Conteas O., Berlin G.W., Lariviere M.J., Pandhumas S.S., Speck C.E., Porschen R., Nakaya T. Examination of the prevalence and seasonal variation of intestinal microsporidiosis in the stools of persons with chronic diarrhea and human immunodeficiency virus infection. Am. J. Trop. Med. Hyg. 1998;58(5):559–561. doi: 10.4269/ajtmh.1998.58.559. [DOI] [PubMed] [Google Scholar]
- Christophers J., Clayton J., Craske J., Ward R., Collins P., Trowbridge M., Darby G. Survey of resistance of herpes simplex virus to acyclovir in northwest England. Antimicrob. Agents Chemother. 1998;42:868–872. doi: 10.1128/aac.42.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy S. Genetics of the influenza virus. Nat. Educ. 2008;1:83. [Google Scholar]
- De Clercq E., Li G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016;29:695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromeans T.L., Kahler A.M., Hill V.R. Inactivation of adenoviruses, enteroviruses, and murine norovirus in water by free chlorine and monochloramine. Appl. Environ. Microbiol. 2010;76:1028–1033. doi: 10.1128/AEM.01342-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croughan W.S., Behbehani A.M. Comparative study of inactivation of herpes simplex virus types 1 and 2 by commonly used antiseptic agents. J. Clin. Microbiol. 1988;26:213–215. doi: 10.1128/jcm.26.2.213-215.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang H.T.T., Tarabara V.V. Virus deposition onto polyelectrolyte-coated surfaces: a study with bacteriophage MS2. J. Colloid Interface Sci. 2019;540:155–166. doi: 10.1016/j.jcis.2018.12.107. [DOI] [PubMed] [Google Scholar]
- Darnell M.E., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhangar K., Kumar M. Tricks and tracks in removal of emerging contaminants from the wastewater through hybrid treatment systems: a review. Sci. Total Environ. 2020;738 doi: 10.1016/j.scitotenv.2020.140320. [DOI] [PubMed] [Google Scholar]
- Dowell S.F. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg. Infect. Dis. 2001;7:369–374. doi: 10.3201/eid0703.010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler, M. (2014). What You Need to Know About Infectious Disease. [PubMed]
- Duffy S. Why are RNA virus mutation rates so damn high? PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.3000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duizer E., Bijkerk P., Rockx B., De Groot A., Twisk F., Koopmans M. Inactivation of caliciviruses. Appl. Environ. Microbiol. 2004;70:4538–4543. doi: 10.1128/AEM.70.8.4538-4543.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoki S., Iino R., Morone N., Kaihatsu K., Sakakihara S., Kato N., Noji H. Label-free single-particle imaging of the influenza virus by objective-type total internal reflection dark-field microscopy. PloS One. 2012;7 doi: 10.1371/journal.pone.0049208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Enriquez, C. (1994). Detection and survival of selected viruses in water.
- Fan D., Tian Y., Han L., Liu Z., Teng Y., Li B. Catalytic electrolysis of sodium chlorite to prepare highly pure chlorine dioxide. J. Funct. Mater. 2017;48:9150–9156. [Google Scholar]
- Fisman D. Seasonality of viral infections: mechanisms and unknowns. Clin. Microbiol. Infect. 2012;18:946–954. doi: 10.1111/j.1469-0691.2012.03968.x. [DOI] [PubMed] [Google Scholar]
- Funderburg S.W., Moore B.E., Sagik B.P., Sorber C.A. Viral transport through soil columns under conditions of saturated flow. Water Res. 1981;15:703–711. [Google Scholar]
- Funke J., Prasse C., Ternes T.A. Identification of transformation products of antiviral drugs formed during biological wastewater treatment and their occurrence in the urban water cycle. Water Res. 2016;98:75–83. doi: 10.1016/j.watres.2016.03.045. [DOI] [PubMed] [Google Scholar]
- Gehrke C., Steinmann J., Goroncy-Bermes P. Inactivation of feline calicivirus, a surrogate of norovirus (formerly Norwalk-like viruses), by different types of alcohol in vitro and in vivo. J. Hosp. Infect. 2004;56:49–55. doi: 10.1016/j.jhin.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Glass J.S., O’Brien R.T. Enterovirus and coliphage inactivation during activated sludge treatment. Water Res. 1980;14:877–882. [Google Scholar]
- Goyal S.M., Gerba C.P. Comparative adsorption of human enteroviruses, simian rotavirus, and selected bacteriophages to soils. Appl. Environ. Microbiol. 1979;38:241–247. doi: 10.1128/aem.38.2.241-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P.M., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1:10. [Google Scholar]
- Haynes A.K., Fowlkes A.L., Schneider E., Mutuc J.D., Armstrong G.L., Gerber S.I. Human metapneumovirus circulation in the United States, 2008 to 2014. Pediatrics. 2016;137 doi: 10.1542/peds.2015-2927. 137. [DOI] [PubMed] [Google Scholar]
- He H., Dong X., Yang M., Yang Q., Duan S., Yu Y., Han J., Zhang C., Chen L., Yang X. Catalytic inactivation of SARS coronavirus, Escherichia coli and yeast on solid surface. Catal. Commun. 2004;5:170–172. doi: 10.1016/j.catcom.2003.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijnen W.A.M., Beerendonk E.F., Medema G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo) cysts in water: a review. Water Res. 2006;40:3–22. doi: 10.1016/j.watres.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Van Der Hoek L., Pyrc K., Jebbink M.F., Vermeulen-Oost W., Berkhout R.J., Wolthers K.C., Wertheim-van Dillen P.M., Kaandorp J., Spaargaren J., Berkhout B. Identification of a new human coronavirus. Nat. Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulkower R.L., Casanova L.M., Rutala W.A., Weber D.J., Sobsey M.D. Inactivation of surrogate coronaviruses on hard surfaces by health care germicides. Am. J. Infect. Control. 2011;39:401–407. doi: 10.1016/j.ajic.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson T.H., Beesley A., Frickers P.E., Readman J.W., Shaw J.P., Straub J.O. Extending the environmental risk assessment for oseltamivir (Tamiflu®) under pandemic use conditions to the coastal marine compartment. Environ. Int. 2009;35:931–936. doi: 10.1016/j.envint.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Hu X., Zhang R., An T., Li Q., Situ B., Ou Z., Wu C., Yang B., Tian P., Hu Y. Impact of heat-inactivation on the detection of SARS-CoV-2 IgM and IgG antibody by ELISA. Clin. Chim. Acta. 2020;509:288–292. doi: 10.1016/j.cca.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin K.K., Renzette N., Kowalik T.F., Jensen J.D. Antiviral drug resistance as an adaptive process. Virus Evol. 2016;2 doi: 10.1093/ve/vew014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Kumar P., Vyas R.K., Pandit P., Dalai A.K. Occurrence and removal of antiviral drugs in environment: a review. Water Air Soil Pollut. 2013;224:1410. [Google Scholar]
- Jelsma T., Wijnker J.J., Smid B., Verheij E., van der Poel W.H., Wisselink H.J. Salt inactivation of classical swine fever virus and African swine fever virus in porcine intestines confirms the existing in vitro casings model. Vet. Microbiol. 2019;238 doi: 10.1016/j.vetmic.2019.108424. [DOI] [PubMed] [Google Scholar]
- Jensen J., Scott-Fordsmand J.J. Ecotoxicity of the veterinary pharmaceutical ivermectin tested in a soil multi-species (SMS) system. Environ. Pollut. 2012;171:133–139. doi: 10.1016/j.envpol.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Jeong E.K., Bae J.E., Kim I.S. Inactivation of influenza A virus H1N1 by disinfection process. Am. J. Infect. Control. 2010;38:354–360. doi: 10.1016/j.ajic.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Jin Y., Chu Y., Li Y. Virus removal and transport in saturated and unsaturated sand columns. J. Contam. Hydrol. 2000;43:111–128. [Google Scholar]
- Johnson D.C., Enriquez C.E., Pepper I.L., Davis T.L., Gerba C.P., Rose J.B. Survival of Giardia, Cryptosporidium, poliovirus and Salmonella in marine waters. Water Sci. Technol. 1997;35:261–268. [Google Scholar]
- John D.E., Rose J.B. Review of factors affecting microbial survival in groundwater. Environ. Sci. Technol. 2005;39:7345–7356. doi: 10.1021/es047995w. [DOI] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuscinski R.B., Mitchell R. Processes controlling virus inactivation in coastal waters. Water Res. 1980;14:363–371. [Google Scholar]
- Khetan S.K., Collins T.J. Human pharmaceuticals in the aquatic environment: a challenge to green chemistry. Chem. Rev. 2007;107:2319–2364. doi: 10.1021/cr020441w. [DOI] [PubMed] [Google Scholar]
- Killerby M.E., Biggs H.M., Haynes A., Dahl R.M., Mustaquim D., Gerber S.I., Watson J.T. Human coronavirus circulation in the United States 2014–2017. J. Clin. Virol. 2018;101:52–56. doi: 10.1016/j.jcv.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley D.H., Hoover D.G., Papafragkou E.F.I., Richards G.P. Inactivation of hepatitis A virus and a calicivirus by high hydrostatic pressure. J. Food Prot. 2002;65:1605–1609. doi: 10.4315/0362-028x-65.10.1605. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpin D.W., Skopec M., Meyer M.T., Furlong E.T., Zaugg S.D. Urban contribution of pharmaceuticals and other organic wastewater contaminants to streams during differing flow conditions. Sci. Total Environ. 2004;328:119–130. doi: 10.1016/j.scitotenv.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Ko W.-C., Rolain J.-M., Lee N.-Y., Chen P.-L., Huang C.-T., Lee P.-I., Hsueh P.-R. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzel A., Todt D., V’kovski P., Steiner S., Gultom M., Thao T.T.N., Ebert N., Holwerda M., Steinmann J., Niemeyer D. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-recommended hand rub formulations and alcohols. Emerg. Infect. Dis. 2020;26:1592–1595. doi: 10.3201/eid2607.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo E., Song E., Yockey L.J., Rakib T., Wong P.W., Homer R.J., Iwasaki A. Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc. Natl. Acad. Sci. 2019;116:10905–10910. doi: 10.1073/pnas.1902840116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaan G.F., Van Amerongen G., Van Riel D., Laman J.D., De Jong T., Van Doornum G., Lim W. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Chaminda G.T., Honda R. Seasonality impels the antibiotic resistance in Kelani River of the emerging economy of Sri Lanka. NPJ Clean Water. 2020;3:1–8. [Google Scholar]
- Kumar M., Kuroda K., Dhangar K. The most eagerly awaited summer of the Anthropocene: a perspective of SARS-CoV-2 decay and seasonal change. Groundw. Sustain. Dev. 2020;11 doi: 10.1016/j.gsd.2020.100400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Kuroda K., Dhangar K., Mazumder P., Sonne C., Rinklebe J., Kitajima M. Potential emergence of antiviral-resistant pandemic viruses via environmental drug exposure of animal reservoirs. Environ. Sci. Technol. 2020;54:8503–8505. doi: 10.1021/acs.est.0c03105. [DOI] [PubMed] [Google Scholar]
- Kumar M., Mohapatra S., Mazumder P., Singh A., Honda R., Lin C., Kumari R., Goswami R., Jha P.K., Vithanage M., Kuroda K. Making Waves Perspectives of Modelling and Monitoring of SARS-CoV-2 in Aquatic Environment for COVID-19 Pandemic. Current Pollution Reports. 2020:1–12. doi: 10.1007/s40726-020-00161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746 doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Taki K., Gahlot R., Sharma A., Dhangar K. A chronicle of SARS-CoV-2: part-I-Epidemiology, diagnosis, prognosis, transmission and treatment. Sci. Total Environ. 2020;734 doi: 10.1016/j.scitotenv.2020.139278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- K’oreje K.O., Demeestere K., Wispelaere P. De, Vergeynst L., Dewulf J., Van Langenhove H. From multi-residue screening to target analysis of pharmaceuticals in water: development of a new approach based on magnetic sector mass spectrometry and application in the Nairobi River basin, Kenya. Sci. Total Environ. 2012;437:153–164. doi: 10.1016/j.scitotenv.2012.07.052. [DOI] [PubMed] [Google Scholar]
- Laine R.F., Albecka A., Van De Linde S., Rees E.J., Crump C.M., Kaminski C.F. Structural analysis of herpes simplex virus by optical super-resolution imaging. Nat. Commun. 2015;6:1–10. doi: 10.1038/ncomms6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landes M.B., Neil R.B., McCool S.S., Mason B.P., Woron A.M., Garman R.L., Smalley D.L. The frequency and seasonality of influenza and other respiratory viruses in T ennessee: two influenza seasons of surveillance data, 2010–2012. Influenza Other Respir. Viruses. 2013;7:1122–1127. doi: 10.1111/irv.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.-M., Lemanske R.F., Jr, Evans M.D., Vang F., Pappas T., Gangnon R., Jackson D.J., Gern J.E. Human rhinovirus species and season of infection determine illness severity. Am. J. Respir. Crit. Care Med. 2012;186:886–891. doi: 10.1164/rccm.201202-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., Feng Z., Rao S., Xiao C., Xue X., Lin Z., Zhang Q., Qi W. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69:1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- Lin M.-H., Moses D.C., Hsieh C.-H., Cheng S.-C., Chen Y.-H., Sun C.-Y., Chou C.-Y. Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antivir. Res. 2018;150:155–163. doi: 10.1016/j.antiviral.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:1–4. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizasoain A., Tort L.F.L., García M., Gillman L., Alberti A., Leite J.P.G., Miagostovich M.P., Pou S.A., Cagiao A., Razsap A. Human enteric viruses in a wastewater treatment plant: evaluation of activated sludge combined with UV disinfection process reveals different removal performances for viruses with different features. Lett. Appl. Microbiol. 2018;66:215–221. doi: 10.1111/lam.12839. [DOI] [PubMed] [Google Scholar]
- Macinga D.R., Sattar S.A., Jaykus L.-A., Arbogast J.W. Improved inactivation of nonenveloped enteric viruses and their surrogates by a novel alcohol-based hand sanitizer. Appl. Environ. Microbiol. 2008;74:5047–5052. doi: 10.1128/AEM.00487-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McChesney E.W. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am. J. Med. 1983;75:11–18. doi: 10.1016/0002-9343(83)91265-2. [DOI] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Meister S., Verbyla M.E., Klinger M., Kohn T. Variability in disinfection resistance between currently circulating enterovirus B serotypes and strains. Environ. Sci. Technol. 2018;52:3696–3705. doi: 10.1021/acs.est.8b00851. [DOI] [PubMed] [Google Scholar]
- Menon N.G., Mohapatra S., Padhye L.P., Tatiparti S.S.V., Mukherji S. Emerging Issues in the Water Environment During Anthropocene. Springer; 2020. Review on occurrence and toxicity of pharmaceutical contamination in Southeast Asia; pp. 63–91. [Google Scholar]
- Mestankova H., Schirmer K., Escher B.I., von Gunten U., Canonica S. Removal of the antiviral agent oseltamivir and its biological activity by oxidative processes. Environ. Pollut. 2012;161:30–35. doi: 10.1016/j.envpol.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Midgley C.M., Haynes A.K., Baumgardner J.L., Chommanard C., Demas S.W., Prill M.M., Abedi G.R., Curns A.T., Watson J.T., Gerber S.I. Determining the seasonality of respiratory syncytial virus in the United States: the impact of increased molecular testing. J. Infect. Dis. 2017;216:345–355. doi: 10.1093/infdis/jix275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez L., Pedelucq J., Farcy E., Ballandonne C., Budzinski H., Halm-Lemeille M.-P. Toxicities of 48 pharmaceuticals and their freshwater and marine environmental assessment in northwestern France. Environ. Sci. Pollut. Res. 2016;23:4992–5001. doi: 10.1007/s11356-014-3662-5. [DOI] [PubMed] [Google Scholar]
- Mitjà O., Clotet B. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob. Health. 2020;8:e639–e640. doi: 10.1016/S2214-109X(20)30114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlunguza N.Y., Ncube S., Mahlambi P.N., Chimuka L., Madikizela L.M. Determination of selected antiretroviral drugs in wastewater, surface water and aquatic plants using hollow fibre liquid phase microextraction and liquid chromatography-tandem mass spectrometry. J. Hazard. Mater. 2020;382 doi: 10.1016/j.jhazmat.2019.121067. [DOI] [PubMed] [Google Scholar]
- Mohapatra S., Menon N.G., Padhye L.P., Tatiparti S.S.V., Mukherji S. Contaminants in Drinking and Wastewater Sources. Springer; 2020. Natural attenuation of pharmaceuticals in the aquatic environment and role of phototransformation; pp. 65–94. [Google Scholar]
- Mohapatra S., Padhye L.P., Mukherji S. Environmental Contaminants. Springer; 2018. Challenges in detection of antibiotics in wastewater matrix; pp. 3–20. [Google Scholar]
- Monto A.S. Epidemiology of viral respiratory infections. Am. J. Med. 2002;112:4–12. doi: 10.1016/s0002-9343(01)01058-0. [DOI] [PubMed] [Google Scholar]
- Morikawa S., Kohdera U., Hosaka T., Ishii K., Akagawa S., Hiroi S., Kase T. Seasonal variations of respiratory viruses and etiology of human rhinovirus infection in children. J. Clin. Virol. 2015;73:14–19. doi: 10.1016/j.jcv.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama M., Hugentobler W.J., Iwasaki A. Seasonality of respiratory viral infections. Annu. Rev. Virol. 2020;7:83–101. doi: 10.1146/annurev-virology-012420-022445. [DOI] [PubMed] [Google Scholar]
- Mourtzoukou E.G., Falagas M.E. Exposure to cold and respiratory tract infections. Int. J. Tuberc. Lung Dis. 2007;11:938–943. [PubMed] [Google Scholar]
- Musharrafieh R., Ma C., Wang J. Discovery of M2 channel blockers targeting the drug-resistant double mutants M2-S31N/L26I and M2-S31N/V27A from the influenza A viruses. Eur. J. Pharm. Sci. 2020;141 doi: 10.1016/j.ejps.2019.105124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannou C., Ofrydopoulou A., Evgenidou E., Heath D., Heath E., Lambropoulou D. Antiviral drugs in aquatic environment and wastewater treatment plants: A review on occurrence, fate, removal and ecotoxicity. Sci. Total Environ. 2020;699 doi: 10.1016/j.scitotenv.2019.134322. [DOI] [PubMed] [Google Scholar]
- Naquin T. Eight lions and tigers at Bronx Zoo test positive for coronavirus. Fox8. 2020 [Google Scholar]
- Ncube S., Madikizela L.M., Chimuka L., Nindi M.M. Environmental fate and ecotoxicological effects of antiretrovirals: a current global status and future perspectives. Water Res. 2018;145:231–247. doi: 10.1016/j.watres.2018.08.017. [DOI] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraia A., Surya K., Wiegand T., Buyukyoruk M., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. MedRxiv. 2020;1 doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngumba E., Gachanja A., Nyirenda J., Maldonado J., Tuhkanen T. Occurrence of antibiotics and antiretroviral drugs in source-separated urine, groundwater, surface water and wastewater in the peri-urban area of Chunga in Lusaka, Zambia. Water SA. 2020;46:278–284. [Google Scholar]
- Ngumba E., Gachanja A., Tuhkanen T. Occurrence of selected antibiotics and antiretroviral drugs in Nairobi River Basin, Kenya. Sci. Total Environ. 2016;539:206–213. doi: 10.1016/j.scitotenv.2015.08.139. [DOI] [PubMed] [Google Scholar]
- Ogata N., Shibata T. Protective effect of low-concentration chlorine dioxide gas against influenza A virus infection. J. Gen. Virol. 2008;89:60–67. doi: 10.1099/vir.0.83393-0. [DOI] [PubMed] [Google Scholar]
- Ogorzaly L., Bertrand I., Paris M., Maul A., Gantzer C. Occurrence, survival, and persistence of human adenoviruses and F-specific RNA phages in raw groundwater. Appl. Environ. Microbiol. 2010;76:8019–8025. doi: 10.1128/AEM.00917-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J. Hosp. Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.A., Kang J.K., Kim S.B. Comparative analysis of bacteriophages and bacteria removal in soils and pyrophyllite-amended soils: column experiments. Water Air Soil Pollut. 2017;228:103. doi: 10.1007/s11270-017-3288-6. [DOI] [Google Scholar]
- Park J.-A., Kim J.-H., Lee I., Kim S.-B. Assessment of viral attenuation in soil using probabilistic quantitative model. J. Korean Soc. Environ. Eng. 2011;33:544–551. [Google Scholar]
- Park S.H., Kim E.J., Yun T.H., Lee J.H., Kim C.K., Seo Y.H., Oh S.A., Choi S.S., Cho S.J., Kim M.S. Human enteric viruses in groundwater. Food Environ. Virol. 2010;2:69–73. [Google Scholar]
- Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. JAMA. 2020;323:707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- Peci A., Winter A.-L., Li Y., Gnaneshan S., Liu J., Mubareka S., Gubbay J.B. Effects of absolute humidity, relative humidity, temperature, and wind speed on influenza activity in Toronto, Ontario, Canada. Appl. Environ. Microbiol. 2019;85 doi: 10.1128/AEM.02426-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S.M., Lai S.T., Poon L.L.M., Guan Y., Yam L.Y.C., Lim W., Nicholls J., Yee W.K.S., Yan W.W., Cheung M.T. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Wang C., Zhang K., Wang Z., Huang Q., Yu Y., Ou W. Profile and behavior of antiviral drugs in aquatic environments of the Pearl River Delta, China. Sci. Total Environ. 2014;466:755–761. doi: 10.1016/j.scitotenv.2013.07.062. [DOI] [PubMed] [Google Scholar]
- Perdiz D., Gróf P., Mezzina M., Nikaido O., Moustacchi E., Sage E. Distribution and repair of bipyrimidine photoproducts in solar UV-irradiated mammalian cells possible role of dewar photoproducts in solar mutagenesis. J. Biol. Chem. 2000;275:26732–26742. doi: 10.1074/jbc.M001450200. [DOI] [PubMed] [Google Scholar]
- Pfaender S., Brinkmann J., Todt D., Riebesehl N., Steinmann J., Steinmann J., Pietschmann T., Steinmann E. Mechanisms of methods for hepatitis C virus inactivation. Appl. Environ. Microbiol. 2015;81:1616–1621. doi: 10.1128/AEM.03580-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasse C., Schlüsener M.P., Schulz R., Ternes T.A. Antiviral drugs in wastewater and surface waters: a new pharmaceutical class of environmental relevance? Environ. Sci. Technol. 2010;44:1728–1735. doi: 10.1021/es903216p. [DOI] [PubMed] [Google Scholar]
- Prasse C., Wagner M., Schulz R., Ternes T.A. Oxidation of the antiviral drug acyclovir and its biodegradation product carboxy-acyclovir with ozone: kinetics and identification of oxidation products. Environ. Sci. Technol. 2012;46:2169–2178. doi: 10.1021/es203712z. [DOI] [PubMed] [Google Scholar]
- Pratelli A. Canine coronavirus inactivation with physical and chemical agents. Vet. J. 2008;177:71–79. doi: 10.1016/j.tvjl.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenau H.F., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H.W. Stability and inactivation of SARS coronavirus. Med. Microbiol. Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race M., Ferraro A., Galdiero E., Guida M., Núñez-Delgado A., Pirozzi F., Siciliano A., Fabbricino M. Current emerging SARS-CoV-2 pandemic: potential direct/indirect negative impacts of virus persistence and related therapeutic drugs on the aquatic compartments. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnesar-Shumate S., Williams G., Green B., Krause M., Holland B., Wood S., Bohannon J., Boydston J., Freeburger D., Hooper I. Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. J. Infect. Dis. 2020;222:214–222. doi: 10.1093/infdis/jiaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanat J.-L., Douki T., Cadet J. Direct and indirect effects of UV radiation on DNA and its components. J. Photochem. Photobiol. B Biol. 2001;63:88–102. doi: 10.1016/s1011-1344(01)00206-8. [DOI] [PubMed] [Google Scholar]
- Robson L., Barnhoorn I.E.J., Wagenaar G.M. The potential effects of efavirenz on Oreochromis mossambicus after acute exposure. Environ. Toxicol. Pharmacol. 2017;56:225–232. doi: 10.1016/j.etap.2017.09.017. [DOI] [PubMed] [Google Scholar]
- Russo D., Siciliano A., Guida M., Galdiero E., Amoresano A., Andreozzi R., Reis N.M., Puma G.L., Marotta R. Photodegradation and ecotoxicology of acyclovir in water under UV254 and UV254/H2O2 processes. Water Res. 2017;122:591–602. doi: 10.1016/j.watres.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Sanderson H., Johnson D.J., Reitsma T., Brain R.A., Wilson C.J., Solomon K.R. Ranking and prioritization of environmental risks of pharmaceuticals in surface waters. Regul. Toxicol. Pharmacol. 2004;39:158–183. doi: 10.1016/j.yrtph.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Sanjuán R., Nebot M.R., Chirico N., Mansky L.M., Belshaw R. Viral mutation rates. J. Virol. 2010;84:9733–9748. doi: 10.1128/JVI.00694-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schijven J.F., Hassanizadeh S.M. Removal of viruses by soil passage: overview of modeling, processes, and parameters. Crit. Rev. Environ. Sci. Technol. 2000;30:49–127. [Google Scholar]
- Seo K., Lee J.E., Lim M.Y., Ko G. Effect of temperature, pH, and NaCl on the inactivation kinetics of murine norovirus. J. Food Prot. 2012;75:533–540. doi: 10.4315/0362-028X.JFP-11-199. [DOI] [PubMed] [Google Scholar]
- Shin G.-A., Sobsey M.D. Inactivation of norovirus by chlorine disinfection of water. Water Res. 2008;42:4562–4568. doi: 10.1016/j.watres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Shoji M., Katayama K., Sano K. Absolute humidity as a deterministic factor affecting seasonal influenza epidemics in Japan. Tohoku J. Exp. Med. 2011;224:251–256. doi: 10.1620/tjem.224.251. [DOI] [PubMed] [Google Scholar]
- Sidhu J.P.S., Toze S., Hodgers L., Barry K., Page D., Li Y., Dillon P. Pathogen decay during managed aquifer recharge at four sites with different geochemical characteristics and recharge water sources. J. Environ. Qual. 2015;44:1402–1412. doi: 10.2134/jeq2015.03.0118. [DOI] [PubMed] [Google Scholar]
- Singer A.C., Colizza V., Schmitt H., Andrews J., Balcan D., Huang W.E., Keller V.D., Vespignani A., Williams R.J. Assessing the ecotoxicologic hazards of a pandemic influenza medical response. Environ. Health Perspect. 2011;119:1084–1090. doi: 10.1289/ehp.1002757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A.C., Nunn M.A., Gould E.A., Johnson A.C. Potential risks associated with the proposed widespread use of Tamiflu. Environ. Health Perspect. 2007;115:102–106. doi: 10.1289/ehp.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skraber S., Ogorzaly L., Helmi K., Maul A., Hoffmann L., Cauchie H.-M., Gantzer C. Occurrence and persistence of enteroviruses, noroviruses and F-specific RNA phages in natural wastewater biofilms. Water Res. 2009;43:4780–4789. doi: 10.1016/j.watres.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Sloan C., Moore M.L., Hartert T. Impact of pollution, climate, and sociodemographic factors on spatiotemporal dynamics of seasonal respiratory viruses. Clin. Transl. Sci. 2011;4:48–54. doi: 10.1111/j.1752-8062.2010.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasfeld L., Chou S. Antiviral drug resistance: mechanisms and clinical implications. Infect. Dis. Clin. 2010;24:809–833. doi: 10.1016/j.idc.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Straub J.O. An environmental risk assessment for oseltamivir (Tamiflu®) for sewage works and surface waters under seasonal-influenza-and pandemic-use conditions. Ecotoxicol. Environ. Saf. 2009;72:1625–1634. doi: 10.1016/j.ecoenv.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Sun X., Wang T., Cai D., Hu Z., Liao H., Zhi L., Wei H., Zhang Z., Qiu Y., Wang J. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamerius J., Nelson M.I., Zhou S.Z., Viboud C., Miller M.A., Alonso W.J. Global influenza seasonality: reconciling patterns across temperate and tropical regions. Environ. Health Perspect. 2011;119:439–445. doi: 10.1289/ehp.1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A.K., Vithanage M., Das D., Kumar M. A review on design, material selection, mechanism, and modeling of permeable reactive materials for community-scale groundwater treatment. Environ. Technol. Innov. 2020;19 [Google Scholar]
- Toranzo A.E., Barja J.L., Hetrick F.M. Mechanism of poliovirus inactivation by cell-free filtrates of marine bacteria. Can. J. Microbiol. 1983;29:1481–1486. doi: 10.1139/m83-228. [DOI] [PubMed] [Google Scholar]
- Toranzo A.E., Metricic F.M. Comparative stability of two salmonid viruses and poliovirus in fresh, estuarine and marine waters. J. Fish Dis. 1982;5:223–231. [Google Scholar]
- Tree J.A., Adams M.R., Lees D.N. Chlorination of indicator bacteria and viruses in primary sewage effluent. Appl. Environ. Microbiol. 2003;69:2038–2043. doi: 10.1128/AEM.69.4.2038-2043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya V., Dhere R., Agnihotri S., Muley R., Patil S., Pawar A. Ultraviolet-C irradiation for inactivation of viruses in foetal bovine serum. Vaccine. 2018;36:4215–4221. doi: 10.1016/j.vaccine.2018.06.008. [DOI] [PubMed] [Google Scholar]
- Vettori C., Gallori E., Stotzky G. Clay minerals protect bacteriophage PBS1 of Bacillus subtilis against inactivation and loss of transducing ability by UV radiation. Can. J. Microbiol. 2000;46:770–773. [PubMed] [Google Scholar]
- Vieno N.M., Tuhkanen T., Kronberg L. Seasonal variation in the occurrence of pharmaceuticals in effluents from a sewage treatment plant and in the recipient water. Environ. Sci. Technol. 2005;39:8220–8226. doi: 10.1021/es051124k. [DOI] [PubMed] [Google Scholar]
- Vorou R. Zika virus, vectors, reservoirs, amplifying hosts, and their potential to spread worldwide: what we know and what we should investigate urgently. Int. J. Infect. Dis. 2016;48:85–90. doi: 10.1016/j.ijid.2016.05.014. [DOI] [PubMed] [Google Scholar]
- van der Voorn T., van den Berg C., Bhattacharya P., Quist J. Never waste a crisis: drawing first lessons from the COVID-19 pandemic to tackle the water crisis. ACS ES&T Water. 2020 [Google Scholar]
- van der Vries E., Ison M.G. Antimicrobial Drug Resistance. Springer; 2017. Antiviral resistance in influenza viruses: clinical and epidemiological aspects; pp. 1165–1183. [Google Scholar]
- Wallensten A., Munster V.J., Latorre-Margalef N., Brytting M., Elmberg J., Fouchier R.A., Fransson T., Haemig P.D., Karlsson M., Lundkvist A. Surveillance of influenza virus A in migratory waterfowl in Northern Europe. Emerg. Infect. Dis. 2007;13:404–411. doi: 10.3201/eid1303.061130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walshe G.E., Pang L., Flury M., Close M.E., Flintoft M. Effects of pH, ionic strength, dissolved organic matter, and flow rate on the co-transport of MS2 bacteriophages with kaolinite in gravel aquifer media. Water Res. 2010;44:1255–1269. doi: 10.1016/j.watres.2009.11.034. [DOI] [PubMed] [Google Scholar]
- Walshe G.E., Pang L., Flury M., Close M.E., Flintoft M. Effects of pH, ionic strength, dissolved organic matter, and flow rate on the co-transport of MS2 bacteriophages with kaolinite in gravel aquifer media. Water Res. 2010;44:1255–1269. doi: 10.1016/j.watres.2009.11.034. [DOI] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-W., Li J.-S., Jin M., Zhen B., Kong Q.-X., Song N., Xiao W.-J., Yin J., Wei W., Wang G.-J. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol. Methods. 2005;126:171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Shen J., Ye D., Yan X., Zhang Y., Yang W., Li X., Wang J., Zhang L., Pan L. Disinfection technology of hospital wastes and wastewater: Suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T.P., Stilianakis N.I. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J. Infect. 2008;57:361–373. doi: 10.1016/j.jinf.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C., Chen Z., Kapes T., Chiossone C., Lukula S., Suchmann D., Nims R., Zhou S.S. Inactivation an d disinfection of Zika virus on a nonporous surface. J. Micro Biochem. Technol. 2016;8:422–427.. [Google Scholar]
- Williamson K.E., Radosevich M., Wommack K.E. Abundance and diversity of viruses in six Delaware soils. Appl. Environ. Microbiol. 2005;71:3119–3125. doi: 10.1128/AEM.71.6.3119-3125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhib T., Silva T.M., Newman R.D., Garcia L.S., Pereira M.L.D., Chaves C.S., Wahlquist S.P., Bryan R.T., Guerrant R.L., de A., Sousa Q. Cryptosporidial and microsporidial infections in human immunodeficiency virus-infected patients in northeastern Brazil. J. Infect. Dis. 1994;170:494–497. doi: 10.1093/infdis/170.2.494. [DOI] [PubMed] [Google Scholar]
- Wurtzer S., Marechal V., Mouchel J.-M., Moulin L. Time course quantitative detection of SARS-CoV-2 in Parisian wastewaters correlates with COVID-19 confirmed cases. MedRxiv. 2020 [Google Scholar]
- Wu F., Xiao A., Zhang J., Gu X., Lee W.L., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. MedRxiv. 2020 doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Ellis A., Magnuson M., Harper W.F., Jr Adsorption of bacteriophage MS2 to colloids: Kinetics and particle interactions. Colloids Surf. A Physicochem. Eng. Asp. 2020;585 doi: 10.1016/j.colsurfa.2019.124099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., He J.-T., Su S.-H., Cui Y.-F., Huang D.-L., Wang G.-C. Occurrence, distribution, and attenuation of pharmaceuticals and personal care products in the riverside groundwater of the Beiyun River of Beijing, China. Environ. Sci. Pollut. Res. 2017;24:15838–15851. doi: 10.1007/s11356-017-8999-0. [DOI] [PubMed] [Google Scholar]
- Yates M.V., Jury W.A. On the use of virus transport modeling for determining regulatory compliance. J. Environ. Qual. 1995;24:1051–1055. [Google Scholar]
- Yates M.V., Yates S.R., Wagner J., Gerba C.P. Modeling virus survival and transport in the subsurface. J. Contam. Hydrol. 1987;1:329–345. [Google Scholar]
- Ye Y., Ellenberg R.M., Graham K.E., Wigginton K.R. Survivability, partitioning, and recovery of enveloped viruses in untreated municipal wastewater. Environ. Sci. Technol. 2016;50:5077–5085. doi: 10.1021/acs.est.6b00876. [DOI] [PubMed] [Google Scholar]
- Yu Y., Huang Q., Wang Z., Zhang K., Tang C., Cui J., Feng J., Peng X. Occurrence and behavior of pharmaceuticals, steroid hormones, and endocrine-disrupting personal care products in wastewater and the recipient river water of the Pearl River Delta, South China. J. Environ. Monit. 2011;13:871–878. doi: 10.1039/c0em00602e. [DOI] [PubMed] [Google Scholar]
- Yu B., Zhou Y., Huang Z.W. Research on simple disinfection system for medical wastewater of Township hospital. Asian J. Chem. 2014;26:3243–3245. [Google Scholar]
- Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 2020;30:1346–1351. doi: 10.1016/j.cub.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:1–18. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Chan J.F., Azhar E.I., Hui D.S., Yuen K.-Y. Coronaviruses—drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimunek J., Van Genuchten M.T., Šejna M. Recent developments and applications of the HYDRUS computer software packages. Vadose Zone J. 2016;15 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material