Abstract
A variety of bis-heterocycles such as bis(pyrimido[4,5-b]quinolone), bis(chromeno[3′,4':5,6]pyrido[2,3-d]pyrimidine), bis(pyrido[2,3-d:6,5-d']dipyrimidine), and bis(benzo[g]pyrimido[4,5-b]quinolone) derivatives were synthesized via one-pot, multi-component reaction of various 6-aminouracils or 6-aminothiouracils, terephthalaldehyde, and CH-acids such as 4-hydroxycoumarin, dimedone, 2-hydroxy-1,4-naphthoquinone, barbituric acid, and thiobarbituric acid in EtOH as a solvent at reflux. The mild conditions, fast rate of reaction, absence of catalyst, different functional group compatibility, simple operation and work-up involving no chromatographic process, are worth mentioning.
Keywords: Organic chemistry, Multi-component reaction, Bis pyridopyrimidine, Pyrimidoquinolone, Aminothiouracils
Organic chemistry, Multi-component reaction, Bis pyridopyrimidine, Pyrimidoquinolone, Aminothiouracils.
1. Introduction
Multi-component reactions (MCRs) have regarded as essential tools for the preparation of biologically active heterocyclic compounds because of their productivity, convergence, simple procedures, and easy execution [1]. The development of MCRs and their applications for the one-pot synthesis of various useful heterocyclic compounds are of remarkable interest in the running research articles [2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13].
Heterocyclic scaffolds are widely distributed in nature and used in structure-based drug design [14]. Therefore, considerable attention has been paid to develop novel approaches to the synthesis of heterocycles [15, 16, 17, 18, 19, 20, 21, 22]. Among them pyridopyrimidine derivatives have attracted wide attention due to their broad biological activities including anticancer [23], antiviral [24], antiallergic [25], anti-HIV [26], anti-inflammatory [27], and antifolate [28] properties; and pyrimidoquinoline derivatives are of importance because of their interesting and diverse pharmacological properties; such as, antimicrobial, anti-inflammatory, anticancer [29], antiallergic [30], anti-HIV, antimalarial [31], and antibacterial [32] activities. Some biologically important pyridopyrimidines [33] and pyrimidoquinolines [34] are shown in Figure 1.
Figure 1.
Some biologically important pyridopyrimidines and pyrimidoquinolines.
Also some bis-heterocyclic compounds exhibit a wide range of biological and pharmacological activities and have received extensive attention in recent decades [35, 36]. In 2013 Nefzi and Murru prepared a new library of oxazol-thiazole bis-heterocycles by a solution and solid-phase parallel synthesis methodology in good to excellent yields.38 Also in 2016 Montano et al. reported synthesis of novel unsymmetrical bis-heterocycles containing the imidazo[2,1-b]thiazole or the benzo[d]imidazo[2,1-b]thiazole frameworks bound with quinolone, chromone, or julolidine via an acid-free Groebke-Blackburn-Bienayme reaction (GBBR) under microwave-heating conditions in good to excellent yields [36].
As part of our continuing interest in the preparation of novel heterocyclic compounds and due to importance of pyridopyrimidine and pyrimidoquinoline derivatives as substructures in a wide range of drug-like compounds, herein we developed synthesis of new bis-pyridopyrimidines and bis-pyrimidoquinolones by one-pot, multi-component reaction of diverse 6-aminouracils, terephthalaldehyde, and CH-acids in EtOH as a solvent at reflux without a catalyst.
2. Experimental
2.1. Reagent and apparatus
The diverse 6-aminouracils, 6-aminothiouracil, terephthalaldehyde, 4-hydroxycoumarin, dimedone, 2-hydroxy-1,4-naphthoquinone, barbituric acid, thiobarbituric acid, and solvents were purchased from Sigma-Aldrich chemical company and were used as received without further purification. Melting points were measured with an electrothermal 9100 apparatus. Infrared (IR) spectra were obtained on a Bruker Tensor 27 spectrometer. Mass spectra recorded with an Agilent 5975C VL MSD with Triple-Axis Detector operating at an ionization potential of 70 eV. Nuclear magnetic resonance (NMR) spectra were recorded with a Bruker DRX-300 Avance instrument (300 MHz for 1H, 68.8 and 125 MHz for 13C) with DMSO as solvent. Chemical shifts are given in parts per million (ppm), and coupling constant (J) are reported in hertz (Hz).
2.2. General procedure for the synthesis of product 4a
A mixture of 6-aminothiouracil (2 mmol, 0.286 g), terephthalaldehyde (1 mmol, 0.134 g), 2-hydroxy-1,4-naphthoquinone (2 mmol, 0.348 g) and 10 mL EtOH in a 50 mL flask was stirred at reflux for 5 min. Upon completion as monitored by TLC (ethyl acetate/n-hexane, 1:1), the reaction mixture was cooled to room temperature and filtered to give the crude product. The resulting solid product was washed with EtOH to give pure product 4a in 76% yields.
2.3. Spectral data
2.3.1. 7,7'-(1,4-Phenylene)bis(10-thioxo-9,10,11,12-tetrahydro-6H-chromeno[3′,4':5,6]pyrido[2,3-d]pyrimidine-6,8(7H)-dione) (4a)
White solid: M.p.: 300–302 °C, yield: 0.510 g (76%). IR (KBr) (νmax/cm−1): 3340, 3220, 1680, 1650, 1220. MS (EI, 70 eV): m/z (%) = 378 (19), 266 (24), 155 (100), 120 (39), 82 (88), 57 (38). 1H NMR (300 MHz, DMSO-d6): δ 5.52 (2H, s, 2CH), 6.74–7.79 (12H, m, Ar), 12.13 (2H, s, 2NH), 12.45 (2H, s, 2NH), 13.49 (2H, s, 2NH). 13C NMR (62.8 MHz, DMSO-d6): δ 35.8 (CH), 92.2 (CC = ONH), 106.3 (CC = OO), 117.5, 118.4, 125.0, 125.6, 127.7, 133.7, 136.7, 153.3, 155.7, 164.6, 165.9 (C=O), 166.5 (C=O), 174.1 (C=S).
2.3.2. 7,7'-(1,4-Phenylene)bis(11,12-dihydro-6H-chromeno[3′,4':5,6]pyrido[2,3-d]pyrimidine-6,8,10(7H,9H)-trione) (4b)
White solid: M.p.: 305–307 °C, yield: 0.435 g (68%). %). IR (KBr) (νmax/cm−1): 3320, 3200, 1687, 1650, 1215. MS (EI, 70 eV): m/z (%) = 251 (5), 162 (74), 120 (100), 92 (88), 63 (30). 1H NMR (300 MHz, DMSO-d6): δ 5.46 (2H, s, 2CH), 6.60–7.80 (12H, m, Ar), 10.72 (2H, s, 2NH), 11.02 (2H, s, 2NH), 14.05 (2H, s, 2NH). 13C NMR (62.8 MHz, DMSO-d6): δ 35.7 (CH), 87.5 (CC = ONH), 106.6 (CC = OO), 117.5, 118.6, 125.0, 125.6, 127.6, 133.6, 137.2, 150.7, 153.3, 156.8, 164.8 (C=O), 166.8 (C=O), 168.3 (C=O).
2.3.3. 7,7'-(1,4-Phenylene)bis(9,11-dimethyl-11,12-dihydro-6H-chromeno[3′,4':5,6]pyrido[2,3-d]pyrimidine-6,8,10(7H,9H)-trione) (4c)
White solid: M.p.: 304–306 °C, yield: 0.508 g (73%). IR (KBr) (νmax/cm−1): 3426, 3328, 3212, 1704, 1653, 1618, 1199. MS (EI, 70 eV): m/z (%) = 404 (40), 292 (20), 180 (26), 155 (97), 120 (100), 82 (74), 57 (39). 1H NMR (300 MHz, DMSO-d6): δ 3.10 (6H, s, 2NCH3), 3.12 (6H, s, 2NCH3), 3.34 (6H, s, 2NCH3), 3.36 (6H, s, 2NCH3), 5.52 (4H, s, 4CH), 6.93–7.81 (24H, m, Ar), 13.98 (4H, s, 4NH). 13C NMR (62.8 MHz, DMSO-d6): δ 29.6 (NCH3), 31.9 (NCH3), 31.3 (NCH3), 37.1 (CH), 38.0 (CH), 88.1 (CC = ONCH3), 92.4 (CC = ONCH3), 105.2 (CC = OO), 106.1 (CC = OO), 117.0, 117.5, 118.3, 120.3, 124.6, 125.0, 125.6, 127.2, 127.6, 128.7, 130.8, 132.7, 133.8, 135.9, 137.0, 138.3, 139.8, 147.5, 151.5, 151.9, 153.3, 153.7, 156.5, 165.1 (C=O), 165.5 (C=O), 166.1 C=O), 167.1 (C=O), 167.5 (C=O).
2.3.4. 5,5'-(1,4-Phenylene)bis(8,8-dimethyl-2-thioxo-2,3,7,8,9,10-hexahydropyrimido[4,5-b]quinoline-4,6(1H,5H)-dione) (4d)
White solid: M.p.: 331–333 °C, yield: 0.496 g (79%). %). IR (KBr) (νmax/cm−1): 3300, 1710, 1680, 1230. MS (EI, 70 eV): m/z (%) = 378 (100), 295 (16), 266 (46), 240 (24), 143 (45), 97 (29), 83 (63), 69 (94), 57 (52). 1H NMR (300 MHz, DMSO-d6): δ 0.99 (12H, s, 4CH3), 1.05 (12H, s, 4CH3), 2.24–2.39 (16H, m, 4CH2), 5.32 (4H, s, 4CH), 6.57 (4H, s, Ar), 6.91 (4H, s, Ar), 12.01 (4H, s, 4NH), 12.25 (8H, s, 8NH). 13C NMR (62.8 MHz, DMSO-d6): δ 28.0 (CH3), 28.3 (CH3), 29.3 (C(CH3)2), 30.1 (C(CH3)2), 32.5 (CH), 33.7 (CH), 51.5 (CH2), 93.5 (CC = ONH), 95.5 (CC = ONH), 112.5, 115.3, 127.3, 127.5, 128.4, 128.6, 129.9, 130.7, 137.4, 138.2, 144.1, 144.9, 150.1, 155.2, 161.5 (C=O), 165.4 (C=O), 174.1 (C=S), 174.7 (C=S), 195.8 (C=O).
2.3.5. 5,5'-(1,4-Phenylene)bis(1,3,8,8-tetramethyl-7,8,9,10-tetrahydropyrimido[4,5-b]quinoline-2,4,6(1H,3H,5H)-trione) (4e)
White solid: M.p.: 273–275 °C, yield: 0.554 g (85%). %). IR (KBr) (νmax/cm−1): 3360, 3190, 1718, 1689, 1240. MS (EI, 70 eV): m/z (%) = 378 (21), 266 (23), 155 (86), 127 (16), 82 (100), 57 (50). 1H NMR (300 MHz, DMSO-d6): δ 1.00 (6H, s, 2CH3), 1.07 (6H, s, 2CH3), 2.18–2.41 (8H, m, 4CH2), 3.06 (6H, s, 2NCH3), 3.25 (6H, s, 2NCH3), 5.38 (2H, s, 2CH), 6.92 (2H, s, Ar), 7.25 (2H, s, Ar), 12.80 (2H, s, 2NH). 13C NMR (62.8 MHz, DMSO-d6): δ 28.5 (CH3), 29.4 (CH3), 30.2 (CH3), 31.7 (NCH3), 32.5 (NCH3), 33.6 (C(CH3)2), 34.0 (CH), 45.0 (CH2), 50.9 (CH2), 89.2 (CC = ONCH3), 115.2, 115.3, 127.1, 127.4, 128.6, 138.0, 151.6, 151.9, 156.0, 161.1 (C=O), 177.3 (C=S), 200.8 (C=O).
2.3.6. 5,5'-(1,4-Phenylene)bis(8,8-dimethyl-7,8,9,10-tetrahydropyrimido[4,5-b]quinoline-2,4,6(1H,3H,5H)-trione) (4f)
White solid: M.p.: 348–350 °C, yield: 0.408 g (65%). 1H NMR (300 MHz, DMSO-d6): δ 0.83 (6H, s, 2CH3), 0.86 (6H, s, 2CH3), 0.97 (12H, s, 4CH3), 1.98–2.40 (16H, m, 8CH2), 4.65 (2H, s, 2CH), 4.67 (2H, s, 2CH), 6.16 (2H, s, Ar), 6.95 (6H, s, Ar), 8.69 (4H, s, 4NH), 10.14 (4H, s, 4NH), 10.67 (4H, s, 4NH).
2.3.7. 5,5'-(1,4-Phenylene)bis(8-thioxo-7,8,9,10-tetrahydropyrido[2,3-d:6,5-d']dipyrimidine-2,4,6(1H,3H,5H)-trione) (4g)
Orange solid: M.p.: >360 °C, yield: 0.544 g (90%). IR (KBr) (νmax/cm−1): 3172, 1701, 1618, 1209. MS (EI, 70 eV): m/z (%) = 407 (2), 379 (3), 279 (6), 183 (7), 149 (38), 111 (34), 85 (61), 57 (100), 43 (92). 1H NMR (300 MHz, DMSO-d6): δ 5.22 (2H, s, 2CH), 6.65 (2H, s, Ar), 6.96 (2H, s, Ar), 10.84 (2H, s, 2NH), 11.13 (2H, s, 2NH), 12.24 (2H, s, 2NH), 12.47 (2H, s, 2NH), 14.82 (2H, s, 2NH). 13C NMR (125 MHz, DMSO-d6): δ 55.8 (CH), 87.7 (CC = ONH), 96.7 (CC = ONH), 126.5, 148.2, 149.5, 157.0, 160.0, 167.4 (C=O), 173.6 (C=S).
2.3.8. 5,5'-(1,4-Phenylene)bis(9,10-dihydropyrido[2,3-d:6,5-d']dipyrimidine-2,4,6,8(1H,3H,5H,7H)-tetraone) (4h)
Yellow solid: M.p.: >360 °C, yield: 0.400 g (70%). IR (KBr) (νmax/cm−1): 3396, 3341, 3205, 1745, 1676, 1625, 1212. MS (EI, 70 eV): m/z (%) = 127 (12), 99 (5), 84 (6), 68 (36), 89 (5), 45 (33), 43 (100). 1H NMR (300 MHz, DMSO-d6): δ 5.19 (2H, s, 2CH), 5.29 (2H, s, 2CH), 6.60–8.26 (8H, m, Ar), 10.77–11.42 (16H, s, 16NH), 14.22 (4H, s, 4NH). 13C NMR (125 MHz, DMSO-d6): δ 58.8 (CH), 91.0 (CC = ONH), 91.5 (CC = ONH), 118.2, 126.8, 134.4, 147.5, 149.6, 150.3, 150.6, 155.2, 162.2 (C=O), 164.1 (C=O).
2.3.9. 5,5'-(1,4-Phenylene)bis(1,3-dimethyl-9,10-dihydropyrido[2,3-d:6,5-d']dipyrimidine-2,4,6,8(1H,3H,5H,7H)-tetraone) (4i)
Yellow solid: M.p.: 262–264 °C, yield: 0.452 g (72%). IR (KBr) (νmax/cm−1): 3430, 3162, 1686, 1615, 1282. MS (EI, 70 eV): m/z (%) = 542 (1), 404 (100), 376 (57), 353 (20), 292 (69), 254 (29), 180 (65), 128 (34), 89 (8), 82 (30), 42 (39). 1H NMR (300 MHz, DMSO-d6): δ 3.04 (6H, s, 2CH3), 3.09 (12H, s, 4CH3), 3.21 (6H, s, 2CH3), 5.29 (4H, s, 4CH), 6.98 (4H, s, Ar), 7.38 (4H, s, Ar), 10.96 (4H, s, 4NH), 11.17 (4H, s, 4NH), 14.13 (4H, s, 4NH). 13C NMR (125 MHz, DMSO-d6): δ 27.0 (NCH3), 28.1 (NCH3), 29.2 (NCH3), 30.4 (NCH3), 32.7 (CH), 88.5 (CC = ONCH3), 90.3 (CC = ONCH3), 126.0, 129.5, 134.0, 136.5, 149.8, 150.0, 155.0, 161.2, 163.9, 167.3.
2.3.10. 5,5'-(1,4-Phenylene)bis(1,3-dimethylbenzo[g]pyrimido[4,5-b]quinoline-2,4,6,11(1H,3H,5H,12H)-tetraone) (4j)
Orange solid: M.p.: 269–271 °C, yield: 0.497 g (69%). IR (KBr) (νmax/cm−1): 3393, 3237, 1703, 1653, 1605, 1254. MS (EI, 70 eV): m/z (%) = 720 (M+, 1), 514 (6), 426 (13), 338 (21), 280 (26), 188 (9), 133 (38), 89 (93), 45 (100). 1H NMR (300 MHz, DMSO-d6): δ 3.09 (6H, s, 2CH3), 3.11 (6H, s, 2CH3), 3.33 (12H, s, 4CH3), 5.77 (4H, s, 4CH), 6.91–8.01 (24H, m, Ar), 13.19 (4H, s, 4NH). 13C NMR (62.8 MHz, DMSO-d6): δ 29.5 (NCH3), 31.4 (NCH3), 31.8 (NCH3), 35.8 (CH), 36.2 (CH), 87.2 (CC = ONH), 125.0, 127.1, 127.5, 127.9, 129.0, 130.8, 132.0, 133.1, 134.1, 134.9, 135.8, 136.9, 138.2, 151.6, 151.9, 155.8, 160.0 (C=O), 165.0 (C=O), 182.5 (C=O), 187.4 (C=O), 193.0 (C=O).
2.4. Supplementary material
Experimental section, general procedure for the synthesis of product 4a, structure of all products, copies of 1H, 13C NMR spectrum, IR spectra, and Mass spectra of selected products are provided.
3. Results and discussion
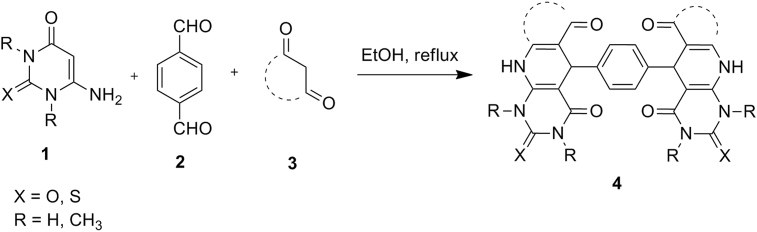
In the current study, synthesis of new functionalized bis-heterocycles (such as bis(pyrimido[4,5-b]quinolone), bis(chromeno[3′,4':5,6]pyrido[2,3-d]pyrimidine), bis(pyrido[2,3-d:6,5-d']dipyrimidine), and bis(benzo[g]pyrimido[4,5-b]quinolone) derivatives) 4 via one-pot, multi-component reaction of various 6-aminouracils or 6-aminothiouracils 1, terephthalaldehyde 2, and CH-acids (such as 4-hydroxycoumarin, dimedone, 2-hydroxy-1,4-naphthoquinone, barbituric acid and thiobarbituric acid) 3 in EtOH as a solvent at reflux without any catalyst is described (Scheme 1).
Scheme 1.
Synthetic scheme for the product 4.
We tested the general scope of this reaction by varying the structure of the 6-aminouracils 1 and CH acids 3. The reaction was completed after 5–30 min, under the same reaction conditions to give corresponding bis-heterocycles 4 in good to high yields (65–90%). The results are shown in Table 1.
Table 1.
One-pot, multi-component synthesis of bis-heterocycles 4a-j.
| Entry | Aminouracila | CH-acida | Product | Time (min) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 6-Aminothiouracil | 2-Hydroxy-1,4-naphthoquinone | 4a | 5 | 76 |
| 2 | 6-Aminouracil | 4-Hydroxycoumarin | 4b | 5 | 68 |
| 3 | 6-Amino-1,3-dimethyluracil | 4-Hydroxycoumarin | 4c | 5 | 73 |
| 4 | 6-Aminothiouracil | Dimedone | 4d | 10 | 79 |
| 5 | 6-Amino-1,3-dimethyluracil | Dimedone | 4e | 10 | 85 |
| 6 | 6-Aminouracil | Dimedone | 4f | 30 | 65 |
| 7 | 6-Aminouracil | Thiobarbituric acid | 4g | 5 | 90 |
| 8 | 6-Aminouracil | Barbituric acid | 4h | 20 | 70 |
| 9 | 6-Amino-1,3-dimethyluracil | Barbituric acid | 4i | 20 | 72 |
| 10 | 6-Amino-1,3-dimethyluracil | 2-Hydroxy-1,4-naphthoquinone | 4j | 10 | 69 |
Various 6-aminouracils (2 mmol), terephthalaldehyde (1 mmol), and CH-acids (2 mmol) were used in EtOH at reflux, without any catalyst.
The structures of products 4a-j were assigned from their IR, mass, 1H NMR, and 13C NMR spectra (see the Supporting Information). The 1H NMR spectrum of 4a exhibited a singlet at δ 5.52 ppm arising from the CH proton, multiplets for aromatic protons in the range of δ 6.74–7.79 ppm as well as three singlets for the NH protons at δ 12.13, 12.45, and 13.49 ppm. Moreover, the 13C NMR spectrum agreed with the proposed structure 4a. Resonances due to CH, CC = ONH, CC = OO, C=O, C=O, and C=S groups appeared at δ 35.8, 92.2, 106.3, 165.9, 166.5, and 174.1 ppm, respectively. Also the mass spectrum of 4a was in agreement with the proposed structure (see the supplementary material).
Compounds 4 have two stereogenic centers, and therefore two diastereoisomers are expected. In some products, one of which is prepared in a highly stereo controlled fashion (for example 4a). Also the 1H- and 13C NMR spectra of the products 4c, 4d, 4f, 4h, 4i, 4j indicated the presence of two diastereoisomers. We were not able to separate these compounds in pure form. However, their NMR data can be extracted from the mixture of the two diastereoisomers and the 1H and 13C NMR spectra of two diastereoisomers are too similar. We did not obtain the good NMR spectra of these samples because of its insolubility in any solvent (All products are very insoluble compounds). The two diastereoisomers of 4c is shown in Scheme 2.
Scheme 2.
The two diastereoisomers of 4c.
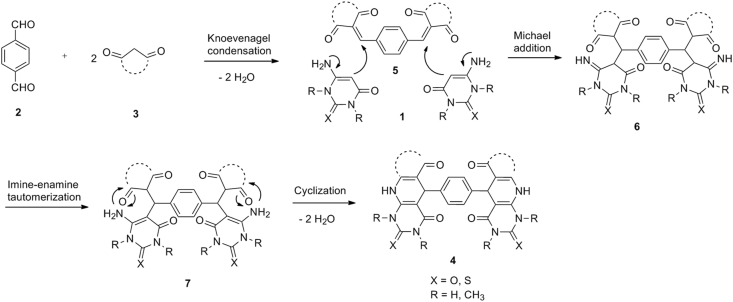
A proposed mechanism for the synthesis of 4 is shown in Scheme 3. To form the product 4, it is possible that initially the formation of the adduct 5 occurs through Knoevenagel condensation between terephthalaldehyde 2 (1 mmol) and CH-acid 3 (2 mmol). Then the Knoevenagel adduct 5 undergoes Michael addition with 6-aminouracil 1 (2 mmol) to give 6. This intermediate is converted into 7 through the imine-enamine tautomerization, followed by N-cyclization to prepare product 4.
Scheme 3.
Proposed mechanism for the formation of product 4.
4. Conclusion
The present study described a simple route for the synthesis of new bis-pyridopyrimidine and bis-pyrimidoquinolone derivatives by the one-pot, multi-component condensation of 6-aminouracils, terephthalaldehyde, and CH-acids in EtOH as a solvent at reflux. The notable advantages of the present work are easy accessibility of reactants, simplicity of the experimental procedures, high atom economy, absence of catalyst, short reaction time, and good product yields. In addition, various functional groups that exist in these heterocycles lead to the pharmacological/biological activities of them.
Declarations
Author contribution statement
Mohammad Bayat: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Fahimeh Sadat Hosseini: Analyzed and interpreted the data; Wrote the paper.
Milad Masoumi: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Afsharnezhad M., Bayat M., Hosseini F.S. Efficient synthesis of new functionalized 2-(alkylamino)-3-nitro-4-(aryl)-4H-benzo[g]chromene-5,10-dione. Mol. Divers. 2019:1. doi: 10.1007/s11030-019-09959-y. [DOI] [PubMed] [Google Scholar]
- 2.del Corte X., de Marigorta E.M., Palacios F., Vicario J. A Brønsted acid-catalyzed multicomponent reaction for the synthesis of highly functionalized γ-lactam derivatives. Molecules. 2019;24:2951. doi: 10.3390/molecules24162951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramos L.M., Rodrigues M.O., Neto B.A.D. Mechanistic knowledge and noncovalent interactions as the key features for enantioselective catalysed multicomponent reactions: a critical review. Org. Biomol. Chem. 2019;17:7260. doi: 10.1039/c9ob01088b. [DOI] [PubMed] [Google Scholar]
- 4.Wiemann J., Fischer L., Kessler J., Strohl D., Csuk R. Ugi multicomponent-reaction: syntheses of cytotoxic dehydroabietylamine derivatives. Bioorg. Chem. 2018;81:567. doi: 10.1016/j.bioorg.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Alvim H.G.O., Correa J.R., Assumpcao J.A.F., da Silva W.A., Rodrigues M.O., de Macedo J.L., Fioramonte M., Gozzo F.C., Gatto C.C., Neto B.A.D. Heteropolyacid-containing ionic liquid-catalyzed multicomponent synthesis of bridgehead nitrogen heterocycles: mechanisms and mitochondrial staining. J. Org. Chem. 2018;83:4044. doi: 10.1021/acs.joc.8b00472. [DOI] [PubMed] [Google Scholar]
- 6.de Marigorta E.M., de los Santos J.M., de Retana A.M.O., Vicario J., Palacios F. Multicomponent reactions in the synthesis of γ-lactams. Synthesis. 2018;50:4539. [Google Scholar]
- 7.Konstantinidou M., Kurpiewska K., Kalinowska-Tluscik J., Domling A. Glutarimide alkaloids through multicomponent reaction chemistry. Eur. J. Org Chem. 2018;2018:6714. [Google Scholar]
- 8.Yu S., Hua R., Fu X., Liu G., Zhang D., Jia S., Qiu H., Hu W. Asymmetric multicomponent reactions for efficient construction of homopropargyl amine carboxylic esters. Org. Lett. 2019;21:5737. doi: 10.1021/acs.orglett.9b02139. [DOI] [PubMed] [Google Scholar]
- 9.Abbas E.M.H., Gomha S.M., Farghaly T.A. Multicomponent reactions for synthesis of bioactive polyheterocyclic ring systems under controlled microwave irradiation. Arabian J. Chem. 2014;7:623. [Google Scholar]
- 10.Sayed A.R., Gomha S.M., Taher E.A., Muhammad Z.A., El-Seedi H.R., Gaber H.M., Ahmed M.M. One-pot synthesis of novel thiazoles as potential anti-cancer agents. Drug Des. Dev. Ther. 2020;14:1363. doi: 10.2147/DDDT.S221263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rashdan H., Gomha S.M., El-Gendey M.S., El-Hashash M.A., Soliman A.M.M. Eco-friendly one-pot synthesis of some new pyrazolo[1,2-b]phthalazinediones with antiproliferative efficacy on human hepatic cancer cell lines. Green Chem. Lett. Rev. 2018;11:264. [Google Scholar]
- 12.Gomha S.M., Muhammad Z., Abdel-aziz M.R., Abdel-Aziz H.M., Gaber H., Elaasser M.M. One-pot synthesis of new thiadiazolyl-pyridines as anticancer and antioxidant agents. J. Heterocycl. Chem. 2018;55:530. [Google Scholar]
- 13.Abdelrazek F.M., Gomha S.M., Shaaban M.E.B., Rabee K.A., El-Shemy H.N., Abdallah A.M., Metz P. One-pot three-component synthesis and molecular docking of some novel 2-thiazolyl pyridines as potent antimicrobial agents. Mini Rev. Med. Chem. 2019;19:527. doi: 10.2174/1389557518666181019124104. [DOI] [PubMed] [Google Scholar]
- 14.Meng L., Lorsbach B.A., Sparks T.C., Fettinger J.C., Kurth M.J. Parallel synthesis of bis-heterocyclic isoxazolylmethyl- and isoxazolinylmethylpyrazoles. J. Comb. Chem. 2010;12:129. doi: 10.1021/cc900133k. [DOI] [PubMed] [Google Scholar]
- 15.Keshipour S., Shaabani S., Shaabani A. A novel one-pot pseudo five-component isocyanide-based reaction: synthesis of 2,6-bis(alkylamino)-benzofuro[5,6-b]furan-4,8-dione derivatives. Tetrahedron Lett. 2012;53:7085. [Google Scholar]
- 16.Keshipour S., Shaabani A., Shojaei S., Nosrati H., Ng S.W. A novel one-pot isocyanide-based three-component reaction: synthesis of highly functionalized imidazo-chromen-4-ones. J. Iran. Chem. Soc. 2015;12:1655. [Google Scholar]
- 17.Shaabani A., Sarvary A., Rezayan A.H., Keshipour S. Synthesis of fully substituted pyrano[2,3-c]pyrazole derivatives via a multicomponent reaction of isocyanides. Tetrahedron. 2009;65:3492. [Google Scholar]
- 18.Abdallah M.A., Gomha S.M., Morad M.A., Elaasser M.M. Synthesis of pyridotriazolopyrimidines as antitumor agents. J. Heterocycl. Chem. 2017;54:1242. [Google Scholar]
- 19.Gomha S.M., Edrees M.M., Faty R.A.M., Muhammad Z.A., Mabkhot Y.N. Microwave-assisted one pot three-component synthesis of some novel pyrazole scafolds as potent anticancer agents. Chem. Cent. J. 2017;11:57. doi: 10.1186/s13065-017-0266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelrazek F.M., Gomha S.M., Abdel-aziz H.M., Farghaly M.S., Metz P., Abdel-Shafy A. Efficient synthesis and in Silico study of some novel pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidine derivatives. J. Heterocycl. Chem. 2020;57:1759. [Google Scholar]
- 21.Chen X., Yang H., Hulsey M.J., Yan N. One-step synthesis of N-heterocyclic compounds from carbohydrates over tungsten-based catalysts. ACS Sustain. Chem. Eng. 2017;5:11096. [Google Scholar]
- 22.Abdella A.M., Abdelmoniem A.M., Abdelhamid I.A., Elwahy A.H.M. Synthesis of heterocyclic compounds via Michael and Hantzsch reactions. J. Heterocycl. Chem. 2020;57:1476. [Google Scholar]
- 23.Abbas S.E.-S., George R.F., M Samir E., Aref M.M.A., Abdel-Aziz H.A. Synthesis and anticancer activity of some pyrido[2,3-d]pyrimidine derivatives as apoptosis inducers and cyclin-dependent kinase inhibitors. Future Med. Chem. 2019;11:2395. doi: 10.4155/fmc-2019-0050. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Aziem A., El-Gendy M.S., Abdelhamid A.O. Synthesis and antimicrobial activities of pyrido[2,3-d]pyrimidine, pyridotriazolopyrimidine, triazolopyrimidine, and pyrido[2,3-d:6,5d']dipyrimidine derivatives. Eur. J. Chem. 2012;3:455. [Google Scholar]
- 25.Mamaghani M., Tabatabaeian K., Araghi R., Fallah A., Hossein Nia R. An efficient, clean, and catalyst-free synthesis of fused pyrimidines using sonochemistry. Org. Chem. Insights. 2014;2014:1. [Google Scholar]
- 26.Verma A.K., Singh A.K., Islam M.M. Synthesis, characterization and evaluation of pyridopyrimidine carboxylate derivatives as potential antimicrobial and anticancer agents. Int. J. Pharm. Sci. 2014;6:341. [Google Scholar]
- 27.El-Shahat M., Elhefny E.A., El-Sayed A.A., Salama M.A.M. A novel fused pyridopyrimidine derivatives: synthesis and characterization. Int. J. Pharm. 2015;5:53. [Google Scholar]
- 28.Rad A.M., Mokhtary M. Efficient one-pot synthesis of pyrido[2,3-d]pyrimidines catalyzed by nanocrystalline MgO in water. Int. Nano Lett. 2015;5:109. [Google Scholar]
- 29.El-Hashash M.A., Sherif S.M., Badawy A.A.E., Rashdan H.R.M. Synthesis of some new antimicrobial 5,6,7,8-tetrahydropyrimido[4,5-b]quinolone derivatives. Der Pharma Chem. 2014;6:23. [Google Scholar]
- 30.Singh R.M., Sharma N., Kumar R., Asthana M., Upadhyay S. An alternative synthesis of pyrimido[4,5-b]quinoline-4-ones via metal-free amination in water and Vilsmeier–Haack cyclization. Tetrahedron. 2012;68:10318. [Google Scholar]
- 31.El-Gazzar A.B.A., Youssef M.M., Youssef A.M.S., Abu-Hashem A.A., Badria F.A. Design and synthesis of azolopyrimidoquinolines, pyrimidoquinazolines as anti-oxidant, anti-inflammatory and analgesic activities. Eur. J. Med. Chem. 2009;44:609. doi: 10.1016/j.ejmech.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Quiroga J., Trilleras J., Insuasty B., Abonía R., Nogueras M., Marchal A., Cobo J. A straightforward synthesis of pyrimido[4,5-b]quinoline derivatives assisted by microwave irradiation. Tetrahedron Lett. 2010;51:1107. [Google Scholar]
- 33.Shamroukh A.H., Rashad A.E., Abdelmegeid F.M.E. The chemistry of pyrido[2,3-d]pyrimidines and their applications. J. Chem. Pharmaceut. Res. 2016;8:734. [Google Scholar]
- 34.Nongthombam G.S., Kharmawlong G.K., Kumar J.E., Nongkhlaw R. UV365 light promoted catalyst-free synthesis of pyrimido[4,5-b]quinoline-2,4-diones in aqueous-glycerol medium. New J. Chem. 2018;42:9436. [Google Scholar]
- 35.Murru S., Nefzi A. Combinatorial synthesis of oxazol-thiazole bis-heterocyclic compounds. ACS Comb. Sci. 2014;16:39. doi: 10.1021/co400133a. [DOI] [PubMed] [Google Scholar]
- 36.Kishore K.G., Jacome A.I., Renteria-Gomez A., Conejo A.S., Basavanag U.M.V., Wrobel K., Gamez-Montano R. Synthesis of unsymmetrical bis-heterocycles containing the imidazo[2,1-b]thiazole framework and their benzo[d]fused analogues by an acid-free Groebke–Blackburn–Bienaymé reaction. Tetrahedron Lett. 2016;57:3556. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.