Abstract
Manganese has recently been a topic of interest among researchers, particularly when 1,752 million tonnes of manganese are expected to be produced by the steel industry in 2020. Manganese discharges from industrial effluents have increased manganese contamination in water sources. Its concentrations of more than 0.2 mg/L in the water sources could have negative impacts on human health and the aquatic ecosystem. Thereby, the available water treatment processes face challenges in effectively removing manganese at low cost. In response to these challenges, adsorption has emerged as one of the most practical water treatment processes for manganese removal. In particular, agricultural waste adsorbents received a lot of attention owing to their low cost and high efficiency (99%) in the removal of manganese. Therefore, this paper reviews the removal of manganese by adsorption process using agricultural waste adsorbents. The factors affecting the adsorption process, the mechanisms, and the performances of the adsorbents are elucidated in detail.
Keywords: Manganese, Adsorption, Agricultural waste adsorbents, Low cost, Mechanisms, Water treatment, Materials chemistry, Environmental science, Environmental hazard, Environmental pollution, Water pollution, Water chemistry, Water quality
Manganese; Adsorption; Agricultural waste adsorbents; Low cost; Mechanisms; Water treatment; Materials chemistry; Environmental science; Environmental hazard; Environmental pollution; Water pollution; Water chemistry; Water quality.
1. Introduction
Water is an essential component of all living organisms and national development (Ahmed et al., 2014). Rapid industrial development has increased the pollution of water resources. Massive wastes discharged from domestic, industrial, and commercial sources ended up in the water bodies (Lee et al., 2017). This includes the discharge of heavy metals directly or indirectly into the water (Jin et al., 2016; Zhao et al., 2016). Heavy metals are natural elements in the environment with a density of more than 5 g/cm3. Heavy metals could pose a risk to humans as well as to the flora and fauna of the receiving water bodies (Omar et al., 2019; Jawed et al., 2020). The pollution of heavy metals has become notable issues due to the disposal of untreated industrial wastes (Sharma et al., 2018). The presence of heavy metals in industrial effluents discharged into the river alarmed the public and waterworks industries (Zou et al., 2016). Approximately 300–400 million tonnes of heavy metals, toxic sludge, solvents, and other hazardous materials from industrial activities were disposed into the water bodies annually (Singh et al., 2018).
Manganese is one of the heavy metals which has been widely used in the steel production. It is an easily oxidised, chemically active, and strong metal with a density of 7.43 g/cm3 (Chen et al., 2018). It can also react with water and iron to form rust and dissolve in acids (Mthombeni et al., 2016). Manganese is a naturally occurring element that can be found extensively in the environment (Milatovic et al., 2017). It is detected in surface and groundwater at various concentrations, mainly due to anthropic activities (Marsidi et al., 2018; Gerke et al., 2016; Bouchard et al., 2018; Dion et al., 2018). Manganese is water soluble and exists as a bivalent ion (Mn2+). Manganese (II) is the most stable oxidation state that appears as a pale pink colour. It is considered to be a pollutant due to its organoleptic properties. Upon oxidation, manganese that dissolved in water becomes insoluble and turn into brownish-red in colour (Ali, 2017; Marsidi et al., 2018). This metal is also known as trace minerals due to the need for a certain amount of trace minerals in the human body as a cofactor for a variety of enzymes in intracellular activity. However, excessive consumption of this mineral may cause manganese toxicity that disturbs the central nervous system (Marcus, 2013).
The World Steel Association (2019) reported that the global demand for steel in 2019 had increased by 1.3% (1,735 million tonnes) from 2018. By 2020, it was predicted that the demand would grow by 1.0%, reaching 1,752 million tonnes. With the growing numbers of steel production worldwide, the manganese contamination in water sources has become a serious issue. The point sources of manganese contamination derived from the wastewater treatment plant, mine quarry operation, and industrial effluent discharge. Manganese is widely used in the manufacturing industries producing steel alloys, batteries, glass, fireworks, fertilizer, stock food additive, and organic synthesis catalysts (Mthombeni et al., 2016). The presence of manganese affects the aesthetics of water quality (Tobiason et al., 2016; Rose et al., 2017). In addition, manganese in the drinking water system also contributes to the formation of oxide layers in corroded pipes. As a result, the water flow is affected, deteriorating water quality and increasing the cost of distribution (Alvarez-Bastida et al., 2018). Excessive presence of manganese in the water may contribute to reddish colour, laundry stains, bad odour and taste (Qiu et al., 2014; Kasim et al., 2017).
Although manganese is an essential mineral, there have been concerns about manganese consumption in drinking water that may lead to neurological adverse effects in terms of intellectual and cognitive development (Rumsby et al., 2014). Gerke et al. (2016) highlighted that overexposure and ingestion of high doses of manganese from drinking water can cause neurological disorders. Excessive accumulation of manganese in specific brain areas has been reported to produce neurotoxicity leading to degenerative brain disorder (Idrees et al., 2018; Milatovic and Gupta, 2018). According to Mthombeni et al. (2016), children who exposed to 240–350 μg/L of manganese in water demonstrated impaired manual dexterity, speed, short-term memory, and visual recognition compared to children exposed to controlled manganese.
The growing attention to manganese contamination in the water has led to the discovery of various treatment technologies (Baysal et al., 2013). Prevalent treatment technologies developed for the treatment of manganese-containing water include chemical precipitation, ion exchange, oxidation, electrochemical treatment, ultraviolet irradiation, ozone and membrane filtration (Jeirani et al., 2015; Ihsanullah et al., 2016; Al-Jubouri and Holmes, 2017; Carolin et al., 2017; Alvarez-Bastida et al., 2018; Fatemeh Seyedpour et al., 2018; Du et al., 2019).
Such water treatment technologies are readily available and capable of removing manganese at a certain rate. However, most of the methods have drawbacks in terms of treatment capacity, space requirements, complex processes, sludge disposal, maintenance increases, and operating costs (Shu et al., 2016; Kale et al., 2017; Marsidi et al., 2018). Adsorption is a trendy and economical water treatment process that has been used to remove different types of heavy metals owing to its efficiency, simplicity and environmental friendliness (Jawed and Pandey, 2019). Nowadays, natural adsorbents made from agricultural wastes have been extensively studied by many researchers in the treatment of manganese-containing water (Gupta et al., 2015). Hence this paper provides insight into the manganese removal by using agricultural wastes adsorbent. The adsorption mechanisms for the removal of manganese and the efficiency of agricultural waste adsorbents in this paper are explained in detail.
2. Adsorption process
The severity of water contaminated with heavy metals has become more challenging due to the rapid development of industries and the high demand for freshwater supplies worldwide (Kobielska et al., 2018). The removal of heavy metals in water before being discharged into the aquatic environment or used for drinking water has indeed become a top priority (Tofighy and Mohammadi, 2011). Hence, the search for low cost and environmentally friendly water and wastewater treatment materials without the generation of hazardous by-products has been extensively studied (Hokkanen et al., 2016).
The adsorption is one of the well-known water treatment technologies that is considered to be efficient, cheap, and safe for the removal of heavy metals compared to other methods (Liu et al., 2013; Ali et al., 2016). The process provides a flexible design and operation that is capable of producing treated effluent free of odour, colour, and sludge. Furthermore, adsorption is also an attractive and economical process, as the adsorbent can be regenerated and reused. It is also interesting that adsorption does not produce secondary waste as no sludge is generated during the removal process of heavy metals, even from the diluted solutions (Goher et al., 2015; Mthombeni et al., 2016; Al-Jubouri and Holmes, 2017). Adsorption also has the ability to remove pollutants at low concentrations with low energy consumption (Anu, 2015; Ahmad et al., 2015).
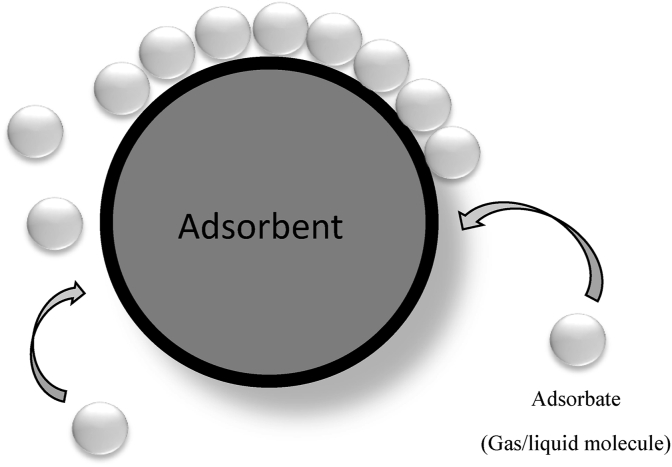
The process of adsorption occurs when a gas or liquid molecules (absorbate) is attached to the surface of a solid or a liquid (adsorbent) and creates a molecular or atomic film as shown in Figure 1 (Lakherwal, 2014). This happens due to the existence of unbalanced or residual forces on the surface of a liquid or solid phase. The residual imbalance forces continue to attract and retain the molecular species as they reach the surface. The adsorbate is absorbed by the adsorbent in which the attraction between adsorbate and adsorbent arises due to the bonding forces such as Van der Waals forces (weak forces) or covalent bond (strong forces) (Kale et al., 2017). According to Rashid and Yaqub (2017), adsorption is an interactive process that binds the liquid phase component to the surface of solid adsorbent by interacting either physically or chemically, depending on the intermolecular forces. This is known as a segregation process that used to isolate the selected metal ion from the reaction mixture and can be carried out either by batch, semi-batch, or continuous experimentation.
Figure 1.
Adsorption process involving adsorbent and adsorbate.
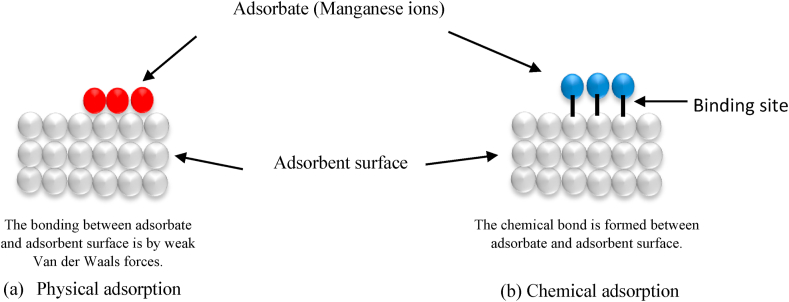
Adsorption can be divided into two forms: physical and chemical adsorption (Kale et al., 2017). Physical adsorption happens when the absorbent and adsorbate undergo weak Van der Waals forces, hydrogen bonding, polarity, and dipole-dipole interactions (Shafiq et al., 2018; Afroze and Sen, 2018). This physical process absorbs metal ions electrostatically across the surface of the materials. Moreover, the process takes place at a lower or almost equal temperature of the adsorbed components. Meanwhile, chemical adsorption is the process between the adsorbate and the surface of the adsorbent by chemical bonding or electron transfer. It is a permanent reaction known as activated adsorption, which requires high activation energy. Unlike the physical adsorption, the process of chemical adsorption is irreversible (Singh and Gupta, 2016; Liu et al., 2019). Figure 2 shows the mechanisms of physical and chemical adsorption between adsorbent and adsorbate.
Figure 2.
The mechanism of (a) physical and (b) chemical adsorption.
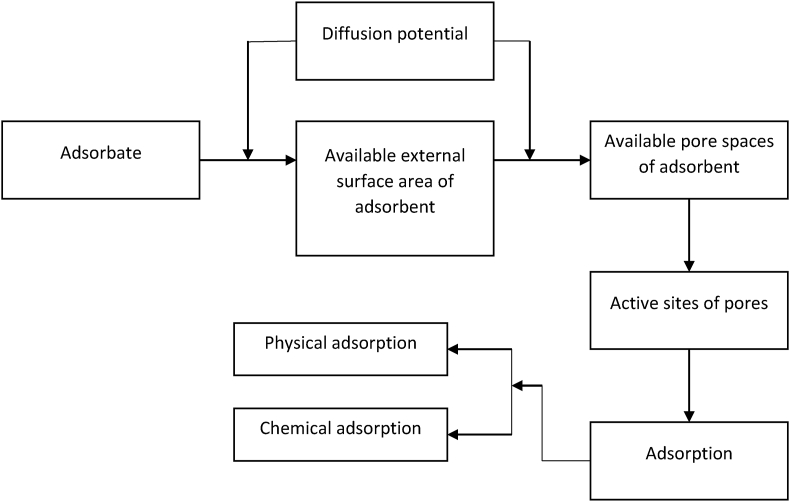
Physical adsorption is capable of forming a multilayer adsorption process that provides high adsorption capacity. On the contrary, chemical adsorption is limited to monolayer adsorption and selectively eliminates trace materials from aqueous solutions. Thus, the regeneration and reusability of the adsorbent is also difficult due to its irreversible reaction (Alaei Shahmirzadi et al., 2018). Figure 3 shows the steps in the adsorption process. Adsorbate is diffuse on the outer surface of the adsorbent due to the diffusion potential that is determined by the concentration of adsorbate and the accessible outer surface area on the adsorbent. Diffusion potential occurs either in a single step or in the combination of the steps such as film or external diffusion, pore diffusion, surface diffusion, and adsorption on the pore surface. After that, the adsorbate is diffused on the available pores of the adsorbent. During the adsorption process, all exposed active sites are occupied by either physical or chemical adsorption (Lata and Samadder, 2016).
Figure 3.
Steps involve in the adsorption process (Lata and Samadder, 2016).
2.1. Factors affecting manganese adsorption process
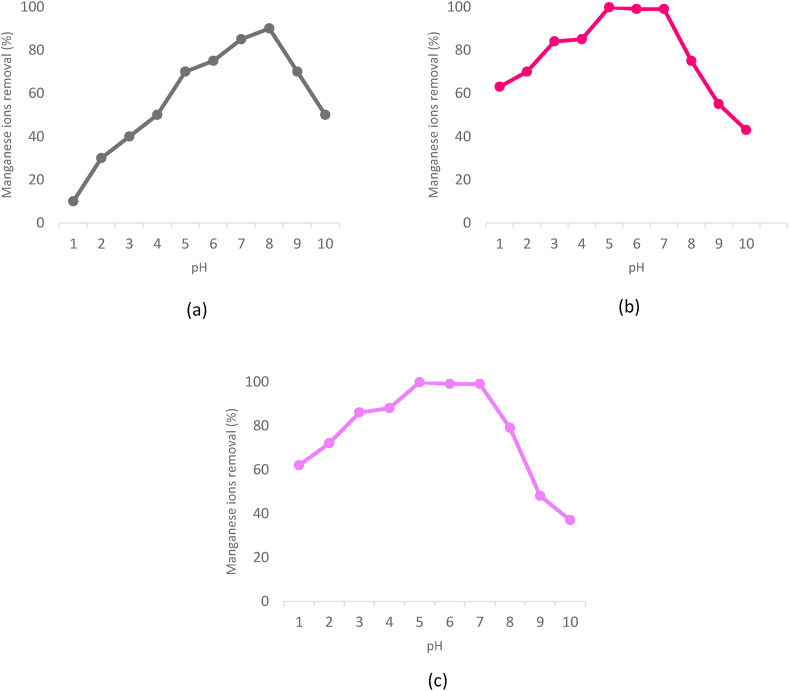
The adsorption process and the removal rate of manganese in the water depends on several factors. These include the pH of the solution, adsorbent dosage, adsorbent particle size, contact time, temperature, and manganese concentration (Kobielska et al., 2018). Table 1 shows the factors affecting the manganese removal rate by the adsorption process. The degree of ionization of adsorbent is determined by the pH of solution due to the presence of weak acid or weak base (Shafiq et al., 2018). Idrees et al. (2018) found that the manganese removal efficiency was more than 80 % at pH 6 using biochar adsorbent. This is due to the low concentration of hydrogen ion which is less competitive with the adsorption of manganese ion on the negatively charged biochar surfaces. Excess of hydrogen ions in acidic solution will surround the binding sites of biochar and reduce the adsorption. Ali (2017) has shown that the optimum pH of grafted banana peel adsorbent (GBPs) adsorbent in the removal of manganese is at 7. However, the adsorption of manganese is not efficient at lower pH levels. Similarly, increased pH ≥ 8 also reduced the adsorption of manganese due to precipitation of hydroxide ions and manganese hydroxide. Other study that utilised corn cob (CC) and Strychnouspotatorum seed powder (SPSP) as adsorbents showed excellent adsorption performance of 99.8 % at pH 5 (Kumar et al., 2018). It was also noticeable that at pH ≥ 8, the precipitation of manganese had reduced the adsorption rate. Figure 4 shows the effects of pH toward manganese removal using GBPs, CC, and SPSP adsorbents. This indicates that the preferred pH for manganese removal is ranges from pH 5 to 7. Higher pH value is not suitable due to precipitate formation of excessive OH ions (Surovka and Pertile, 2017).
Table 1.
Factors affecting the adsorption process for manganese removal.
| Adsorbent | Manganese removal (%) | pH | Contact time (min) | Particle size (μm) | Adsorbent dosage (g) | Temperature (oC) | Manganese concentration (mg/L) | References |
|---|---|---|---|---|---|---|---|---|
| Biochar | >80 | 6 | 180 | - | 0.25 | 24.85 | 8 mg/L | (Idrees et al., 2018) |
| Rice husk ash | 100 | 3 | 100 | 300 | 0.5 | 30 | 3.8 mg/L | (Adekola et al., 2016) |
| Peanut husk | 100 | 6 | 180 | - | 5 | 25 | 20 mg/L | (Abdelfattah et al., 2016) |
| Sunflower seed shell | 81.6 | 8 | 120 | - | 0.15 | - | - | (Feizi and Jalali, 2015) |
| Potato peel | 79.8 | 7 | 120 | - | 0.15 | - | - | (Feizi and Jalali, 2015) |
| Canola flower | 81.8 | 8 | 200 | - | 0.15 | - | - | (Feizi and Jalali, 2015) |
| Walnut shell | 96.5 | 8 | 200 | - | 0.15 | - | - | (Feizi and Jalali, 2015) |
| Polyvinyl alcohol/chitosan (PVA/CS) | 84.5 | 5 | 120 | 0.158 | 0.8 | 30 | 20 mg/L | (Abdeen et al., 2015) |
| Untreated banana peel (UTBP) | >80 | 6 | 60 | - | 4 | 25 | 10 mg/L | (Ali and Saeed, 2015) |
| Sugarcane bagasse | 99 | 4.5 | - | 25–30 | 15 | 23 | 12 mg/L | (Esfandiar et al., 2014) |
| White rice husk ash | 26.6 | 7 | 480 | 50 | 2.5 | 34.85 | 100 mg/L | (Tavlieva et al., 2015) |
| Tamarind fruit shell | 74 | 3 | 60 | - | 1.2 | 30 | 0.1 g/L | (Bangaraiah, 2018) |
| Yam peel | 30.5 | 6.8 | 120 | 106 | 1 | 30 | 50 mg/L | (Isagba et al., 2017) |
| Orange peel | 96 | 6 | - | - | - | 45 | 300 mg/L | (Surovka and Pertile, 2017) |
| Modified tangerine peel | 92.48 | 5 | 30 | - | 0.3 | 30 | - | (Abdić et al., 2018) |
| Moringa oleifera seed | 95 | 4–6 | 5 | 500–1000 | 0.5 | - | 4 mg/L | (Marque et al., 2013) |
| Corn cob and Strychnous Potatorum seed powder | 99.8 | 5 | 60 | - | 0.4 | 40 | 10 mg/L | (Kumar et al., 2018) |
| Tea waste | 95.5 | - | 60 | - | 3 | - | 2.2 mg/L | (Badrealam et al., 2019) |
| Grafted banana peels (GBPs) | 94 | 7 | 60 | - | 4 | 25 | 400 mg/L | (Ali, 2017) |
| Peanut husk | 61 | - | 240 | - | - | 25 | - | (Zaini et al., 2019) |
| Banana peels | 97.4 | 5 | 120 | - | 0.8 | 25 | 20 mg/L | (Mahmoud, 2014) |
| Sugarcane Bagasse | 62.5 | 6 | 150 | 750 | 1.5 | 30 | 2 mg/L | (Ahmed et al., 2015) |
| Beet pulp | 86.4 | 6 | 90 | 750 | 1 | 30 | 2 mg/L | (Ahmed et al., 2015) |
| Moringa oleifera seed pods (MSP) | >40 | 8 | 60 | 100 | 1 | 35 | 3 mg/g | (Maina et al., 2016) |
| Sclerocarya birrea nut shells (MNS) | >40 | 8 | 120 | 100 | 2 | 35 | 3 mg/g | (Maina et al., 2016) |
| Unripe plantain peels | >32 | 6 | 60 | - | 2 | - | 50 mg/L | (Leizou et al., 2018) |
Figure 4.
The effects of pH toward manganese removal using (a) Grafted banana peel adsorbent (GBPs), (b) corn cob (CC), and (c) Strychnouspotatorum seed powder (SPSP) adsorbents (Ali, 2017; Kumar et al., 2018).
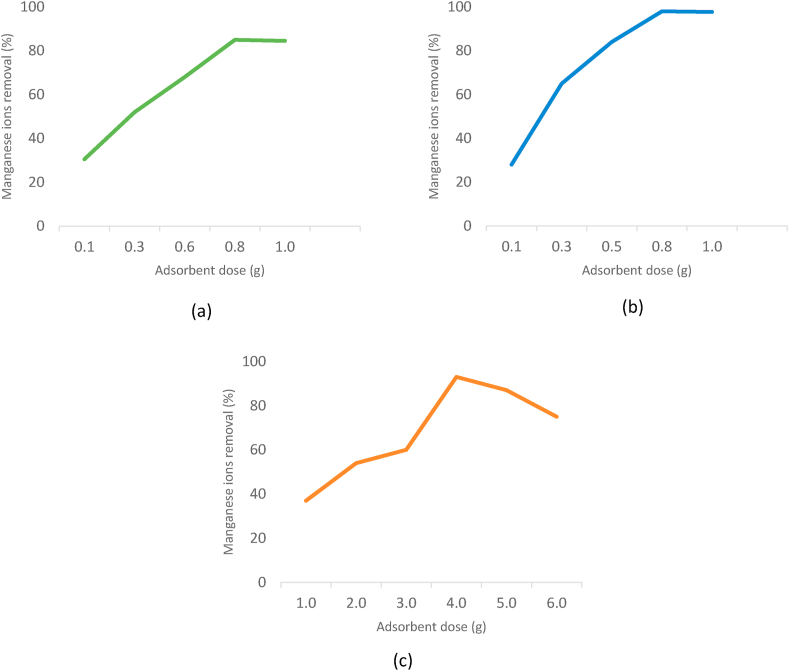
Generally, high adsorbent dosage provides more active exchangeable adsorption sites. However, excessive adsorbent dosage also could decrease the adsorption rate due to interference caused by the interaction of active sites of the adsorbent (Iftekhar et al., 2018). Abdeen et al. (2015) revealed that the increase of polyvinyl alcohol/chitosan (PVA/CS) dosage from 0.1 g/100 ml to 1.0 g/100 ml resulted in an increase of manganese removal efficiency from 30.5 % to 84.5 %. The increase in adsorption capacity is mainly due to the higher surface area of PVA/CS which provides a greater amount of adsorption surface sites. Other studies that utilised untreated banana peels (UTBP) as adsorbents have also shown that increasing adsorbent dosage has improved the manganese adsorption efficiency. It was reported that only 37 % of manganese were adsorbed at a low adsorbent dosage of 1 g/L, while 93 % of manganese were successfully removed using a high adsorbent dosage of 4 g/L (Ali and Saeed, 2015). Abdić et al. (2018) have shown that the increase in the amount of tangerine peels adsorbent has increased the efficiency of manganese removal. This is because increasing the adsorbent dosage ensures greater surface area and greater availability of metal binding sites. Therefore, the rate of manganese adsorption was increased even when the initial metal concentration remained constant (Esfandiar et al., 2014).
In addition, Mahmoud (2014) found that an increase in adsorbent dosage of banana peels activated carbon (BPAC) from 0.1 to 1.0 g boosted manganese removal efficiency from 28.0 to 97.70% due to the increase of binding sites which available for better adsorption. However, a further increase in adsorbent dosage did not significantly alter the adsorption capacity. This is due to the binding of almost all manganese to the adsorbent surface that reaches an equilibrium state. Figure 5 shows the effects of adsorbent dosage on manganese removal using PVA/CS, BPAC, and UTBP adsorbents.
Figure 5.
The effects of adsorbent dosage toward manganese removal using (a) Polyvinyl alcohol/chitosan (PVA/CS), (b) Banana peels activated carbon (BPAC), and (c) Untreated banana peels (UTBP) adsorbents (Abdeen et al., 2015; Ali and Saeed, 2015; Mahmoud, 2014).
The smaller particle size of the adsorbent capable of achieving maximum adsorption due to the ability to reduce internal diffusion and mass transfer limitations to the adsorbate attachment. However, the limitation of adsorbents with a small surface area is that larger molecules have difficulty entering small pores. This leads to a longer contact time in order to obtain the same desired outcome as the diffusion occurs only through aggregate particles (Shafiq et al., 2018). Maina et al. (2016) showed high manganese removal using Moringa oleifera seedpods (MSP) and Sclerocarya birrea nutshells (MNS) adsorbents with particles less than 100 μm in size compared to larger particles. The smaller the particle size, the higher the surface area per unit weight of adsorbent. The removal rate for MSP and MNS was increased from 85 % to ~90 % and 15 %–~50 %, respectively, as the particle size decreased from 500 μm to 100 μm.
Contact time also plays a vital role in the adsorption process. According to Shafiq et al. (2018), a shorter interaction time in attaining equilibrium adsorption indicates the efficacy of the adsorbent. In the water treatment process, shorter contact time is favourable as this reduces the operational cost and the operating time. Marque et al. (2013) revealed that the manganese removal increased with the increase in contact time until it reaches equilibrium using Moringa oleifera seeds adsorbent. The optimum contact time was 5 min with 95% removal of manganese. Leizou et al. (2018) showed that the removal of manganese using unripe plantain adsorbent was increased from 32 % to 36 % in 60 min. In addition, Adeogun et al. (2013) reported that maize husk adsorbent was able to remove 88 % manganese and reach equilibrium within 30 min.
Temperature is another factor in the adsorption process that provides an estimate of thermodynamic parameters. Based on the thermodynamic parameters, the physical or chemical adsorption mechanism can be predicted and the temperature range in which the adsorption is promising or not can be examined. The value of enthalpy change, ΔH° of 2.1–20.9 kJ/mol is physical adsorption, while the value ΔH° of 80–200 kJ/mol is chemical adsorption. The positive value of ΔH° indicates the endothermic nature that is caused by a higher temperature, which increases the adsorption process. On the contrary, the negative value of ΔH° reveals exothermic nature, in which the adsorption capacity will decreases at a higher temperature (Anastopoulos et al., 2019a). The van't Hoff equation is commonly used in the adsorption studies to calculate ΔG° as shown in Eqs. (1) and (2).
| ΔG° = -RT ln K | (1) |
| ΔG° = ΔH° - TΔS° | (2) |
where ΔG° is the Gibbs energy change, ΔH° is the enthalpy change, ΔS° is the entropy change, constant R is the universal gas constant (8.314 J/mol K), T is the absolute temperature in Kelvin and K is the thermodynamic equilibrium constant.
Idrees et al. (2018) found that a lower temperature of 24.85 °C results in a higher rate of manganese removal by more than 85 % while, higher temperature of 41.85 °C leads to lower manganese removal of less than 47 %. This process is known as an exothermic reaction. This is because higher temperature leads to the higher kinetic energy of manganese ions, which weakens the electrostatic forces between manganese and the biochar-derived adsorbent. However, Tavlieva et al. (2015) reported that manganese adsorption increases with temperature (14.85 °C, 24.85 °C, and 34.85 °C) using white rice husk ash adsorbent, indicating that the endothermic reaction is occurred. As temperature increases, the mobility of manganese ions increases and the retardant forces acting on the diffusing ions decrease. Thereby, the adsorption capacity of the adsorbent is increasing. The adsorption of the manganese ions was enhanced by the release of two hydrogen ions in the solution from the hydroxide groups of silica. The prepared white rice husk ash adsorbent was almost pure amorphous silica produced by the pyrolysis process. In addition, Adekola et al. (2016) examined the effect of temperatures ranging from 30 °C to 50 °C toward manganese removal using rice husk adsorbent. The decrease in adsorption with the rise of temperature might due to the formation of the adsorbate–adsorbent complex, which becomes unstable and results in an escape from the solid phase to the bulk solution.
Apart from that, the rate of adsorption is a function of manganese concentration. The initial metal ions concentration provides an important driving force to overcome all the mass transfer resistance between the solution and solid phases (Mahmoud, 2014). The increase in the initial metal ions concentration enhances the interaction between the metal ions in the aqueous phase and the adsorbent surface. Consequently, a higher initial concentration of metal ions increases the rate of adsorption (Das et al., 2014). Adekola et al. (2016) reported that the amount of manganese adsorbed using rice husk ash adsorbent was increased from 0 to 3.21 mg/g with respect to the increased of manganese ions concentration from 0 to 100 mg/L. The increase in the initial manganese concentration was due to the increase in the driving force of the concentration gradient.
However, studies also shows that the accumulation of metal ions on the vacant sites results in a limited mass transfer of the adsorbate from the bulk liquid to the external surface of the adsorbent (Gedam and Dongre, 2015). Abdelfattah et al. (2016) stated that the percentage of manganese ions removed had decreased as the initial concentration of manganese ions increased. The maximum removal of peanut husk powder (PHP) adsorbent was 100% at 20 mg/L manganese ions concentration. The increase of manganese concentration to 120 mg/L reduced the removal rate to 58%. Tavlieva et al. (2015) also reported that the higher manganese concentration in the solution decreased the rate of manganese removal. The reduction of white rice husk ash adsorbent was decreased by 2.5 times with a tenfold rise in manganese concentration. This occurs due to the lack of available active sites that are rapidly saturated at higher manganese concentrations, as the amount of adsorbent dosage remained constant.
3. Development of agricultural waste as low-cost adsorbents
Adsorbents produced from agricultural wastes are gaining attention in the water treatment process due to their ability to remove various types of heavy metals (Leizou et al., 2018). There are variety of agricultural wastes that have been intensively studied as potential low-cost adsorbent. These adsorbents can be classified into two types, conventional and non-conventional adsorbents. Conventional adsorbents are commercial adsorbent such as activated carbons, polymeric organic resins, activated alumina, silica gel, zeolites, etc. While non-conventional adsorbents are adsorbents made from low-cost waste materials. These include industrial (e.g., blast furnace sludge, slag, flue dust, sawdust, fly ash, black liquor lignin, red mud), agricultural wastes (e.g., rice husk, peanut husk, sunflower seed shell, potato peel, walnut shell, sugarcane bagasse), biomass, etc (Crini et al., 2019). Most of the non-conventional adsorbents have a high surface area-to-volume ratio and a large number of active binding sites (e.g., –COOH, –NH2, –OH, –SH groups) that can effectively bind and remove heavy metals (Ahmed and Ahmaruzzaman, 2016; Jacob et al., 2018; Anastopoulos et al., 2019a).
Among the non-conventional adsorbents, agricultural wastes have been widely used for the water treatment process (Shafiq et al., 2018). Agricultural wastes are organic by-product of agricultural activities such as crop residues, animal, and poultry fertilizer (Dai et al., 2018). The generation of agricultural waste was approximately 2 billion tonnes worldwide, mainly derived from plants such as corncob, oil palm empty fruit bunch, rice husk, rice straw, sugarcane bagasse, and wheat straw, as shown in Table 2 (Millati et al., 2019). These wastes have caused significant environmental problems. Instead of disposing of such agricultural waste in landfills, it can be used to manufacture low-cost and environmentally friendly adsorbents (Ali et al., 2016). The use of agricultural waste as adsorbents provides a sustainable alternative to the water treatment process and leads to good waste-to-wealth practices (Afroze and Sen, 2018).
Table 2.
Production of agricultural residue worldwide (Millati et al., 2019).
| Types | Production (million tonnes) | Country of origin |
|---|---|---|
| Corncob | 81 | USA |
| 49 | China | |
| 20 | Brazil | |
| 13 | EU | |
| 9 | Argentina | |
| Oil palm empty fruit bunch | 37 | Indonesia |
| 19 | Malaysia | |
| 2 | Thailand | |
| 1 | Colombia | |
| 0.9 | Nigeria | |
| Rice husk | 39 | China |
| 30 | India | |
| 10 | Indonesia | |
| 9 | Bangladesh | |
| 8 | Vietnam | |
| Rice straw | 149 | China |
| 114 | India | |
| 39 | Indonesia | |
| 36 | Bangladesh | |
| 30 | Vietnam | |
| Sugarcane bagasse | 94 | Brazil |
| 93 | India | |
| 55 | EU | |
| 38 | Thailand | |
| 29 | China | |
| Wheat straw | 128 | EU |
| 110 | China | |
| 40 | USA | |
| 25 | Canada | |
| 22 | Pakistan |
The agriculture wastes adsorbents are the most economical adsorbents that available locally with abundant sources. Sulyman et al. (2017) concluded that economic adsorbent is a by-product or waste from production that requires less processing or has little or no economic value. Accordingly, the current trend calls for a new alternative process in accordance with the principles of “Green Chemistry’’ or “Sustainable Chemistry’’. These include the reduction of toxic chemicals reagents; the prevention of wastes generated that cannot be recycled; the reuse of reagents; the reduction of energy consumption; the adoption of an environmentally friendly analytical system for the detection of analytes; and the use of automation and microscale developments (Anastopoulos et al., 2019b).
The use of low-cost adsorbents produced from agricultural waste is of high demand in the current market owing to the benefits of the water treatment process and solid waste management (Afroze and Sen, 2018). Varieties of low-cost adsorbents have been developed and tested for the removal of heavy metals. And thereby, the efficiency of adsorption still depends on the type of adsorbent (Fu and Wang, 2011). High content of carbon or oxygen in the adsorbent moiety is important to ensure an excellent adsorption performance. For instance, lignocellulosic materials have a very complex configuration and contain a variety of active sites that capable of adsorbing manganese from water. Apart from that, the characteristics that should present in any adsorbent are high abrasion resistance, high thermal stability, and small pore diameters that will increased the surface area of the adsorption process (Ali et al., 2012).
3.1. Adsorption mechanism of agricultural waste adsorbents for manganese removal
Adsorbents from agricultural wastes can either be used in their natural form or undergo physical and chemical modification (Alaei Shahmirzadi et al., 2018). The use of natural agricultural waste usually contributes to problems such as high chemical oxygen demand, biological oxygen demand, total organic carbon, and low adsorption capacity. This is due to the release of soluble organic compounds in the plant resulting in secondary pollution (Acharya et al., 2018). Modification of adsorbents by physical or chemical treatments is necessary in order to improve the adsorption capacity and also to prevent secondary pollution caused by the release of soluble organic compounds from the plant materials (Acharya et al., 2018).
Physical modification includes heating/boiling, freezing/thawing, drying, autoclaving, and lyophilization (Gupta et al., 2015). While, chemical treatment is by washing raw adsorbent materials with detergents and treated with organic or inorganic solutions such as acid, caustic, methanol, formaldehyde, etc. Chemical treatment will remove the surface impurities and develop the reactive functional groups on the surface such as carbonyl (ketone), phenolic, acetamido, alcoholic, amino, sulfhydryl group, etc (Renu et al., 2017; Afroze and Sen, 2018). These functional groups are able to bind to metal ions by substituting hydrogen ions with metal ions in solution or by donating electron pairs to form complexes between functional groups and metal ions. This mechanism can be described as physisorption, chemisorption, complexation, ion exchange, or chelation/coordination (Gupta et al., 2015). Other than that, the chemical treatment also enables the extraction of soluble organic compounds, enhance chelating efficiency, reduces the coloration of aqueous solutions and increases the efficiency of heavy metals adsorption (Acharya et al., 2018). In addition, pre-filtration during the adsorption process is required due to the presence of suspended particles, oils, and greases which reduce the adsorption efficiency (Ali and Gupta, 2007).
The adsorption mechanism is a complex process that involving the binding of metal ions and adsorbents by physical or chemical bonding, chelation, reduction, precipitation, and complexation (Kanamarlapudi et al., 2018). The sorption mechanism can be one or a combination of several phenomena, including the formation of a chemical complex at the surface of the adsorbent, electrical attraction (involved in almost all chemical mechanisms), and the exclusion of the adsorbate from the bulk solution (Patil et al., 2016). The mechanism of the adsorption process determines the adsorbent efficiency and the rate of manganese removal (Singh et al., 2020). Table 3 shows the modification and mechanism of agricultural waste adsorbents for manganese removal.
Table 3.
Modification and mechanism of agricultural waste adsorbents for manganese removal.
| Adsorbent | Modification | Mechanism | Manganese removal (%) | References |
|---|---|---|---|---|
| Green tomato husk | Formaldehyde | Ion exchange and complexation | 84.8 | (García-Mendieta et al., 2012) |
| Ziziphus spina-christi seeds | Activated carbon | Ion exchange | >80 | (Omri and Benzina, 2012) |
| Olive stones | Oxidation with concentrated nitric acid | Ion exchange and complexation | 41 | (Ak et al., 2013) |
| Rice husk ash | Drying | Physical in nature | >95 | (Zhang et al., 2014) |
| Sugarcane bagasse | Hydrochloric acid | Ion exchange | 99 | (Esfandiar et al., 2014) |
| Modified tangerine peel | 0.25 M of nitric acid and 0.1 M of sodium hydroxide | Ion exchange | 92.48 | (Abdić et al., 2018) |
| Moringa oleifera seed | 0.1 M of sodium hydroxide | Ion exchange and complexation | 95 | (Marque et al., 2013) |
| Moringa oleifera seed pods and Sclerocarya birrea nut shells | 0.4 M Nitric acid | Electrostatic interactions, ion exchange, complexation, microprecipitation, and acid-base interactions | 40–90 | (Maina et al., 2016) |
| Sunflower, potato, canola, and walnut shell | Drying | Electrostatic interactions, ion exchange and complexation | 79–97 | (Feizi and Jalali, 2015) |
Modification of the carbon surface through oxidation with concentrated nitric acid introduces a variety of carbon-oxygen functional groups of acidic nature in olive stones adsorbent. The possible interaction was acquired from the Fourier transform infrared (FTIR) spectra results. The exchange of ions between hydrogen ions released from the carboxylic and phenolic surface groups contributed to the improvement of manganese removal. While carbonyl and/or lactonic groups are more likely to be involved in a complex formation of manganese ions (Ak et al., 2013).
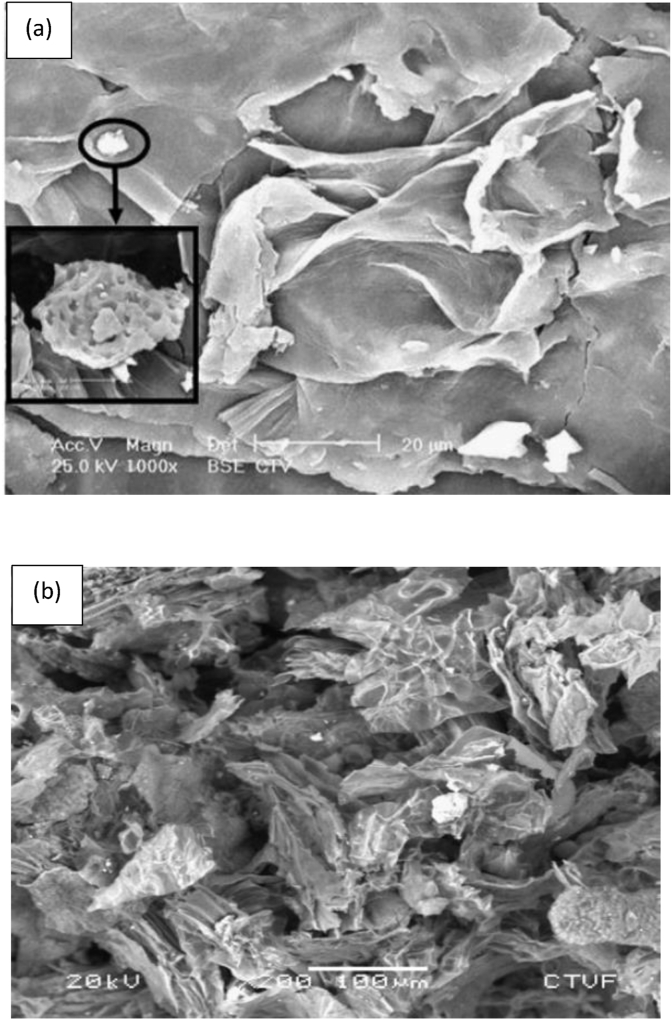
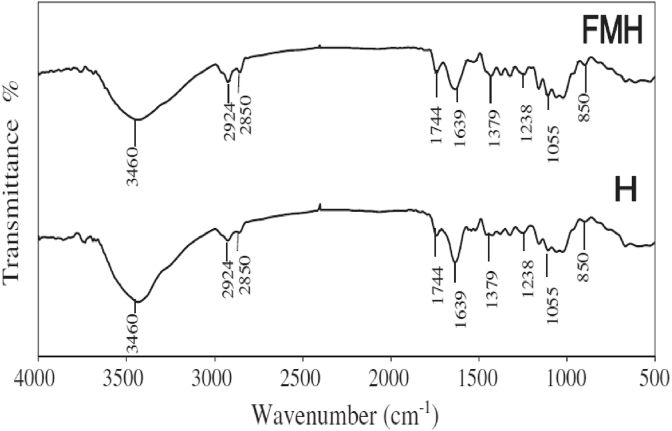
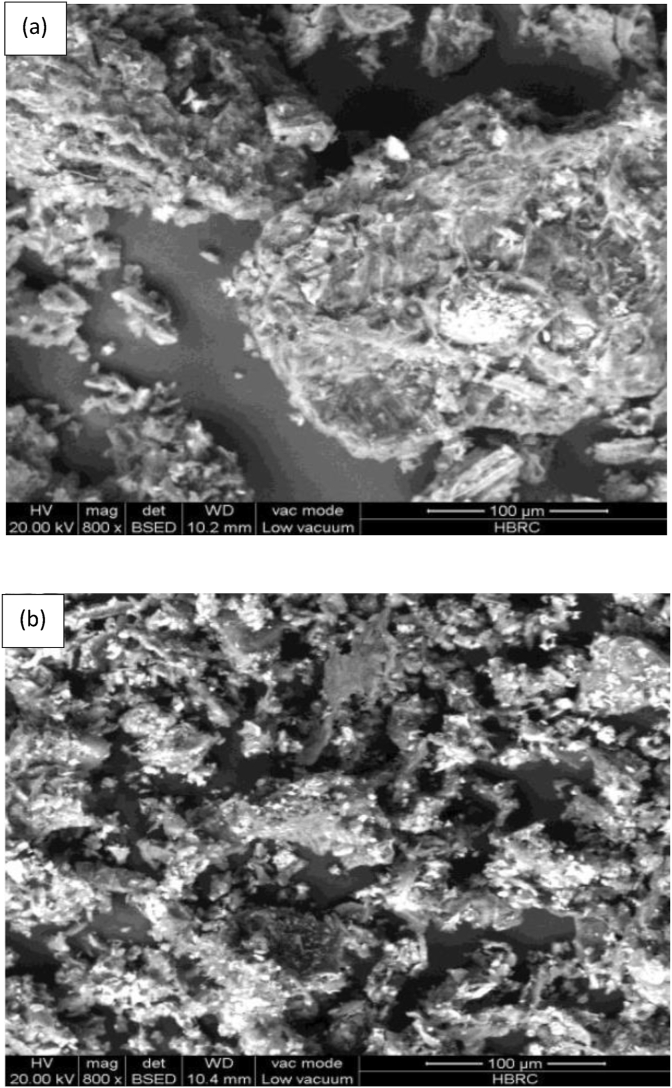
Apart from that, Garcia-Mendieta et al. (2012) presented the adsorption process of formaldehyde modified green tomato husk (FMH) adsorbent in removing manganese. Figure 6 shows the SEM images of raw green tomato husk (H) and FMH adsorbents. The morphology of the H adsorbent shows more agglomeration of the particles than the FMH adsorbent. This could be due to the weight loss of the FMH adsorbent during the modification process. On the other hand, Figure 7 shows the FTIR results of the H and FMH adsorbents where both spectra had similar absorption bands. This indicates that the modification of the green tomato husk with formaldehyde does not alter the structure of the adsorbent. It was discovered that ion exchange and complexation are the main adsorption mechanisms responsible for the manganese removal due to the cation balance and the interactions between the manganese ions and the organic functional groups (amino, carbonyl, carboxyl, ester, hydroxyl, sulfonate, phenols, and alcohol) (Garcia-Mendieta et al., 2012).
Figure 6.
SEM images of (a) raw green tomato husk and (b) formaldehyde green tomato husk adsorbents (García-Mendieta et al., 2012).
Figure 7.
FTIR spectra of green tomato hush (H) and formaldehyde modified green tomato husk (FMH) adsorbents (García-Mendieta et al., 2012).
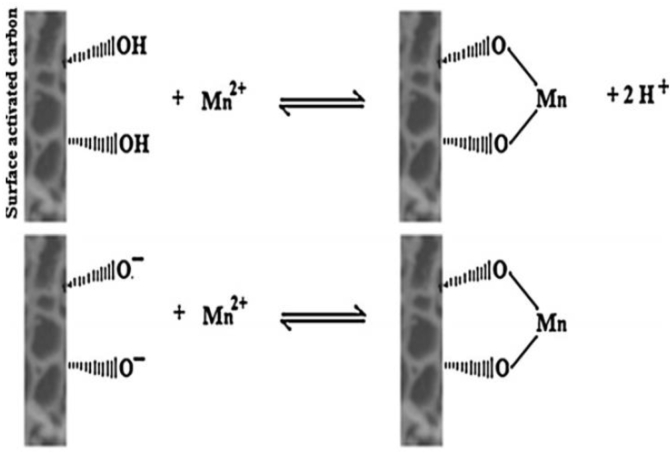
Omri and Benzina (2012) have revealed that the surface of Ziziphus spina-christi seeds activated carbon adsorbent contains functional groups such as oxygen and aromatic compounds. These groups were involved in chemical bonding and responsible for the cation exchange capacity of the adsorbent. Figure 8 shows the ion exchange occurs when a manganese ion is attached to two adjacent hydroxyl groups and two-oxyl groups, which could donate two pairs of electrons to the metal ions. This consists of four coordination number of compounds and two hydrogen ions released into the solution.
Figure 8.
Ion exchange mechanism of Ziziphus spina-christi seeds activated carbon (Omri and Benzina, 2012).
The mean free energy (E) could also provide information on the adsorption mechanism. The E value between 8 and 16 kJ/mol indicates that the adsorption mechanism occurs by chemical ion exchange in nature. Whereas, the E value of less than 8 kJ/mol indicates that the adsorption mechanism occurs as physical reactions in nature. Zhang et al. (2014) have shown that the mechanism of rice husk ash (RHA) adsorbent for manganese removal is physical in nature with an E value of 2.27 kJ/mol.
3.2. The performance of manganese removal using agricultural waste adsorbents
Many types of agricultural wastes have been used to remove manganese from the water. The performance of agricultural waste adsorbents is shown in Table 4. The RHA adsorbent was able to remove 3.81 mg/L of manganese by using 0.5 g of the adsorbent under acidic condition and within 100 min contact time. The adsorbents were pre-treated with 0.3 M of nitric acid to increase the efficacy of manganese adsorption (Adekola et al., 2016). The functional groups observed in the RHA adsorbents include silanol (Si–O – H) groups, alkanes, aromatic groups, lactones, aliphatic C–H bonds in CH2, and CH3. All of these linkages that present on the adsorbent surface are responsible for the manganese adsorption (Adekola et al., 2016).
Table 4.
The performance of agricultural waste adsorbents for manganese removal.
| Adsorbent | Manganese removal (%) | References |
|---|---|---|
| Rice husk ash | 100 | (Adekola et al., 2016) |
| Peanut husk | 38 | (Abdelfattah et al., 2016) |
| Sunflower seed shell | 81.6 | (Feizi and Jalali, 2015) |
| Potato peel | 79.8 | (Feizi and Jalali, 2015) |
| Canola flower | 81.8 | (Feizi and Jalali, 2015) |
| Walnut shell | 96.5 | (Feizi and Jalali, 2015) |
| Maize husk | 88.4 | (Adeogun et al., 2013) |
| Sugarcane bagasse | 99 | (Esfandiar et al., 2014) |
| White rice husk ash | 26.6 | (Tavlieva et al., 2015) |
| Yam peel | 30.5 | (Isagba et al., 2017) |
| Orange peel | 96 | (Surovka and Pertile, 2017) |
| Modified tangerine peel | 92.48 | (Abdić et al., 2018) |
| Moringa oleifera seed | 95 | (Marque et al., 2013) |
| Corn cob and Strychnous Potatorum seed powder | 99.8 | (Kumar et al., 2018) |
| Tea waste | 95.5 | (Badrealam et al., 2019) |
| Grafted banana peels | 94 | (Ali, 2017) |
| Peanut husk | 61 | (Zaini et al., 2019) |
| Banana peels | 97.4 | (Mahmoud, 2014) |
| Sugarcane Bagasse | 62.5 | (Ahmed et al., 2015) |
| Beet pulp | 86.4 | (Ahmed et al., 2015) |
| Moringa oleifera seed pods | 40–80 | (Maina et al., 2016) |
| Sclerocarya birrea nut shells | 40–90 | (Maina et al., 2016) |
| Unripe plantain peels | 32–94 | (Leizou et al., 2018) |
Among the agricultural wastes, banana waste has shown high performances in the adsorption process. The banana wastes are derived from various parts that can be used as adsorbents including the banana peels, trunks, pseudo-stems, leaves, and piths. The banana peels are wastes generated in large quantities due to the consumption of the fruits. These wastes contain carbon-rich organic compounds such as cellulose, hemicellulose, pectin substances, chlorophyll pigments, and some other low molecular weight compounds (Ahmad and Danish, 2018).
Mahmoud (2014) shows the efficiency of banana peels activated carbon (BPAC) adsorbent up to 97.4% in removing manganese. Figure 9 shows the surface morphology of the BPAC adsorbent before and after manganese adsorption. It can be seen that manganese was adsorbed to the BPAC after the adsorption process. The FTIR spectra confirmed that the carboxylic acid and the hydroxyl groups played a major role in manganese removal.
Figure 9.
SEM images of BPAC adsorbent (a) before and (b) after the manganese adsorption process (Mahmoud, 2014).
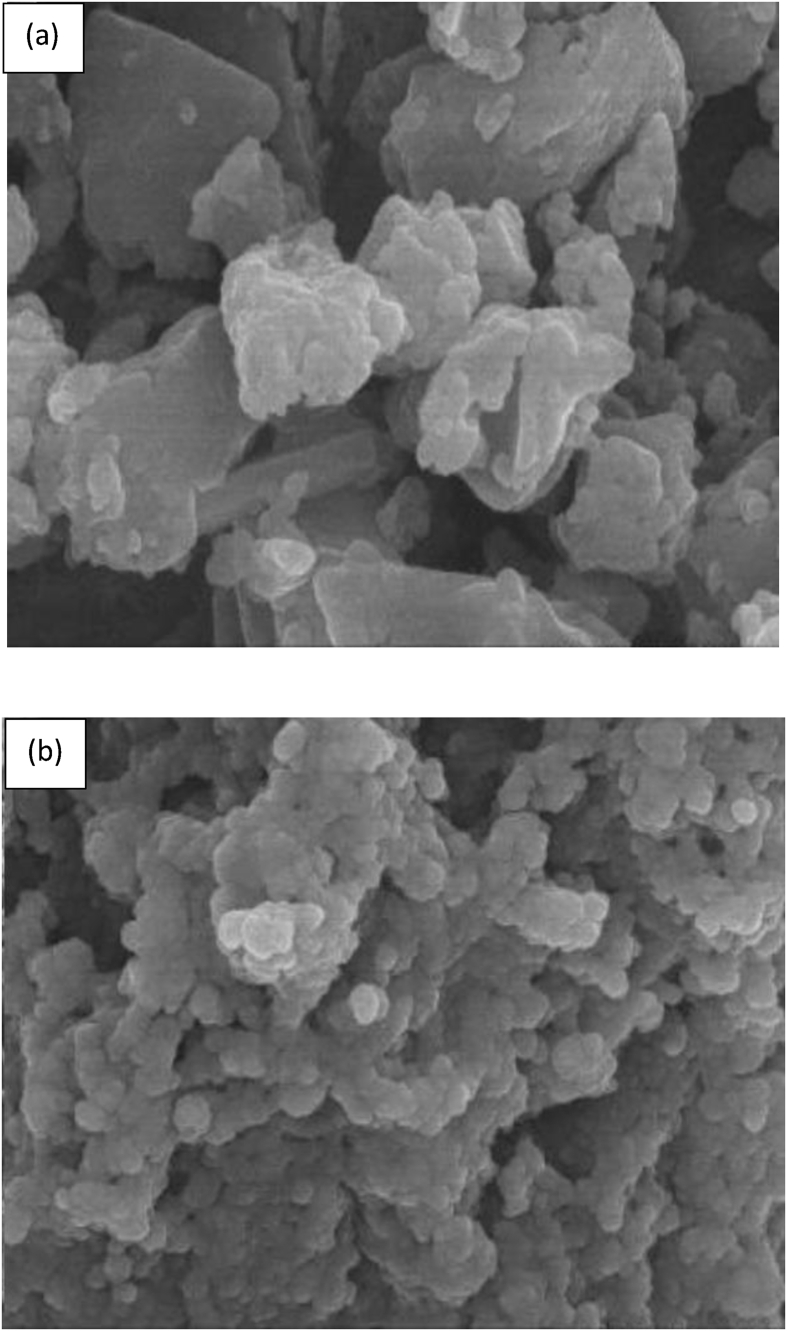
Ali (2017) synthesised grafted banana peels (GBPs) adsorbents via hydrolysation of alkali followed by bleaching with sodium chlorate. The highest manganese adsorption of 94% onto the GBPs was recorded at the optimum condition of 400 mg/L manganese concentration at pH 7 with 4 g of dosage within 60 min of contact time. The chemical treatment of the GBPs was able to remove viscous compounds such as lignin and pectin, as well as expose the active functional groups of cellulosic skeletons including hydroxyl, carboxyl, epoxy, and carboxylic groups. The scanning electron microscopy (SEM) images in Figure 10 showed the surface of GBPs adsorbents before and after the adsorption process. Before the adsorption, the surface of GBPs shows few open pores and fibres with rough surfaces. After the adsorption process, a smooth surface was observed as the pores and caves were filled with manganese ions.
Figure 10.
SEM images of GBPs adsorbent (a) before and (b) after the manganese adsorption process (Ali, 2017).
Abdić et al. (2018) presented that the untreated tangerine peels adsorbent had low manganese removal of 30%. Nonetheless, threefold increase in adsorption was achieved by using treated tangerine peels adsorbent with nitric acid and sodium hydroxide serves as a chemical modification to the adsorbent surface. The chemical treatment of adsorbent tangerine peels has improved the adsorption process by removing up to 92.48% of manganese. In addition, the adsorbent synthesised from sugarcane bagasse (SCB) also recorded remarkable performance in removing manganese at an initial concentration of 12 mg/L and pH 4.5 from 63% up to 99%. The SCB has been treated with hydrochloric acid, which leads to the increase of anionic groups such as carboxyl and hydroxyl on the adsorbent surface, thus increasing the efficiency of the adsorption process (Esfandiar et al., 2014).
Apart from that, the use of corn cob and Strychnouspotatorum seed adsorbents in manganese removal without any chemical treatment showed excellent manganese adsorption of 99.8% at an optimum dose of 0.4 g, pH 5 and 60 min contact time (Kumar et al., 2018). The FTIR spectra showed that both adsorbents contain phenolic, hydroxyl, carbonyl, and lactone groups which play a vital role in the removal of manganese.
4. Cost analysis of adsorbents
Cost analysis is crucial in determining the practicality of the adsorption process for water treatment in industrial scale. Generally, the operational cost is related to the production cost of adsorbents. The most common and well-known commercial adsorbents are activated carbon (~543 USD – 1086 USD per kg), iron oxide (more than 1086 USD per ton), and activated alumina (~325 USD – 543 USD per ton) (Iakovleva and Sillanpää, 2020). Afroze and Sen (2018) have mentioned that the cost estimation for adsorbent comprise of several factors. This includes the wide availability of raw materials at local whether from natural, industrial, agricultural, domestic waste, by-product, or synthesised products. Apart from that, the cost estimation involves the process requirement, condition during treatment, ability for recycling, and long-term effects. In addition, the cost is also subjected to where and when the adsorbents were originally produced, whether in (or for) developed, or underdeveloped countries.
According to Demis et al. (2015), the total cost estimate for low-cost adsorbents can be calculated by adding fixed capital with initial working capital. Fixed capital is the cost of acquiring or constructing equipment and installations. While, the initial cost of working capital is required in order for the production to achieve a normal level of operation, which covers the cost of raw and auxiliary materials, wages and salaries, the cost of product delivery as well as the cost of operating tests. The comparative distribution of various operating components which affect the calculation of cost estimation shall therefore include raw and auxiliary materials, energy balance (energy cost and mill power), insurance, depreciation, labour costs, regular and damage maintenance, administrative costs, interest (loan and initial working capital), taxes, general costs (research and development, and sales promotion), and raw materials (adsorbents).
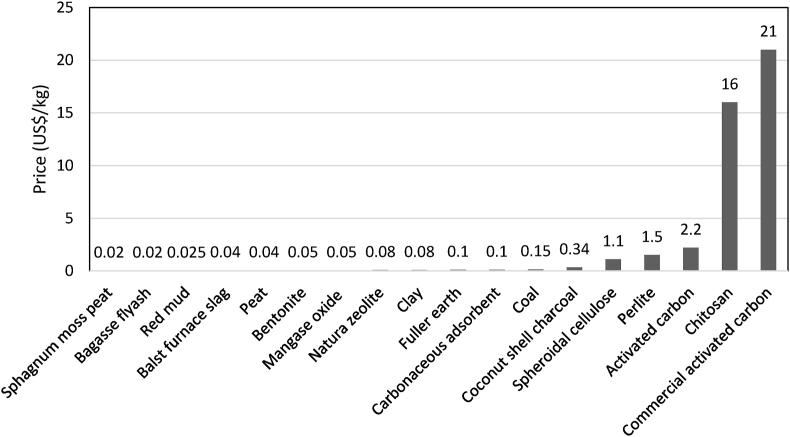
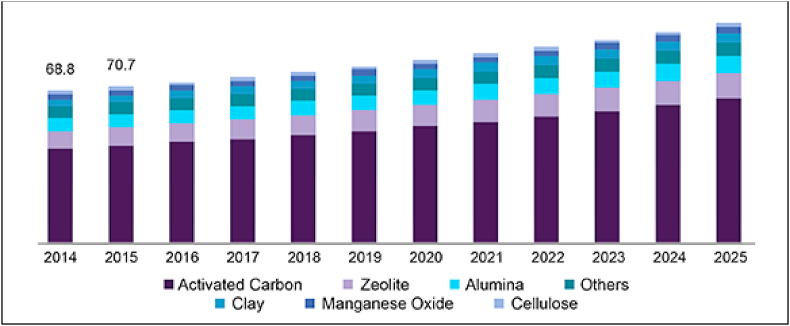
Low-cost adsorbents are cost-effective technologies for the water treatment industry to treat water containing different concentrations of heavy metals. Figure 11 shows the cost of several adsorbents available on the market (Bello et al., 2013). The commercial activated carbon is the most expensive at 21 USD per kg. This is followed by chitosan, worth 16 USD per kg, and others. Agricultural waste adsorbents such as sphagnum moss peat and bagasse fly ash have been shown to be the most economical adsorbents with only 0.02 USD per kg. Not only affordable, but these adsorbents also contain cellulosic compounds that have been shown to be effective in the adsorption process. Figure 12 shows the adsorbent market size from 2014 to 2025. The adsorbents were estimated at USD 459 million in 2018 and are expected to grow at a compound annual growth rate (CAGR) of 4.0% from 2019 to 2025 (Grand View Research, 2019). However, numerous adsorbents have varied drawbacks and limitations when implemented in the industry. As mentioned earlier in this paper, modifications in the properties of adsorbents may help to improve the efficacy of metal binding in the removal of manganese. Even so, the consequences of such alterations could increase the overall cost of the process, which is almost the price of commercial ion-exchange resins (Gupta et al., 2015).
Figure 11.
Cost of adsorbents in the market (Bello et al., 2013).
Figure 12.
Adsorbent market size from 2014-2025 (USD million) (Grand View Research, 2019).
The cost for adsorbent regeneration is also a key factor that affecting the operational cost. The possibility of reusing adsorbent is an attractive advantage for cost-effective adsorbents (kyzas2014). Moreover, most of the adsorption process is reversible in nature as the adsorbent can be regenerated several times. The process of desorption is simple, efficient, and involves low-cost maintenance (Hua et al., 2012). When the demand for low-cost adsorbents becomes higher than the supply, it will cause a problem to the industries that manufactured adsorbents. This consequently affects the operation of the water treatment plant due to the inadequate supply of adsorbents (Lim and Aris, 2014). According to a report by Markets and Markets (2020), the global adsorbent market is projected to worth USD 4.3 billion by 2020, with a CAGR of 6.3% between 2015 and 2020. The growth value of adsorbent in the market is expected to increase due to the increasing level of pollution and stringent environmental regulations on water and wastewater treatment. Moreover, the low-cost adsorbent process is not in competition with other conventional water treatment technologies such as reverse osmosis and ion exchange, as the current markets are searching for a cost-effective alternative with high efficiency in the removal of heavy metals (Gupta et al., 2015).
A number of studies have reported the performances of the agricultural waste adsorbents (Ali, 2017; Surovka and Pertile, 2017; Bangaraiah, 2018; Kumar et al., 2018; Leizou et al., 2018; Badrealam et al., 2019). However, these studies are limited to the laboratory scale, which is unable to estimate the real operational cost. Thus, a pilot plant study should be carried out prior to the large scale commercialisation (Bello et al., 2013). Anastopoulos et al. (2019b) also agreed that continuing to work with continuous systems and scaling up the adsorption process is crucial, since most studies are conducted on a laboratory scale only.
5. Conclusion
The presence of manganese mostly from the industrial effluents in the water sources has deteriorated the water quality. Although manganese is a trace element, prolonged exposure could lead to many negative impacts. Adsorption has proven to be the most practical method of water treatment owing to its efficiency, low cost, and simple process. In particular, adsorbents produced from agricultural waste have shown excellent performances in the removal of manganese. Controlling the factors that govern the adsorption process is a key strategy for achieving the optimum conditions for the highest manganese removal in the process. The adsorption mechanism of manganese could occur through a complex formation and/or ion exchange that depends entirely on the types of adsorbents. A thorough understanding of the adsorption process that occurs between the adsorbent and manganese makes adsorption technological improvements possible. Modification of adsorbent by physical or chemical treatment results in the presence of active functional groups on the adsorbent surface that significantly increase the manganese removal. The total cost of adsorbent production includes fixed and working capital which requires an in-depth costs’ analysis. Overall, the agricultural waste adsorbents are the most cost-effective adsorbents with a high potential for manganese removal in the water treatment process. It can be further developed for commercialisation and large-scale applications in the treatment of manganese-containing water for the water industry.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
The authors wish to thank Ministry of Higher Education (MOHE), Malaysia for the financial support from Fundamental Research Grant Scheme grant (K219) (FRGS/1/2019/TK10/UTHM/03/3) and Universiti Tun Hussein Onn Malaysia for GPPS grant (H597).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abdeen Z., Mohammad S.G., Mahmoud M.S. Adsorption of Mn (II) ion on polyvinyl alcohol/chitosan dry blending from aqueous solution. Environ. Nanotech. Monit. Manag. 2015;3:1–9. [Google Scholar]
- Abdelfattah I., Ismail A.A., Al Sayed F. Biosorption of heavy metals ions in real industrial wastewater using peanut husk as efficient and cost -effective adsorbent. Environ. Nanotech. Monit. Manag. 2016;6:176–183. [Google Scholar]
- Abdić, Memić M., Šabanović E. Adsorptive removal of eight heavy metals from aqueous solution by unmodified and modified agricultural waste: tangerine peel. Int. J. Environ. Sci. Technol. 2018;15:2511–2518. [Google Scholar]
- Acharya J., Kumar U., Rafi P.M. Removal of heavy metal ions from wastewater by chemically modified agricultural waste material as potential adsorbent-A review. Int. J. Curr. Eng. Technol. 2018;8:526–530. [Google Scholar]
- Adekola F.A., Hodonou D.S.S., Adegoke H.I. Thermodynamic and kinetic studies of biosorption of iron and manganese from aqueous medium using rice husk ash. Appl. Water Sci. 2016;6:319–330. [Google Scholar]
- Adeogun A.I., Idowu M.A., Ofudje A.E. Comparative biosorption of Mn(II) and Pb(II) ions on raw and oxalic acid modified maize husk: kinetic, thermodynamic and isothermal studies. Appl. Water Sci. 2013;3:167–179. [Google Scholar]
- Afroze S., Sen T.K. A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water, air. Soil Pollut. 2018;229:225. [Google Scholar]
- Ahmad M., Ahmed S., Swami B.L., Ikram S. Adsorption of heavy metal Ions : role of chitosan and cellulose for water treatment. Int. J. Pharmacogn. 2015;2:280–289. [Google Scholar]
- Ahmad T., Danish M. Prospects of banana waste utilization in wastewater treatment: a review. J. Environ. Manag. 2018;206:330–348. doi: 10.1016/j.jenvman.2017.10.061. [DOI] [PubMed] [Google Scholar]
- Ahmed F., Siwar C., Begum R.A. Water resources in Malaysia: issues and challenges. J. Food Agric. Environ. 2014;12:1100–1104. [Google Scholar]
- Ahmed M.J.K., Ahmaruzzaman M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Process Eng. 2016;10:39–47. [Google Scholar]
- Ahmed S.A., El-Roudi A.M., Salem A.A.A. Removal of Mn(II) from ground water by solid wastes of sugar industry. J. Environ. Sci. Technol. 2015;8:338–351. [Google Scholar]
- Ak M.A., Yousef A.M., AbdElnasser S. Removal of iron and manganese in water samples using activated carbon derived from local agro-residues. J. Chem. Eng. Process Technol. 2013;4 [Google Scholar]
- Al-Jubouri S.M., Holmes S.M. Hierarchically porous zeolite X composites for manganese ion-exchange and solidification: equilibrium isotherms, kinetic and thermodynamic studies. Chem. Eng. J. 2017;308:476–491. [Google Scholar]
- Alaei Shahmirzadi M.A., Hosseini S.S., Luo J., Ortiz I. Significance, evolution and recent advances in adsorption technology, materials and processes for desalination, water softening and salt removal. J. Environ. Manag. 2018;215:324–344. doi: 10.1016/j.jenvman.2018.03.040. [DOI] [PubMed] [Google Scholar]
- Ali A. Removal of Mn(II) from water using chemically modified banana peels as efficient adsorbent. Environ. Nanotech. Monit. Manag. 2017;7:57–63. [Google Scholar]
- Ali A., Saeed K. Decontamination of Cr (VI) and Mn (II) from aqueous media by untreated and chemically treated banana peel: a comparative study. Desalin. Water Treat. 2015;53:3586–3591. [Google Scholar]
- Ali I., Asim M., Khan T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012;113:170–183. doi: 10.1016/j.jenvman.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Ali I., Gupta V.K. Advances in water treatment by adsorption technology. Nat. Protoc. 2007;1:2661–2667. doi: 10.1038/nprot.2006.370. [DOI] [PubMed] [Google Scholar]
- Ali R.M., Hamad H.A., Hussein M.M., Malash G.F. Potential of using green adsorbent of heavy metal removal from aqueous solutions: adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016;91:317–332. [Google Scholar]
- Alvarez-Bastida C., Martínez-Miranda V., Solache-Ríos M. Drinking water characterization and removal of manganese. Removal of manganese from water. J. Environ. Chem. Eng. 2018;6:2119–2125. [Google Scholar]
- Anastopoulos I., Pashalidis I., Hosseini-Bandegharaei A. Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. J. Mol. Liq. 2019;111684 [Google Scholar]
- Anastopoulos I., Pashalidis I., Hosseini-Bandegharaei A. Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. J. Mol. Liq. 2019;295 [Google Scholar]
- Anu Y. Bioremediation of wastewater using various sorbents and vegetable enzymes. Res. Biotechnol. 2015;6:16–23. [Google Scholar]
- Badrealam S., Darrell V.C., Dollah Z. Adsorption of manganese and zinc in synthetic wastewater by tea waste (TW) as a low cost adsorbent. J. Phys. Conf. Ser. 2019;1349 [Google Scholar]
- Bangaraiah P. Biosorption of manganese using tamarind fruit shell powder as a biosorbent. Res. J. Pharm. Technol. 2018;11:4313. [Google Scholar]
- Baysal A., Ozbek N., Akm S. Waste Water - Treatment Technologies and Recent Analytical Developments. InTech; 2013. Determination of trace metals in waste water and their removal processes; p. 13. [Google Scholar]
- Bello O.S., Adegoke K.A., Olaniyan A.A., Abdulazeez H. Dye adsorption using biomass wastes and natural adsorbents: overview and future prospects. Desalin. Water Treat. 2013;53:1292–1315. [Google Scholar]
- Bouchard M.F., Surette C., Cormier P., Foucher D. Low level exposure to manganese from drinking water and cognition in school-age children. Neurotoxicology. 2018;64:110–117. doi: 10.1016/j.neuro.2017.07.024. [DOI] [PubMed] [Google Scholar]
- Carolin C.F., Kumar P.S., Saravanan A. Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J. Environ. Chem. Eng. 2017;5:2782–2799. [Google Scholar]
- Chen P., Bornhorst J., Aschner M. Manganese metabolism in humans. Front. Biosci. Landmark. 2018;23:1655–1679. doi: 10.2741/4665. [DOI] [PubMed] [Google Scholar]
- Crini G., Lichtfouse E., Wilson L.D., Morin-Crini N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019;17:195–213. [Google Scholar]
- Dai Y., Sun Q., Wang W. Utilizations of agricultural waste as adsorbent for the removal of contaminants: a review. Chemosphere. 2018;211:235–253. doi: 10.1016/j.chemosphere.2018.06.179. [DOI] [PubMed] [Google Scholar]
- Das B., Mondal N.K., Bhaumik R., Roy P. Insight into adsorption equilibrium, kinetics and thermodynamics of lead onto alluvial soil. Int. J. Environ. Sci. Technol. 2014;11(4):1101–1114. [Google Scholar]
- Demis S., Tapali J.G., Papadakis V.G. Plant design and economics of rice husk ash exploitation as a pozzolanic material. Waste Biomass Valoriz. 2015;6:843–853. [Google Scholar]
- Dion L.A., Saint-Amour D., Sauvé S. Changes in water manganese levels and longitudinal assessment of intellectual function in children exposed through drinking water. Neurotoxicology. 2018;64:118–125. doi: 10.1016/j.neuro.2017.08.015. [DOI] [PubMed] [Google Scholar]
- Du X., Zhang K., Xie B. Peroxymonosulfate-assisted electro-oxidation/coagulation coupled with ceramic membrane for manganese and phosphorus removal in surface water. Chem. Eng. J. 2019:334–343. [Google Scholar]
- Esfandiar N., Nasernejad B., Ebadi T. Removal of Mn(II) from groundwater by sugarcane bagasse and activated carbon (a comparative study): application of response surface methodology (RSM) J. Ind. Eng. Chem. 2014;20:3726–3736. [Google Scholar]
- Fatemeh Seyedpour S., Rahimpour A., Mohsenian H., Taherzadeh M.J. Low fouling ultrathin nanocomposite membranes for efficient removal of manganese. J. Membr. Sci. 2018;549:205–216. [Google Scholar]
- Feizi M., Jalali M. Removal of heavy metals from aqueous solutions using sunflower, potato, canola and walnut shell residues. J. Taiwan Inst. Chem. Eng. 2015;54:125–136. [Google Scholar]
- Fu F., Wang Q. Removal of heavy metal ions from wastewaters: a review. J. Environ. Manag. 2011;92:407–418. doi: 10.1016/j.jenvman.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Garcia-Mendieta A., Olguín M.T., Solache-Ríos M. Biosorption properties of green tomato husk (Physalis philadelphica Lam) for iron, manganese and iron-manganese from aqueous systems. Desalination. 2012;284:167–174. [Google Scholar]
- Gedam A.H., Dongre R.S. Adsorption characterization of Pb (II) ions onto iodate doped chitosan composite: equilibrium and kinetic studies. RSC Adv. 2015;5(67):54188–54201. [Google Scholar]
- Gerke T.L., Little B.J., Barry Maynard J. Manganese deposition in drinking water distribution systems. Sci. Total Environ. 2016;541:184–193. doi: 10.1016/j.scitotenv.2015.09.054. [DOI] [PubMed] [Google Scholar]
- Goher M.E., Hassan A.M., Abdel-Moniem I.A. Removal of aluminum, iron and manganese ions from industrial wastes using granular activated carbon and Amberlite IR-120H. Egypt J. Aquat. Res. 2015;41:155–164. [Google Scholar]
- Grand View Research . Mark. Res. Rep. 2019. Drinking Water Adsorbents Market Size, Share & Trends Analysis Report by Product, (Activated Carbon, Zeolite, Alumina, Clay, Manganese Oxide, Cellulose), by Region, and Segment Forecasts, 2019 - 2025.https://www.grandviewresearch.com/industry-analysis/drinking-water-adsorbents-market [Google Scholar]
- Gupta V.K., Nayak A., Agarwal S. Bioadsorbents for remediation of heavy metals: current status and their future prospects. Environ. Eng. Res. 2015;20:1–18. [Google Scholar]
- Hokkanen S., Bhatnagar A., Sillanpää M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016;91:156–173. doi: 10.1016/j.watres.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Hua M., Zhang S., Pan B. Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J. Hazard Mater. 2012;211–212:317–331. doi: 10.1016/j.jhazmat.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Iakovleva E., Sillanpää M. Novel sorbents from low-cost materials for water treatment. In: Treatment A.W., editor. Advanced Water Treatment. first ed. Elsevier; United States: 2020. pp. 265–359. [Google Scholar]
- Idrees M., Batool S., Ullah H. Elsevier B.V; 2018. Adsorption and Thermodynamic Mechanisms of Manganese Removal from Aqueous media by Biowaste-Derived Biochars. [Google Scholar]
- Iftekhar S., Ramasamy D.L., Srivastava V. Understanding the factors affecting the adsorption of Lanthanum using different adsorbents: a critical review. Chemosphere. 2018;204:413–430. doi: 10.1016/j.chemosphere.2018.04.053. [DOI] [PubMed] [Google Scholar]
- Ihsanullah, Abbas A., Al-Amer A.M. Heavy metal removal from aqueous solution by advanced carbon nanotubes: critical review of adsorption applications. Separ. Purif. Technol. 2016;157:141–161. [Google Scholar]
- Isagba E.S., Kadiri S., Ilaboya I.R. Yam peels as adsorbent for the removal of copper (Cu) and manganese (Mn) in waste water. Niger. J. Environ. Sci. Technol. 2017;1:230–243. [Google Scholar]
- Jacob J.M., Karthik C., Saratale R.G. Biological approaches to tackle heavy metal pollution: a survey of literature. J. Environ. Manag. 2018;217:56–70. doi: 10.1016/j.jenvman.2018.03.077. [DOI] [PubMed] [Google Scholar]
- Jawed A., Pandey L.M. Application of bimetallic Al-doped ZnO nano-assembly for heavy metal removal and decontamination of wastewater. Water Sci. Technol. 2019:1–12. doi: 10.2166/wst.2019.393. [DOI] [PubMed] [Google Scholar]
- Jawed A., Saxena V., Pandey L.M. Engineered nanomaterials and their surface functionalization for the removal of heavy metals: a review. J. Water Process Eng. 2020;33:101009. [Google Scholar]
- Jeirani Z., Sadeghi A., Soltan J. Effectiveness of advanced oxidation processes for the removal of manganese and organic compounds in membrane concentrate. Separ. Purif. Technol. 2015;149:110–115. [Google Scholar]
- Jin W., Du H., Zheng S., Zhang Y. Electrochemical processes for the environmental remediation of toxic Cr(VI): a review. Electrochim. Acta. 2016;191:1044–1055. [Google Scholar]
- Kale A.E., Mandake M.B., Chitodkar V.D. Removal of heavy metals using adsorption process- A review. Int. J. Adv. Eng. Res. Dev. 2017;4:1–4. [Google Scholar]
- Kanamarlapudi S.L.R.K., Chintalpudi V.K., Muddada S. Biosorption; 2018. Application of Biosorption for Removal of Heavy Metals from Wastewater. [Google Scholar]
- Kasim N., Mohammad A.W., Abdullah S.R.S. Iron and manganese removal by nanofiltration and ultrafiltration membranes: influence of pH adjustment. Malaysian J. Anal. Sci. 2017;21:149–158. [Google Scholar]
- Kobielska P.A., Howarth A.J., Farha O.K., Nayak S. Metal–organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018;358:92–107. [Google Scholar]
- Kumar G.V.S.R.P., Rao K.S., Yadav A. Biosorption of copper (II) and manganese (II) from waste water using low cost bio adsorbents. J. Indian Chem. Soc. 2018;95:1–8. [Google Scholar]
- Lakherwal D. Adsorption of heavy metals: a review. Int. J. Environ. Res. Dev. 2014;4:41–48. [Google Scholar]
- Lata S., Samadder S.R. Removal of arsenic from water using nano adsorbents and challenges: a review. J. Environ. Manag. 2016;166:387–406. doi: 10.1016/j.jenvman.2015.10.039. [DOI] [PubMed] [Google Scholar]
- Lee I., Hwang H., Lee J. Modeling approach to evaluation of environmental impacts on river water quality: a case study with Galing River, Kuantan, Pahang, Malaysia. Ecol. Model. 2017;353:167–173. [Google Scholar]
- Leizou Elijah K., Ashraf M.A., Ahmad Jalal, Khan Chowdhury H.R. Adsorption studies of Pb2+ and Mn2+ ions on low-cost adsorbent: unripe plantain (Musa paradisiaca) peel biomass. Acta. Chem. Malaysia. 2018;2:11–15. [Google Scholar]
- Lim A.P., Aris A.Z. A review on economically adsorbents on heavy metals removal in water and wastewater. Rev. Environ. Sci. Biotechnol. 2014;13:163–181. [Google Scholar]
- Liu D., Li Z., Li W. Adsorption behavior of heavy metal ions from aqueous solution by soy protein hollow microspheres. Ind. Eng. Chem. Res. 2013;52:11036–11044. [Google Scholar]
- Liu Y., Hu L., Tan B. Adsorption behavior of heavy metal ions from aqueous solution onto composite dextran-chitosan macromolecule resin adsorbent. Int. J. Biol. Macromol. 2019;141:738–746. doi: 10.1016/j.ijbiomac.2019.09.044. [DOI] [PubMed] [Google Scholar]
- Mahmoud M.S. Banana peels as an eco-sorbent for manganese ions. Int. J. Agric. Biosyst. Eng. 2014;8:1183–1189. [Google Scholar]
- Maina I.W., Obuseng V., Nareetsile F. Use of Moringa oleifera (Moringa) seed pods and Sclerocarya birrea (Morula) nut shells for removal of heavy metals from wastewater and borehole water. J. Chem. 2016 2016. [Google Scholar]
- Marcus J.B. Culinary Nutrition. 2013. Vitamin and mineral basics: the ABCs of healthy foods and beverages, including phytonutrients and functional foods; pp. 279–331. [Google Scholar]
- Markets and Markets . Mark. Mark. (Adsorption Mark) 2020. Adsorbent market by type (molecular sieves, activated carbon, silica gel, activated alumina, clay, and others), by application (petroleum refining, chemicals/petrochemicals, gas refining, water treatment, air separation & drying, packaging, and others), a.https://www.marketsandmarkets.com/Market-Reports/adsorption-market-1173.html [Google Scholar]
- Marque T.L., Alves V.N., Coelho L.M., Coelho N.M.M. Assessment of the use of Moringa oleifera seeds for removal of manganese ions from aqueous systems. Bioresour. 2013;8:2738–2751. [Google Scholar]
- Marsidi N., Abu Hasan H., Sheikh Abdullah S.R. A review of biological aerated filters for iron and manganese ions removal in water treatment. J. Water Process Eng. 2018;23:1–12. [Google Scholar]
- Milatovic D., Gupta R.C. third ed. Elsevier Inc; 2018. Manganese. [Google Scholar]
- Milatovic D., Gupta R.C., Yin Z. Manganese. In: Gupta R.C., editor. Reproductive and Developmental Toxicology. second ed. Elsevier Inc.; KY, United States: 2017. pp. 567–581. [Google Scholar]
- Millati R., Cahyono R.B., Ariyanto T. Sustainable Resource Recovery and Zero Waste Approaches. Elsevier B.V.; 2019. Agricultural, industrial, Municipal, and forest wastes; pp. 1–22. [Google Scholar]
- Mthombeni N.H., Mbakop S., Onyango M.S. Adsorptive removal of manganese from industrial and mining wastewater. Annu. Conf. Sustain. Res. Innov. 2016:4–6. 2016. [Google Scholar]
- Omar S., Muhamad M.S., Te Chuan L. A review on lead sources, occurrences, health effects, and treatment using hydroxyapatite (HAp) adsorbent made from fish waste. Water Air Soil Pollut. 2019;230 [Google Scholar]
- Omri A., Benzina M. Removal of manganese(II) ions from aqueous solutions by adsorption on activated carbon derived a new precursor: Ziziphus spina-christi seeds. Alexandria Eng. J. 2012;51:343–350. [Google Scholar]
- Patil D.S., Chavan S.M., Oubagaranadin J.U.K. A review of technologies for manganese removal from wastewaters. J. Environ. Chem. Eng. 2016;4:468–487. [Google Scholar]
- Qiu Y.R., Mao L.J., Wang W.H. Removal of manganese from waste water by complexation-ultrafiltration using copolymer of maleic acid and acrylic acid. Trans. Nonferrous Met. Soc. China. 2014 English Ed 24:1196–1201. [Google Scholar]
- Rashid H., Yaqub G. Bioadsorbents and filters for removal of heavy metals in different environmental samples-A brief review. Nat. Environ. Pollut. Technol. 2017;16:1157–1164. [Google Scholar]
- Renu M.A., Singh K., Upadhyaya S., Dohare R.K. Removal of heavy metals from wastewater using modified agricultural adsorbents. Mater. Today Proc. 2017;4:10534–10538. [Google Scholar]
- Rose P., Hager S., Glas K. Coating techniques for glass beads as filter media for removal of manganese from water. Water Sci. Technol. Water Supply. 2017;17:95–106. [Google Scholar]
- Rumsby P., Rockett L., Clegg H. Speciation of manganese in drinking water. Toxicol. Lett. 2014;229 [Google Scholar]
- Shafiq M., Alazba A.A., Amin M.T. Removal of heavy metals from wastewater using date palm as a biosorbent: a comparative review. Sains Malays. 2018;47:35–49. [Google Scholar]
- Sharma S., Tiwari S., Hasan A. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. Biotech. 2018;8 doi: 10.1007/s13205-018-1237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu J., Liu R., Liu Z. Simultaneous removal of ammonia and manganese from electrolytic metal manganese residue leachate using phosphate salt. J. Clean. Prod. 2016;135:468–475. [Google Scholar]
- Singh N., Gupta D.S.K. Adsorption of heavy metals: a review. Int. J. Innov. Res. Sci. Eng. Technol. 2016;5:41–48. [Google Scholar]
- Singh N.B., Nagpal G., Agrawal S. Environmental technology & innovation water purification by using adsorbents: a review. Environ. Technol. Innov. 2018;11:187–240. [Google Scholar]
- Singh S., Kumar V., Datta S. Current advancement and future prospect of biosorbents for bioremediation. Sci. Total Environ. 2020;709:135895. doi: 10.1016/j.scitotenv.2019.135895. [DOI] [PubMed] [Google Scholar]
- Sulyman M., Namiesnik J., Gierak A. Low-cost adsorbents derived from agricultural by-products/wastes for enhancing contaminant uptakes from wastewater: a review. Pol. J. Environ. Stud. 2017;26:479–510. [Google Scholar]
- Surovka D., Pertile E. Sorption of iron, manganese, and copper from aqueous solution using orange peel: optimization, isothermic, kinetic, and thermodynamic studies. Pol. J. Environ. Stud. 2017;26:795–800. [Google Scholar]
- Tavlieva M.P., Genieva S.D., Georgieva V.G., Vlaev L.T. Thermodynamics and kinetics of the removal of manganese(II) ions from aqueous solutions by white rice husk ash. J. Mol. Liq. 2015;211:938–947. [Google Scholar]
- Tobiason J.E., Bazilio A., Goodwill J. Manganese removal from drinking water sources. Curr. Pollut. Reports. 2016;2:168–177. [Google Scholar]
- Tofighy M.A., Mohammadi T. Adsorption of divalent heavy metal ions from water using carbon nanotube sheets. J. Hazard Mater. 2011;185:140–147. doi: 10.1016/j.jhazmat.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Worldsteel Association . World Steel Assoc. AISBL. 2019. Global steel demand continues to grow in slowing economic environment.https://www.worldsteel.org/media-centre/press-releases/2019/worldsteel-short-range-outlook-april-2019.html [Google Scholar]
- Zaini H., Sami M., Arifin R. Activated variation of adsorbent and variation of contact time effects on manganese (II) in groundwater by column system using peanut shell as bioadsorbent. IOP Conf. Ser. Mater. Sci. Eng. 2019;536 [Google Scholar]
- Zhang Y., Zhao J., Jiang Z. Biosorption of Fe(II) and Mn(II) ions from aqueous solution by rice husk ash. BioMed Res. Int. 2014;1–10 doi: 10.1155/2014/973095. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Xu Y., Zhang C. New trends in removing heavy metals from wastewater. Appl. Microbiol. Biotechnol. 2016;100:6509–6518. doi: 10.1007/s00253-016-7646-x. [DOI] [PubMed] [Google Scholar]
- Zou Y., Wang X., Khan A. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: a review. Environ. Sci. Technol. 2016;50:7290–7304. doi: 10.1021/acs.est.6b01897. [DOI] [PubMed] [Google Scholar]