Abstract
Vitamin D deficiency has been associated with human abdominal aortic aneurysm (AAA); however, its role in AAA pathogenesis is unclear. The aim of the present study was to investigate the effect of vitamin D deficiency on AAA development and examine if administering cholecalciferol (CCF) could limit growth of established AAA within the angiotensin-II (AngII) infused apolipoprotein E-deficient mouse model. Mice were rendered vitamin D deficiency through dietary restriction and during AngII infusion developed larger AAAs as assessed by ultrasound and ex vivo morphometry that ruptured more commonly (48% vs. 19%; P=0.028) than controls. Vitamin D deficiency was associated with increased aortic expression of osteopontin and matrix metalloproteinase-2 and -9 than controls. CCF administration to mice with established aortic aneurysms limited AAA growth as assessed by ultrasound (P<0.001) and ex vivo morphometry (P=0.036) and reduced rupture rate (8% vs. 46%; P=0.031). This effect was associated with up-regulation of circulating and aortic sclerostin. Incubation of human aortic smooth muscle cells with 1,25-dihyroxyvitamin D3 (the active metabolite of vitamin D) for 48 h induced up-regulation of sclerostin (P<0.001) and changed the expression of a range of other genes important in extracellular matrix remodeling. The present study suggests that vitamin D deficiency promotes development of large rupture-prone aortic aneurysms in an experimental model. CCF administration limited both growth and rupture of established aneurysms. These effects of vitamin D appeared to be mediated via changes in genes involved in extracellular matrix remodeling, particularly sclerostin.
Keywords: Abdominal aortic aneurysm, sclerostin, vitamin D
Introduction
Abdominal aortic aneurysm (AAA) is an important cause of death in older adults due to aortic rupture [1]. Currently, there are no drug therapies that have been demonstrated to limit AAA progression and rupture [2]. Administration of the vitamin D receptor (VDR) agonist 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) has been reported to limit AAA development in a mouse model [3]. Short-term paricalcitol administration limits severity of inflammation within human AAA samples [4]. Vitamin D deficiency is clinically managed by cholecalciferol (CCF) supplementation in order to raise circulating 25-hydroxyvitamin D (25(OH)D) [5]. It remains unknown whether vitamin D supplementation limits progression of pre-established AAA. A relatively low circulating concentration of 25(OH)D has been associated with human AAA in a number of cross-sectional studies [6,7]. Contrary to this evidence, it has been reported that the prevalence of AAA is high in Australasia where levels of ultraviolet exposure (and thus likely vitamin D) are very high [1]. Excess vitamin D has also been reported to have potential pathological effects such as inhibiting production of elastin by vascular smooth muscle cells (VSMCs) [8]. Further experimental research is needed to resolve the importance of vitamin D in AAA pathogenesis.
Vitamin D plays an important role in the control of bone mineralization [9]. A number of bone proteins have been implicated in AAA pathogenesis [10–12]. Aortic expression of the bone protein sclerostin (SOST) is reduced in both experimental mouse and human AAA [11]. Mice in which SOST was over-expressed were protected from AAA induction by angiotensin II (AngII) infusion due to the ability of SOST to block the canonical wingless-type mouse mammary virus integration site (Wnt)/β-catenin pathway [11]. Vitamin D has been reported to up-regulate the expression of SOST in osteoblasts [13]; however, its effect on the aorta and VSMCs is unknown. It was hypothesized that vitamin D protected against AAA pathogenesis through up-regulation of SOST.
In the present study, the effects of vitamin D on AAA development, growth and rupture were investigated in vivo using the Apolipoprotein E (ApoE−/−) mouse model in which AAA was induced by AngII infusion. The study also investigated the effect of vitamin D on human aortic VSMCs in vitro. The study had four aims: (1) to examine whether the active metabolite of vitamin D, 1,25(OH)2D3, induced increased SOST expression in VSMCs in vitro; (2) to investigate whether diet-induced vitamin D deficiency reduced circulating levels of SOST in ApoE−/− mice; (3) to investigate if diet-induced deficiency of vitamin D promoted AAA development and rupture; and (4) to examine if raising circulating 25(OH)D levels through CCF supplementation limited progression of established AAA.
Materials and methods
Animals
Male apolipoprotein E deficient (ApoE−/−) mice (C57BL/6J background) were obtained from the Animal Resource Centre (WA, Australia) and maintained in individually ventilated, temperature/humidity-controlled cages (Aero IVC Green Line; Tecniplast). All mice were housed under a 12-h light–dark cycle (relative humidity: 55–60%; temperature: 22 ± 1°C). All animal procedures were approved by the James Cook University Animal Ethics Committee (A1970 and A2354), adhered to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, USA) and were performed at James Cook University animal facility.
Design of mouse studies
A number of animal studies were included (see Supplementary Figure S1):
Study 1
This was a pilot study to investigate whether vitamin D deficiency affected circulating levels of 25(OH)D and SOST. At the age of 13 weeks, mice were randomly split into two groups (n = 8 mice/group) [vitamin D sufficient (VDS) and vitamin D deficient groups (VDD)]. The VDS group was allocated to a vitamin D3 enriched diet (2,200 IU/ kg of feed) while the VDD group was maintained on a CCF depleted diet (0 IU/kg of feed). The diets were formulated by Specialty Feeds (WA, Australia) to ensure all other nutrients were balanced between the two diets, including mineral, fats, proteins and overall energy intake (Supplementary Table S1). Peripheral blood was collected by tail bleeding prior to starting the diet and every month thereafter. The mice were maintained on their respective diets for 5 months. All mice completed the study protocol.
Study 2
In order to examine if VDD promoted AAA development and rupture, 13-week-old mice were allocated to VDD (n=25) or VDS (n=24) groups and maintained on the diet for 8 weeks prior to induction of AAA. Consistent with ethical guidelines, three mice from the VDS group were killed due to poor health (unrelated to AAA) within the first week of diet administration. After 8 weeks on the VDS diet (n=21) or VDD diet (n=25), mice commenced subcutaneous AngII (1 µg/kg/min) infusion for 28 days while continuing on their allocated diets. Necroscopy was performed on all mice that died during the AngII infusion period to assess the cause of death.
Study 3
The present study examined if vitamin D administration could slow progression of established AAAs. AAA was initially induced in 13-week-old mice (n=32) by infusion of AngII (1 µg/kg/min for 4 weeks followed by 0.5 µg/kg/min for another 4 weeks). Two weeks after commencing the AngII infusion, surviving mice were allocated into two groups [intervention (n=12) and control (n=13)] with equivalent median [interquartile range] supra-renal (SRA) diameter of 1.80 [1.60–1.80] mm as assessed by ultrasound. The two groups of mice were subjected to oral gavage administration of either CCF (9446 IU/Kg/week, OsteVit-D®) or vehicle control (0.1% carboxymethyl cellulose; Sigma Aldrich) for 6 weeks. The CCF dose was aimed to increase circulating 25(OH)D levels to ≥75 nmol/l using a published formula [5]: Loading dose of cholecalciferol (IU) = 40 × (75 − baseline circulating 25(OH)D) × body weight]. The average mouse body weight was assumed to be 30 g and the average baseline circulating 25(OH)D from study 2 was used.
All surgical procedures were performed under general anesthesia induced with isoflurane inhalation (4% isoflurane/O2 inhaled) as previously described [11]. At the end of the animal studies, mice were killed by CO2 asphyxiation and relevant samples collected (Supplementary Figure S1).
Assessment of AAA severity
Maximum SRA diameter was measured using ultrasound as previously reported [14]. In study 2, the ultrasound was performed before the diet was introduced (baseline), before AngII infusion (day 0), and at day 14 and 28 after the AngII infusion was commenced. In study 3, ultrasound was performed prior to AngII infusion (baseline) and then every 14 days for 56 days (Supplementary Figure S1). Prior to ultrasound scanning, hair was removed from the abdomen and then mice were sedated by inhalation of 2.5% isoflurane. Mice were then held in a dorsal position and scans were performed using a MyLab™ 70 VETXV platform (Esaote, Italy) with a 40 mm linear transducer at an operating frequency of 12 MHz (LA435; Esaote, Italy) to provide a sagittal image of the SRA. The maximum SRA diameter in the orthogonal plane was measured from outer wall to outer wall at peak systole using an inbuilt caliper feature [14,15].
Maximum diameters of four segments (arch, thoracic aorta, SRA and infra-renal aorta) of the excised aortas were measured using an established protocol [11]. During dissection, phosphate buffered saline (PBS) was perfused through the aortas at physiological pressure for complete removal of blood clots. The aorta was then carefully excised using a dissecting microscope, ensuring complete removal of perivascular fat. The entire aorta along with the brachiocephalic artery was then placed next to a graduated template on a black background and digitally photographed (Coolpix 4500, Nikon). These photographs were used to measure maximum outer to outer orthogonal diameter of the four aortic segments using a computer software program (Adobe® Photoshop® CS5 Extended version 12, Adobe Systems Incorporated). Morphometric analyses were only performed on the aortas of mice that were alive until the completion of the experiment (i.e. ruptured aortas were excluded).
Both ultrasound and morphometric measurements have been found to have excellent inter-observer reproducibility in the investigators prior studies [11,14,15].
Non-invasive tail-cuff blood pressure measurements
Systolic blood pressure (SBP) measurements were performed using a computerized non-invasive tail-cuff system (CODA Monitor, Kent Scientific) [15]. Mice were habituated to the device by handling them on a daily basis for 1 week to minimize restraining disturbance. Mice were allowed to settle for 2 h at room temperature prior to assessment. Excellent reproducibility of this technique has been previously reported [15].
Plasma analyses
Blood collection was performed by tail bleeding during the experiments and at the dissection end-point by cardiac apex puncture. The blood was collected into heparin-coated tubes (BD Microtainer). Plasma was isolated by centrifugation at 6400 × g for 10 min at 4°C and then stored at −80°C until analyzed. For the 25(OH)D assay, plasma was diluted in RPMI Media 1640 (1:3) and sent on dry ice to be assayed at the Centre for Metabolomics (WA, Australia) which has clinical accreditation for this assay [16]. The assay was performed using two-dimensional ultra-performance liquid chromatography (UPLC) separation coupled tandem mass spectrometry (MS/MS) as previously described [16]. The lower detectable limit for 25(OH)D3 was 5.0 nmol/l. Plasma SOST was measured using a Quantikine® enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems; cat#: MSST00) according to the manufacturer’s instructions. Plasma samples required a 2-fold dilution using the calibrator diluent (RD6-12) provided by the manufacturer. All samples and standards were performed in duplicates. Mean intra-assay and inter-assay coefficient of variation (CV) were 5.1% and 5.8%, respectively.
Histological analyses
Segments of harvested SRA were stored in OCT (ProSciTech) and kept at −80°C until assessment. Serially cut 5 μM thick cryostat sections were processed for each of the following histological evaluations: Elastin Van Giessen (EVG) staining and picrosirius red staining. All sections were mounted in Entellan mounting medium (Electron Microscopy Sciences) and digitally photographed using a Nikon Eclipse 50i microscope fitted with a CCD Camera (DSFi1) attached to a PC supported with NIS Elements application (version F2.30). Elastin filament degradation was assessed at 40 × magnifications using a previously reported semi-quantitative grading system as follows: (1) no elastin filament degradation; (2) mild elastin filament distension; (3) moderate-to-severe elastin filament degradation; and (4) severe elastin filament degradation [11]. Collagen content was assessed in sections stained with picrosirius red [11]. The images were captured using bright field and polarization filters integrated into the microscope with identical exposure settings (Zeiss) to reveal collagen by yellow or red birefringence. The percentage of the field area that stained for collagen was determined for 4 fields assessed from four sections per sample; and the mean value was calculated as previously reported [11].
SRA tissue protein assays
SRA segments were cleared of OCT and homogenized in the presence of the radio-immunoprecipitation assay buffer (1 × RIPA buffer; 50 mM Tris-HCl pH 7.4, 150 mM NaCl; 2 mM EDTA, 1% TritonX-100, 0.1% SDS, and 0.1% sodium deoxycholate) supplemented with protease and phosphatase inhibitors (Roche). SOST was quantified using a Quantikine® ELISA (R&D systems). Phosphorylated glycogen synthase kinase-3α and β isoforms (phospho-GSK-3α/β) protein levels were determined using a DuoSet® IC ELISA (R&D systems) following the manufacture’s instruction. All samples and standards were assayed in duplicates with a mean intra-assay CV of 4.7% and 4.9% for SOST and phospho-GSK-3α/β assays, respectively.
Analysis of gene expression by quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from samples stored in RNA-later (Qiagen) using TRIzol reagents (Qiagen) and RNeasy Mini kit (Qiagen). RNA was quantified using a Nanodrop2000. qRT-PCR analyses were performed using the QuantiTect SYBR Green one-step RT-PCR assay (Qiagen) according to the manufacturer’s instructions. RT-PCR reactions were run in duplicate using RotorGeneQ (Qiagen) and analyzed using the Rotor-Gene Q operating software (version 2.0.24). Relative gene expression in each sample was calculated using a concentration-Ct-standard curve and normalized to relative expression of GAPDH as a housekeeping gene. All primers were purchased from commercial suppliers (Sigma Aldrich and Qiagen). A list of primers used in this study is shown in Supplementary Table S2.
VSMC studies
Commercially available human aortic VSMCs were purchased from Lonza (CC-2571). Cells were originally isolated from healthy young donors. All cell culture procedures were performed in a laminar flow hood under sterile conditions. Experiments were performed on cells from passage 6 to 9. Cells were plated into six separate 6-well cultures plates at 1 × 105 cells/ml. About 70–80% confluency was confirmed after 72 h of cell platting. Cells were then synchronized to the G0 phase by draining out the initial media and supplementation of fetal calf serum (FCS) poor media for 12 h. The FCS-poor medium was then drained out and cells were incubated with 1α,25(OH)2D3 (Sigma) at 0, 0.1, 1, 10 and 100 nM for 48 h.
For immunostaining, cells were rinsed with PBS and fixed using 70% methanol for 10 min. For α-smooth muscle actin (SMA) staining, cells were rinsed 3× with PBS and incubated for 20 min at RT with 10% normal goat serum (Vector Laboratory, CA) in PBS to block non-specific binding. Cells were then rinsed twice with PBS and incubated with 1 µg/ml α-SMA antibody in PBS-BSA solution for 1 h at RT. Following three washes with PBS, the cells were incubated with 1:250 (or 4 µ/ml) a fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG (Santa Cruz Biotechnology) for 1 h. The cells were then washed three times with PBS and the coverslips were removed from the plate wells and mounted inverted using ∼10 µl 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) medium. Micrographs were captured using polarization filters integrated into the microscope with identical exposure settings (Zeiss) to reveal cytoskeleton α-SMA filaments (green) and Blue (DAPI staining). Gene expression was assessed by RT-PCR to quantify calponin 1 (CNN1), myosin heavy chain 11 (MYH11), smoothelin (SMTN), caldesmon (CALD1), transgelin (TAGLN), α-SMA (ACTA2), matrix metalloproteinase-2 (MMP-2), the vitamin D receptor (VDR), 25-hydroxyvitamin D-24-hydroxylase (CYP24A1), osteocalcin (OCN), 25-hydroxyvitamin D3 1-alpha-hydroxylase (CYP27B1), osteopontin (OPN), β-catenin (CTNNB1), the angiotensin-II receptor type 1 (AGTR1) and monocyte chemoattractant protein 1 (MCP-1).
Statistical analysis
The D’Agostino and Pearson test was used to test the normality of the data. Results were expressed as median and interquartile range (IQR) for non-normally distributed data and as mean ± SEM for normally distributed data. For non-normally distributed data, comparisons were made using Mann–Whitney U-test or Kruskal–Wallis test followed by Dunn’s multiple comparisons test, where appropriate. Normally distributed data were compared using ANOVA followed by Bonferroni’s multiple comparisons test. Repeated measures were analyzed using by two-way repeated measures ANOVA. Mortality rates due to aortic rupture were compared using the Kaplan–Meier survival curves and log rank tests. Data analyses were performed using GraphPad Prism 5 software (GraphPad Software, Inc., U.S.A.). Differences were considered statistically significant at P<0.05.
Results
1,25(OH)2D3 modulated human aortic VSMC gene expression
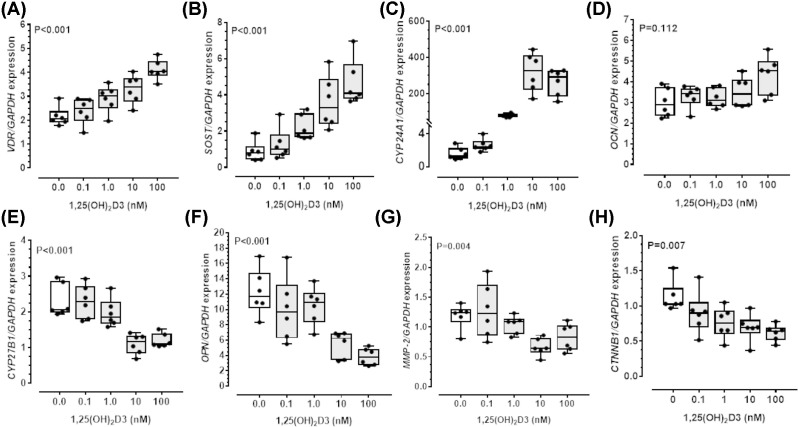
Human aortic VSMC cultures at passage 6-9 expressed α-SMA on immunofluorescence staining (Supplementary Figure S2). Incubating human aortic VSMC with 1,25(OH)2D3 resulted in dose-dependent up-regulation of key VSMCs contractile genes, including CNN1, MYH11, SMTN, CALD1, TAGLN and ACTA2 (Table 1). Incubation of VSMCs with increasing concentrations of 1,25(OH)2D3 also dose-dependently increased gene expression for the VDR, SOST and CYP24A1 but not OCN (Figure 1). In contrast, expression of CYP27B1, OPN, MMP-2, CTNNB1, AGTR1 and MCP-1 were dose-dependently down-regulated by 1,25(OH)2D3 (Figure 1 and Table 1).
Table 1. Relative expression of human aortic VSMCs genes following incubation with 1,25(OH)2D3 over 48 h.
| Gene | 1,25(OH)2D3 Concentration (nM) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.1 | 1.0 | 10 | 100 | P | |
| AGTR1 | 4.32 [3.29–5.43] | 2.35 [1.71–2.68] | 1.45 [0.74–1.77] | 1.82 [1.23–2.23] | 1.68 [0.90–2.28] | 0.002 |
| MCP-1 | 4.06 [3.52–5.34] | 1.49 [1.08–1.68] | 0.69 [0.37–0.86] | 1.16 [0.78–1.66] | 1.33 [0.72–1.65] | <0.001 |
| CNN1 | 0.67 [0.55–0.79] | 0.81 [0.59–0.92] | 0.74 [0.58–0.91] | 0.99 [0.78–1.12] | 1.18 [1.09–1.42] | 0.002 |
| MYH11 | 0.05 [0.04–0.06] | 0.05 [0.04–0.09] | 0.12 [0.11–0.14] | 0.14 [0.12–0.17] | 0.13 [0.11–0.19] | <0.001 |
| SMTLN | 0.95 [0.76–1.14] | 0.80 [0.60–0.98] | 0.82 [0.59–1.22] | 1.55 [1.05–1.74] | 1.58 [1.30–1.70] | 0.002 |
| ACTA2 | 0.41 [0.33–0.52] | 0.53 [0.33–0.84] | 0.74 [0.43–0.80] | 0.89 [0.64–1.41] | 1.20 [0.72–1.41] | 0.007 |
| CALD1 | 0.93 [0.76–0.95] | 0.73 [0.59–0.91] | 0.84 [0.57–1.00] | 1.30 [1.07–1.57] | 1.21 [1.09–1.53] | 0.001 |
| TGLN | 0.76 [0.64-–0.91] | 0.79 [0.55–1.14] | 0.83 [0.60–0.98] | 1.22 [1.14–1.47] | 1.37 [1.35–1.60] | 0.001 |
Data expressed as median (interquartile range) mRNA expression relative to ‘house-keeping’ gene GAPDH for six repeat cultures. P value calculated using Kruskal–Wallis test. Abbreviations: VSMC, vascular smooth muscle cell; AngII, Angiotensin 2; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; AGTR1, angiotensin-II receptor type 1; MCP-1, monocyte chemoattractant protein 1; CNN1, calponin 1; MYH11, myosin heavy chain 11; SMTLN, smoothelin; ACTA2, α-smooth muscle actin; CALD1, caldesmon 1; TGLN, transgelin.
Figure 1. 1,25(OH)2D3 up-regulated SOST and modified VSMC gene expression.
Human aortic vascular smooth muscle cells (VSMC) were incubated with increasing concentration of 1,25-dihydroxyvitamin D3 [1,25 (OH)2D3; 0, 0.1, 1, 10, 100 nmol/l] over 48 h. Up-regulation in gene expression for vitamin D receptor (VDR), sclerostin (SOST) and 25-hydroxyvitamin D-24-hydroxylase (CYP24A1), not osteocalcin (OCN) (A–D). Dose-dependent down-regulation in gene expression for 25-hydroxyvitamin D3 1-alpha-hydroxylase (CYP27B1), osteopontin (OPN), matrix metalloproteinase-2 (MMP-2) and β-catenin (CTNNB1) (E–H). Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for expression relative to GAPDH (n = 6/group); P-value calculated by Kruskal–Wallis test.
Circulating levels of 25(OH)D and SOST were decreased by dietary restriction of CCF in ApoE−/− mice
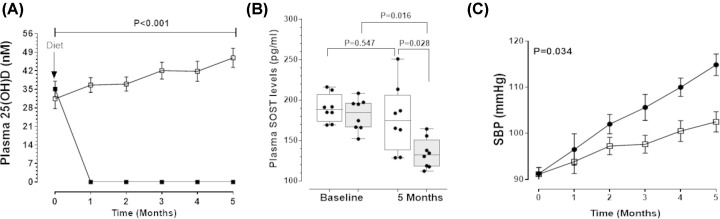
The first animal experiment investigated whether VDD diet reduced plasma concentrations of 25(OH)D and SOST in ApoE−/− mice (Supplementary Figure S1). At baseline, there was no difference in the plasma concentrations of 25(OH)D between groups (Figure 2A). After one month, the median [interquartile range (IQR)] plasma concentration of 25(OH)D was 35.45 [31.11–38.47] nmol/l in mice allocated the VDS control diet, whereas the plasma concentration of 25(OH)D was no longer measurable in mice receiving the VDD diet (Figure 2A). Circulating SOST concentrations did not change significantly during the experiment in mice receiving the VDS diet (Figure 2B). At 5 months, plasma SOST was significantly lower in mice given the VDD diet compared with baseline and to mice receiving the VDS diet (Figure 2B). Mice that received the VDD diet had significantly higher SBP than those given the VDS diet (Figure 2C). Relative gene and protein expression were examined in SRA segments recovered from mice completing this experiment. Mice receiving the VDD diet had significantly lower aortic expression of Sost and higher expression of Opn than controls (Table 2). Median protein concentration of SOST within the SRA was less in mice receiving the VDD diet than those given the VDS diet (Table 3). In contrast, relative concentrations of p-GSK-3α/β protein, was higher within the SRA of mice receiving the VDD diet compared with those given the VDS diet (Table 3). The SRA expression of Cyp27b1, Ocn and Ctnnb1 was higher in mice fed the VDD diet than controls (Table 2), while expression of osteoprotegerin (Opg), Vdr and Dickkopf Wnt signalling pathway inhibitor 1 (Dkk-1) did not differ significantly between the groups. Additionally, SRA expression of the AGTR1 and MCP-1 were significantly greater in the VDD group compared with the VDS mice (Table 2).
Figure 2. Vitamin D deficiency was associated with decreased circulating SOST and elevated blood pressure in ApoE−/− mice.
(A) Depleted circulating 25-hydroxyivtamin D [25(OH)D] after one month in ApoE−/-mice fed vitamin D-deficient diet (VDD; solid square) compared with mice fed vitamin D-sufficient diet (VDS; open square). Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for concentration (nmol/l). P-value calculated for difference between groups by two-way ANOVA. (B) Plasma sclerostin (SOST) concentrations were significantly lower in mice fed VDD (gray) compared with VDS diet (white) after 5 months. Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for concentration (pg/ml). P-value for inter-group comparison was calculated using Mann–Whitney U-test and intra-group comparison was calculated using Wilcoxon paired test. (C) Significantly higher systolic blood pressure (SBP) maintained over 5 months in mice fed VDD diet (solid square) compared with mice receiving VDS diet (open square). Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for pressure (mmHg). P-value calculated for difference between groups by two-way repeated measures ANOVA; n = 8 mice/group.
Table 2. Effect of dietary restriction or supplementation of CCF on gene and protein expression and structure of the suprarenal aorta of ApoE−/− mice.
| Study 1: Dietary restriction of CCF | ||||
|---|---|---|---|---|
| Protein/Gene | VDS (n=8) | VDD (n=8) | Expression | P |
| Sost | 1.32 [1.06–2.02] | 0.14 [0.07–0.30] | ↓ | <0.001 |
| Opg | 0.07 [0.07–0.08] | 0.08 [0.06–0.08] | ↔ | 0.863 |
| Opn | 1.25 [0.76–1.81] | 4.67 [2.97–8.26] | ↑ | <0.001 |
| Vdr | 0.42 [0.26–0.89] | 0.53 [0.18–0.91] | ↔ | 0.959 |
| Cyp27b1 | 0.35 [0.24–0.44] | 0.55 [0.47–0.77] | ↑ | 0.001 |
| Dkk-1 | 0.47 [0.32–0.66] | 0.24 [0.17–0.40] | ↔ | 0.065 |
| Ocn | 0.71 [0.44–1.07] | 1.10 [0.91–1.31] | ↑ | 0.028 |
| Ctnnb1 | 0.69 [0.33–0.99] | 1.11 [1.02–1.26] | ↑ | 0.007 |
| Agtr1 | 0.59 [0.37–0.80] | 0.83 [0.70–0.97] | ↑ | 0.038 |
| Mcp-1 | 0.16 [0.13–0.26] | 0.39 [0.30–0.47] | ↑ | 0.001 |
| Study 2: Dietary restriction of CCF during AngII-infusion | ||||
|---|---|---|---|---|
| Gene | VDS (n=8) | VDD (n=7) | Expression | P |
| Sost | 2.25 [1.27–4.11] | 1.22 [0.99–1.35] | ↓ | 0.029 |
| Opg | 2.49 [0.82–3.73] | 2.01 [0.74–4.44] | ↔ | 1.000 |
| Opn | 8.35 [6.08–19.36] | 29.51 [9.05–59.09] | ↑ | 0.040 |
| Mmp-9 | 1.45 [0.28–3.76] | 13.95 [9.07–21.98] | ↑ | 0.001 |
| Mmp-2 | 0.62 [0.09–1.82] | 6.54 [1.61–9.38] | ↑ | 0.021 |
| Vdr | 0.71 [0.29–1.32] | 0.30 [0.12–0.58] | ↔ | 0.152 |
| Cyp27b1 | 0.81 [0.46–1.87] | 2.40 [1.72–3.51] | ↑ | 0.009 |
| Dkk-1 | 0.91 [0.44–2.07] | 0.46 [0.36–0.97] | ↔ | 0.281 |
| Ocn | 0.93 [0.53–1.84] | 2.35 [1.39–3.43] | ↑ | 0.029 |
| Ctnnb1 | 1.60 [0.60–4.37] | 4.35 [3.11–8.84] | ↑ | 0.040 |
| Agtr1 | 0.71 [0.35–0.88] | 1.13 [0.92–1.84] | ↑ | 0.004 |
| Mcp-1 | 0.30 [0.23–0.45] | 0.52 [0.43–0.75] | ↑ | 0.029 |
| Study 3: CCF supplementation during AngII-infusion | ||||
|---|---|---|---|---|
| Gene | Control (n=7) | CCF (n=11) | Expression | P |
| Sost | 1.05 [0.55–1.91] | 2.71 [1.53–5.97] | ↑ | 0.035 |
| Opg | 1.10 [0.97–1.49] | 1.19 [0.95–1.46] | ↔ | 0.724 |
| Opn | 57.96 [35.14–82.32] | 24.07 [13.68–48.24] | ↓ | 0.035 |
| Mmp-9 | 12.49 [8.43–34.06] | 7.36 [5.05–9.20] | ↓ | 0.020 |
| Mmp-2 | 5.47 [2.94–13.68] | 2.27 [1.86–3.56] | ↓ | 0.035 |
| Vdr | 0.72 [0.21–0.97] | 1.30 [0.75–2.23] | ↑ | 0.027 |
| Cyp27b1 | 1.33 [1.27–2.41] | 0.77 [0.43–1.04] | ↓ | <0.001 |
| Dkk-1 | 0.76 [0.51–0.95] | 1.81 [1.16–2.27] | ↑ | <0.001 |
| Ocn | 1.17 [0.97–1.88] | 1.22 [0.97–2.57] | ↔ | 0.930 |
| Ctnnb1 | 4.59 [3.66–8.28] | 2.12 [1.79–4.02] | ↓ | 0.015 |
| Agtr1 | 1.95 [1.39–2.44] | 0.76 [0.28–1.09] | ↓ | 0.002 |
| Mcp-1 | 1.05 [0.86–1.46] | 0.85 [0.51–0.99] | ↓ | 0.035 |
Shown are relative gene expression as median (inter-quartile range) relative to glyceraldehyde 3-phosphate dehydrogenase (Gapdh) expression. In all studies, samples were randomly selected using random generated numbers. Inter-group comparison was analyzed using Mann–Whitney U-test. Abbreviations: CCF, cholecalciferol; Sost, sclerostin; Opg, osteoprotegerin; Opn, osteopontin; Vdr, vitamin D receptor; Cyp27b1, gene encoding for 25-hydroxvitamin D3 hydrolase; Dkk, Wnt inhibitor Dickkopf-1; Ocn, osteocalcin (bone-Gla matrix); Ctnnb1, gene encoding for β-catenin; Mmp-2/9, matrix metalloproteinase 2/9; Agtr1, angiotensin-II receptor 1; Mcp-1, monocyte chemoattractant protein-1.
Table 3. Effect of dietary restriction or supplementation of CCF on SRA protein expression and histopathological structure.
| Study 1: Dietary restriction of CCF | ||||
|---|---|---|---|---|
| Protein | VDS (n=8) | VDD (n=8) | Expression | P |
| SOST (pg/ml) | 215.3 [135.6–230.6] | 125.4 [84.8–152.8] | ↓ | 0.015 |
| p-GSK-3α/β (pg/ml) | 6.4 [0–17.7] | 46.0 [29.8–56.7] | ↑ | 0.001 |
| Study 2: Dietary restriction of CCF during AngII-infusion | ||||
|---|---|---|---|---|
| Protein | VDS (n=8) | VDD (n=7) | Expression | P |
| SOST (pg/ml) | 123.8 [91.8–226.4] | 75.3 [46.5–98.3] | ↓ | 0.028 |
| p-GSK-3α/β (pg/ml) | 39.5 [11.0–51.7] | 72.7 [65.1–108.9] | ↑ | <0.001 |
| Study 3: CCF supplementation during AngII-infusion | ||||
|---|---|---|---|---|
| Gene | Control (n=7) | CCF (n=11) | Expression | P |
| SOST (pg/ml) | 230.3 [154.1–290.9] | 420.1 [240.8–482.8] | ↑ | 0.012 |
| p-GSK-3α/β (pg/ml) | 92.2 [71.1–133.3] | 40.2 [19.1–57.2] | ↓ | 0.003 |
| Picrosirius red | 31.7 [21.9–64.0] | 70.4 [52.3–78.9] | ↑ | 0.015 |
| EVG | 2 [1–3] | 3 [3–4] | ↑ | 0.033 |
Shown are protein expression as median (inter-quartile range) measured from homogenized SRA tissue. Quantikine ELISA was used to measure SRA SOST protein and p-GSK-3α/β was measured using sandwiched ELISA. Histopathological assessments in study 2 and 3 were performed to assess SRA collagen content (picrosirius red); elastin degradation (EVG); monocytes/macrophages infiltration (MOMA-2) and smooth muscle marker by α- SMA antibody. Samples were randomly selected using random generated numbers. Inter-group comparison was analyzed using Mann–Whitney U-test. Representative images for all histopathology assessments are provided in Figure 5. Abbreviations: CCF, cholecalciferol; SRA, suprarenal aorta, SOST, sclerostin; p-GSK-3α/β, phosphorylated glycogen synthase kinase-3α and β isoforms; EVG, elastin Verhoeff-van Gieson.
Dietary restriction of CCF promoted development of larger rupture-prone AAAs in the AngII-infused ApoE−/− mouse model
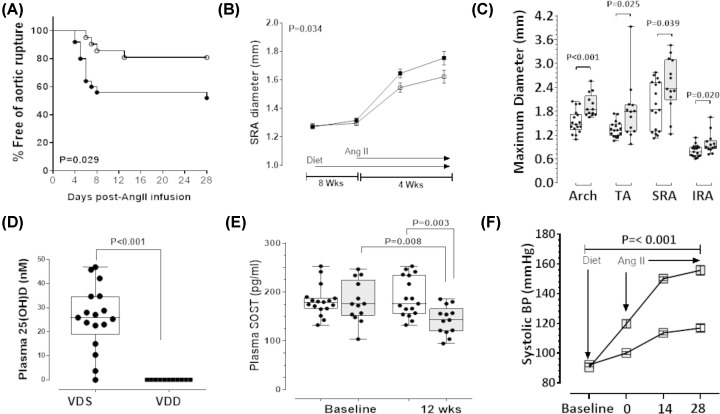
Mice received VDD or VDS diets for 8 weeks and then AAA was induced by AngII infusion for a period of 4 weeks during which time the diets were continued (Supplementary Figure S1). In mice allocated VDD diet (n=25), a total of 12 (48%) experienced aortic rupture [aortic arch rupture in four mice and SRA rupture in eight mice]. In mice receiving VDS diet (n=21), 4 (19%) experienced aortic rupture [aortic arch rupture in two mice and SRA rupture in two mice]. By Kaplan–Meier analysis and log rank test, the incidence of aortic rupture was greater in mice allocated VDD diet than those receiving VDS diet (Figure 3A). The maximum diameter of the SRA was measured in vivo using ultrasound. Mice administered the VDD diet exhibited a greater rate of SRA expansion during the Ang II infusion compared with VDS diet administered control mice (Figure 3B). Mice fed VDD diet that reached the end of the experimental period had larger maximum SRA diameter than those receiving VDS diet when measured by morphometry (Supplementary Figure S3; Figure 3C).
Figure 3. Vitamin D deficiency promoted larger rupture prone AAAs in AngII-infused ApoE−/− mice.
The 28-day infusion period for AngII commenced eight weeks into the 12-week diet period. (A) Kaplan–Meier curves illustrating survival data for mice fed VDD diet (solid circle) compared with VDS diet (open circle); P-value calculated using Log-rank (Mantel-Cox) test. (B) Ultrasound measurement of maximum in vivo suprarenal aortic (SRA) diameter in mice receiving VDD diet (solid square) compared with VDS diet (open square) over the 12-week diet period. Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for diameter (mm); P-value calculated for difference between groups by two-way ANOVA based on the interaction between time and dietary allocation. (C) Maximum ex vivo SRA diameter at the end of the study in mice receiving VDD diet compared with VDS diet. Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for diameter (mm). (D) Endpoint circulating 25-hydroxyvitamin D [25(OH)D] concentrations in mice fed VDD versus VDS diet. Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for concentration (nmol/l). (E) Plasma SOST concentrations in mice fed VDD (gray) compared with VDS diet (white) after 12 weeks. Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for concentration (pg/ml). P-value calculated for difference between groups by Mann–Whitney U-test and within groups by Wilcoxon paired test. (F) Systolic blood pressure (SBP) in mice receiving VDD diet (solid square) compared with VDS diet (open square) over the 12-week diet period. Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for pressure (mmHg). After 8 weeks on the VDS diet (n=21) or VDD diet (n=25), mice commenced subcutaneous AngII (1 µg/kg/min) infusion for 28 days while continuing on their allocated diets. In mice allocated VDD diet (n=25), a total of 12 (48%) experienced aortic rupture before completing the study. In mice receiving VDS diet (n=21), 4 (19%) experienced aortic rupture before completing the study. P-value calculated for difference between groups by two way repeated measures ANOVA.
Dietary restriction of CCF was associated with lower circulating SOST and higher blood pressure
At the end of the second experiment, median [IQR] plasma concentrations of 25(OH)D in mice receiving VDS diet was 25.90 [18.80–34.75] nmol/l, but was not detectable in VDD diet-fed mice (Figure 3D). At the same time point, plasma SOST was significantly lower in mice administered the VDD diet compared with baseline and to mice receiving the VDS diet (Figure 3E). Furthermore, as in study 1, mice that were fed the VDD diet had significantly higher SBP compared with those fed the VDS diet (Figure 3F). Relative gene expression analysis performed on SRA segments recovered from surviving mice showed that mice receiving the VDD diet had significantly lower expression of Sost, and significantly higher expression of Cyp27b1, Opn, Ocn, Ctnnb1, Mmp-2 and Mmp-9 than controls (Table 2). There was no difference between groups in the expression of Opg, Vdr or Dkk-1. Median protein concentration of SOST within the SRA was significantly less in mice receiving the VDD diet than those receiving VDS diet (Table 3). Protein expression of p-GSK-3α/β was higher within the SRA of mice receiving the VDD diet compared with the VDS diet (Table 3). Finally, gene expression of AGTR1 and MCP-1 were significantly upregulated in the SRA of the VDD group compared with VDS mice (Table 2).
CCF administration increased circulating levels of 25(OH)D and SOST in AngII infused ApoE−/− mice with established AAAs
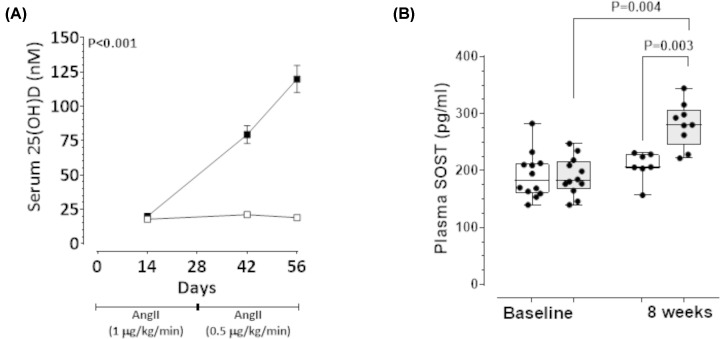
The efficacy of CCF to limit progression of pre-established AAA was examined in a third animal study. Previous human studies have shown AAA patients have lower 25(OH)D concentrations but they are not usually within the deficient range suggesting that high circulating 25(OH)D might protect against AAA [6,7]. Therefore unlike animal studies 1 and 2, in study 3 the effect of up-titrating circulating 25(OH)D concentrations to >75 nmol/l was studied. Firstly, AAAs were established in 32 mice by infusion of AngII during which time 7 mice died from aortic rupture and were excluded. Two weeks after commencing AngII infusion, mice were allocated to receive CCF by oral gavage at a dose aimed to up-titrate circulating 25(OH)D concentrations to >75 nmol/l (9446 IU/kg/week; n=12) or vehicle control (0.1% carboxymethyl cellulose oral gavage; n=13) groups. Both groups received a VDS diet. The experiment continued for a further 6 weeks during which time AngII infusion was continued. Baseline median [IQR] plasma 25(OH)D was similar in both groups (CCF: 17.26 [13.47–21.52 nmol/l] vs control: 19.61 [16.57–23.15] nmol/l; P=0.242; Figure 4A). Mice receiving CCF exhibited a time-dependent increase in plasma levels of 25(OH)D at concentrations significantly higher than controls (Figure 4A). Median [IQR] plasma SOST was similar in both groups at baseline (CCF: 182.80 [167.70–216.30] pg/ml vs control: 182.40 [160.80–212.30] pg/ml; P=0.832; Figure 4B]. At the end of the 8-week experiment, median plasma SOST was significantly higher in mice administered CCF compared with baseline and with control mice (Figure 4B). SRA protein concentration of SOST was also higher in mice receiving CCF compared with controls (Table 3). In contrast, SRA concentration of p-GSK-3α/β was lower within the CCF group compared with the control group (Table 3).
Figure 4. Cholecalciferol (CCF) supplementation increased circulating levels of 25(OH)D and SOST in AngII-infused ApoE−/− mice.
CCF (9446 IU/kg) or control (0.1 carboxymethyl cellulose) administered weekly by oral gavage, starting 14 days after commencement of AngII infusion. (A) Plasma 25(OH)D at baseline (14 days after commencing AngII infusion), 4 and 6 weeks of supplementation (endpoint) compared between CCF (solid square) and control groups (open square). Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for concentration (nmol/L); P-value calculated for difference between groups by two way repeated measures ANOVA. (B) Plasma SOST concentrations in CCF-supplemented mice (gray) compared with controls (white) after 8 weeks. AAA was initially induced in 32 mice by infusion of AngII (1 µg/kg/min for 4 weeks followed by 0.5 µg/kg/min for another 4 weeks). Two weeks after commencing the AngII infusion, surviving mice were allocated into two groups [intervention (n=12) and control (n=13)] with equivalent median [interquartile range] supra-renal (SRA) diameter 1.80 [1.60–1.80] mm as assessed by ultrasound. Six of 13 control mice (46%) exhibited aortic rupture compared with one of 12 mice (8%) administered CCF prior to competing the experiment. Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for concentration (pg/ml). P-value calculated for difference between groups by Mann–Whitney U-test and within group comparisons by Wilcoxon paired test.
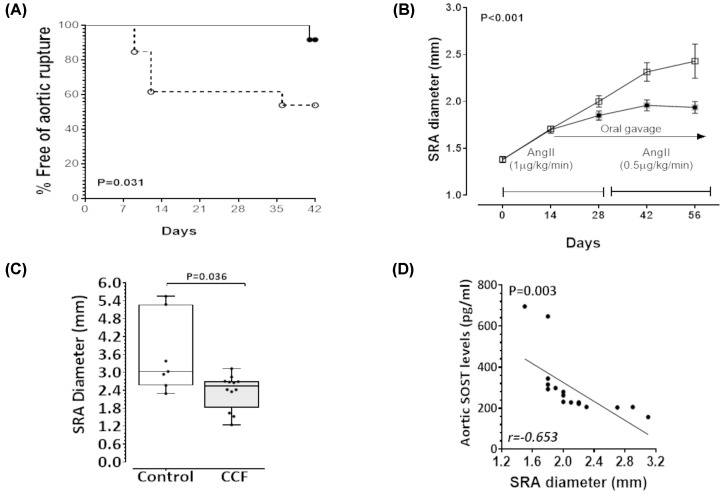
CCF limited growth and rupture of pre-established AAAs in AngII-infused ApoE−/− mice
Supplementation with CCF significantly limited rupture of established AAA in the AngII-infused ApoE−/− mice (Figure 5A). Six of 13 control mice (46%) exhibited aortic rupture compared with one of 12 mice (8%) administered CCF (Figure 5A). SRA dilatation was monitored fortnightly by ultrasound. A time-dependent increase in maximum SRA diameter in response to AngII was found (Figure 5B). Mice given CCF exhibited significantly smaller increases in SRA diameter compared with controls (Figure 5B). Morphometric analysis of aortas at completion of the study (excluding ruptures) confirmed significantly smaller maximum SRA diameter in mice receiving CCF compared with controls (Figure 5C and Supplementary Figure S4). An inverse correlation was demonstrated between circulating SOST concentrations and maximum SRA diameter measured by ultrasound after 56 days (r = −0.653, P=0.003; Figure 5D). Relative gene expression analysis of SRA segments from surviving mice showed significantly higher expression of Sost, Vdr and Dkk-1 and significantly lower expression of Cyp27b1, Opn, Ctnnb1, Mmp-2 and Mmp-9 in the CCF group compared with the control group (Table 2). There was no significant difference in Opg and Ocn expression between the groups. SRA expression of Agtr1 and Mcp-1 were down-regulated in the CCF group compared with control mice (Table 2). Additionally, histological and histopathological assessments of the SRA sections showed that mice receiving CCF had higher collagen and less elastin fragmentation (Table 3 and Supplementary Figure S5).
Figure 5. Cholecalciferol (CCF) supplementation reduced growth and rupture of established AAAs.
(A) Kaplan–Meier curves illustrating survival data for mice supplemented with CCF (solid circle) compared with control (open circle); P-value calculated using Log rank test. (B) Ultrasound measurement of maximum SRA diameter in mice administered CCF over 6 weeks, starting 14 days after commencement of AngII infusion (solid square) compared with control (open square). Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for diameter (mm); P-value calculated for difference between groups by two way repeated measures ANOVA. (C) Maximum SRA diameter at completion of the study determined by morphometry in mice administered CCF compared with control. Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for diameter (mm). (D) There was an inverse correlation between plasma SOST and AAA diameter measured by ultrasound at the end of the experiment (r = −0.653). AAA was initially induced in 32 mice by infusion of AngII (1 µg/kg/min for 4 weeks followed by 0.5 µg/kg/min for another 4 weeks). Two weeks after commencing the AngII infusion, surviving mice were allocated into two groups [intervention (n=12) and control (n=13)] with equivalent median [interquartile range] supra-renal (SRA) diameter 1.80 [1.60–1.80] mm as assessed by ultrasound. Six of 13 control mice (46%) exhibited aortic rupture compared with one of 12 mice (8%) administered CCF prior to competing the experiment.
Discussion
The main findings of the present study were that within the AngII induced mouse model, diet-induced vitamin D deficiency promoted development of large AAAs which were prone to rupture. CCF supplementation to achieve high circulating concentrations of 25(OH)D limited growth and rupture of pre-established AAAs. These effects appeared to be linked to changes in circulating and aortic expression of SOST, along with effects on the Wnt/β-catenin signaling pathway and VSMC gene expression within the aorta. These findings support those from a previous study, which suggested that transgenic up-regulation and parenteral administration of SOST protected against AAA development in the same mouse model through competitive inhibition of the Wnt/β-catenin signaling pathway [11].
Guidelines recommend that circulating 25(OH)D concentrations of >75 nmol/l are optimal [5]. In a previous community screening study in older men, it was reported that men with plasma concentrations of 25(OH)D in the lowest quartile were more than five times as likely to have an AAA measuring >40 mm compared with men with 25(OH)D concentrations in the upper quartile [7]. In keeping with those clinical findings in the present study, ApoE−/− mice with no detectable plasma 25(OH)D were predisposed to development of large AAA which were more likely to rupture during AngII infusion. While AAA diagnosis has been associated with lower circulating concentrations of 25(OH)D, most patients that develop AAA do not have 25(OH)D concentrations within the deficient range of <50 nmol/l [6,7]. In the present study raising 25(OH)D concentrations to >75 nmol/l limited growth and rupture of pre-established AAA within the AngII mouse model. Supplementation of mice with CCF was associated with up-regulation of the aortic expression of the VDR and down-regulation of CYP27B1. Findings were similar within an in vitro experiment involving VSMCs in which incubation of cells with 1,25(OH)2D3 led to a dose-dependent upregulation of VDR and CYP24A1 while CYP27B1 was dose-dependently down-regulated.
Incubation of osteoblasts with 1,25(OH)2D3 has been reported to induce SOST expression [13]. Ergocalciferol administration has previously been reported to increase circulating SOST concentrations in patients with osteoporosis [17]. In the present study, incubation of VSMCs with 1,25(OH)2D3 led to a dose-dependent increase in SOST expression. In the mouse experiments, a VDD diet led to a reduction in plasma and aortic SOST while supplementation with CCF increased plasma and aortic SOST. Aortic concentrations of p-GSK-3α/β, which is indicative of Wnt/β-catenin signaling activity, were also lower in mice receiving CCF. Phosphorylation of GSK-3α/β leads to stabilization of the β-catenin complex and consequent translocation to the nucleus. The Wnt/β-catenin signaling pathway has been implicated in stimulating the activation of an inflammatory cascade in a number of autoimmune diseases [18]. In the present study CCF administration up-regulated aortic expression of Dkk-1, another Wnt pathway inhibitor [18]. In line with these findings, a VDD diet led to increased aortic expression of the Wnt pathway-signaling gene Ctnnb1 whereas CCF supplementation led to decreased aortic expression of Ctnnb1. In vitro experiments showed that CTNNB1 was dose-dependently down-regulated in response to 1,25(OH)2D3 administration, suggesting inhibition of Wnt/β-catenin signaling. This was associated with down-regulation of AGTR1 and MCP-1 as well as up-regulation of key contractile markers in VSMCs. These responses were plausibly attributable to up-regulation in SOST expression. It was previously reported that SOST up-regulation in a mouse model reduced phosphorylation of GSK-3β and consequent β-catenin accumulation in SRA tissue [11]. The present study suggests that CCF supplementation up-regulates SOST and inhibits experimental AAA progression by inhibiting Wnt/βcatenin signaling.
Degradation of the extracellular matrix has been strongly implicated in AAA pathogenesis. In the present study, mice administered a VDD diet had greater aortic expression of Mmp-2 and Mmp-9 compared with controls. Similarly, supplementation with CCF in mice with established AAA led to down-regulation of aortic Mmp-2 and Mmp-9 expression compared with controls. Taken together, these findings suggest that CCF supplementation limited proteolytic degradation within the aorta. These findings are consistent with those of a previous publication in which it was reported that parenteral administration of 1,25(OH)2D3 (1 μg/kg) down-regulated aortic Mmp-2 and Mmp-9 and up-regulated endogenous tissue inhibitor of Mmp-1 expression in AngII-infused ApoE−/− mice [3]. VDR activation has also been reported to down-regulate MMP-2 and MMP-9 activity and expression in cultured human VSMC [19]. OPN, while initially described as a bone protein, has been strongly implicated in AAA [12,20]. It has previously been reported that plasma concentrations of OPN are increased in patients with AAA and positively correlated with AAA growth rates [12]. Within the AngII mouse model, it has been reported that OPN deficiency protects against AAA formation [21]. Findings from the present study suggest that a VDD diet up-regulated aortic expression of Opn, while supplementation with CCF down-regulated Opn in comparison with controls. In vitro incubation of VSMC with 1,25(OH)2D3 dose-dependently down-regulated OPN expression. The effect of CCF supplementation on MMP-2, MMP-9 and OPN may, at least in part, explain its ability to limit AAA progression.
Hypertension is a risk factor for AAA diagnosis and rupture [20,22]. SBP has previously been reported to rise in mice receiving AngII-infusion although it is not thought to be responsible for the induction of AAA in this model [23]. A VDD diet promoted higher SBP in ApoE−/− mice. Vitamin D has been reported to suppress renin expression [24,25] and administration of CCF (25000 IU/week) has been reported to attenuate hypertension in patients [25]. AngII is not thought to induce AAA via increases in SBP and therefore it is unlikely that the link between vitamin D and SBP is responsible for the findings in the present study [23].
A number of strengths and limitations of the present study should be acknowledged. Strengths include the use of multiple mouse experiments designed to examine the link between vitamin D and AAA, in addition to an in vitro study. Most previous rodent AAA studies have focused on the ability of interventions to limit AAA development; however, in the present study the ability of CCF supplementation to limit growth of established AAAs was also investigated. The design of animal experiment 3 aimed to simulate a clinically relevant scenario where patients present with small established AAAs. The loading dose of CCF required to rapidly achieve >75 nmol/l was estimated using a formula proposed by van Groningen et al. [5]. Furthermore, circulating 25(OH)D were measured using a highly sensitive mass spectrometry assay in a validated laboratory. The study was, however, limited to investigating the AngII-infused ApoE−/− mouse model and human VSMCs. It is possible that other vascular cells might respond to 1,25(OH)2D3 differently. Furthermore, analysis of tissue samples was limited to RT-PCR and ELISAs. Insufficient samples were available for other analyses such as zymography or Western blotting. Finally, insufficient samples were available for assessment of serum calcium concentrations or tissue Von Kossa staining.
In conclusion, the present study suggests that within a mouse model, dietary vitamin D deficiency promotes formation of larger AAAs that were more prone to rupture. CCF supplementation limited growth and rupture of established AAAs within the same model. Vitamin D appears to act via changes in SOST, the Wnt pathway, OPN and other phenotypic changes in VSMCs to limit extracellular matrix degradation promoted by MMP-2 and MMP-9. Further research is needed to examine the ability to translate these findings to AAA patients.
Clinical perspectives
Previous studies suggest that people with abdominal aortic aneurysm are more likely to have vitamin D insufficiency.
The present study demonstrated that mice rendered vitamin D deficient developed larger aneurysms that were more likely to rupture than controls. Administration of cholecalciferol to mice with already formed aneurysms improved vitamin D status, limited the progressive increase in aneurysm size and reduced the number of ruptures. These effects appeared to be driven by the ability of vitamin D to modify a range of vascular smooth muscle cell genes, such as sclerostin, implicated in controlling extracellular matrix remodeling.
The findings suggest that optimizing levels of vitamin D could limit AAA progression.
Supplementary Material
Acknowledgements
All data has been included in the paper and supplement. Requests to use data should be made to the corresponding author.
Abbreviations
- AAA
abdominal aortic aneurysm
- AngII
angiotensin-II
- CCF
cholecalciferol
- SOST
suprarenal aorta
- SRA
sclerostin
- VSMC
vascular smooth muscle cell
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work is funded in part by grants from the National Health and Medical Research Council [grant numbers 1098717 and 1079193]. J. Golledge holds a Practitioner Fellowship from the National Health and Medical Research Council, Australia [grant number 1117061], and a Senior Clinical Research Fellowship from the Queensland Government. V. Nsengiyumva was supported by the Research Training Scheme (formerly known as Australian Post-Graduate Award) and JCU College of Medicine Dentistry Funding. The funding bodies played no role in generation of the data presented in this publication.
Open Access
Open access for this article was enabled by the participation of James Cook University in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with CAUL.
Author Contribution
Intellectual ideas and experimental design: J.G., S.W.S., S.K., V.N. Experimental procedures: V.N., S.K., C.S.M., S.K.M., M.W.C. Statistical analysis: V.N., J.V.M., S.K.M., J.G. Manuscript writing: V.N., J.G. Manuscript editing and revisions: All authors.
References
- 1.Sampson U.K.A., Norman P.E., Fowkes F.G.R., Aboyans V., Song Y., Harrell F.E. Jr et al. (2014) Estimation of Global and Regional Incidence and Prevalence of Abdominal Aortic Aneurysms 1990 to 2010. Global Heart 9, 159–170 10.1016/j.gheart.2013.12.009 [DOI] [PubMed] [Google Scholar]
- 2.Golledge J. and Norman P.E. (2011) Current status of medical management for abdominal aortic aneurysm. Atherosclerosis 217, 57–63 10.1016/j.atherosclerosis.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 3.Martorell S., Hueso L., Gonzalez-Navarro H., Collado A., Sanz M.J. and Piqueras L. (2016) Vitamin D receptor activation reduces angiotensin-ii-induced dissecting abdominal aortic aneurysm in apolipoprotein E-knockout mice. Arterioscler. Thromb. Vasc. Biol. 36, 1587–1597 10.1161/ATVBAHA.116.307530 [DOI] [PubMed] [Google Scholar]
- 4.Nieuwland A.J., Kokje V.B., Koning O.H., Hamming J.F., Szuhai K., Claas F.H. et al. (2016) Activation of the vitamin D receptor selectively interferes with calcineurin-mediated inflammation: a clinical evaluation in the abdominal aortic aneurysm. Lab. Invest. 96, 784–790 10.1038/labinvest.2016.55 [DOI] [PubMed] [Google Scholar]
- 5.van Groningen L., Opdenoordt S., van Sorge A., Telting D., Giesen A. and de Boer H. (2010) Cholecalciferol loading dose guideline for vitamin D-deficient adults. Eur. J. Endocrinol. 162, 805–811 10.1530/EJE-09-0932 [DOI] [PubMed] [Google Scholar]
- 6.Takagi H., Umemoto T. and Alice (All-Literature Investigation of Cardiovascular Evidence) group (2017) Vitamins and abdominal aortic aneurysm. Int. Angiol. 36, 21–30 [DOI] [PubMed] [Google Scholar]
- 7.Wong Y.Y., Flicker L., Yeap B.B., McCaul K.A., Hankey G.J. and Norman P.E. (2013) Is hypovitaminosis D associated with abdominal aortic aneurysm, and is there a dose-response relationship? Eur. J. Vasc. Endovasc. Surg. 45, 657–664 10.1016/j.ejvs.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 8.Norman P.E., Wysocki S.J. and Lamawansa M.D. (1995) The role of vitamin D3 in the aetiology of abdominal aortic aneurysms. Med. Hypotheses 45, 17–20 10.1016/0306-9877(95)90193-0 [DOI] [PubMed] [Google Scholar]
- 9.Christakos S., Dhawan P., Verstuyf A., Verlinden L. and Carmeliet G. (2016) Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 96, 365–408 10.1152/physrev.00014.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moran C.S., McCann M., Karan M., Norman P, Ketheesan N. and Golledge J. (2005) Association of osteoprotegerin with human abdominal aortic aneurysm progression. Circulation 111, 3119–3125 10.1161/CIRCULATIONAHA.104.464727 [DOI] [PubMed] [Google Scholar]
- 11.Krishna S.M., Seto S.W., Jose R.J., Li J., Morton S.K., Biros E. et al. (2017) Wnt signaling pathway inhibitor sclerostin inhibits angiotensin II-Induced aortic aneurysm and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 37, 553–566 10.1161/ATVBAHA.116.308723 [DOI] [PubMed] [Google Scholar]
- 12.Golledge J., Muller J., Shephard N., Clancy P., Smallwood L., Moran C. et al. (2007) Association between osteopontin and human abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 27, 655–660 10.1161/01.ATV.0000255560.49503.4e [DOI] [PubMed] [Google Scholar]
- 13.Wijenayaka A.R., Yang D., Prideaux M., Ito N., Kogawa M., Anderson P.H. et al. (2015) 1α,25-dihydroxyvitamin D3 stimulates human SOST gene expression and sclerostin secretion. Mol. Cell. Endocrinol. 413, 157–167 10.1016/j.mce.2015.06.021 [DOI] [PubMed] [Google Scholar]
- 14.Moran C.S., Seto S.W., Biros E., Krishna S.M., Morton S.K., Kleinschnitz C. et al. (2020) Factor XII blockade inhibits aortic dilatation in angiotensin II-infused apolipoprotein E-deficient mice. Clin. Sci. (Lond.) 134, 1049–1061 10.1042/CS20191020 [DOI] [PubMed] [Google Scholar]
- 15.Seto S.W., Krishna S.M., Moran C.S., Liu D. and Golledge J. (2014) Aliskiren limits abdominal aortic aneurysm, ventricular hypertrophy and atherosclerosis in an apolipoprotein-E-deficient mouse model. Clin. Sci. (Lond.) 127, 123–134 10.1042/CS20130382 [DOI] [PubMed] [Google Scholar]
- 16.Clarke M.W., Tuckey R.C., Gorman S., Holt B. and Hart P.H. (2013) Optimized 25-hydroxyvitamin D analysis using liquid–liquid extraction with 2D separation with LC/MS/MS detection, provides superior precision compared to conventional assays. Metabolomics 9, 1031–1040 10.1007/s11306-013-0518-9 [DOI] [Google Scholar]
- 17.Sankaralingam A., Roplekar R., Turner C., Dalton R.N. and Hampson G. (2014) Changes in Dickkopf-1 (DKK1) and sclerostin following a loading dose of vitamin D 2 (300,000 IU). J. Osteoporos. 2014, 682763 10.1155/2014/682763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nusse R. and Clevers H. (2017) Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 10.1016/j.cell.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 19.Aoshima Y., Mizobuchi M., Ogata H., Kumata C., Nakazawa A., Kondo F. et al. (2012) Vitamin D receptor activators inhibit vascular smooth muscle cell mineralization induced by phosphate and TNF-alpha. Nephrol. Dial. Transplant. 27, 1800–1806 10.1093/ndt/gfr758 [DOI] [PubMed] [Google Scholar]
- 20.Golledge J., Muller J., Daugherty A. and Norman P. (2006) Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler. Thromb. Vasc. Biol. 26, 2605–2613 10.1161/01.ATV.0000245819.32762.cb [DOI] [PubMed] [Google Scholar]
- 21.Bruemmer D., Collins A.R., Noh G., Wang W., Territo M., Arias-Magallona S. et al. (2003) Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J. Clin. Invest. 112, 1318–1331 10.1172/JCI200318141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweeting M.J., Thompson S.G., Brown L.C., Powell J.T. and RESCAN collaborators (2012) Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br. J. Surg. 99, 655–665 10.1002/bjs.8707 [DOI] [PubMed] [Google Scholar]
- 23.Cassis L.A., Gupte M., Thayer S., Zhang X., Charnigo R., Howatt D.A. et al. (2009) ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am. J. Physiol. Heart Circ. Physiol. 296, H1660–H1665 10.1152/ajpheart.00028.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajabshir S., Asif A. and Nayer A. (2014) The effects of vitamin D on the renin-angiotensin system. J. Nephropathol. 3, 41–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrara D., Bernini M., Bacca A., Rugani I., Duranti E., Virdis A. et al. (2014) Cholecalciferol administration blunts the systemic renin-angiotensin system in essential hypertensives with hypovitaminosis D. J. Renin Angiotensin Aldosterone Syst. 15, 82–87 10.1177/1470320312471149 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.