Abstract
Background: Exclusive breastfeeding (EBF) during the first six months of life is critical for child’s linear growth. While there is strong evidence in favor of EBF, the evidence with regards to other interventions for linear growth is unclear. We evaluated intervention domains of micronutrients, food supplements, deworming, maternal education, water sanitation and hygiene (WASH), and kangaroo care, for their comparative effectiveness on linear growth.
Methods: For this review, we searched for randomized clinical trials (RCTs) of the interventions provided to infants aged 0-6 months and/or their breastfeeding mothers in low- and middle-income countries reporting on length-for-age z-score (LAZ), stunting, length, and head circumference. We searched for reports published until September 17 th, 2019 and hand-searched bibliographies of existing reviews. For LAZ and stunting, we used network meta-analysis (NMA) to compare the effects of all interventions except for kangaroo care, where we used pairwise meta-analysis to compare its effects versus standard-of-care. For length and head circumference, we qualitatively summarized our findings.
Results: We found 29 RCTs (40 papers) involving 35,119 mother and infant pairs reporting on the effects of aforementioned interventions on linear growth outcomes. Our NMA on LAZ found that compared to standard-of-care, multiple micronutrients administered to infants (MMN-C) improved LAZ (mean difference: 0.20; 95% credible interval [CrI]: 0.03,0.35), whereas supplementing breastfeeding mothers with MMN did not (MMN-M, mean difference: -0.02, 95%CrI: -0.18,0.13). No interventions including MMN-C (relative risk: 0.74; 95%CrI: 0.36,1.44) reduced risk for stunting compared to standard-of-care. Kangaroo care, on the other hand, improved head circumference (mean difference: 0.20 cm/week; 95% confidence intervals [CI]: 0.09,0.31 cm/week) and length (mean difference: 0.23 cm/week; 95%CI: 0.10,0.35 cm/week) compared to standard-of-care.
Conclusion: Our study found important improvements for kangaroo care, but we did not find sufficient evidence for other interventions.
Registration: PROSPERO CRD42018110450; registered on 17 October 2018.
Keywords: Exclusive breastfeeding, linear growth, stunting, low- and middle-income countries, network meta-analysis
Introduction
In past decades, important progress achieved in maternal, newborn, and child health (MNCH) have led to substantial reductions in maternal and child mortality rates 1, 2. However, many children still fail to reach their linear growth potential, particularly those living in low- and middle-income countries (LMICs) 3. Linear growth in early childhood is a marker of healthy development that is closely linked with neurodevelopment 4. The first six months of age (birth to 6 months), known as the exclusive breastfeeding period, is a critical life stage for early child development. There is a strong evidence to support the benefits of exclusive breastfeeding during this life stage 5– 7. As such, mechanisms and resources to facilitate appropriate self-care in addition to psycho-social support for breastfeeding mothers is necessary to improve both health outcomes of mothers and babies. For instance, poor maternal nutrition could lead to lactation issues creating barriers for mothers to exclusively breastfeed 3. Inadequate care, poor hygiene, and control of diseases for infants and mothers may also inadvertently limit the growth of infants who are adequately breastfed 3, 8, 9.
The current evidence for other interventions, such as micronutrients, food supplements, deworming, maternal education, and kangaroo care (i.e. early skin-to-skin care) interventions is unclear for the exclusive breastfeeding life stage. Although there are numerous published reviews aimed to assess the effectiveness of these interventions that can be provided during exclusive breastfeeding period ( Table 1), their scope has been limited to summarize the comparative effectiveness of a single intervention or interventions within a single domain only. Given that determinants of linear growth for exclusive breastfeeding period is multi-faceted, there is a need to summarize the evidence base of interventions from multiple intervention domains, since multi-domain intervention solutions are likely needed to tackle this problem.
Table 1. Existing reviews on interventions for exclusive breastfeeding period.
| Review ID | Title | Interventions | No of
studies |
Included study
types |
|---|---|---|---|---|
| Kramer 2012 5 | Optimal duration of exclusive breastfeeding
(Review). |
Exclusive breastfeeding
vs complementary food introduction at 4 months |
23 | Randomized trials |
| Lumbiganon
2016 10 |
Antenatal breastfeeding education for increasing
breastfeeding duration. |
Breastfeeding education for
increasing breastfeeding duration |
24 | Randomized trials |
| Haroon 2013 11 | Breastfeeding promotion interventions and
breastfeeding practices: a systematic review. |
Breastfeeding education or
support |
110 | Randomized
trials and quasi- experimental studies |
| Balogun 2016 12 | Interventions for promoting the initiation of
breastfeeding. |
Breastfeeding education,
support groups |
28 | Randomized trials |
| Giugliani 2015 13 | Effect of breastfeeding promotion interventions
on child growth: a systematic review and meta- analysis. |
Breastfeeding promoting
interventions |
35 | Randomized trials |
| Abe 2016 14 | Supplementation with multiple micronutrients for
breastfeeding women for improving outcomes for the mother and baby. |
Micronutrients mothers | 2 | Randomized trials |
| Ndikom 2014 15 | Extra fluids for breastfeeding mothers for
increasing milk production. |
Forced fluids | 1 | Randomized trials |
| Martin 2016 16 | Review of Infant Feeding: Key Features of Breast
Milk and Infant Formula. |
Infant nutrition | 6 | Randomized trials |
| Fleith 2005 17 | Dietary PUFA for Preterm and Term Infants:
Review of Clinical Studies |
Infant nutrition | 28 | Randomized trials |
| Conde-Agudelo
2016 18 |
Kangaroo mother care to reduce morbidity and
mortality in low birthweight infants. |
Kangaroo care | 21 | Randomized trials |
| Moore 2016 19 | Early skin-to-skin contact for mothers and their
healthy newborn infants. |
Kangaroo care | 46 | Randomized trials |
| Delgado-
Noguera 2015 20 |
Supplementation with long chain polyunsaturated
fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. |
Long chain polyunsaturated
fatty acids supplements |
8 | Randomized trials |
| Thiele 2013 21 | Maternal vitamin D supplementation to meet the
needs of the breastfed infant: a systematic review. |
Vitamin D supplements | 3 | Randomized trials |
| Becker 2016 22 | Methods of milk expression for lactating women. | Methods of lactation | 41 | Randomized trials |
This article uses a comprehensive literature review for multiple intervention domains of micronutrient, food supplements, deworming, maternal education, water sanitation and hygiene (WASH), and kangaroo care to summarize their effects on linear growth for LMIC-based infants in the exclusive breastfeeding period. For our quantitative summary, we have used network meta-analysis for all interventions except for kangaroo care to summarize their effects on LAZ and stunting outcomes; kangaroo care was assessed using pairwise meta-analysis. As the data was too sparse to facilitate meta-analysis, we qualitatively summarized the evidence base for outcomes length and head circumference.
Methods
Our analysis and report was designed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) extension to network meta-analysis 23. The protocol for this study was registered in PROSPERO ( CRD42018110450).
Search strategy and selection criteria
Our search strategy was developed after first reviewing the papers published in the Lancet 2013 Maternal and Child Nutrition series 3, 24, inclusive of the umbrella review by Bhutta and colleagues 7, for an overview of the literature. Specifically, we hand-searched the bibliography of Bhutta et al. 7 for relevant systematic reviews, global health guidelines, and LMIC-based trials. We also performed additional searches in PubMed and the Cochrane Database of Systematic Reviews for more recent trials and other reviews published after 2013. The list of published reviews relevant to this study is provided in Table 1.
For our systematic literature search, we scanned the following databases from inception to August 28, 2019: the Cochrane Central Register of Controlled Trials, Embase, and MEDLINE ( Extended data, Supplementary Tables 1–3) 25. To increase the sensitivity of our search, we complemented our database searches with relevant trials identified from bibliographies of prior reviews. Table 3 describes the Population, Intervention, Comparator, Outcome, and Study Design (PICOS) criteria used to guide the study selection for our systematic literature review. We included randomized clinical trials on interventions of the following domains: Micronutrient supplements; Food supplements; Deworming for mothers; Maternal and breastfeeding education, and promotion; WASH; and kangaroo care (i.e. skin-to-skin care). The outcomes of interest included change in LAZ, proportions of participants with stunting (defined by LAZ below -2SD), change in length, and change in head circumference. For all intervention domains, except for kangaroo care, we excluded studies that did not report the effects of their respective interventions for at least three months. For kangaroo care, there was no restriction for time of follow-up given short duration nature of this intervention. We excluded non-English language studies.
A team of four reviewers (JJHP, ES, LD, and RM) independently reviewed all abstracts and proceedings identified in the literature searches. The same team independently conducted relevant full-text reviews of relevant papers. If any discrepancies occurred between the studies selected by the same reviewers, a third investigator (KT) provided arbitration.
Using a standardized data sheet in Microsoft Excel, four investigators (JJHP, VJ, NEZ, and HG) independently extracted data for study characteristics, interventions used, patient characteristics at baseline, and outcomes from the final list of selected eligible studies. Any discrepancies observed during data extraction were resolved through discussion between the investigators until consensus was reached.
Evidence synthesis and data analysis
When sufficient data was available for quantitative assessment, a network meta-analysis or pairwise meta-analysis approach was applied. For all domains of interventions except for kangaroo care, we performed a network meta-analysis for LAZ and stunting. There was a limited number of studies that reported on length and head circumference, so we qualitatively synthesized findings from these trials as an alternative to quantitative analysis. We did not consider kangaroo care as part of the network meta-analysis since these trials involved a shorter intervention duration and follow-up (median follow-up of 2 weeks).
We performed a network meta-analysis within the Bayesian framework in R using the R2WinBUGS v14 package 26, 27. Bayesian models were performed according to the National Institute for Health and Care Excellence (NICE) in their Technical Support Document 2 (TSD2) 28. Estimates of comparative effectiveness were measured using mean differences in LAZ with the associated 95% credible intervals (95% CrI). In all models, we used an empirically informative heterogeneity prior distribution, as suggested by Rhodes et al. 2016 29 for LAZ and Turner et al. 2015 30 for stunting. This was done to stabilize the estimation of heterogeneity in the face of low number of trials per comparison in the network. Our model selection was informed by using the deviance information criterion and the deviance-leverage plots that could help identify outlier(s) in terms of model fit, in accordance with the NICE TSD2 recommendations 28.
For our primary network meta-analysis, we included both cluster and non-cluster randomized clinical trials (with the unit of randomization set at the individual level). To adjust for clustering effects of the cluster trials, we assumed a conservative intra-cluster correlation coefficient (ICC) of 0.05, and we inflated variances accordingly for continuous outcomes and adjusted the sample sizes and the number of cases for dichotomous outcomes, as recommended by Uhlmann et al. 31 We performed a sensitivity analysis by excluding cluster randomized clinical trials in our network meta-analysis. For our pairwise meta-analysis on kangaroo care, we performed a random-effects model using the Metafor R package (in R2WinBUGS v14) 32. For our network meta-analysis, the estimates of effectiveness were measured using mean differences or relative risk with accompanying 95% credible intervals (CrIs). The estimates of effectiveness were measured using mean differences with accompanying 95% confidence intervals (CIs) for our pairwise meta-analysis on kangaroo care. As no kangaroo care trials involved cluster randomization, our pairwise meta-analysis did not need to adjust for the clustering effect.
Risk of bias within and across studies
Each full text article was evaluated for reporting quality according to the Cochrane Risk of Bias Tool 33. The risk of bias assessment within and across studies are provided in the Extended data (Supplementary Table 8) 25.
Results
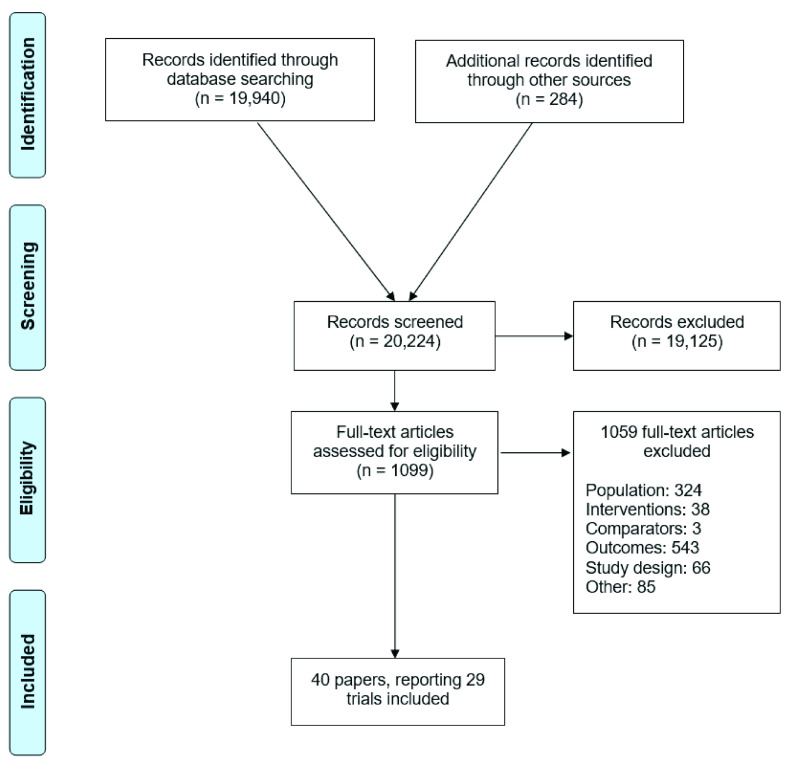
We identified 20,224 abstracts from our database searches and hand searching of reference lists from published reviews ( Figure 1). Of these, 1099 studies underwent a full-text review, and 40 papers reporting on 29 trials met our inclusion criteria. In total, these trials pertained to 35,119 participants that were randomized to 73 unique interventions ( Figure 2). The list of the final subset is provided in Table 2, and the list of excluded studies ( Extended data, Supplementary Table 5) 25 is provided in the online appendix.
Figure 1. Study selection.
Figure 2. Overall network of the comparisons between interventions for exclusive breastfeeding period.
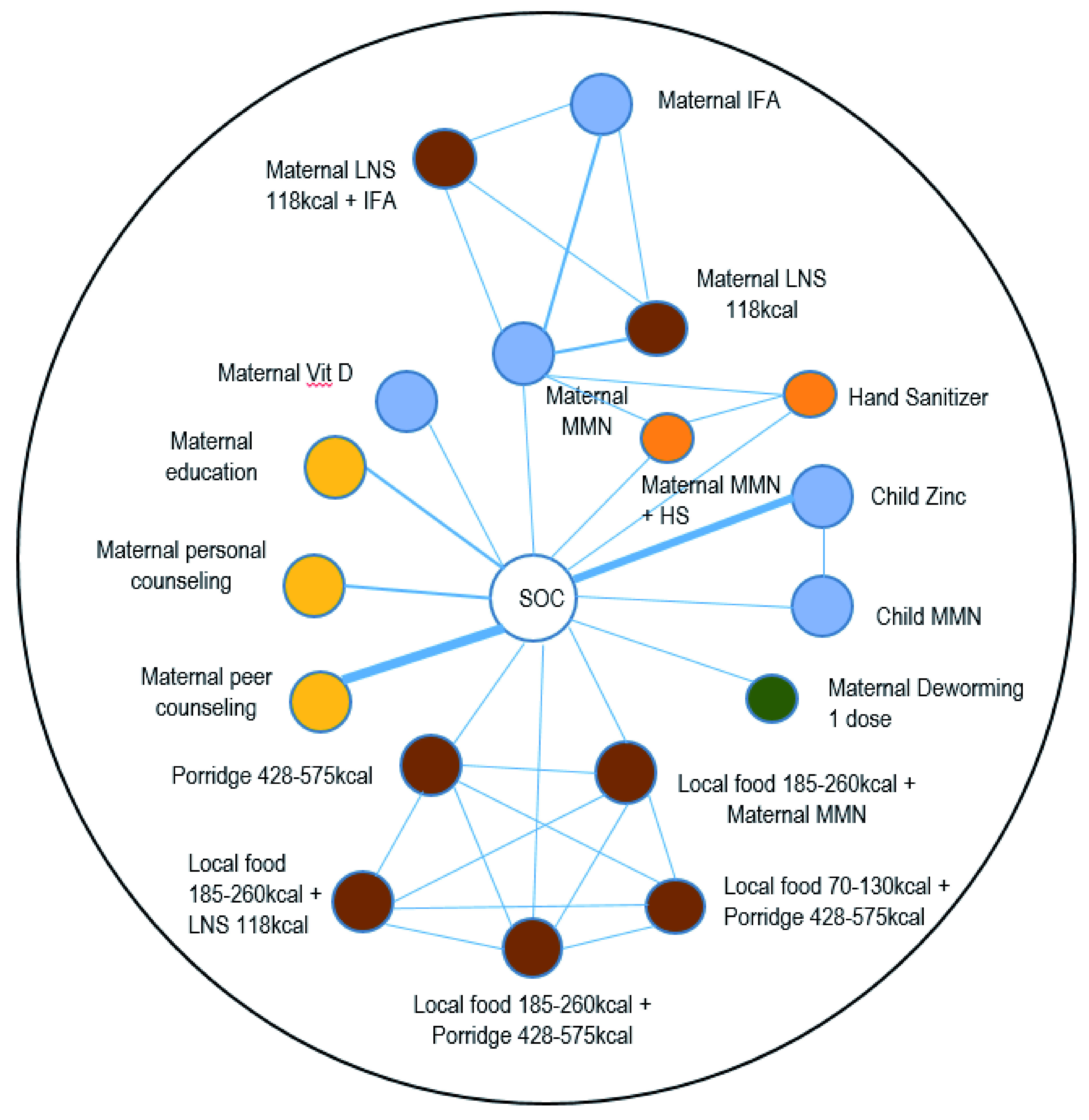
Each node (circle) represents an intervention with each line representing a direct comparison between interventions (i.e. these interventions have been compared directly in a head-to-head randomized clinical trial). The width of the lines represents the numbers of trials with comparison in question. The white circle shows standard-of-care; blue circles represent micronutrient interventions; brown circles represent balanced energy protein or food supplements that are fortified and not; yellow circles represent education and counseling interventions; green circle represents deworming intervention; and orange circles represents WASH interventions. Fort, fortified; IFA, iron + folic acid; LNS, lipid based nutrient supplements; MMN, multiple micronutrients; SOC, standard of care; Vit, vitamin; HS, hand sanitizer.
Table 2. The list of included studies.
| Trial ID | Registry number | First author, year | Title |
|---|---|---|---|
| Acharya 2014 41 | NR | Acharya 2014 | Randomized Control Trial of Kangaroo Mother Care in Low
Birth Weight Babies at a Tertiary Level Hospital |
| Adu-Afarwuah 2016 34, 42 | NCT00970866 | Adu-Afarwuah 2016 | Small-quantity, lipid-based nutrient supplements provided to
women during pregnancy and 6 mo postpartum and to their infants from 6 mo of age increase the mean attained length of 18-mo-old children in semi-urban Ghana: a randomized controlled trial |
| Adu-Afarwuah 2017 | Maternal supplementation with small-quantity lipid-based
nutrient supplements compared with multiple micronutrients, but not with iron and folic acid, reduces the prevalence of low gestational weight gain in semi-urban ghana: A randomized controlled trial |
||
| Ashorn 2015A 35, 43, 44 | NCT01239693 | Ashorn 2015A | Supplementation of Maternal Diets during Pregnancy and
for 6 Months Postpartum and Infant Diets Thereafter with Small-Quantity Lipid-Based Nutrient Supplements Does Not Promote Child Growth by 18 Months of Age in Rural Malawi: A Randomized Controlled Trial |
| Ashorn 2015B | The impact of lipid-based nutrient supplement provision
to pregnant women on newborn size in rural Malawi: a randomized controlled trial |
||
| Adu-Afarwuah 2018 | From the field: Improving fetal and infant growth in
vulnerable populations |
||
| Boo 2007 45 | NR | Boo 2007 | Short duration of skin-to-skin contact: effects on growth and
breastfeeding |
| CARING trial 46 | ISCRTN51505201 | Nair 2017 | Effect of participatory women's groups and counselling
through home visits on children's linear growth in rural eastern india (caring trial): A cluster-randomised controlled trial |
| Feliciano 1994 47 | NR | Feliciano 1994 | Seasonal and Geographical Variations in the Growth Rate of
Infants in China Receiving Increasing Dosages of Vitamin D Supplements |
| Gathwala 2010 48 | NR | Gathwala 2010 | Effect of Kangaroo Mother Care on physical growth,
breastfeeding and its acceptability |
| Goodstart 49, 50 | NR | Tomlinson 2011 | An effectiveness study of an integrated, community-based
package for maternal, newborn, child and HIV care in South Africa: study protocol for a randomized controlled trial |
| NR | Tomlinson 2014 | Goodstart: a cluster randomised effectiveness trial of
an integrated, community-based package for maternal and newborn care, with prevention of mother-to-child transmission of HIV in a South African township |
|
| Habib 2015 51 | NCT01229579 | Habib 2015 | Zinc supplementation fails to increase the immunogenicity of
oral poliovirus vaccine: A randomized controlled trial |
| Hamadani 2001 52 | NR | Hamadani 2001 | Randomized controlled trial of the effect of zinc
supplementation on the mental development of bangladeshi infants |
| JiVitA-3 53, 54 | NCT00860470 | Christian 2016 | Effects of prenatal multiple micronutrient supplementation
on growth and cognition through 2 y of age in rural Bangladesh: the JiVitA-3 Trial |
| West 2014 | Effect of maternal multiple micronutrient vs iron-folic acid
supplementation on infant mortality and adverse birth outcomes in rural Bangladesh: the JiVitA-3 randomized trial. |
||
| Kumbhojkar 2016 55 | NR | Kumbhojkar 2016 | Kangaroo Mother Care (KMC): An Alternative to
Conventional Method of Care for Low Birth Weight Babies |
| Le Roux 2013 56, 57 | NCT00996528 | Le Roux 2013 | Outcomes of home visits for pregnant mothers and their
infants: a cluster randomized controlled trial |
| Rotheram-Borus
2014 |
A Cluster Randomised Controlled Effectiveness Trial
Evaluating Perinatal Home Visiting among South African Mothers/Infants |
||
| Locks 2016 58, 59 | NCT00421668 | Locks 2016 | Effect of zinc and multivitamin supplementation on the
growth of Tanzanian children aged 6–84 wk: a randomized, placebo-controlled, double-blind trial |
| Locks L 2015 | Effect of zinc & multiple micronutrient supplements on
growth in tanzanian children |
||
| Lonnerdal 2017 60 | NCT00970398 | Lonnerdal 2017 | Growth, Nutrition, and Cytokine Response of Breast-
fed Infants and Infants Fed Formula With Added Bovine Osteopontin |
| LUCOMAI 61 | NCT01977365 | Nikiema 2017 | Effectiveness of facility-based personalized maternal
nutrition counseling in improving child growth and morbidity up to 18 months: A cluster-randomized controlled trial in rural Burkina Faso |
| MDIG 62 | NCT01924013 | Roth 2018 | Vitamin D Supplementation in Pregnancy and Lactation and
Infant Growth |
| Mofid 2017 38 | NCT01748929 | Mofid 2017 | A Double-Blind Randomized Controlled Trial of Maternal
Postpartum Deworming to Improve Infant Weight Gain in the Peruvian Amazon |
| Osendarp 2002 63 | NR | Osendarp 2002 | Effect of zinc supplementation between 1 and 6 mo of life on
growth and morbidity of Bangladeshi infants in urban slums on the mental development of Bangladeshi infants |
| Ostadrahimi 2017 64 | NR | Ostadrahimi 2017 | The effect of perinatal fish oil supplementation on
neurodevelopment and growth of infants: a randomized controlled trial |
| PROCOMIDA 37 | NCT01072279 | Olney 2018 | Procomida, a food-assisted maternal and child health and
nutrition program, reduces child stunting in guatemala: A cluster-randomized controlled intervention trial |
| PROMISE EBF 65– 67 | NCT00397150 | Engebretsen 2014 | Growth effects of exclusive breastfeeding promotion by peer
counsellors in sub-Saharan Africa: the cluster-randomised PROMISE EBF trial |
| Fadnes 2016 | Effects of an exclusive breastfeeding intervention for six
months on growth patterns of 4–5 year old children in Uganda: the cluster-randomised PROMISE EBF trial |
||
| Tylleskar 2011 | Exclusive breastfeeding promotion by peer counsellors in
sub-Saharan Africa (PROMISE-EBF): a cluster-randomised trial. |
||
| RDNS 36, 68 | NCT01715038 | Dewey 2017 | Lipid-based nutrient supplementation in the first 1000 d
improves child growth in Bangladesh: a cluster-randomized effectiveness trial |
| Mridha 2016 | Lipid-based nutrient supplements for pregnant women
reduce newborn stunting in a cluster-randomized controlled effectiveness trial in Bangladesh |
||
| Shafique 2016 39 | NCT01455636 | Shafique 2016 | Mineral- and vitamin-enhanced micronutrient powder
reduces stunting in full-term low-birth-weight infants receiving nutrition, health, and hygiene education: A 2 × 2 factorial, cluster-randomized trial in bangladesh |
| Simondon 1996 40 | NR | Simondon 1996 | Effect of early, short-term supplementation on weight and
linear growth of 4-7-mo-old infants in developing countries: a four-country randomized triaI |
| Suman 2008 69 | NR | Suman 2008 | Kangaroo mother care for low birth weight infants: a
randomized controlled trial |
| Urban 2008 70 | NR | Urban 2008 | Growth of infants born to HIV infected women when fed
a biologically acidified starter formula with and without probiotics |
| Vazir 2013 71 | NR | Vazir 2013 | Cluster-randomized trial on complementary and responsive
feeding education to caregivers found improved dietary intake, growth, and development among rural Indian toddlers |
| Velaphi 2008 72 | NR | Velaphi 2008 | Growth and metabolism of infants born to women infected
with human immunodeficiency virus and fed acidified whey- adapted starter formulas |
Table 3. Population, interventions, comparator, outcomes, and study design criteria.
| Category | Inclusion criteria |
|---|---|
| Population | Infants of age 0 to 6 months, living in low- and middle-income countries |
| Intervention | • Micronutrient & calcium supplementation to mothers or infants
• Food supplementation to mothers or infants • Kangaroo care* • Deworming • Maternal and breastfeeding education and promotion • Water, sanitation and hygiene (WASH) intervention |
| Comparators | • Placebo
• Standard-of-care (if applicable) • No intervention • Any of the interventions listed above as monotherapy or in combination that can be used for indirect comparison |
| Outcomes | At least one of the following outcomes (reported after at least 2 months, *except for kangaroo care):
• Length for age z-score (LAZ) • Proportion of stunted (LAZ < -2SD) • Length or height • Head circumference |
| Study Design | Randomized clinical trials |
| Other | Published in the English language |
The trial characteristics of the included studies ( Extended data, Supplementary Table 6) 25 are provided in the online appendix. Of the 29 included trials, ten were cluster randomized trials (1156 clusters; 24,389 mother-infant dyads). The majority of trials were conducted in Southeastern Asian (n = 14) and African (n = 10) countries, and involved individual randomization (i.e. non-cluster trials, n = 19) and were open-label trials (n = 9). Several trials (n = 24) focused on a single domain of interventions, with micronutrient (n = 11) and food supplements (n = 9) being the most common intervention domains investigated. There were four trials that investigated interventions from two different intervention domains 34– 37, but the scope of these trials was still limited to nutritional (micronutrient and food) supplementations. There was one trial reporting on deworming study 38 and another on WASH intervention 39, and there were five trials on kangaroo care. There were 24 trials that investigated other intervention domains (non-kangaroo care trials), the median duration of interventions was 24 weeks (IQR: 12, 24 weeks). The kangaroo care trials entailed short follow-ups, with intervention durations that varied between one to two weeks.
The patient baseline characteristics are provided in the online appendix ( Extended data, Supplementary Table 7) 25. The median age of mothers at enrollment was 25.4 years (ranging from 21.8 to 29.8 years). For infants, the majority of trials enrolled participants from birth (after follow-up of the mother) or within the first month of life, except one trial 40 that investigated the effects of food supplements for an early weaning off breastfeeding enrolled patients at 4 months of age (up to 7 months of age). The proportion of boys included in these trials was 51.3% on average, ranging from 36.6% 39 to 73% 41.
Network meta-analysis on LAZ
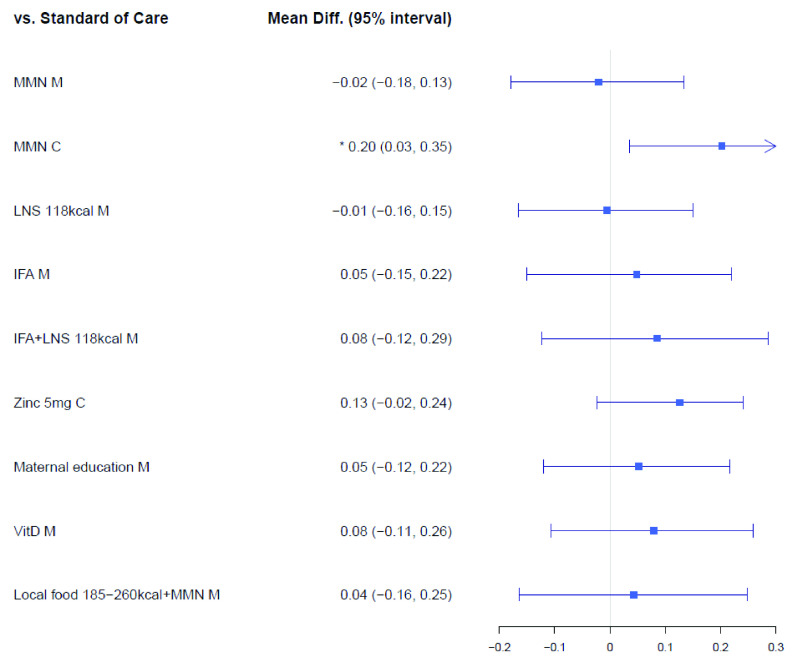
The LAZ network ( Extended data, Supplementary Figure 1) 25 included 18 trials consisting of 27,896 mother-infant dyads randomized to 52 intervention arms. The results of our primary analysis on LAZ that included both cluster and non-cluster randomized clinical trials are illustrated in Figure 3. Among micronutrient supplements, multiple micronutrients supplementation (MMN) provided to infants improved LAZ relative to standard-of-care (MMN-C, mean difference: 0.20, 95% CrI: 0.03, 0.35), whereas supplementing breasting mothers with MMN did not improve LAZ (MMN-M, mean difference: -0.02, 95% CrI: -0.18, 0.13). Compared to standard of care, other micronutrient supplements to infants, such as zinc 5 mg (zinc 5 mg C) showed a trend towards improved LAZ, but its CrIs overlapped the null of effect of 0.00 (Mean difference: 0.13; 95% CrI: -0.02, 0.24). Also, other micronutrients to breastfeeding mothers, such as iron and folic acid (IFA-M, mean difference: 0.05, 95% CrI: -0.15, 0.22) and vitamin D (Vit D-M: mean difference: 0.08, 95% CrI: -0.11, 0.26), did not improve LAZ in comparison to standard of care. Similarly, both food supplements and maternal education interventions did not improve LAZ; for instance, in comparison to standard-of-care, combination of IFA and 118 kcal of lipid-based nutrient supplements (IFA+LNS 118 kcal-M) showed a mean difference of 0.08 cm (95% CrI: -0.12, 0.29) for LAZ, where maternal education showed a mean difference of 0.05 cm (95% CrI: -0.12, 0.22 cm). No deworming or WASH interventions showed improvements on LAZ.
Figure 3. Forest plot for the effects of interventions on LAZ (mean difference in cm), cluster & non-cluster trials.
Vit, vitamin; IFA, iron and folic Acid; LNS, lipid-based nutrient supplements; Fort, fortification; MMN, multiple micronutrients; M, maternal; C, child.
Network meta-analysis on stunting
The stunting network ( Extended data, Supplementary Figure 3) 25 included 18 trials that consisted of 27,896 mother-infant dyads randomized to 52 intervention arms. The results of our primary analysis that included both cluster and non-cluster randomized clinical trials are illustrated in Extended data, Supplementary Figure 9 25. While supplementations of zinc to infants (Zinc 5 mg-C, relative risk [RR]: 0.82, 95% CrI: 0.56, 1.18) and local food supplement within the caloric range of 185–260 kcal fortified with MMN for mothers (Local food 185–260 kcal + MMN M, RR: 0.85, 95% CrI: 0.46, 1.46) showed a trend towards reduced risk of stunting, their CrIs contained the null effect of 1.00. In fact, no interventions demonstrated any improvements towards reducing the risk of stunting.
Sensitivity analyses on LAZ and stunting
Our sensitivity analyses were limited to individually (non-cluster) randomized clinical trials only. The network diagrams for LAZ ( Extended data, Supplementary Figure 2) 25 and stunting ( Extended data, Supplementary Figure 4) 25 can be found online along with forest plots ( Extended data, Supplementary Figures 10 and 11) 25 and cross-tables ( Extended data, Supplementary Table 9 and 10) 25. In our sensitivity analysis on LAZ, no interventions showed improvements for LAZ when compared to standard-of-care, and similarly for stunting, no interventions showed reduced risks for stunting. This is likely due to very few studies being available for sensitivity analyses; only nine trials were available for LAZ and stunting analyses.
Kangaroo care
Five randomized clinical trials investigating the effects of kangaroo care on linear growth of newborns were included in the pairwise meta-analysis 41, 45, 48, 55, 69. The outcome reporting of these kangaroo care trials was limited to growth velocity of head circumference and length (cm per week). All kangaroo care trials were conducted in Southeastern Asian countries (i.e. India, Malaysia, and Nepal), in hospital settings and involved low birthweight neonates. Kangaroo care consisted of skin-to-skin contact between the mothers’ breasts, where infants in the control group were kept under either a warmer or incubator. The effects of kangaroo care on head circumference and length growth velocities are shown in Extended data, Supplementary Figures 12 and 13 25, respectively. All studies except for Acharya 2014 41 showed improvements in head circumference (Mean (SD): 31.5 (1.4) 95%CI -0.5, 0.6 45; Kangaroo mother care (KMC): 0.75 cm vs conventional method of care (CMC): 0.49 cm p<0.001 55;) and length (KMC: 0.99 cm vs CMC 0.70 cm p<0.001 55). The pooled estimates of growth velocities for head circumference and length showed improvements for kangaroo care in comparison to the control. Relative to the control, kangaroo care showed an improved mean difference of 0.20 cm/week (95% CI: 0.09, 0.31 cm/week) for head circumference, and for length, a mean difference of 0.23 cm/week (95% CI: 0.10, 0.35 cm/week).
Qualitative summary of trials reporting on length and head circumference
There were twelve trials available for our qualitative summary 37, 38, 40, 47, 51, 53, 56, 60, 63– 65, 71. Of these trials, three were cluster randomized clinical trials that investigated interventions related to maternal education and breastfeeding promotion: Le Roux 56 Vazir 71, and PROMISE EBF 65 did not find differences in their maternal education and breastfeeding promotion interventions. In this three-arm trial conducted in India, mothers in the Complementary Feeding group (n = 202; 20 clusters) received nutrition education messages on breastfeeding and complementary feeding from CHWs, and the mothers in the Complementary Feeding + Play group (n = 195; 20 clusters) received messages on psychosocial stimulation in addition to the same nutritional messages received by the women in the complementary feeding group (the control group received local standard of care; n = 202; 20 clusters). The mothers were approached by the trial investigators during pregnancy, and the interventions began when their child was three months old. By the age of six months, this trial found no differences in terms of length between the three groups (Mean ± SD: Control group: 64.2 ± 2.3; Complementary Feeding group: 64.4 ± 2.5 cm; and Complementary Feeding + Play group: 64.2 ± 2.3).
Additionally, nine trials investigated the effect of nutritional interventions (four trials on micronutrient supplements, three on food supplements, one on both, and one other for deworming) on the incidence of changes in head circumference, or changes in length 37, 38, 40, 47, 53, 60, 63, 64, 71. Of these nine trials, JiVitA-3 trial 53, 54, 73 and Ostadrahimi 64 provided supplements to mothers from pregnancy into postpartum, where the other five trials provided supplements to children. PROCOMIDA 37 provided food to the entire family. Ostadrahimi 64 enrolled pregnant women from the 20 th week of gestational age and were provided daily fish oil supplements (120 mg docosahexaenoic acid and 180 mg eicosapentaenoic acid) or placebo up to 1 month into the postpartum, with their child being followed-up up to six months of age. At the 6-month assessment of this trial, there were no differences found in neither length (mean difference: 0.12, 95% CI: -0.52, 0.76) or head circumference (mean difference: -0.03, 95% CI: -0.38, 0.30) between the fish oil and placebo groups. Mofid 38 found that deworming interventions provided to mothers who tested positive for soil-transmitted helminth infection at baseline had a positive impact on mean length gain (Mean difference: 0.8; 95% CI: 0.1, 1.4) and LAZ (mean difference:0.5; 95% CI: 0.2, 0.8) of infants at six months of age.
Three of the five trials investigated the effectiveness of micronutrient supplements administered directly to children 47, 60, 63. In Feliciano 47, three different dosages of Vitamin D supplements (daily dose of 100, 200, and 400 IU) were provided to Chinese infants from birth up to six months of age; at the 6-months assessment, differences in length between the three groups were observed. Another placebo-controlled trial 63 conducted in Bangladesh found that daily zinc supplements (5 mg) to children between the age of one month to six months did not change the length or head circumference.
There were two trials that explored the role of food supplements to children. Simondon et al. 40 was a multi-national trial (Congo, Sengal, Bolivia, and New Caledonia) that randomized four-month old infants to either cereal-based precooked porridge fortified with MMN or the control group consisting of local food. The mean consumption of supplement varied from 133 to 189 kcal/day. There were no differences in length (cm) between the supplemented and control groups in all four countries at six months of age. In Lonnerdal et al. 60, one-month old infants of non-breastfeeding mothers were randomized to receive regular formula or formula fortified with bovine osteopontin (65 or 130 mg/L). There were no differences in length or head circumference between children who were randomized to different formula groups. This trial also recruited infants whose mothers had expressed the desire to exclusively breastfeed up to six months of age and used this breastfeeding group as a non-randomized control. The breastfeeding group had a higher mean head circumference but similar length at six months of age.
Discussion
Despite recent global achievements towards improved MNCH, the existing evidence on exclusive breastfeeding period interventions for linear growth remains unclear. Our study aimed to improve the current evidence base by assessing the comparative effectiveness of interventions across several domains: micronutrients, food supplements, maternal education, WASH, deworming, and kangaroo care. Both network meta-analysis and pairwise meta-analysis techniques were undertaken to appraise and synthesize findings from relevant studies reporting the desired outcomes for infants of age 0–6 months in LMICs (i.e. LAZ and proportion of stunted), and due to limited number of studies, length and head circumference were summarized qualitatively.
We found that MMN supplementation to infants (i.e. MMN-C) was the only intervention that showed important improvement for linear growth during the exclusive breastfeeding period. However, this finding was limited to only one trial in the study 58. Our analysis of kangaroo care also exhibited important improvements in growth in terms of increased head circumference and length growth velocity. However, kangaroo care interventions were excluded from the network meta-analysis and were analyzed separately via pairwise meta-analysis. This was due to the specific nature of this type of intervention, consisting of skin-to-skin contact between mothers’ breasts during a precise period for a limited duration (of between 1 and 6 weeks). In relation to this point was the observed heterogeneity in the intervention duration between included studies, generally, creating an added challenge when making comparisons across interventions. Deworming and WASH interventions did not show any improvements in both LAZ and stunting.
The main strength of this study was the use of network meta-analysis to assess the effectiveness of different interventions from a large network of evidence compared to standard-of-care 74. Previous reviews have focused only on intervention(s) within a single domain ( Table 1). We used a broad evidence base that included multiple interventions from different domains to simultaneously analyze all potential treatment options and make full use of the available evidence within a single analysis 75, 76. Additionally, appropriate statistical adjustments were made for clustering effects of cluster randomized clinical trials to enable the convergence of cluster and non-cluster trials for our network meta-analysis. Nevertheless, the narrow parameters of our PICOS criteria may have limited the breadth of our evidence base. Ethical and resource challenges associated with conducting clinical trials with neonates may have influenced investigators’ decision to undertake other non-randomized methodological approaches, such as observational studies. Additionally, since our population of interest focused on newborns living in LMICs, this prevented the inclusion of several trials conducted in non-LMICs. A number of studies assessed the effectiveness of long chain poly unsaturated fatty acids 77– 79; an intervention that has demonstrated some promise for improving linear growth in neonates compared to standard of care. As these trials were limited to high income settings, we were unable to incorporate this data into our analyses.
In general, our analysis revealed that the existing evidence base for improving linear growth during the exclusive breastfeeding period is limited. Our scan and appraisal of the evidence resulted in a paucity of studies focused on this early life stage. The scarcity of evidence for this early life stage could be explained by several factors. Generally, clinical trials involving neonates are considerably more difficult to perform due to a range of ethical, physiological, pharmacometric, and economic challenges 80. Obtaining ethical clearance for enrolling neonates can be extremely tasking, particularly with the need to preserve equipoise between intervention arms through balancing risk factors across intervention groups 80. Such complexities can complicate both the study design and recruitment, especially as it pertains to trials conducted in resource scarce settings 81. These reasons may explain to why the current evidence base for exclusive breastfeeding period is limited.
More clinical trial research is needed for the EBF period. To enhance the quality of evidence, it will beneficial if trials in the future will utilize more efficient trial designs, such as adaptive trial designs, that can better manage the range of uncertainties that may be associated with investigations focused on neonates 82, 83. It is important for mothers and infants living in resource limited settings that our assessment of interventions is thorough and appropriate for diverse contexts and settings. This will be a critical step to achieve the global goal of achieving a 40% reduction in the number of stunted children <5 years by 2025 84.
Data availability
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
Extended data
Open Science Framework: Interventions to improve linear growth during exclusive breastfeeding life-stage for children aged 0–6 months living in low- and middle-income countries: a systematic review and network and pairwise meta-analyses. https://doi.org/10.17605/OSF.IO/46HMQ 25
File ‘EBF period NMA - Supplementary tables and figures - v2.0’ contains the following extended data:
Appendix 1. Literature search strategy. (Contains Supplementary Tables 1–3.)
Appendix 2. Details to our statistical analysis.
Appendix 3. List of included and excluded studies after full-text review. (Contains Supplementary Tables 4 and 5.)
Appendix 4. Details of the evidence base. (Contains Supplementary Tables 6 and 7.)
Appendix 5. Bias Assessment. (Contains Supplementary Table 8.)
Appendix 6. The intervention networks for LAZ and stunting. (Contains Supplementary Figures 1–4.)
Appendix 7. Primary analysis leverage and consistency plots. (Contains Supplementary Figures 5–8.)
Appendix 8. Forest plots, cluster and non-cluster trials. (Contains Supplementary Figures 9–11.)
Appendix 9. Forest plots for kangaroo care (Contains Supplementary Figures 12 and 13.)
Appendix 10. Cross tables for LAZ and stunting. (Contains Supplementary Tables 9–12.)
Reporting guidelines
Open Science Framework: PRISMA checklist for “Interventions to improve linear growth during exclusive breastfeeding life-stage for children aged 0–6 months living in low- and middle-income countries: a systematic review and network and pairwise meta-analyses.” https://doi.org/10.17605/OSF.IO/46HMQ 25.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Funding Statement
This study was funded by the Bill and Melinda Gates Foundation (Contract Number: 52565).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 1 approved, 1 approved with reservations]
References
- 1. Alkema L, Chou D, Hogan D, et al. : Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet. 2016;387(10017):462–74. 10.1016/S0140-6736(15)00838-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu L, Oza S, Hogan D, et al. : Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027–35. 10.1016/S0140-6736(16)31593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Black RE, Victora CG, Walker SP, et al. : Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–51. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- 4. Suchdev PS, Boivin MJ, Forsyth BW, et al. : Assessment of Neurodevelopment, Nutrition, and Inflammation From Fetal Life to Adolescence in Low-Resource Settings. Pediatrics. 2017;139(Suppl 1):S23–S37. 10.1542/peds.2016-2828E [DOI] [PubMed] [Google Scholar]
- 5. Kramer MS, Kakuma R: Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012; (8):CD003517. 10.1002/14651858.CD003517.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brownell EA, Hagadorn JI, Lussier MM, et al. : Optimal periods of exclusive breastfeeding associated with any breastfeeding duration through one year. J Pediatr. 2015;166(3):566–70 e1. 10.1016/j.jpeds.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 7. Bhutta ZA, Das JK, Rizvi A, et al. : Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382(9890):452–77. 10.1016/S0140-6736(13)60996-4 [DOI] [PubMed] [Google Scholar]
- 8. Campbell OM, Benova L, Gon G, et al. : Getting the basic rights - the role of water, sanitation and hygiene in maternal and reproductive health: a conceptual framework. Trop Med Int Health. 2015;20(3):252–67. 10.1111/tmi.12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campbell SJ, Nery SV, Wardell R, et al. : Water, Sanitation and Hygiene (WASH) and environmental risk factors for soil-transmitted helminth intensity of infection in Timor-Leste, using real time PCR. PLoS Negl Trop Dis. 2017;11(3):e0005393. 10.1371/journal.pntd.0005393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lumbiganon P, Martis R, Laopaiboon M, et al. : Antenatal breastfeeding education for increasing breastfeeding duration. Cochrane Database Syst Rev. 2016;12:CD006425. 10.1002/14651858.CD006425.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haroon S, Das JK, Salam RA, et al. : Breastfeeding promotion interventions and breastfeeding practices: a systematic review. BMC Public Health. 2013;13 Suppl 3:S20. 10.1186/1471-2458-13-S3-S20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balogun OO, O'Sullivan EJ, McFadden A, et al. : Interventions for promoting the initiation of breastfeeding. Cochrane Database Syst Rev. 2016;11:CD001688. 10.1002/14651858.CD001688.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giugliani ER, Horta BL, Loret de Mola C, et al. : Effect of breastfeeding promotion interventions on child growth: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):20–9. 10.1111/apa.13160 [DOI] [PubMed] [Google Scholar]
- 14. Abe SK, Balogun OO, Ota E, et al. : Supplementation with multiple micronutrients for breastfeeding women for improving outcomes for the mother and baby. Cochrane Database Syst Rev. 2016;2:CD010647. 10.1002/14651858.CD010647.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ndikom CM, Fawole B, Ilesanmi RE: Extra fluids for breastfeeding mothers for increasing milk production. Cochrane Database Syst Rev. 2014; (6):CD008758. 10.1002/14651858.CD008758.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin CR, Ling PR, Blackburn GL: Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients. 2016;8(5): pii: E279. 10.3390/nu8050279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fleith M, Clandinin MT: Dietary PUFA for preterm and term infants: review of clinical studies. Crit Rev Food Sci Nutr. 2005;45(3):205–29. 10.1080/10408690590956378 [DOI] [PubMed] [Google Scholar]
- 18. Conde-Agudelo A, Diaz-Rossello JL: Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2016; (8):CD002771. 10.1002/14651858.CD002771.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moore ER, Bergman N, Anderson GC, et al. : Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. 2016;11:CD003519. 10.1002/14651858.CD003519.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Delgado-Noguera MF, Calvache JA, Bonfill Cosp X, et al. : Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst Rev. 2015; (7):CD007901. 10.1002/14651858.CD007901.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thiele DK, Senti JL, Anderson CM: Maternal vitamin D supplementation to meet the needs of the breastfed infant: a systematic review. J Hum Lact. 2013;29(2):163–70. 10.1177/0890334413477916 [DOI] [PubMed] [Google Scholar]
- 22. Becker GE, Smith HA, Cooney F: Methods of milk expression for lactating women. Cochrane Database Syst Rev. 2016;9:CD006170. 10.1002/14651858.CD006170.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hutton B, Salanti G, Caldwell DM, et al. : The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 24. Ruel MT, Alderman H, Maternal and Child Nutrition Study Group: Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? Lancet. 2013;382(9891):536–51. 10.1016/S0140-6736(13)60843-0 [DOI] [PubMed] [Google Scholar]
- 25. Park JJ, Siden E, Harari O, et al. : Interventions to improve linear growth during exclusive breastfeeding life-stage for children aged 0-6 months living in low- and middle-income countries: a systematic review and network and pairwise meta-analyses.2019. 10.17605/OSF.IO/46HMQ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. R Development Core Team: R: A Language Environment for Statistical Computing. In: Team RC, editor. Vienna, Austria: R Foundation for Statistical Computing;2017. [Google Scholar]
- 27. Sturtz S, Ligges U, Gelman A: R2OpenBUGS: a package for running OpenBUGS from R. J Stat Softw. 2005;12(3):1–16. Reference Source [Google Scholar]
- 28. Dias S, Sutton AJ, Ades AE, et al. : Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607–17. 10.1177/0272989X12458724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rhodes KM, Turner RM, White IR, et al. : Implementing informative priors for heterogeneity in meta-analysis using meta-regression and pseudo data. Stat Med. 2016;35(29):5495–511. 10.1002/sim.7090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Turner RM, Jackson D, Wei Y, et al. : Predictive distributions for between-study heterogeneity and simple methods for their application in Bayesian meta-analysis. Stat Med. 2015;34(6):984–98. 10.1002/sim.6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Uhlmann L, Jensen K, Kieser M: Bayesian network meta-analysis for cluster randomized trials with binary outcomes. Res Synth Methods. 2017;8(2):236–50. 10.1002/jrsm.1210 [DOI] [PubMed] [Google Scholar]
- 32. Viechtbauer W: Metafor: meta-analysis package for R. R package version. 2010;2010:1–0. [Google Scholar]
- 33. Higgins JPT, Chandler J, Cumpston M, et al. : Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane Reviews;2019. Reference Source [Google Scholar]
- 34. Adu-Afarwuah S, Lartey A, Okronipa H, et al. : Small-quantity, lipid-based nutrient supplements provided to women during pregnancy and 6 mo postpartum and to their infants from 6 mo of age increase the mean attained length of 18-mo-old children in semi-urban Ghana: a randomized controlled trial. Am J Clin Nutr. 2016;104(3):797–808. 10.3945/ajcn.116.134692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ashorn P, Alho L, Ashorn U, et al. : Supplementation of Maternal Diets during Pregnancy and for 6 Months Postpartum and Infant Diets Thereafter with Small-Quantity Lipid-Based Nutrient Supplements Does Not Promote Child Growth by 18 Months of Age in Rural Malawi: A Randomized Controlled Trial. J Nutr. 2015;145(6):1345–53. 10.3945/jn.114.207225 [DOI] [PubMed] [Google Scholar]
- 36. Mridha MK, Matias SL, Chaparro CM, et al. : Lipid-based nutrient supplements for pregnant women reduce newborn stunting in a cluster-randomized controlled effectiveness trial in Bangladesh. Am J Clin Nutr. 2016;103(1):236–49. 10.3945/ajcn.115.111336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olney DK, Leroy J, Bliznashka L, et al. : PROCOMIDA, a Food-Assisted Maternal and Child Health and Nutrition Program, Reduces Child Stunting in Guatemala: A Cluster-Randomized Controlled Intervention Trial. J Nutr. 2018;148(9):1493–1505. 10.1093/jn/nxy138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mofid LS, Casapia M, Aguilar E, et al. : A Double-Blind Randomized Controlled Trial of Maternal Postpartum Deworming to Improve Infant Weight Gain in the Peruvian Amazon. PLoS Negl Trop Dis. 2017;11(1):e0005098. 10.1371/journal.pntd.0005098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shafique S, Sellen DW, Lou W, et al. : Mineral- and vitamin-enhanced micronutrient powder reduces stunting in full-term low-birth-weight infants receiving nutrition, health, and hygiene education: a 2 × 2 factorial, cluster-randomized trial in Bangladesh. Am J Clin Nutr. 2016;103(5):1357–69. 10.3945/ajcn.115.117770 [DOI] [PubMed] [Google Scholar]
- 40. Simondon KB, Gartner A, Berger J, et al. : Effect of early, short-term supplementation on weight and linear growth of 4-7-mo-old infants in developing countries: a four-country randomized trial. Am J Clin Nutr. 1996;64(4):537–45. 10.1093/ajcn/64.4.537 [DOI] [PubMed] [Google Scholar]
- 41. Acharya N, Singh RR, Bhatta NK, et al. : Randomized Control Trial of Kangaroo Mother Care in Low Birth Weight Babies at a Tertiary Level Hospital. Journal of Nepal Paediatric Society. 2014;34(1). 10.3126/jnps.v34i1.8960 [DOI] [Google Scholar]
- 42. Adu-Afarwuah S, Lartey A, Okronipa H, et al. : Maternal Supplementation with Small-Quantity Lipid-Based Nutrient Supplements Compared with Multiple Micronutrients, but Not with Iron and Folic Acid, Reduces the Prevalence of Low Gestational Weight Gain in Semi-Urban Ghana: A Randomized Controlled Trial. J Nutr. 2017;147(4):697–705. 10.3945/jn.116.242909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ashorn P, Alho L, Ashorn U, et al. : The impact of lipid-based nutrient supplement provision to pregnant women on newborn size in rural Malawi: a randomized controlled trial. Am J Clin Nutr. 2015;101(2):387–97. 10.3945/ajcn.114.088617 [DOI] [PubMed] [Google Scholar]
- 44. Adu-Afarwuah S: From the Field: Improving Fetal and Infant Growth in Vulnerable Populations. Food Nutr Bull. 2018;39(2_suppl):S60–S8. 10.1177/0379572118773035 [DOI] [PubMed] [Google Scholar]
- 45. Boo NY, Jamli FM: Short duration of skin-to-skin contact: effects on growth and breastfeeding. J Paediatr Child Health. 2007;43(12):831–6. 10.1111/j.1440-1754.2007.01198.x [DOI] [PubMed] [Google Scholar]
- 46. Nair N, Tripathy P, Sachdev H, et al. : Effect of participatory women's groups and counselling through home visits on children's linear growth in rural eastern India (CARING trial): a cluster-randomised controlled trial. Lancet Glob Health. 2017;5(10):e1004–e16. 10.1016/S2214-109X(17)30339-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feliciano ES, Ho ML, Specker BL, et al. : Seasonal and Geographical Variations in the Growth Rate of Infants in China Receiving Increasing Dosages of Vitamin D Supplements. J Trop Pediatr. 1994;40(3):162–5. 10.1093/tropej/40.3.162 [DOI] [PubMed] [Google Scholar]
- 48. Gathwala G, Singh B, Singh J: Effect of Kangaroo Mother Care on physical growth, breastfeeding and its acceptability. Trop Doct. 2010;40(4):199–202. 10.1258/td.2010.090513 [DOI] [PubMed] [Google Scholar]
- 49. Tomlinson M, Doherty T, Jackson D, et al. : An effectiveness study of an integrated, community-based package for maternal, newborn, child and HIV care in South Africa: study protocol for a randomized controlled trial. Trials. 2011;12:236. 10.1186/1745-6215-12-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tomlinson M, Doherty T, Ijumba P, et al. : Goodstart: a cluster randomised effectiveness trial of an integrated, community-based package for maternal and newborn care, with prevention of mother-to-child transmission of HIV in a South African township. Trop Med Int Health. 2014;19(3):256–66. 10.1111/tmi.12257 [DOI] [PubMed] [Google Scholar]
- 51. Habib MA, Soofi S, Sheraz A, et al. : Zinc supplementation fails to increase the immunogenicity of oral poliovirus vaccine: a randomized controlled trial. Vaccine. 2015;33(6):819–25. 10.1016/j.vaccine.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 52. Hamadani JD, Fuchs GJ, Osendarp SJ, et al. : Randomized controlled trial of the effect of zinc supplementation on the mental development of Bangladeshi infants. Am J Clin Nutr. 2001;74(3):381–6. 10.1093/ajcn/74.3.381 [DOI] [PubMed] [Google Scholar]
- 53. Christian P, Kim J, Mehra S, et al. : Effects of prenatal multiple micronutrient supplementation on growth and cognition through 2 y of age in rural Bangladesh: the JiVitA-3 Trial. Am J Clin Nutr. 2016;104(4):1175–82. 10.3945/ajcn.116.135178 [DOI] [PubMed] [Google Scholar]
- 54. West KP, Jr, Shamim AA, Mehra S, et al. : Effect of maternal multiple micronutrient vs iron-folic acid supplementation on infant mortality and adverse birth outcomes in rural Bangladesh: the JiVitA-3 randomized trial. JAMA. 2014;312(24):2649–58. 10.1001/jama.2014.16819 [DOI] [PubMed] [Google Scholar]
- 55. Kumbhojkar S, Mokase Y, Sarawade S: Kangaroo Mother Care (KMC): An Alternative to Conventional Method of Care for Low Birth Weight Babies. International Journal of Health Sciences and Research. 2016;6(3):36–42. Reference Source [Google Scholar]
- 56. le Roux IM, Tomlinson M, Harwood JM, et al. : Outcomes of home visits for pregnant mothers and their infants: a cluster randomized controlled trial. AIDS. 2013;27(9):1461–71. 10.1097/QAD.0b013e3283601b53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rotheram-Borus MJ, Tomlinson M, le Roux IM, et al. : A cluster randomised controlled effectiveness trial evaluating perinatal home visiting among South African mothers/infants. PLoS One. 2014;9(10):e105934. 10.1371/journal.pone.0105934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Locks LM, Manji KP, McDonald CM, et al. : Effect of zinc and multivitamin supplementation on the growth of Tanzanian children aged 6-84 wk: a randomized, placebo-controlled, double-blind trial. Am J Clin Nutr. 2016;103(3):910–8. 10.3945/ajcn.115.120055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Locks L, Manji K, McDonald C, et al. : Effect of Zinc & Multiple Micronutrient Supplements on Growth in Tanzanian Children. FASEB J. 2015;29(1_supplement):729.1 Reference Source [Google Scholar]
- 60. Lönnerdal B, Kvistgaard AS, Peerson JM, et al. : Growth, Nutrition, and Cytokine Response of Breast-fed Infants and Infants Fed Formula With Added Bovine Osteopontin. J Pediatr Gastroenterol Nutr. 2016;62(4):650–7. 10.1097/MPG.0000000000001005 [DOI] [PubMed] [Google Scholar]
- 61. Nikièma L, Huybregts L, Martin-Prevel Y, et al. : Effectiveness of facility-based personalized maternal nutrition counseling in improving child growth and morbidity up to 18 months: A cluster-randomized controlled trial in rural Burkina Faso. PLoS One. 2017;12(5):e0177839. 10.1371/journal.pone.0177839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Roth DE, Morris SK, Zlotkin S, et al. : Vitamin D Supplementation in Pregnancy and Lactation and Infant Growth. N Engl J Med. 2018;379(6):535–46. 10.1056/NEJMoa1800927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Osendarp S: Effect of zinc supplementation between 1 and 6 mo of life on growth and morbidity of Bangladeshi infants in urban slums. Am J Clin Nutr. 2002;76(6):1401–8. 10.1093/ajcn/76.6.1401 [DOI] [PubMed] [Google Scholar]
- 64. Ostadrahimi A, Salehi-Pourmehr H, Mohammad-Alizadeh-Charandabi S, et al. : The effect of perinatal fish oil supplementation on neurodevelopment and growth of infants: a randomized controlled trial. Eur J Nutr. 2017;57(7):2387–2397. 10.1007/s00394-017-1512-1 [DOI] [PubMed] [Google Scholar]
- 65. Engebretsen IM, Jackson D, Fadnes LT, et al. : Growth effects of exclusive breastfeeding promotion by peer counsellors in sub-Saharan Africa: the cluster-randomised PROMISE EBF trial. BMC Public Health. 2014;14:633. 10.1186/1471-2458-14-633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fadnes LT, Nankabirwa V, Engebretsen IM, et al. : Effects of an exclusive breastfeeding intervention for six months on growth patterns of 4-5 year old children in Uganda: the cluster-randomised PROMISE EBF trial. BMC Public Health. 2016;16(1):555. 10.1186/s12889-016-3234-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tylleskär T, Jackson D, Meda N, et al. : Exclusive breastfeeding promotion by peer counsellors in sub-Saharan Africa (PROMISE-EBF): a cluster-randomised trial. Lancet. 2011;378(9789):420–7. 10.1016/S0140-6736(11)60738-1 [DOI] [PubMed] [Google Scholar]
- 68. Dewey KG, Mridha MK, Matias SL, et al. : Lipid-based nutrient supplementation in the first 1000 d improves child growth in Bangladesh: a cluster-randomized effectiveness trial. Am J Clin Nutr. 2017;105(4):944–57. 10.3945/ajcn.116.147942 [DOI] [PubMed] [Google Scholar]
- 69. Suman RP, Udani R, Nanavati R: Kangaroo mother care for low birth weight infants: a randomized controlled trial. Indian Pediatr. 2008;45(1):17–23. [PubMed] [Google Scholar]
- 70. Urban M: Growth of infants born to HIV-infected women when fed a biologically acidified starter formula with and without probiotics. S Afr J Clin Nutr. 2008;21(1):28–32. 10.1080/16070658.2008.11734148 [DOI] [Google Scholar]
- 71. Vazir S, Engle P, Balakrishna N, et al. : Cluster-randomized trial on complementary and responsive feeding education to caregivers found improved dietary intake, growth and development among rural Indian toddlers. Matern Child Nutr. 2013;9(1):99–117. 10.1111/j.1740-8709.2012.00413.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Velaphi SC, Cooper PA, Bolton KD, et al. : Growth and metabolism of infants born to women infected with human immunodeficiency virus and fed acidified whey-adapted starter formulas. Nutrition. 2008;24(3):203–11. 10.1016/j.nut.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 73. Christian P, Shaikh S, Shamim AA, et al. : Effect of fortified complementary food supplementation on child growth in rural Bangladesh: a cluster-randomized trial. Int J Epidemiol. 2015;44(6):1862–76. 10.1093/ije/dyv155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mills EJ, Thorlund K, Ioannidis JP: Demystifying trial networks and network meta-analysis. BMJ. 2013;346:f2914. 10.1136/bmj.f2914 [DOI] [PubMed] [Google Scholar]
- 75. Kanters S, Ford N, Druyts E, et al. : Use of network meta-analysis in clinical guidelines. Bull World Health Organ. 2016;94(10):782–4. 10.2471/BLT.16.174326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hutton B, Salanti G, Chaimani A, et al. : The quality of reporting methods and results in network meta-analyses: an overview of reviews and suggestions for improvement. PLoS One. 2014;9(3):e92508. 10.1371/journal.pone.0092508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gibson RA, Neumann MA, Makrides M: Effect of increasing breast milk docosahexaenoic acid on plasma and erythrocyte phospholipid fatty acids and neural indices of exclusively breast fed infants. Eur J Clin Nutr. 1997;51(9):578–84. 10.1038/sj.ejcn.1600446 [DOI] [PubMed] [Google Scholar]
- 78. Helland IB, Saugstad OD, Smith L, et al. : Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatrics. 2001;108(5):E82. 10.1542/peds.108.5.e82 [DOI] [PubMed] [Google Scholar]
- 79. Lucia Bergmann R, Bergmann KE, Haschke-Becher E, et al. : Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy? J Perinat Med. 2007;35(4):295–300. 10.1515/JPM.2007.085 [DOI] [PubMed] [Google Scholar]
- 80. Kern SE: Challenges in conducting clinical trials in children: approaches for improving performance. Expert Rev Clin Pharmacol. 2009;2(6):609–17. 10.1586/ecp.09.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Coppini R, Simons SHP, Mugelli A, et al. : Clinical research in neonates and infants: Challenges and perspectives. Pharmacol Res. 2016;108:80–7. 10.1016/j.phrs.2016.04.025 [DOI] [PubMed] [Google Scholar]
- 82. Park JJ, Thorlund K, Mills EJ: Critical concepts in adaptive clinical trials. Clin Epidemiol. 2018;10:343–51. 10.2147/CLEP.S156708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Thorlund K, Haggstrom J, Park JJ, et al. : Key design considerations for adaptive clinical trials: a primer for clinicians. BMJ. 2018;360:k698. 10.1136/bmj.k698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bhutta ZA: Nutrition: How will the next 'Decade of Nutrition' be different from the past one? Nat Rev Gastroenterol Hepatol. 2016;13(8):441–2. 10.1038/nrgastro.2016.102 [DOI] [PubMed] [Google Scholar]