Abstract
Objectives
The emergency department (ED) is a challenging setting to conduct pharmacogenomic studies and integrate that data into fast-paced and potentially life-saving treatment decisions. Therefore our objective is to present the methods and feasibility of a pilot pharmacogenomic study set in the ED that measured pediatric bronchodilator response (BDR) during acute asthma exacerbations.
Methods
This is an exploratory pilot study that collected buccal swabs for DNA and measured bronchodilator response during ED encounters for pediatric asthma exacerbations. We evaluated the study’s feasibility with a qualitative analysis of ED provider surveys and quantitatively by the proportion of eligible patients enrolled.
Results
We enrolled 59 out of 90 patients (65%) that were identified and considered eligible during a 5-month period (target enrollment 60 patients over 12 months). The median patient age was 7 years (interquartile range 4–9 years), 61% (N=36) were male, and 92% (N=54) were African American. Quality DNA collection was successful for all 59 patients. ED provider survey response rate was 100%. Most ED providers reported the study did not impact their workflow (98% of physicians, 88% of nurses, and 90% of respiratory therapists). ED providers did report difficulties with spirometry in the younger age group.
Conclusions
Pharmacogenomic studies can be conducted in the ED setting, and enroll a younger patient population with a high proportion of minority participants. By disseminating this study’s methods and feasibility analysis, we aim to increase interest in pharmacogenomic studies set in the ED and aimed towards future ED-based pharmacogenomic decision-making.
Keywords: Pediatric, Asthma, Bronchodilator Response, Pharmacogenomics, Emergency Department
Introduction
There are emergencies for which pharmacogenomics could make a life-saving difference (e.g., bronchodilator response (BDR) for asthma exacerbations). Yet, pharmacogenomic-driven decisions are typically employed in settings with a relative luxury of time (e.g., outpatient clinics).1,2 For pharmacogenomics to realize its full practice-changing potential, initiatives must expand to new and faster-paced areas such as emergency medicine. For example, albuterol, codeine, metoclopramide, pantoprazole, succinylcholine, tramadol, and many other medications frequently used in the emergency department (ED) have known pharmacogenomic interactions.3 However, even widespread CYP2D6 testing for anti-coagulant therapy has turnaround times too long to be practically employed in the ED.4 For ED providers and patients to benefit from advances in pharmacogenomics, rapid point-of-care testing must be developed and implemented, along with extensive education and clinical decision support.
As a first step, conducting pharmacogenomic research in the ED setting can lay the foundation for those future steps and initiatives. Currently in the broader precision medicine world, some biobanks enroll emergency department (ED) patients (including children).5–7 However, there are few studies on pharmacogenomic-driven ED therapy.8 The ED is a challenging environment for research studies in general given the rapidity, acuity, and unpredictability of patient presentation. However, the ED setting can be even more challenging for pharmacogenomic studies which may require serial and time-sensitive measurements. Further, eventual implementation of pharmacogenomic testing and clinical decision-making in the ED would require rapid point-of-care testing. Therefore, we present the methods and feasibility of a pilot (early stage/discovery) pharmacogenomics study of BDR in children with asthma exacerbations set in a pediatric ED. Our goal is to disseminate methods by which others may begin ED-based pharmacogenomic studies and future clinical implementation initiatives.
Methods
Study Rationale
Nearly 7 million children in the United States have asthma and each on average has at least one exacerbation per year. 9–13 First-line treatment for asthma exacerbations are inhaled beta-2-agonist bronchodilators (e.g., albuterol),14 yet individual BDR varies.15 Several single nucleotide polymorphisms (SNPs) are associated with low BDR in children.16,17 In racially diverse populations, up to 50% of subjects tested exhibited low BDR.18 However, those studies measured BDR when patients were not experiencing an exacerbation.18,19,20 During an asthma exacerbation the albuterol dose-response curve shifts to the right, requiring larger doses to achieve the same effect.21 Inflammatory and epigenetic confounders may also change what SNPs are associated with BDR. Therefore, the SNP or SNPs that actually determine BDR when it matters most (i.e., during an asthma exacerbation) remain unknown. The objective of this exploratory pilot study was to test what SNPs (Table 1) are associated with BDR during an asthma exacerbation. To do so, we measured BDR and collected demographic, clinical, environmental, and genetic data for pediatric ED patients experiencing an asthma exacerbation. As this was a pilot study, we aimed to i) first determine its feasibility and ii) determine if there is a signal for significant and/or new SNP information to justify expansion of the sample population beyond the pilot.
Table 1:
SNPs potentially associated with BDR
| SNP | Chr | Allele: | Gene | MAF | BDR | Potential Mechanism | Reference | |
|---|---|---|---|---|---|---|---|---|
| Effect | Ref | |||||||
| rs28450894 | 4 | T | C | NFKB1 | 0.09 | Low | Bronchial smooth muscle regulation | 18 |
| rs9507294 | 13 | C | T | SPATA13-AS1 | 0.19 | Low | Unknown, anti-sense RNA, may regulate smooth muscle contraction | 19 |
| rs912142 | 13 | G | A | “ “ | 0.41 | |||
| rs2248119 | 13 | A | G | “ “ | 0.42 | |||
| rs9551086 | 13 | T | C | “ “ | 0.19 | |||
| rs9553225 | 13 | A | G | “ “ | 0.14 | |||
| rs1042713 | 5 | A | G | ADRB2 | 0.45 | Low | Related to beta-2 adrenergic receptors | 22, 23 |
| rs73650726 | 9 | G | A | * | 0.05 | High | Unknown; Related to NO signaling pathways & albuterol response | 20 |
| rs7903366 | 10 | T | C | PRKG1 | 0.46 | High | ||
| rs7070958 | 10 | G | A | PRKG1 | 0.46 | Low | ||
| rs7081864 | 10 | A | G | PRKG1 | 0.46 | High | ||
Does not map to gene, BDR = bronchodilator response, Chr = Chromosome, MAF = mean allele frequency, SNP = single nucleotide polymorphism
Study Start-Up
The University of Florida (UF) Institutional Review Board approved this study. This study was conducted in the UF Health Jacksonville Pediatric ED, an inner-city pediatric ED that sees an estimated 15,000 encounters annually, of which approximately 10% are for asthma. The ED employs research coordinators who enroll patients 7 days a week from 8am – 11pm. Our target enrollment was 60 patients over 12 months.
Pre-enrollment, the principal investigator conducted training sessions with research coordinators, pediatric ED nurses (RNs), and pediatric respiratory therapists (RTs). Those sessions explained the purpose of the study and conducted training with hand-held spirometers (Spirobank II, Medical International Research, New Berlin, WI), and buccal swab DNA collection kits (Isohelix SK-1S Buccal-Prep Plus DNA Isolation Kit, Boca Scientific, Dedham, MA). Study presentations and announcements were presented in multiple forums (lectures, meetings) to all ED physicians and staff. Study flyers were posted throughout the Pediatric ED.
Pediatric ED Workflow & Study Protocol
Pediatric asthma patients are typically brought immediately to a resuscitation bed or a private room. The triage RN performs an assessment including the Pediatric Asthma Severity Score (PASS),24 and i) notifies a pediatric ED physician, ii) initiates a treatment of nebulized albuterol and ipratropium bromide, and iii) calls the designated pediatric RT to the bedside. After a focused history and physical exam, that team of physicians, RNs, and RTs formulate a treatment plan that typically includes 1–3 treatments of albuterol (2.5 mg for < 10 kg, 5 mg for ≥ 10kg) with ipratropium bromide (0.5 mg), systemic corticosteroids, and other therapies as indicated. That initial assessment and treatment planning typically occurs within 5–10 minutes of patient arrival. The study added PASS scores before and after each bronchodilator treatment (performed by RNs). For patients ages 7 and older who could perform hand-held spirometry, the study also added spirometry before and after each bronchodilator treatment (performed by RNs or RTs). PASS scores and spirometry were recorded in the electronic medical record (EMR). All other usual ED treatments and procedures remained unchanged.
ED providers paged research coordinators using an EMR order ‘Page Emergency Medicine Research’ (this order was separate from other asthma-related orders). After patient stabilization, research coordinators determined subject eligibility using EMR information and data from the treating ED providers. If patients were deemed eligible, research coordinators then obtained informed consent of legal guardians (or from patients aged 18 years), and informed assent of patients aged 12 – 17 years in private / closed ED rooms. Research coordinators collected real-time ED clinical data, and demographic / environmental data from patients and caregivers (e.g., self-identified race / ethnicity, tobacco exposure, history of allergies / atopy, etc.). Deoxyribonucleic acid (DNA) collection with buccal swabs (Qiagen Buccal Cell Kit, Vento, Netherlands) was performed by either research coordinators or RNs (1 swab per cheek for 2 total per patient). We chose buccal swabs for DNA collection as it is less invasive than phlebotomy, and many pediatric ED asthma patients do not have an intravenous catheter placed. Genomic DNA was extracted from buccal swabs and quantified using a Qiagen extraction kit and Qubit Fluorometer (ThermoFisher Scientific, Waltham, MA). An assay for the SNPs from Table 1 was performed using a targeted AmpliSeq library prep on a NextSeqDx 550 (both Illumina, San Diego, CA). Hand-held spirometers were kept in the Pediatric ED, and buccal swab DNA collection kits were kept in the research coordinator office. Coordinators reported consent and data collection in the ED took between 25–35 minutes, collection and labeling of biospecimens took approximately 10 minutes, and remaining data collection/entry into REDCap™ took approximately 15 minutes. Patient’s participation concluded during the ED visit, and there were no follow-up study visits.
Study Feasibility Analysis
We measured study feasibility through paper surveys administered to the front-line physician (resident, fellow, or attending), bedside ED RN, and bedside RT immediately after each patient encounter (i.e., after clinical care for that patient concluded but during the same shift). Surveys asked if/how the study and its specific procedures impacted workflow with opportunities for free text responses (Table 2). Surveys were de-identified to both the provider and patient (i.e., anonymous). We analyzed surveys using descriptive statistics (count and frequency) and a word cloud of single words, word pairs, and phrases from free text responses.
Table 2:
Survey questions by ED provider type*
| Question | MD | RT | RN |
|---|---|---|---|
| Did enrolling patients in this study impact your workflow? | X | X | X |
| Did you collect buccal swabs for the patient? | X | ||
| Did you perform spirometry with the patient? | X | X | |
| Did any other study procedures impact your workflow? | X | X | |
| Do you feel you understand the purpose of this study? | X | X | X |
All questions had opportunity for free text response to elaborate on yes/no answer.
We compared the number of patients enrolled to the total number of eligible pediatric asthma patients from the same period. Eligible patients were ages 2 – 18 years with ED encounter international classification diagnosis–10 code indicating an asthma exacerbation (J45.21, J45.22, J45.31, J45.32, J45.41, J45.42, J45.51, J45.52, J45.901, J45.902) identified by our institution’s cohort discovery software.25
Results
We enrolled 59 patients from October 11, 2019 to March 7, 2020. Enrollment was paused March 7, 2020 prior to reaching the 60-patient goal due to safety concerns amidst the COVID-19 pandemic. The median patient age was 7 years (interquartile range 4–9 years), 61% (N=36) were male, and 92% (N=54) were African American. Info on BDR. All 59 buccal swab samples after DNA extraction passed quality assurance and had enough quantity to run assays for all included SNPs.
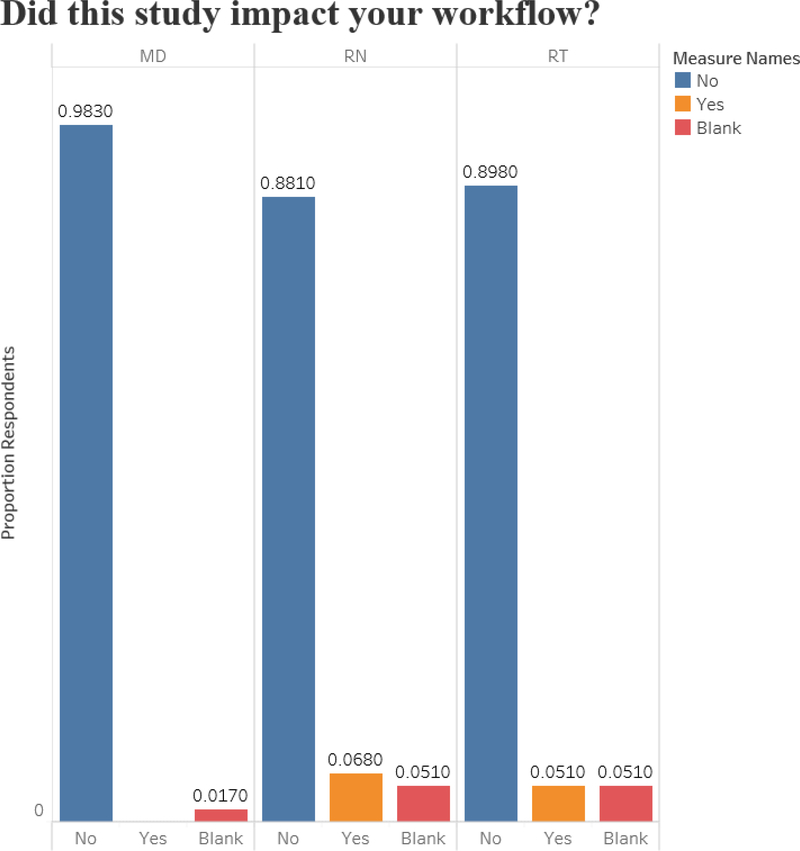
Surveys were completed by all ED providers for all 59 patients (i.e., N=59 surveys for MDs, N=59 for RNs, and N=59 for RTs). The majority reported that the study did not impact their workflow (Figure 1). Most RNs (95%, N=56) and MDs (95%, N=56) reported they felt they understood the study’s purpose. RTs were unable to perform spirometry in 35 patients (N=59%), perhaps due to the median patient age being the lower limit for attempting spirometry per study protocol. Serial PASS scores were completed for all 59 patients.
Figure 1:
Frontline Emergency Department Provider Survey Responses regarding Study Impact on Workflow
Figure 2 displays a word cloud of free text comments. Those comments cite patient cooperation, delaying other procedures, and spirometry as barriers to completing the study simultaneously with patient care. We approached 90 patients for enrollment during research coordinator hours; 20 of those patients were screened but deemed ineligible (return visit, severe respiratory distress, and/or not having a legal guardian present), and an additional 11 patients declined to participate, leaving a final pilot study enrollment of 59 patients (65% of N=90). Using i2b2, there were a potential estimated 192 patients during that time period (includes overnight, weekends, and holidays).
Figure 2:
Word Cloud of Free Text Comments in Front-line Staff Surveys
Delayed treatment (N=5), Distracted Patient/Parent (N=2), Machine did not work (N=2), Patient unable to complete (N=2), Patient was in distress (N=2), Too young (N=2), Not Cooperating (N=1), Not Needed (N=1), Parent Refused (N=1), Easy (N=1)
Discussion
We describe the methods and feasibility of a pharmacogenomics study of children with asthma exacerbations in the ED setting. With an original goal of 60 patients enrolled over 12 months, we reached 59 patients in 5 months. Our protocol emphasized teamwork and involved physicians, RNs, and RTs in the study (as opposed to research coordinators identifying all subjects and performing all study-related procedures). Survey responses from 98% of MDs, 88% of RNs, and 90% of RTs stating that the study had no impact on their workflow highlight that our multidisciplinary approach can facilitate successful integration of a pharmacogenomics study with ED workflow. Notably, we enrolled a younger age range than previous pediatric asthma pharmacogenomic studies.18–20 Additionally, most patients were African American. As EDs increasingly provide safety-net care for underserved and minority populations, involving those populations in pharmacogenomics studies could be accomplished through ED enrollment.26
A minority of survey responses indicated the study impacted ED workflow, more so for RNs and RTs than physicians. Those responses cited patient distraction and cooperation, which may be due to this study’s younger demographic. Many of those comments revealed difficulties with spirometry which is not unexpected given the younger median age of the study (7 years), and the short window of time during which spirometry could be attempted (i.e., a few minutes while nebulized treatments were prepared for administration). This study attempted spirometry in patients aged 7–18 years, however other outpatient studies have performed spirometry in younger children.27 Children of all ages in this study were likely performing spirometry for the first time, as spirometry is not routinely practiced at home.27 Therefore, understanding instructions is crucial and may have been hampered by respiratory distress. Some children may have difficulty complying with technical spirometry requirements (e.g., completing a full forced expiration).28 Coaching spirometry was also a new practice for RNs. Therefore, spirometry may have contributed to the few survey responses that cited treatment delays, since baseline spirometry was encouraged prior to the first bronchodilator treatment. Future studies can optimize the integration of spirometry, and/or use substitutes for BDR such as peak flow meters, or rely on the PASS score and need for other second-line ED treatments such as magnesium in evaluating BDR.24,27 We chose spirometry so we could directly compare results with other pharmacogenomic BDR studies,16–20 and because spirometry can significantly differ from peak expiratory flow.29 It is important to note that spirometry (and PASS scores) are measurements specific to asthma studies, and not to pharmacogenomic studies in general, and therefore not necessarily applicable to others considering pharmacogenomic studies in the ED setting.
There were unforeseen issues worthy of mention. Some patients were excluded from enrollment due to a return visit within 7 days for the same exacerbation. However, that patient population may have more severe or refractory asthma and should be included in future studies. Lastly, we likely would have exceeded our target enrollment of 60 patients had the COVID-19 pandemic not halted enrollment.
Limitations
Survey data do not completely capture staff sentiment regarding the study’s feasibility. This pilot study was conducted at a single institution which may limit generalizability. Regarding the enrollment rate, we enrolled almost two-thirds of patients approached for the study. Enrollment may have been limited by the need for a separate EMR order to page research, and future efforts to boost enrollment could link that order to orders for albuterol. Conversely, the i2b2 estimate of total potentially eligible patients does not accurately capture all elements of enrollment feasibility, as i2b2 includes patients presenting outside of research coordinator hours (on nights, weekend hours, holidays), and may include critically ill patients who were not eligible for the study. i2b2 provides de-identified aggregate patient numbers and so we were unable to ascertain the arrival times and conditions of those 192 patients.25 Another aspect of this study that may limit generalizability is the use of research coordinators. Dedicated research coordinators undoubtedly shoulder some study burdens from clinical staff (e.g., screening patients, ensuring all study measures are recorded properly rather than relying on retrospective EMR data). Although not mentioned in any of the survey comments, it is possible that the presence of the research coordinators impacted ED provider workflow.
Conclusion
We describe a pilot pharmacogenomic study successfully conducted in the pediatric ED setting while emergency asthma treatment is rendered. We also demonstrate that enrollment in the ED setting can capture unique populations such as children and minorities for pharmacogenomic studies. Next, we will analyze physiologic and genomic data, disseminate results, and work to integrate BDR pharmacogenomics into routine pediatric ED asthma exacerbation treatment. By reporting this study’s methods, we aim to generate interest in initiating pharmacogenomic studies in the ED setting for ED patients/conditions. Our future work will build towards the eventual use of point-of-care pharmacogenomic BDR testing in the emergency setting to optimize emergency asthma treatment for children.
Acknowledgements
We acknowledge Ashley Norse, MD, Joe Tucker, RN, David Meysenberg, RN, UF Jacksonville Department of Emergency Medicine Division of Research coordinators, UF Health Jacksonville Pediatric ED nurses, and UF Health Jacksonville respiratory therapists for their assistance with this study.
Both REDCap™ and i2b2 support for University of Florida were supported by a Clinical and Translational Science Institute (CTSI) grant (NIH National Center for Advancing Translational Sciences (NCATS) grant UL1 TR000064 and UL1TR001427.
Funding: Research reported in this publication was supported by the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1 TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Ashley EA. “Towards precision medicine.” Nat Rev Genet. 2016;17:507–22. [DOI] [PubMed] [Google Scholar]
- 2.Farrugia G, Weinshilboum RM. “Challenges in implementing genomic medicine: the Mayo Clinic Center for Individualized Medicine.” Clin Pharmacol Ther. 2013;94(2):204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration, Table of Pharmacogenetic Associations. Available at: https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations. Accessed May 14, 2020.
- 4.Cavallari LH, Van Driest SL, Prows CA, et al. , IGNITE Network. “Multi-site investigation of strategies for the clinical implementation of CYPD2D6 genotyping to guide drug prescribing.” Genet Med. 2019;21(10):2255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourgeouis FT, Avillach P, Kong SW, et al. “Development of the Precision Link Biobank at Boston Children’s Hospital: Challenges and Opportunities.” J Pers Med. 2017;7(4):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saben JL, Shelton SK, Hopkinson AJ, et al. “The Emergency Medicine Specimen Bank: An Innovative Approach to Biobanking in Acute Care.” Acad Emerg Med. 2019;26(6):639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bochud M, Currat C, Chapatte L, Roth C, Mooser V. “High participation rate among 25,721 patients with broad age range in a hospital-based research project involving whole-genome sequencing – the Lausanne Institutional Biobank.” Swiss Med Wkly. 2017. DOI: 10.4414/smw.2017.14528. [DOI] [PubMed] [Google Scholar]
- 8.Seymour CW, Gomez H, Chang CH, et al. “Precision medicine for all? Challenges and opportunities for a precision medicine approach to critical illness.” Critical Care 2017;;21:257 DOI 10.1186/s13054--017--1836--5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asthma Facts: CDC’s National Asthma Control Program Grantees. 2013. Available at https://www.cdc.gov/asthma/pdfs/asthma_facts_program_grantees.pdf. Accessed September 26, 2018.
- 10.Centers for Disease Control and Prevention. 2015. National Health Interview Survey Data. Available at: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed September 26, 2018.
- 11.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011;12(32):1–14. [PubMed] [Google Scholar]
- 12.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123 Suppl 3:S131–45. [DOI] [PubMed] [Google Scholar]
- 13.Akimbami L “Asthma prevalence, health care use and mortality: United States, 2003–05.” 2015. Available from: http://www.cdc.gov/nchs/data/hestat/asthma03-05/asthma03-05.htm. Accessed April 13, 2019.
- 14.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. [DOI] [PubMed] [Google Scholar]
- 15.Sneller H, Carroll CL, Welch K, Sturm J. “Differentiating non-responders from responders in children with moderate and severe asthma exacerbations.” J Asthma. DOI: 10.1080/02770903.2019.1579343. [DOI] [PubMed] [Google Scholar]
- 16.McGeachie MJ, Stahl EA, Himes BE, et al. “Polygenic heritability estimates in pharmacogenetics: focus on asthma and related phenotypes.” Pharmacogenet Genomics. 2013;23:324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naqvi M, Thyne S, Choudhry S, et al. Ethnic-specific differences in bronchodilator responsiveness among African Americans, Puerto Ricans, and Mexicans with asthma. J Asthma. 2007;44(8):639–48. [DOI] [PubMed] [Google Scholar]
- 18.Mak ACY, White MJ, Eckalbar WL, et al. “Whole-genome sequencing of pharamacogenetic drug response in racially diverse children with asthma.” Am J Respir Crit Care Med. 2018;197(12):1552–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padhukasahasram BK, Yang JJ, Levin AM, et al. “Gene-based association identifies SPATA13-AS1 as a pharmacogenomic predictor of inhaled short-acting beta-agonist response in multiple population groups.” Pharmacogenomics J. 2014;14(4):365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spear ML, Hu D, Pino-Yanes M, et al. “A genome-wide association and admixture mapping study of bronchodilator drug response in African Americans with asthma.” Pharmacogenomics J. 2018. DOI: 10.1038/s41397-018-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blake K, Madabushi R, Derendorf H, Lima J. “Population pharmacodynamic model of bronchodilator response to inhaled albuterol in children and adults with asthma.” Chest. 2008;134(5):981–9. [DOI] [PubMed] [Google Scholar]
- 22:Ducharme FM, Zemek R, Gravel J, et al. “Determinants of oral corticosteroid responsiveness in wheezing asthmatic youth (DOORWAY): protocol for a prospective multicentre cohort study of children with acute moderate-to-severe asthma exacerbations.” BMJ Open. 2014;4:e004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23:Martinez FD, Graves PE, Baldini M, et al. “Association between genetic polymorphisms of the beta2-adrenoceptor and response to albuterol in children with and without a history of wheezing.” J Clin Invest. 1997;100:3184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorelick MH, Stevens MW, Schultz TR, Scribano PV. “Performance of a novel clinical score, the Pediatric Asthma Severity Score (PASS), in the evaluation of acute asthma.” Acad Emerg Med. 2004;11(1):10–8. [DOI] [PubMed] [Google Scholar]
- 25.UF Health i2b2. Available at: https://idr.ufhealth.org/i2b2/. Accessed March 10, 2020.
- 26.Popejoy AB. Diversity in precision medicine and pharmacogenomics: Methodological and conceptual considerations for broadening participation. Pharmgenomics Pers Med. 2019;14(12):257–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayuk AC, Uwaezouke SN, Ndukwu CI, Ndu IK, Iloh KK, Okoli CV. “Spirometry in Asthma Care: A Review of the Trends and Challenges in Pediatric Practice.” Clinical Medicine Insights: Pediatrics. 2017;11:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Observations, Opinions, and Ideas about Pulmonary Function Testing. Available at: https://www.pftforum.com/blog/top-10-spirometry-errors-and-mistakes/. Accessed April 2, 2020.
- 29.Agarwal D, Gupta PP. “A comparison of peak expiratory flow measured from forced vital capacity and peak flow meter manouevres in healthy volunteers.” Ann Thorac Med. 2007;2(3):103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]