Abstract
Background:
Deep gluteal syndrome (DGS) is an uncommon source of buttock and groin pain, resulting from entrapment of the sciatic nerve in the deep gluteal space. The incidence and risk factors of postoperative DGS after primary hip arthroscopic surgery are currently unknown.
Purpose:
To investigate the incidence and risk factors of postoperative DGS after primary hip arthroscopic surgery.
Study Design:
Case-control study; Level of evidence, 3.
Methods:
This study reviewed 1167 patients who underwent arthroscopic surgery between 2010 and 2018 by a single surgeon at a single center in Japan. DGS was defined using the seated piriformis stretch test, active hamstring test, and evidence of a hypertrophic sciatic nerve on magnetic resonance imaging. Overall, 11 of 1167 patients were diagnosed with DGS postoperatively. The DGS group (n = 11) was compared with the non-DGS group (n = 1156). Patient age, sex, body mass index (BMI), generalized joint laxity (GJL; Beighton score >6), number of hip arthroscopic procedures, and radiographic parameters including lateral center-edge angle, Sharp angle, vertical center anterior angle, Tönnis angle, alpha angle, ischiofemoral distance, ischiofemoral space, and quadratus femoris space were compared. The prevalence of developmental dysplasia of the hip (DDH) and borderline DDH (BDDH) was also compared. Logistic regression analysis was conducted to identify potential predictors for a postoperative DGS diagnosis.
Results:
The incidence of postoperative DGS in our study was 0.9%. Female sex (male:female ratio: 0:11 in DGS group vs 568:588 in non-DGS group; P < .01), mean number of hip surgical procedures (1.8 ± 0.9 in DGS group vs 1.1 ± 0.4 in non-DGS group; P < .01), and GJL (P < .01) were significantly higher in the DGS group, while the mean BMI was significantly lower in the DGS group (19.8 ± 1.8 vs 22.7 ± 3.6 kg/m2, respectively; P < .01). Radiographic parameters were not significantly different between groups. Logistic regression analysis revealed that female sex (odds ratio [OR], 22.0 [95% CI, 1.29-374.56]), multiple surgical procedures (OR, 7.8 [95% CI, 2.36-25.95]), GJL (OR, 40.9 [95% CI, 8.74-191.70]), lower BMI (OR, 0.77 [95% CI, 0.644-0.914]), and DDH/BDDH (OR, 18.1 [95% CI, 2.30-142.10]) were potential predictors of postoperative DGS.
Conclusion:
The incidence of postoperative DGS in our study was 0.9%. The predictors for postoperative DGS after hip arthroscopic surgery were female sex, GJL, multiple hip surgical procedures, and DDH/BDDH. Although hip arthroscopic surgery can provide favorable clinical outcomes, surgeons should be aware of the risk factors for DGS as a complication of hip arthroscopic surgery.
Keywords: hip arthroscopic surgery, deep gluteal syndrome, piriformis syndrome, sciatic nerve entrapment, deep gluteal pain
Deep gluteal syndrome (DGS) is an uncommon source of buttock and groin pain, resulting from entrapment of the sciatic nerve and the posterior femoral cutaneous nerve in the deep gluteal space.6,18,20 Anatomic variances and abnormalities between musculotendinous structures and nerves can sometimes cause entrapment of the nerve, resulting in DGS, and may vary among patients.6 Other potential causes of DGS are space-occupying lesions as well as posttraumatic or postoperative scarring surrounding the piriformis muscle, obturator internus muscle, proximal hamstring tendon, and sciatic nerve.13,18
Diagnostic and surgical techniques have been evolving to demonstrate varieties of structures entrapping the sciatic nerve and the posterior femoral cutaneous nerve: fibrous bands containing blood vessels, gluteal muscles, and hamstring muscles; the gemelli–obturator internus complex; bone structures; vascular abnormalities; ischiofemoral impingement; greater trochanteric impingement; and space-occupying lesions.13,18 Considering the variations of anatomic entrapment, the term “deep gluteal syndrome” is utilized to describe entrapment of the sciatic nerve, the posterior femoral cutaneous nerve, the superior gluteal nerve, and the inferior gluteal nerve. Entrapment can occur in more than 1 or 2 places in the deep gluteal space.13,17,18 The first-line treatment for DGS is nonsurgical treatment, including rest, nonsteroidal anti-inflammatory agents, ultrasound-guided injections, and physical therapy.13,17,18 Only patients with failed nonoperative treatment are candidates for operative treatment. Several studies have shown the effectiveness of open and endoscopic decompression of these nerves.17,19
Previous reports have demonstrated that postoperative DGS occurs after hip surgery, including total hip arthroplasty.8 In our practice, we have also encountered several slender female patients with DGS after hip arthroscopic surgery, despite no symptoms being present before surgery. However, the incidence and risk factors of postoperative DGS after hip arthroscopic surgery are unclear. The purpose of this study was to investigate the prevalence and risk factors of postoperative DGS associated with hip arthroscopic surgery. It was hypothesized that female sex, multiple number of surgical procedures, lower body mass index (BMI), and generalized joint laxity (GJL) are risk factors for postoperative DGS.
Methods
Design and Setting
This cross-sectional study was approved by the local institutional review board (No. H30-106) and was conducted in accordance with the Declaration of Helsinki. A retrospective review of 1237 hip arthroscopic surgical procedures, including hip arthroscopic labral preservation, such as labral repair and/or reconstruction, cam osteoplasty, and capsular plication, performed by a single surgeon (S.U.) between 2010 and 2018, was conducted. Overall, 70 hips with preoperative DGS, a lumbar spinal disorder, pelvic trauma, a mental disorder, and welfare aid were excluded. The remaining 1167 patients were enrolled (Figure 1).
Figure 1.
Flowchart. DGS, deep gluteal syndrome.
The indications for hip arthroscopic surgery were persistent hip symptoms and intra-articular abnormalities demonstrated on 3.0-T magnetic resonance imaging (MRI) that were not refractory to nonsurgical treatment. Contraindications for hip arthroscopic surgery included osteoarthritis (Tönnis grades >2-3 on radiographs); additional severe bone abnormalities, such as Perthes disease; severe hip dysplasia; and lateral migration of the femoral head.
The diagnosis of DGS was based on a detailed clinical history, including a description of the current condition, date of onset, mechanism of injury (traumatic or nontraumatic), factors that increased or decreased pain, previous consultations, previous surgical interventions, verbal analog pain level, and narcotics use. Patients presenting with posterior hip pain, buttock pain, and pain radiating to the posterior thigh underwent additional provocative tests when the pain was unexplained.17
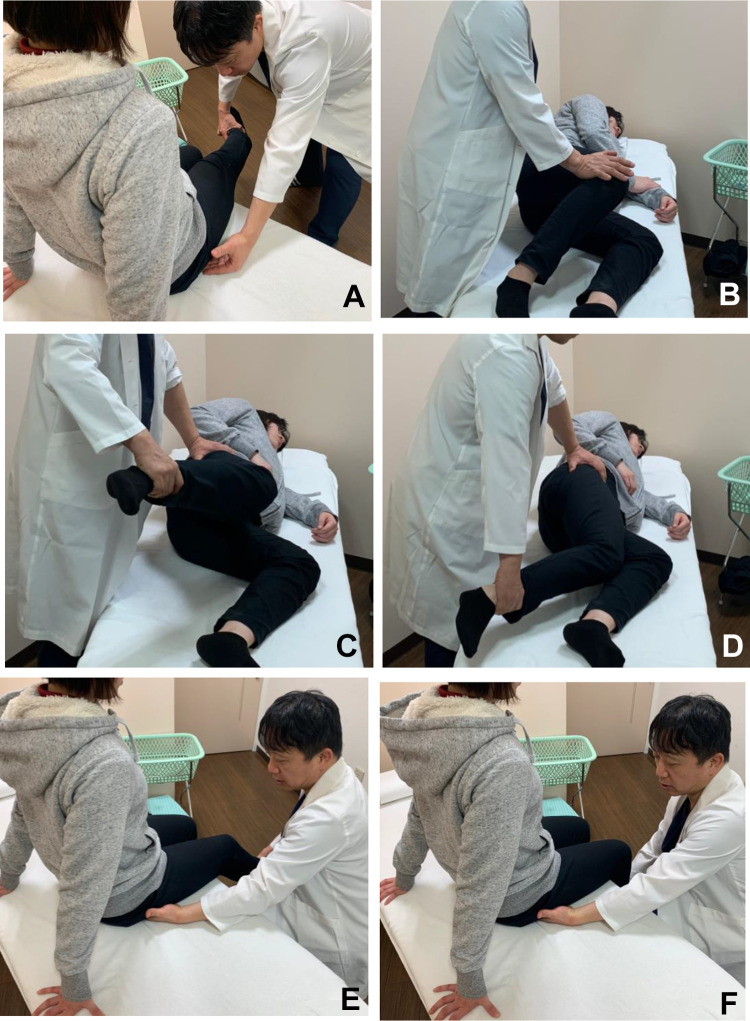
A comprehensive back and hip physical examination ruled out the lumbar spine, sacroiliac joint, and intra-articular lesions as sources of posterior hip pain. In addition, in many cases, the spine was excluded as the principal source of pain by a neurological consultation and MRI. Related symptoms were recorded, including pain when sitting, nighttime pain, back pain, and paresthesia or radicular pain. Each consecutive patient was evaluated by a single examiner (S.U.) using a standardized physical examination protocol. As noted, patients presenting with pain that could not be explained underwent special provocative tests to assess DGS. The seated piriformis stretch test consisted of hip flexion/adduction with internal rotation performed with the patient in the seated position.18,19 The examiner extended the patient’s knee and passively moved the flexed hip into adduction with internal rotation while palpating 1 cm lateral to the ischium (with an index finger) and proximally at the sciatic notch. A positive test finding was defined as the re-creation of posterior pain18,19 (Figure 2A).
Figure 2.
Provocative test for the diagnosis of deep gluteal syndrome. (A) Seated piriformis stretch test. (B) Active piriformis test. (C, D) Ishiofemoral impingement test. (E) Active hamstring test at 30° of knee flexion. (F) Active hamstring test at 90° of knee flexion.
The active piriformis test was performed with the patient in the lateral decubitus position and the involved side up. The patient pushed his or her heel down into the table and actively abducted with external rotation against resistance. The examiner palpated at the level of the piriformis; a positive test finding re-created posterior hip pain18 (Figure 2B). The ischiofemoral impingement test was performed with the patient in the lateral decubitus position. The symptomatic hip was passively taken into extension. A positive test finding was the reproduction of posterior hip pain and numbness in extension with a neutral or adducted hip. The alleviation of pain with abduction during passive hip extension constituted a positive examination finding1,15 (Figure 2, C and D).
The active hamstring test was performed with the patient in a sitting position. It showed marked muscle weakness and pain at 30° of knee flexion, whereas muscle weakness and pain were improved at 90° of knee flexion6,18 (Figure 2, E and F). All patients were evaluated using MRI and ultrasound-guided local anesthesia injections (Figure 3). If MRI scans showed a hypertrophic sciatic nerve or abnormal proximal hamstring tendon, we considered the patients to have DGS.
Figure 3.
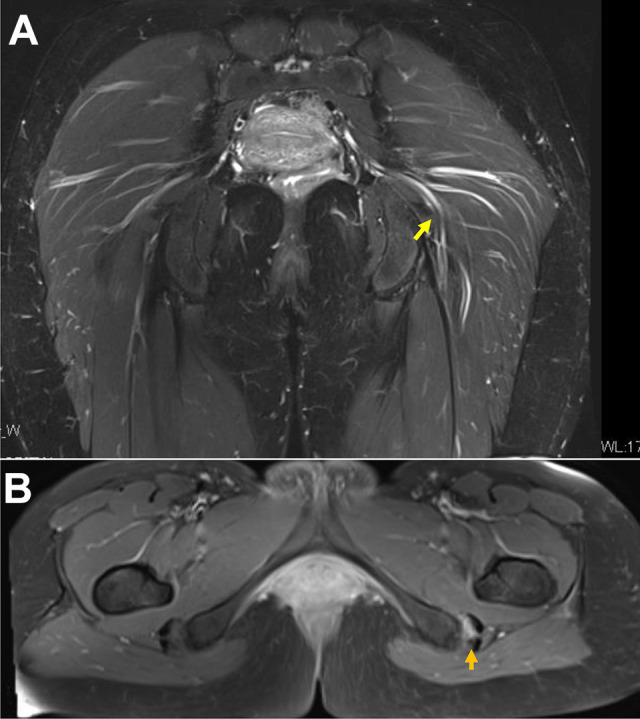
Magnetic resonance imaging scans of a 32-year-old female patient with postoperative deep gluteal syndrome. (A) T2-weighted coronal view showing a hypertrophic sciatic nerve and double-split piriformis proximally at the distal border (yellow arrow). (B) T2-weighted fat-suppressed axial view showing a detached semimembranosus (arrow) from the ischial tuberosity, suggesting proximal hamstring syndrome.
The diagnosis criteria for DGS were composed of history taking (posterior hip pain and difficulty sitting for 30 minutes); physical examinations (tenderness over the deep gluteal space, seated piriformis stretch test, active piriformis test, and active hamstring test); and imaging, including ultrasound-guided local anesthesia injections.
All patients were evaluated for postoperative DGS within 3 months after initial hip arthroscopy surgery. Patients with a positive seated piriformis stretch test, active piriformis test, and active hamstring test finding and/or persistent pain for at least 1 month were considered to have postoperative DGS. Patients with transient symptoms of <1-month duration were excluded because they were considered traction-related nerve palsy.
Overall, 11 (0.9%) of the 1167 patients were diagnosed with postoperative DGS after a primary hip arthroscopic procedure. Patients were divided into 2 groups (DGS group: 11 patients; non-DGS group: 1156 patients). Patient characteristics, including age, sex, BMI, number of hip surgical procedures, and GJL, were also assessed. GJL was defined as a Beighton score of >6 points.2
Radiographic Parameters
All radiographic measurements were manually performed by 2 authors (K.K., F.H.) using a picture archiving and communication system (PACS). We determined the lateral center-edge angle (LCEA), Tönnis angle, femoral neck-shaft angle, presence of a broken Shenton line on pelvic anterior-posterior (AP) views, vertical center anterior (VCA) angle on false profile views, and alpha angle on cross-table lateral or modified Dunn views.4,11,23,31
The LCEA was utilized to define lateral coverage of the acetabulum.31 The femoral neck-shaft angle was calculated as the angle formed by a line through the center of the neck and center of the head and a line parallel to the femoral shaft, as determined by the direction of the shaft below the lesser trochanter.1 The Tönnis angle was utilized as a measure of acetabular inclination.27 The Sharp angle was also utilized as a measure of the acetabular index.25 The VCA angle was utilized as a measure of anterior coverage of the acetabulum.10 The alpha angle was measured to identify cam-type impingement. The presence of a cam deformity was defined as an alpha angle >55° on plain radiographs. We used the highest alpha angle on 2 views, AP pelvic and lateral views, for each hip.21 A broken Shenton line was indicative of superior femoral head subluxation or dislocation, strongly suggesting developmental dysplasia of the hip (DDH). We determined the presence of a broken Shenton line if the inferior femoral neck projection was cephalad to the superior arch of the obturator foramen on standing AP pelvic radiographs.23 The ischiofemoral distance (IFD) was measured on standing AP pelvic views. We determined the IFD as the smallest distance between the ischium and lesser trochanter on standing AP pelvic views.22 We also evaluated all preoperative and yearly radiographic conditions for osteoarthritic changes using the Tönnis classification system.27
The patients were divided into 3 categories according to the LCEA: DDH (LCEA < 20°), borderline DDH (BDDH; 20° ≤ LCEA < 25°), and other (LCEA ≥ 25°).
All MRI examinations were performed according to our department protocol as the following sequences: axial fast spin echo proton density–weighted imaging (repetition time/echo time [TR/TE], 1100/29; matrix, 256 × 320; slice thickness, 1.5 mm; field of view [FOV], 200 × 200 mm) and axial T2-weighted imaging (TR/TE, 4550/67; matrix, 256 × 320; slice thickness, 4.0 mm; FOV, 380 × 380 mm). Measurements were obtained using the length tool of the PACS workstation. The ischiofemoral space was measured at the smallest distance between the lateral cortex of the ischial tuberosity and the medial cortex of the lesser trochanter. The quadratus femoris space (QFS) was measured at the smallest space for passage of the quadratus femoris muscle defined by the superolateral surface of the hamstring tendon and the posteromedial surface of the iliopsoas tendon of the lesser trochanter.22 A study has shown the cutoff values of <17 mm for the IFD and <8 mm for the QFS in patients with symptomatic ischiofemoral impingement.29 The prevalence of patients with an IFD <17 mm was evaluated. The prevalence of patients with a QFS <8 mm was evaluated.
The interobserver and intraobserver reproducibility of these radiographic parameters was investigated. For intraobserver reliability, a single hip surgeon (S.U.) measured each radiograph 3 times with an interval of at least 1 week between measurements. For interobserver reliability, 2 hip surgeons (K.K., F.H.) performed a radiograph review independently and were blinded to clinical data and details of radiology reports. Intraclass correlation coefficients (ICCs) and corresponding 95% CIs were calculated to quantify interobserver and intraobserver reliability for continuous variables. ICCs of 1.00 were indicative of perfect agreement. The strength of agreement was interpreted as having the following ICC values: >0.80, almost perfect agreement; 0.61-0.80, substantial agreement; 0.41-0.60, moderate agreement; and 0.21-0.40, fair agreement. Based on the standards for the kappa statistic proposed by Landis and Koch,9 our measurements were in substantial agreement.
Clinical Outcome Variables
Patients were assessed preoperatively. We obtained clinical follow-up information for all patients. Patients completed patient-reported outcome measures, including the modified Harris Hip Score (mHHS) and Non-Arthritic Hip Score (NAHS).3
Statistical Analysis
The Student unpaired t test was utilized to compare the patient characteristics (age and BMI), number of hip arthroscopic procedures (if patients had undergone 1 previous surgical procedure before the final procedure, they were counted 2 times), and surgery time. Radiographic parameters, including the LCEA, Sharp angle, VCA angle, Tönnis angle, and alpha angle, as well as patient-reported outcome scores (mHHS and NAHS) between the DGS and non-DGS groups were assessed. The Fisher exact test was utilized for categorical variables such as sex and GJL. Logistic regression analysis was conducted to identify predictors of postoperative DGS. For logistic regression analysis, the number of hip arthroscopic procedures was categorized into a binomial variable as single or multiple surgical procedures. We used the statistical software XLSTAT-Biomed (Version 2019.3.2; Addinsoft) for all analyses. We considered a P value <.05 statistically significant. Data were presented as the mean ± SD or odds ratio (OR) with 95% CI unless otherwise stated.
Results
A total of 1167 patients were included in this study. Overall, 11 patients were diagnosed with postoperative DGS, and 1156 patients were not (Figure 1). Patient details are shown in Tables 1 and 2.
TABLE 1.
Patient Characteristicsa
| DGS (n = 11) | Non-DGS (n = 1156) | P Value | |
|---|---|---|---|
| Age, y | 37.5 ± 15.9 | 34.6 ± 9.5 | .54b |
| Sex, male:female, n | 0:11 | 568:588 | <.01c |
| BMI, kg/m2 | 19.8 ± 1.8 | 22.7 ± 3.6 | <.01b |
| No. of hip arthroscopic procedures | 1.8 ± 0.9 | 1.1 ± 0.4 | <.01b |
| GJL, positive:negative, n | 9:2 | 115:1041 | <.01c |
aValues are presented as mean ± SD unless otherwise indicated. BMI, body mass index; DGS, deep gluteal syndrome; GJL, generalized joint laxity.
bThe unpaired t test was used.
cThe Fisher exact test was used.
TABLE 2.
Specific Patient Characteristics Associated With a Postoperative Deep Gluteal Syndrome Diagnosisa
| Case | Age, y | Sex | BMI, kg/m2 | No. of Hip Arthroscopic Procedures | GJL | Diagnosis |
|---|---|---|---|---|---|---|
| 1 | 39 | Female | 18.3 | 2 | Negative | DDH |
| 2 | 24 | Female | 21.3 | 3 | Positive | BDDH |
| 3 | 40 | Female | 17.2 | 2 | Positive | BDDH |
| 4 | 24 | Female | 17.7 | 2 | Positive | BDDH |
| 5 | 49 | Female | 20.3 | 1 | Positive | BDDH |
| 6 | 22 | Female | 20.7 | 1 | Positive | FAI |
| 7 | 29 | Female | 18.3 | 1 | Positive | BDDH |
| 8 | 34 | Female | 21.8 | 3 | Positive | FAI |
| 9 | 32 | Female | 20.8 | 3 | Positive | BDDH |
| 10 | 41 | Female | 20.7 | 2 | Positive | DDH |
| 11 | 48 | Female | 22.5 | 1 | Negative | DDH |
aBDDH, borderline developmental dysplasia of the hip; BMI, body mass index; DDH, developmental dysplasia of the hip; FAI, femoroacetabular impingement; GJL, generalized joint laxity.
There was no significant difference in the patients’ mean age at the time of hip arthroscopic surgery between the groups. Women were more predominant in the DGS group than in the non-DGS group (Table 1).
The mean BMI was significantly lower in the DGS group than in the non-DGS group (P < .01). The number of hip arthroscopic procedures was significantly higher in the DGS group (P < .01). The proportion of patients with GJL was also significantly higher in the DGS group (P < .01) (Table 2). There were no significant differences in preoperative mHHS or NAHS scores between the 2 groups (P = .37 and P = .31, respectively) (Table 3).
TABLE 3.
Preoperative Patient-Reported Outcome Scoresa
| DGS | Non-DGS | P Value | |
|---|---|---|---|
| mHHS | 55.4 ± 12.3 | 60.6 ± 17.2 | .37 |
| Adjusted mHHS | 61.0 ± 13.5 | 66.7 ± 18.9 | .37 |
| NAHS | 42.8 ± 15.2 | 47.9 ± 15.3 | .31 |
| Adjusted NAHS | 53.5 ± 19.0 | 59.9 ± 19.2 | .31 |
aValues are presented as mean ± SD. The unpaired t test was used. DGS, deep gluteal syndrome; mHHS, modified Harris Hip Score; NAHS, Non-Arthritic Hip Score.
Interobserver and intraobserver reliability analysis of the radiographic measurements was conducted. The interobserver/intraobserver ICCs of the LCEA were 0.938/0.989. The interobserver/intraobserver ICCs of the Tönnis and Sharp angles were 0.692/0.854 and 0.787/0.891, respectively. The interobserver/intraobserver ICCs of the alpha and VCA angles were 0.787/0.991 and 0.982/0.977, respectively. There were no significant differences in the radiographic parameters, including the LCEA, Sharp angle, VCA angle, alpha angle, or Tönnis angle. There was no significant difference in the ischiofemoral space between the 2 groups. The mean IFD was significantly smaller in the DGS group than in the non-DGS group. In addition, the mean QFS was significantly smaller in the DGS group than in the non-DGS group (Table 4). There was a trend toward an increasing prevalence of patients with an IFD <17 mm in the DGS group, but there was no significant difference (4/7 in the DGS group vs 155/1001 in the non-DGS group; P = .077). There was no significant difference in the prevalence of patients with a QFS <8 mm between the 2 groups. There was also no significant difference in the mean surgery time between the 2 groups (155.5 ± 53.2 minutes in the DGS group vs 129.3 ± 57.1 minutes in the non-DGS group; P = .13).
TABLE 4.
Preoperative Radiographic Measurementsa
| DGS | Non-DGS | P Value | |
|---|---|---|---|
| LCEA, deg | 25.8 ± 11.0 | 27.2 ± 11.1 | .69 |
| Sharp angle, deg | 44.5 ± 3.8 | 38.9 ± 12.1 | .13 |
| VCA angle, deg | 24.9 ± 13.0 | 23.4 ± 15.4 | .74 |
| Alpha angle, deg | 49.7 ± 21.6 | 54.4 ± 27.2 | .57 |
| Tönnis angle, deg | 8.5 ± 9.6 | 8.0 ± 7.1 | .83 |
| IFD, mm | 16.71 ± 7.74 | 26.05 ± 8.54 | <.001 |
| Ischiofemoral space, mm | 20.77 ± 9.07 | 24.26 ± 9.56 | .229 |
| QFS, mm | 14.42 ± 9.07 | 21.77 ± 9.08 | .008 |
aValues are presented as mean ± SD. The unpaired t test was used. DGS, deep gluteal syndrome; IFD, ischiofemoral distance; LCEA, lateral center-edge angle; QFS, quadratus femoris space; VCA, vertical center anterior.
The prevalence of DDH/BDDH was significantly higher in the DGS group than in the non-DGS group (9/11 vs 415/1156, respectively; P < .01) (Table 5).
TABLE 5.
Primary Diagnosesa
| DGS | Non-DGS | |
|---|---|---|
| BDDH | 6 | 155 |
| DDH | 3 | 260 |
| FAI | 2 | 692 |
| Other (eg, synovial chondromatosis, snapping hip) | 0 | 60 |
aValues are presented as No. The Fisher exact test was used. BDDH, borderline developmental dysplasia of the hip; DDH, developmental dysplasia of the hip; DGS, deep gluteal syndrome; FAI, femoroacetabular impingement.
On logistic regression analysis, female sex (OR, 22.0 [95% CI, 1.29-374.56]; P = .03), GJL (OR, 40.9 [95% CI, 8.74-191.70]; P < .01), multiple surgical procedures (OR, 7.8 [95% CI, 2.36-25.95]; P = .01), DDH/BDDH (OR, 18.1 [95% CI, 2.30-142.10]; P < .01), and lower BMI (OR, 0.77 [95% CI, 0.644-0.914]; P < .01) were identified as potential predictive factors for postoperative DGS (Table 6).
TABLE 6.
Logistic Regression Analysis for Predicting Postoperative Deep Gluteal Syndromea
| Odds Ratio (95% CI) | P Value | |
|---|---|---|
| Female sex | 22.0 (1.29-374.56) | .03 |
| GJL | 40.9 (8.74-191.70) | <.01 |
| Multiple surgical procedures | 7.8 (2.36-25.95) | .01 |
| BMI | 0.77 (0.644-0.914) | <.01 |
aBMI, body mass index; GJL, generalized joint laxity.
The mean BMI of women was significantly lower than that of men (21.5 ± 3.1 vs 23.9 ± 3.7 kg/m2, respectively; P < .01). Furthermore, GJL was more frequently seen in women (P < .01). To control for confounding variables, multiple logistic regression analysis was conducted. GJL (adjusted OR, 11.36 [95% CI, 2.87-45.00]) and multiple surgical procedures (adjusted OR, 3.08 [95% CI, 0.95-10.04]) retained significance as predictive factors for postoperative DGS, whereas lower BMI became insignificant (adjusted OR, 0.79 [95% CI, 0.62-1.01]; P = .06) (Table 7).
TABLE 7.
Multiple Logistic Regression Analysis to Control for Confounding Variablesa
| Odds Ratio (95% CI) | P Value | |
|---|---|---|
| GJL | 11.36 (2.87-45.00) | <.01 |
| Multiple surgical procedures | 3.08 (0.95-10.04) | .06 |
| BMI | 0.79 (0.62-1.01) | .06 |
aBMI, body mass index; GJL, generalized joint laxity.
Based on the number of these 3 predictors (female sex, multiple surgical procedures, and GJL), all participants had a score of 0 to a maximum of 3, and the prevalence of postoperative DGS for each score was calculated. If a patient was female with a history of multiple surgical procedures of the hip and GJL, she was given a score of 3. The prevalence of postoperative DGS was 0.0% (0/491) for score 0, 0.2% (1/490) for score 1, 3.4% (5/143) for score 2, and 13.2% (5/33) for score 3 (Table 8).
TABLE 8.
Scoring With 3 Predictorsa
| DGS, n | Non-DGS, n | Prevalence of Postoperative DGS, % | |
|---|---|---|---|
| Score 0 | 0 | 516 | 0.0 |
| Score 1 | 1 | 489 | 0.2 |
| Score 2 | 5 | 143 | 3.4 |
| Score 3 | 5 | 33 | 13.2 |
| Score ≥2 | 10 | 176 | 5.7 |
aDGS, deep gluteal syndrome.
When the cutoff was set at a score ≥2, the positive likelihood ratio for postoperative DGS was 6.10, with a sensitivity of 0.91 and specificity of 0.85. A score of ≥2 indicated a significantly increased risk of postoperative DGS, with an OR of 57.1 (Table 9).
TABLE 9.
Sensitivity, Specificity, Predictive Value, Positive Likelihood Ratio, and Odds Ratio of Proposed Scoring Systema
| Sensitivity | Specificity | PPV | NPV | Positive Likelihood Ratio | |
|---|---|---|---|---|---|
| Score ≥1 | 1.000 | 0.437 | 0.016 | 1.000 | 1.776 |
| Score ≥2 | 0.909 | 0.851 | 0.054 | 0.999 | 6.100 |
| Score ≥3 | 0.455 | 0.972 | 0.132 | 0.995 | 16.267 |
| Odds Ratio (95% CI) | P Value | ||||
| Score ≥2 | 57.1 (10.24-318.33) | <.01 | |||
| Score ≥3 | 29.0 (8.85-94.90) | <.01 | |||
aNPV, negative predictive value; PPV, positive predictive value.
Discussion
This study identified 4 major risk factors of postoperative DGS after primary hip arthroscopic procedures: female sex, number of hip arthroscopic procedures, lower BMI, DDH/BDDH, and GJL. The diagnosis and management of posterior hip pain have evolved because hip biomechanics and clinical anatomy have become increasingly defined.18 However, the prevalence of postoperative DGS has not been clearly understood. In this study, we demonstrated that the prevalence of postoperative DGS was 0.9%.
A systematic review examining the surgical management of DGS across 28 studies described the causes, surgical indications, patient-reported outcomes, and complications in patients with DGS.7 Some studies revealed that women were more prevalent, while others indicated that men were more prevalent among patients with DGS.19,28,32 Sex differences as a predictor of postoperative DGS are still conflicting. In this study, all patients with postoperative DGS were female.
Femoral torsion can also influence hip spine factors in the loss of terminal hip extension or premature flexion coupling of the hip joint, producing secondary load transfer and pain opposite the site of abnormal loading. A cadaveric study performed by Martin et al14 showed significant effects of simulation, producing decreased femoral version on sciatic nerve strain during hip flexion and abduction. The protective increase in muscular tone can contribute to a loss of sciatic neural mobility because of anatomic piriformis and sciatic nerve variants; the sciatic nerve passes through the piriformis muscle, the division of the nerve passing through and below, above and below, is present in up to 17% of patients. The anatomic basis of DGS development after hip arthroscopic surgery is most likely related to the combination of these differences in piriformis muscle and tendon orientation with increased hip range of motion. The orientation of the tendon or muscle surrounding this area may make a difference in resulting DGS postoperative concerns.
Some studies have shown that ischiofemoral impingement is one of the symptoms of DGS.18 In this study, therefore, the IFD, ischiofemoral space, and QFS were assessed.18 In fact, our findings demonstrated that the mean IFD and QFS were significantly smaller in the DGS group than in the non-DGS group. A smaller IFD and QFS may make sciatic and posterior femoral cutaneous nerves immobile, resulting in DGS. However, there was no significant difference in the prevalence of patients with an IFD <17 mm and a QFS <8 mm between the 2 groups. Apparently, our findings suggest that there is little potential risk of patients with an IFD <17 mm and a QFS <8 mm. In addition, Ohnishi et al22 revealed that the IFD and QFS are significantly smaller in patients with DDH and BDDH than in those with femoroacetabular impingement. It may be one of the reasons why there was a significant difference in the IFD and QFS between the 2 groups.
A further potential cause for the induction of DGS after hip arthroscopic surgery is the traction applied to perform surgery. The amount of traction is variable, with the distraction occurring over a nonstandardized time. The length of time and amount of traction affect the rate of operatively induced sciatic dysfunction, but again, these are mostly related to patient-specific anatomic and biomechanical factors.16,26 Sciatic nerve changes can occur quickly in certain abnormalities, which raises the question of neural monitoring in all patients.26 Even with changes in intraoperative nerve monitoring, the issue is not recognized by patients because they are under general anesthesia during surgery. This lends support for traction time as the most influential factor in neural injuries. Our findings revealed that female patients with GJL frequently had hyperextension of the knee in traction, prompting elongation of the sciatic nerve during hip arthroscopic surgery. Intraoperative nerve monitoring may be helpful to understand the condition of nerves during surgery.
GJL was a potential predictive factor of postoperative DGS. GJL was more prevalent in women than in men. GJL is a relatively common entity in healthy populations, with an overall prevalence of 26.2%.24 Crude logistic regression analysis showed that GJL was a potential predictive factor of postoperative DGS. In addition, it was also associated with DDH. Our findings showed that 6 of 11 patients were diagnosed as having BDDH, and 3 of these 11 patients had DDH. DDH is not usually an indication for hip arthroscopic labral preservation surgery.30 However, BDDH is a relative indication for hip arthroscopic surgery.5 In fact, many practitioners perform hip arthroscopic surgery to treat symptomatic patients in the setting of borderline hip dysplasia. Some practitioners attempt to perform hip arthroscopic surgery combined with periacetabular osteotomy.12,24 Therefore, our findings would be helpful for these surgeons who perform hip arthroscopic surgery in the setting of hip dysplasia.
Undergoing multiple hip arthroscopic procedures was a potential predictive factor of postoperative DGS. Several studies revealed that hip surgery, including total hip arthroplasty or acetabular osteotomy, can cause hematoma or scarring of tissue surrounding the sciatic nerve, resulting in entrapment of the sciatic nerve in the deep gluteal space.18 Therefore, we consider that multiple surgical procedures may increase the potential risk of postoperative DGS.
Some limitations need to be considered. Anatomic variations between the sciatic nerve and piriformis should be considered predictors of postoperative DGS. However, it is difficult to determine anatomic variations between the piriformis and sciatic nerve by preoperative imaging, including MRI or ultrasound. Further investigation and evolution of MRI and ultrasound will be needed. Furthermore, we were unable to evaluate whether traction time may be a potential predictor of postoperative DGS because it was not recorded. Traction time is usually associated with surgery time. Moreover, we should always attempt to reduce traction time to within 60 minutes.
The present study evaluated postoperative DGS that occurred within 90 days after surgery to determine whether complications were mainly associated with intraoperative management. Thus, late complications outside of this window, such as those after sports activities, were not evaluated. Additional studies with a long-term follow-up are needed to assess long-term complications and quality of life.
Conclusion
Overall, 11 (0.9%) of the 1167 patients were diagnosed with postoperative DGS. The predictors for postoperative DGS after hip arthroscopic surgery were female sex, GJL, lower BMI, DDH/BDDH, and multiple hip arthroscopic procedures. Caution is warranted for those with the aforementioned risk factors that are predictive of postoperative DGS.
Acknowledgment
The authors thank Editage (www.editage.jp) for editing and reviewing this article for the English language. They are grateful for the invaluable assistance of Drs Hajime Utsunomiya, Shinichiro Takada, Keisuke Nakayama, Miki Hayashi, Naoya Masumoto, and Kazuaki Miyamoto.
Footnotes
Final revision submitted March 20, 2020; accepted April 7, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: S.U. has received research support from Smith & Nephew, Pfizer, and Johnson & Johnson and consulting fees from Smith & Nephew, Zimmer Biomet, and ConMed. H.D.M. has received consulting fees from Smith & Nephew. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the University of Occupational and Environmental Health (No. H30-106).
References
- 1. Agricola R, Heijboer MP, Ginai AZ, et al. A cam deformity is gradually acquired during skeletal maturation in adolescent and young male soccer players: a prospective study with minimum 2-year follow-up. Am J Sports Med. 2014;42:798–806. [DOI] [PubMed] [Google Scholar]
- 2. Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Ann Rheum Dis. 1973;32:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Christensen CP, Althausen PL, Mittleman MA, Lee JA, McCarthy JC. The Nonarthritic Hip Score: reliable and validated. Clin Orthop Relat Res. 2003;406:75–83. [DOI] [PubMed] [Google Scholar]
- 4. Clohisy JC, Nunley RM, Carlisle JC, Schoenecker PL. Incidence and characteristics of femoral deformities in the dysplastic hip. Clin Orthop Relat Res. 2009;467:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hatakeyama A, Utsunomiya H, Nishikino S, et al. Predictors of poor clinical outcome following arthroscopic labral preservation, capsular plication and cam osteoplasty in the setting of borderline hip dysplasia. Am J Sports Med. 2017;46:135–143. [DOI] [PubMed] [Google Scholar]
- 6. Hernando MF, Cerezal L, Perez-Carro L, Abascal F, Canga A. Deep gluteal syndrome: anatomy, imaging, and management of sciatic nerve entrapments in the subgluteal space. Skeletal Radiol. 2015;44:919–934. [DOI] [PubMed] [Google Scholar]
- 7. Kay J, de Sa D, Morrison L, et al. Surgical management of deep gluteal syndrome causing sciatic nerve entrapment: a systematic review. Arthroscopy. 2017;33:2263–2278.e1. [DOI] [PubMed] [Google Scholar]
- 8. Kumar V, Rushton N. Results of total hip arthroplasty in Gaucher’s disease patients. Hip Int. 2007;17:164–169. [DOI] [PubMed] [Google Scholar]
- 9. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 10. Lequesne M, Berdah L, Gerentes I. Efficacy and tolerance of diacerhein in the treatment of gonarthrosis and coxarthrosis. Rev Prat. 1998;48(17)(suppl):S31–S35. [PubMed] [Google Scholar]
- 11. Lequesne MG, Laredo JD. The faux profil (oblique view) of the hip in the standing position: contribution to the evaluation of osteoarthritis of the adult hip. Ann Rheum Dis. 1998;57:676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maldonado DR, LaReau JM, Perets I, et al. Outcomes of hip arthroscopy with concomitant periacetabular osteotomy, minimum 5-year follow-up. Arthroscopy. 2019;35:826–834. [DOI] [PubMed] [Google Scholar]
- 13. Martin HD, Gómez-Hoyos J. Deep gluteal syndrome In: Martin HD, Gómez-Hoyos J, eds. Posterior Hip Disorders: Clinical Evaluation and Management. Springer; 2019:167–187. [Google Scholar]
- 14. Martin HD, Kboury AN, Schroder R, et al. The effects of hip abduction on sciatic nerve during terminal hip flexion. J Hip Preserv Surg. 2017;4:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin HD, Kelly BT, Leunig M, et al. The pattern and technique in the clinical evaluation of the adult hip: the common physical examination tests of hip specialists. Arthroscopy. 2010;26:161–172. [DOI] [PubMed] [Google Scholar]
- 16. Martin HD, Palmer IJ, Champlin K, Kaiser B, Kelly BT, Leunig M. Physiological changes as a result of hip arthroscopy performed with traction. Arthroscopy. 2012;28:1365–1372. [DOI] [PubMed] [Google Scholar]
- 17. Martin HD, Palmer IJ, Hatem M. Monopolar radiofrequency use in deep gluteal space endoscopy: sciatic nerve safety and fluid temperature. Arthroscopy. 2014;30:60–64. [DOI] [PubMed] [Google Scholar]
- 18. Martin HD, Reddy M, Gomez-Hoyos J. Deep gluteal syndrome. J Hip Preserv Surg. 2015;2:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin HD, Shears SA, Johnson JC, Smathers AM, Palmer IJ. The endoscopic treatment of sciatic nerve entrapment/deep gluteal syndrome. Arthroscopy. 2011;27:172–181. [DOI] [PubMed] [Google Scholar]
- 20. McCrory P, Bell S. Nerve entrapment syndromes as a cause of pain in the hip, groin and buttock. Sports Med. 1999;27(4):261–274. [DOI] [PubMed] [Google Scholar]
- 21. Nötzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84:556–560. [DOI] [PubMed] [Google Scholar]
- 22. Ohnishi Y, Suzuki H, Nakashima H, et al. Radiologic correlation between the ischiofemoral space and morphologic characteristics of the hip in hips with symptoms of dysplasia. AJR Am J Roentgenol. 2018;210:608–614. [DOI] [PubMed] [Google Scholar]
- 23. Rhee PC, Woodcock JA, Clohisy JC, et al. The Shenton line in the diagnosis of acetabular dysplasia in the skeletally mature patient. J Bone Joint Surg Am. 2011;93(suppl 2):35–39. [DOI] [PubMed] [Google Scholar]
- 24. Ricciardi BF, Mayer SW, Fields KG, Wentzel C, Kelly BT, Sink EL. Patient characteristics and early functional outcomes of combined arthroscopic labral refixation and periacetabular osteotomy for symptomatic acetabular dysplasia. Am J Sports Med. 2016;44(10):2518–2525. [DOI] [PubMed] [Google Scholar]
- 25. Sharp IK. Acetabular dysplasia: the acetabular angle. J Bone Joint Surg Br. 1961;43(2):268–272. [Google Scholar]
- 26. Telleria JJM, Safran MR, Harris AH, Garidi JH, Glick JM. Risk of sciatic nerve traction injury during hip arthroscopy: is it the amount or duration? An intraoperative nerve monitoring study. J Bone Joint Surg Am. 2012;94:2025–2032. [DOI] [PubMed] [Google Scholar]
- 27. Tonnis D. Normal values of the hip joint for the evaluation of X-rays in children and adults. Clin Orthop Relat Res. 1976;119:39–47. [PubMed] [Google Scholar]
- 28. Topuz K, Kutlay M, Simsek H, Atabey C, Demircan M, Senol Guney M. Early surgical treatment protocol for sciatic nerve injury due to injection: a retrospective study. Br J Neurosurg. 2011;25:509–515. [DOI] [PubMed] [Google Scholar]
- 29. Torriani M, Souto SC, Thomas BJ, Ouellette H, Bredella MA. Ischiofemoral impingement syndrome: an entity with hip pain and abnormalities of the quadratus femoris muscle. AJR Am J Roentgenol. 2009;193:186–190. [DOI] [PubMed] [Google Scholar]
- 30. Uchida S, Utsunomiya H, Mori T, et al. Clinical and radiographic predictors for worsened clinical outcomes after hip arthroscopic labral preservation and capsular closure in developmental dysplasia of the hip. Am J Sports Med. 2016;44:28–38. [DOI] [PubMed] [Google Scholar]
- 31. Wiberg G. The anatomy and roentgenographic appearance of a normal hip joint. Acta Chir Scand. 1939;83(suppl 58):7–38. [Google Scholar]
- 32. Young IJ, van Riet RP, Bell SN. Surgical release for proximal hamstring syndrome. Am J Sports Med. 2008;36:2372–2378. [DOI] [PubMed] [Google Scholar]