Abstract
We implemented a collaborative diagnostic program in Lahore (Pakistan) aiming to establish the genetic diagnosis, and to asses diagnostic yield and clinical impact in patients with suspected genetic diseases. Local physicians ascertained pediatric patients who had no previous access to genetic testing. More than 1586 genetic tests were performed in 1019 individuals (349 index cases, 670 relatives). Most frequently performed tests were exome/genome sequencing (ES/GS, 284/78 index cases) and specific gene panels (55 index cases). In 61.3% of the patients (n = 214) a genetic diagnosis was established based on pathogenic and likely pathogenic variants. Diagnostic yield was higher in consanguineous families (60.1 vs. 39.5%). In 27 patients, genetic diagnosis relied on additional biochemical testing, allowing rapid assessment of the functional effect of the variants. Remarkably, the genetic diagnosis had a direct impact on clinical management. Most relevant consequences were therapy related such as initiation of the appropriated treatment in a timely manner in 51.9% of the patients (n = 111). Finally, we report 12 candidate genes among 66 cases with no genetic diagnosis. Importantly, three of these genes were validated as ‘diagnostic’ genes given the strong evidence supporting causality derived from our data repository (CAP2-dilated cardiomyopathy, ITFG2-intellectual disability and USP53-liver cholestasis). The high diagnostic yield, clinical impact, and research findings demonstrate the utility of genomic testing, especially when used as first-line genetic test. For patients with suspected genetic diseases from resource-limited regions, ES can be considered as the test of choice to achieve genetic diagnosis.
Subject terms: Molecular medicine, Genetics research, Medical genetics, Molecular medicine, Genetics research
Introduction
Genetic diseases are often severely disabling and have detrimental impact on patients’ physical and cognitive abilities. Such lifelong impairments also have a considerable impact on affected families1. Genetic testing aimed at establishing a precise diagnosis in patients with genetic diseases is extremely important in order to alleviate the disease burden in these families. An accurate diagnosis will guide clinical decisions, allowing for personalized medical management, monitoring and more accurate prognosis. It is of maximum relevance in cases with treatable genetic diseases, where establishing a specific treatment program can make a major difference in outcome2–5. For the families, establishing a diagnosis allows for genetic counseling, including information on recurrence risk, and facilitates family planning and reproductive choices. An accurate diagnosis also advances access to information and assistance from patient support groups as well as access to the education, health, and social care systems, ultimately forming the basis for research into new therapies6.
Unfortunately, access to genetic services and genetic testing is limited in developing countries7. Recently introduced diagnostic technologies, such as exome and genome sequencing (ES/GS), which have a high diagnostic and clinical utility8, are limited for patients in these countries. We implemented a collaborative diagnostic program in Lahore, Pakistan. Diagnostic testing was offered to patients suffering from (suspected) genetic diseases who have limited or no access to genetic testing due to economical or geographical reasons. Through this collaboration program, local clinicians selected patients based on clinical presentation and suspicion of an underlying genetic disease. For 270 index patients (77.4%) ES was performed as the first-line genetic test. GS was indicated as reflex testing in complex cases where ES testing produced no diagnosis.
Here we report the results from 1586 genetic tests performed on 1019 individuals, aimed at establishing the genetic diagnoses. The high diagnostic yield, clinical utility, and relevant research findings demonstrate the usefulness of genomic testing, especially when applied as first-line genetic test.
Results
A total of 245 patients presented with a clinical phenotype at a very young age, having an early disease onset (from prenatal to 5 years old). Approximately half of the patients had a positive family history suggesting a genetic etiology (n = 179). In addition, 295 families reported parental consanguinity, which was expected given the geographical origin of the patients, where intra-familiar marriages are more commonplace. The demographics of all 349 index cases are summarized in Table 1.
Table 1.
Demographics of the complete cohort (349 patients) and cases with genetic diagnosis (P/LP variants identified), uncertain (VUS) and no diagnoses (no relevant variant identified).
| Features | All index cases n = 349 |
Patients with genetic diagnosis n = 214 | Patients with uncertain diagnosis n = 69 | Patients with no diagnosis n = 66 | Chi-square p value |
|---|---|---|---|---|---|
| (% relative to total number of cases in the category) | (% relative to total number of cases in the category) | (% relative to total number of cases in the category) | |||
| Age at onset | |||||
| Prenatal | 45 | 29 (64.4%) | 10 (22.2%) | 6 (13.3%) | 0.352 |
| 0–5 years old | 200 | 119 (59.5%) | 43 (21.5%) | 38 (19.0%) | |
| >5 years old | 33 | 21 (63.6%) | 3 (9.1%) | 9 (27.3%) | |
| Not provided | 71 | 45 | 13 | 13 | |
| Family history | |||||
| Positive | 179 | 109 (60.9%) | 38 (21.2%) | 32 (17.9%) | 0.886 |
| Negative | 150 | 92 (61.4%) | 29 (19.3%) | 29 (19.3%) | |
| Unknown | 20 | 13 | 2 | 5 | |
| Consanguinity | |||||
| Yes | 295 | 186 (60.1%) | 57 (19.3%) | 52 (17.6%) | 0.02 |
| No | 38 | 15 (39.5%) | 12 (31.6%) | 11 (28.9%) | |
| Unknown | 16 | 13 | 0 | 3 | |
As early disease onset, positive family history, and parental consanguinity can indicate a genetic origin of the disease, we evaluated if any of these factors influenced diagnostic yield (Table 1). Indeed, diagnostic yield was higher in consanguineous families, while family history and age at disease onset had no effect (Table 1).
Motive of referral
To assess clinical presentation and motive of referral, we analyzed clinical information based on HPO terms. The top reported disease categories were abnormality of the metabolism and abnormality of the digestive system. Similarly, most reported HPOs were hepatomegaly (69 index cases), splenomegaly (39 cases), elevated hepatic transaminase (34 cases), abdominal distention (31 cases), and jaundice (24 cases). This is corresponding with the main referral clinical department (Gastroenterology). The second most reported disease category was abnormality of the nervous system, with motor delay (51 cases), delayed speech and language development (38 cases), developmental regression (36 cases), and global developmental delay (30 cases) being the top reported HPOs. A summary of the reported phenotypes and corresponding HPOs are depicted in Supplementary Fig. 1.
Diagnostic yield
For ES, trio testing was performed (parents and affected child). In 15 families, biological material from deceased children with suspected genetic diseases was not available. Genetic testing was performed on the parents, aiming to identify variants that could explain the phenotype of their deceased children.
ES was the most frequently chosen method. For 270 patients (77.4%), ES was selected as the first-line test, followed by GS in 78 cases. A summary of the testing strategy in 349 index cases is presented in Supplementary Fig. 2.
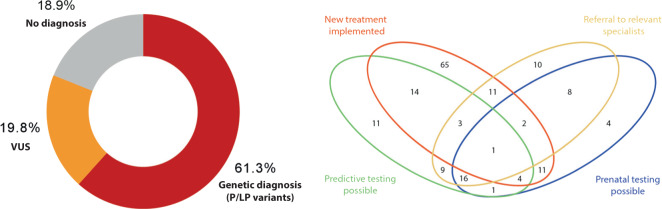
Pathogenic and likely pathogenic variants were identified in 61.3% of the patients (n = 214), establishing a genetic diagnosis. In addition, in 19.8% (69 patients), VUS were identified (Fig. 1a). The majority of the VUS were formally classified as such, but with strong evidence supporting pathogenicity and clinical interpretation consistent with a ‘potential’ genetic diagnosis (46 patients).
Fig. 1. High diagnostic yield and clinical utility.
a High diagnostic yield obtained in 349 index patients. In 214 patients (61.3%) a genetic diagnosis was established based on pathogenic/likely pathogenic variants. b High clinical impact of genetic testing with 170 patients (79.4%) reported with a change in clinical management after establishment of the genetic diagnosis. Venn diagram representing the main categories evaluated and the number of index cases per category/combination.
The most frequently detected type of variants were single nucleotide variants (SNVs), with 191 P/LP variants reported. These included recurrent disease-causing variants for glycogen storage disease IIIa/IIIb (AGL), progressive familial intrahepatic cholestasis type 1 (ATP8B1), and Niemann–Pick disease type A/B (SMPD1). In addition, 20 P/LP copy number variants (CNVs) were reported. Two recurrent CNVs were detected, the SMN1 deletion of exon 7–8 and a duplication within PRSS1 (detected in three cases each). CNV detection was the main reason leading to GS-based diagnosis after inconclusive ES (mainly involving 1–2 exons). CNVs including large chromosomal areas were detected in three patients with Turner syndrome, partial chromosome 22q11.2 deletion syndrome, and partial trisomy 11q. All 179 unique P/LP variants are listed in Supplementary Table 1.
We also explored the mode of inheritance of diseases identified in patients with consanguineous parents, compared to patients without or unknown consanguinity. As expected, a larger proportion of autosomal recessive diseases were diagnosed in the consanguineous group (88.7 vs. 57.1%). They presented less autosomal dominant (AD) and X-linked (XL) diseases compared to the rest of the cases (8.6% AD–1.6% XL vs. 28.6% AD–10.7% XL).
Main diagnosed diseases
Corresponding with main motive of referral, 100 patients (46.7%) were diagnosed with genetic metabolic diseases, including glycogen storage disease, Niemann–Pick disease, biotinidase deficiency, mucopolysaccharidosis, and tyrosinemia. Fifty-two cases (24.4%) were diagnosed with genetic disease of the digestive system, such as early onset hereditary pancreatitis and familial intrahepatic cholestasis. In 41 patients (19.2%), multisystem genetic diseases were diagnosed, including Kleefstra syndrome, Noonan syndrome, Hennekam syndrome, Cantu syndrome, and Raynaud-Claes syndrome. Neurological diseases were detected in 18 patients (8.4%) (e.g., early infantile epileptic encephalopathy, spastic paraplegia). Three patients presented deletions/duplication involving a large chromosomal region (1.4%). A list of all diagnosed diseases is presented in Supplementary Table 2.
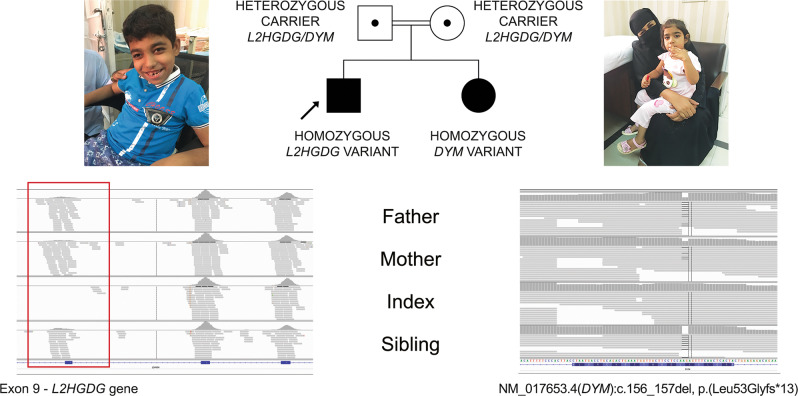
An interesting family with the diagnosis of two distinct genetic diseases is presented in Fig. 2. Parents were consanguineous, with two affected children. Clinical presentations indicated divergent phenotypes in the siblings. The male index had neurodevelopmental delay, seizures, ataxia, and leukodystrophy. The female sibling presented neurodevelopmental delay, regression, and microcephaly. Using ES, we identified a homozygous pathogenic deletion encompassing exon 9 of the L2HGDH in the index, and a homozygous LP frameshift variant in DYM (NM_017653.4:c.156_157del, p.(Leu53Glyfs*13)) in the affected sibling. These findings allowed diagnoses of L-2-hydroxyglutaric aciduria in the index and Dyggve–Melchior–Clausen disease in the affected sister to be established. Parents were confirmed as heterozygous carriers of both variants (Fig. 2).
Fig. 2. Two distinct genetic diseases in one family.
Left: Upper body photograph of male index patient at age of 10 years, with mild dysmorphic features: narrow forehead, thick eyebrows, long eyelashes, mildly upslanted eyes, short palpebral fissure, broad nasal bridge, low hanging columella, short philtrum, thin upper lip and wide mouth, dental malalignment with delayed eruption. A homozygous pathogenic deletion of exon 9 in the L2HGDH gene was detected in this patient by ES. IGV image showing the corresponding region of L2HGDH, with the absence of reads in index patient (area corresponding to exon 9 in red box). Parents and siblings have reduced number of reads consistent with a heterozygous deletion (exon 8 and 10: 100–195 reads, exon 9: 23–35 reads). The findings were confirmed by qPCR. Right: Photograph of female sibling at age of 5 years. A homozygous likely pathogenic variant in the DYM gene was detected in the affected sibling (variant quality score (QS) = 1072, reference value >21538). Carrier status of both parents was confirmed. The variant was validated by Sanger sequencing. Parents of the patients provided written informed consent for the use of patient images for scientific publication.
Furthermore, dual genetic diagnoses were made in 2.1% of the index patients (6/285 index cases with ES/GS). Clinical and genetic details of these patients are summarized in Supplementary Table 3. In four of these cases, one of the diagnosis relates to genetic disorders that are relatively frequent in the studied population (chronic pancreatitis and G6PD deficiency).
Strength of clinical, genetic, and biochemical data combination
In 27 patients, the genetic diagnosis was established using a combination of clinical, genetic, and biochemical data (Table 2). Biochemical testing allowed for supporting evidence for variant pathogenicity, classification, and diagnosis. This was especially relevant for nine patients with novel or very rare variants that otherwise would be classified as VUS due to the lack of functional evidence (MAN2B1, NAGLU, NPC1, SMPD1, GLB1 genes). In other cases, parallel biochemical testing confirmed variant classification based on previous publications or upgraded the classification from LP to P. Most frequently diagnosed diseases were Niemann–Pick disease type A/B and type C1, Mucopolysaccharidosis type IIIB and type IVA, and GM1-gangliosidosis type I (Table 2).
Table 2.
Homozygous variants detected in 27 patients with subsequent abnormal biochemical results.
| Gene | DNA changea | Protein change | Disease (OMIM®) | Biochemical test result | PMID | ACMG criteria |
|---|---|---|---|---|---|---|
| MAN2B1 | NC_000019.9(NM_000528.3):c.1644+4A>G | Splicing | Alpha-Mannosidosis (248500) | Alpha mannosidase < 1.9 (LOD) µmol/l/h | Very rare, first time as homozygous | PS3*, PM2, PP3, PP4 |
| GBA | NM_000157.3:c.1448T>C | p.(Leu483Pro) | Gaucher disease, type I (230800) | Beta-glucocerebrosidase < 1 (LOQ) μmol/L/h; lyso-Gb1 = 401 ng/mL | PMIDs 2880291, 8294487, 8160756 | PS1, PS3, PM2, PP3, PP4 |
| NAGLU | NM_000263.3:c.1336G>A | p.(Glu446Lys) | Mucopolysaccharidosis type IIIB (252920) | Alpha-N-acetylglucosaminidase < 0.3 (LOQ) μmol/L/h | PMID 14984474 | PS1, PS3, PM2, PP3, PP4 |
| NAGLU | NM_000263.3:c.291T>G | p.(Cys97Trp) | Mucopolysaccharidosis type IIIB (252920) | Alpha-N-acetylglucosaminidase < 0.3 (LOQ) μmol/L/h | Very rare, first time as homozygous | PS3*, PM2, PP3, PP4 |
| NAGLU | NM_000263.3:c.701G>C | p.(Arg234Pro) | Mucopolysaccharidosistype IIIB (252920) | Alpha-N-acetylglucosaminidase < 0.3 (LOQ) μmol/L/h | PMID 9832037 | PS1, PS3, PM2, PP3, PP4 |
| NPC1 | NM_000271.4:c.1097C>G | p.(Ser366*) | Niemann-Pick disease type C1 (257220) | Lyso-SM-509 = 1.7 ng/mL; lyso-SM-465 = 14.5 ng/mL | Novel variant | PVS1, PS3, PM2 |
| NPC1 | NM_000271.4:c.2608T>A | p.Ser870Thr | Niemann-Pick disease type C1 (257220) | Lyso-SM-509 = 2.4 ng/mL; lyso-SM-465 = 22.9 ng/mL | Novel variant | PS3*, PM2, PP3 |
| NPC1 | NM_000271.4:c.2978dup | p.(Asp994Argfs*13) | Niemann-Pick disease type C1 (257220) | Lyso-SM-509 = 3.8 ng/mL; lyso-SM-465 = 17.1 ng/mL | Very rare, first time as homozygous | PVS1, PS3, PM2 |
| NPC1 | NM_000271.4:c.3020C>T | p.(Pro1007Leu) | Niemann-Pick disease type C1 (257220) | Lyso-SM-509 = 2.7 ng/mL; lyso-SM-465 = 24.3 ng/mL | PMIDs 17160617, 26666848 | PS1, PS3, PM2, PM3, PM5, PP3 |
| NPC1 | NM_000271.4:c.3503G>A | p.(Cys1168Tyr) | Niemann-Pick disease type C1 (257220) | Lyso-SM-509 = 3.2 ng/mL; lyso-SM-465 = 30 ng/mL | PMID 11333381 | PS3*, PM2, PP3, PP5 |
| HEXA | NM_000520.5:c.109T>A | p.(Tyr37Asn) | Tay-Sachs disease (272800) | Hexosaminidase A < 0.09 (LOD) µmol/L/h; total hexosaminidase = 13.7 μmol/L/h | PMID 18358410 | PS3*, PM2, PM3, PP5 |
| HEXA | NM_000520.5:c.902T>G | p.(Met301Arg) | Tay-Sachs disease (272800) | Hexosaminidase A < 0.09 (LOD) μmol/L/; total hexosaminidase = 7.3 μmol/L/h | PMIDs 8044648, 18490185 | PS3*, PM2, PP3, PP4, PP5 |
| HEXA | NM_000520.4:c.1274_1277dup | p.(Tyr427Ilefs*5) | Tay-Sachs disease (272800) | Hexosaminidase A < 0.09 (LOD) μmol/L/h; total hexosaminidase = 16.2 μmol/L/h | PMIDs 2848800, 21228398, 22975760 | PVS1, PS3, PM2, PP5 |
| HEXB | NM_000521.3:c.1597C>T | p.(Arg533Cys) | Sandhoff disease (268800) | Hexosaminidase A = 0.5 μmol/L/h; total hexosaminidase = 0.6 μmol/L/h | PMIDs 21567908, 22848519 | PS3, PM2, PM3, PM5, PP3, PP5 |
| HEXB | NM_000521.3:c.850C>T | p.(Arg284*) | Sandhoff disease (268800) | Beta-hexosaminidase A = 0.4 μmol/L/h; total hexosaminidase = 0.8 μmol/L/h | PMIDs 8162015, 24613245, 25525159 | PVS1, PS3, PM2, PM3, PP5 |
| SMPD1 | NM_000543.4:c.1382_1383del | p.(His461Argfs*3) | Niemann-Pick disease type A/B (257200/607616) | Acidic sphingomyelinase < 0.4 (LOD) μmol/l/h, lyso-SM-509 = 6.3 ng/mL; lyso-SM-465 = 747 ng/mL | Very rare, homozygote patient in CentoMD® with increased biomarker | PVS1, PS3, PM2, PP4 |
| SMPD1 | NM_000543.4:c.1493G>A | p.(Arg498His) | Niemann-Pick disease type A/B (257200/607616) | Acidic sphingomyelinase < 0.4 (LOD) μmol/L/h; lyso-SM-509 = 2.9 ng/mL, lyso-SM-465 = 534 ng/mL | PMID 15221801 | PS1, PS3, PM2, PP3, PP4 |
| SMPD1 | NM_000543.4:c.1624C>T | p.(Arg542*) | Niemann-Pick disease type A/B (257200/607616) | Acidic sphingomyelinase < 0.4 (LOD) μmol/L/h; lyso-SM-509 = 5.5 ng/mL; lyso-SM-465 = 1480 ng/mL | PMIDs 22796693, 23188845 | PVS1, PS3, PM2, PM3, PP5 |
| SMPD1 | NM_000543.4:c.1624C>T | p.(Arg542*) | Niemann-Pick disease type A/B (257200/607616) | Acidic sphingomyelinase < 0.4 (LOD) μmol/L/h; lyso-SM-509 = 6.3 ng/mL; lyso-SM-465 = 675 ng/mL | PMIDs 22796693, 23188845 | PVS1, PS3, PM2, PM3, PP5 |
| SMPD1 | NM_000543.4:c.1624C>T | p.(Arg542*) | Niemann-Pick disease type A/B (257200/607616) | Acidic sphingomyelinase < 0.4 (LOD) μmol/L/h; lyso-SM-509 = 5.4 ng/mL; lyso-SM-465 = 870 ng/mL | PMIDs 22796693, 23188845 | PVS1, PS3, PM2, PM3, PP5 |
| SMPD1 | NM_000543.4:c.314T>C | p.(Leu105Pro) | Niemann-Pick disease type A/B (257200/607616) | Acidic sphingomyelinase 3.9 μmol/L/h; lyso-SM-509 = 3.2 ng/mL; lyso-SM-465 = 401 ng/mL | PMIDs 15221801, 16010684 | PS1, PS3, PM2, PP3 |
| SMPD1 | NM_000543.4:c.748A>C | p. (Ser250Arg) | Niemann-Pick disease type A/B (257200/607616) | Acidic sphingomyelinase < 0.4 (LOD) μmol/L/h; lyso-SM-509 = 10.4 ng/mL; lyso-SM-465 = 885 ng/mL | PMIDs 12556236 | PS3, PM2, PM3, PP1, PP3, PP5 |
| GLB1 | NM_001317040.1:c.1025_1026delAT | p.Tyr342fs | GM1-gangliosidosis type I (230500) | Beta-galactosidase < 3.3 (LOQ) μmol/L/h | Very rare, 2 unrelated homozygous patients in CentoMD® | PVS1, PS3, PM2 |
| GLB1 | NM_001317040.1:c.1398C>G | p.Tyr466* | GM1-gangliosidosis type I (230500) | Beta-galactosidase = 4.1 µmol/L/h | Novel variant | PVS1, PS3, PM2 |
| GLB1 | NM_001317040.1:c.1399C>T | p.(Arg467Trp) | GM1-gangliosidosis type I (230500) | Beta-galactosidase < 1 (LOD) μmol/L/h | Very rare, first time as homozygous | PS3*, PM2, PP3, PP4 |
| GALNS | NM_001323544.1:c.470C>T | p.(Pro157Leu) | Mucopolysaccharidosis type IVA (253000) | Galactosamine-6-sulfate sulfatase < 0.1 (LOD) μmol/L/h | PMIDs 7633425, 8651279, 22940367 | PS3, PM2, PM5, PP3, PP4 |
| GALNS | NM_001323544.1:c.516C>G | p.(His172Gln) | Mucopolysaccharidosis type IVA (253000) | Galactosamine-6- sulfate sulfatase = 1.7 µmol/L/h | PMIDs 9375852, 22940367 | PS3, PM2, PM5, PP3, PP4 |
PS3* Classification raised from LP to P given the clearly pathological biochemical result.
Reference values: alpha mannosidase ≥ 16.2 µmol/L/h, lyso-SM-509 ≤ 0,9 ng/mL, beta-glucocerebrosidase ≥ 4.1 μmol/L/h; lyso-Gb1 ≤ 6,8 ng/mL; alpha-N-acetylglucosaminidase ≥ 1.5 μmol/L/h; lyso-SM-465 = 46.3 ng/mL; hexosaminidase A ≥ 2.0 μmol/L/h; total hexosaminidase ≥ 4.5 μmol/L/h; acidic sphingomyelinase ≥ 1.7 μmol/L/h; beta-galactosidase ≥ 28.5 μmol/L/h; galactosamine-6-sulfate sulfatase ≥ 2.0 μmol/L/h.
All variants were classified as pathogenic based on abnormal enzyme and/or biomarker results, previous publication(s), and/or additional patients in our data repository.
LOD lower limit of detection, LOQ lower limit of quantification.
aNomenclature of DNA variants according to HGVS recommendations, including intronic variants (NG_008318.1(NM_000528.3):c.1644+4A>G). All variants were checked with Mutalyzer to ensure correct nomenclature.
Clinical utility
Aiming to evaluate clinical utility of the genetic testing, referring physicians reported the main changes on clinical management of the patients and families that had received a genetic diagnosis. For all patients (n = 214) changes in general management were reported (e.g. modification in life style, avoidance of decompensating agents, initiatiation of special surveillance). In 79.4% of the patients (n = 170) more specific measures were reported. Therapy related changes, such as initiation of the appropriated treatment were reported in 51.9% (n = 111). Relevant examples included: initiating treatment with nitisinone in a patient with tyrosinemia type I and bone marrow transplantation in several patients with immunological diseases (immunodeficiencies, lymphoproliferative syndrome, agammaglobulinemia). Furthermore, referral to other relevant medical specialties was reported in 28.0% of the patients (n = 60). Establishment of the genetic diagnosis guided further screening/surveillance of other affected or at-risk individuals (27.6%, n = 59) or made prenatal testing possible in following pregnancies (22.0%, n = 47). Detailed results are shown in Fig. 1b and Supplementary Table 2.
Research findings in cases with no genetic diagnosis and validation of ‘research’ genes
A total of 337 families consented to extensive evaluation of the genetic data beyond the known ‘diagnostic’ genes with established genotype–phenotype relationship. Aiming to identify new candidate genes, we performed further analysis of the sequencing data in 66 patients with no diagnosis after ES/GS testing. Remarkably, in 12 cases (18.2% of the patients with no diagnosis), we identified variants in candidate genes, illustrating the importance of this cohort for research and new gene discovery.
All relevant research findings and the respective patient’s phenotype are summarized in Supplementary Table 4. Selected variants were novel or very rare, mainly homozygous, and with high predicted impact on protein function (e.g., nonsense, frameshift, splicing). Co-segregation in the family (e.g., similarly affected siblings), published evidence on gene function, animal models, or previous case reports were considered. Next, we analyzed phenotype–genotype data from our repository, searching for additional cases supporting the causal role of the variants in the respective genes. Twelve candidate genes were identified for several early onset diseases (Supplementary Table 4). Importantly, three of these candidate genes were validated and confirmed as ‘diagnostics’ considering previous publications and our newly identified patients (ITFG2, USP53, and CAP2).
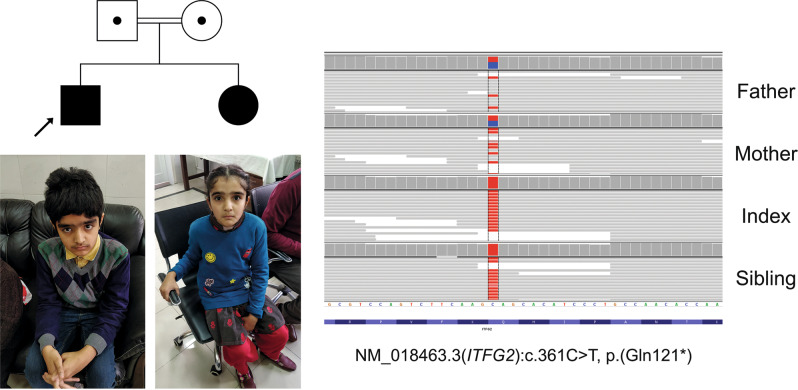
One relevant example relates to the ITFG2 gene. The male index of consanguineous parents, presented with NDD, seizures, developmental regression, and ataxia. A female sibling was similarly affected. Despite clear suspicion of a genetic disease, no relevant variant was identified in known ‘diagnostic’ genes. Additional evaluation focusing on regions of homozygosity shared by the patients identified a homozygous nonsense variant in ITFG2 (Fig. 3). The gene was considered the top candidate based on a previous report of a homozygous nonsense variant in patients with intellectual disability from an unrelated consanguineous family9. Subsequent analysis of our data repository identified three additional unrelated patients with overlapping phenotypes, including NDD and ataxia, with homozygous loss of function variants in ITFG2 (NM_018463.3:c.848-1G>A; NM_018463.3:c.704dupC, p.(Ala236fs), NM_018463.3:c.1000_1001delAT, p.(Ile334fs)). This finding established the genetic diagnosis, producing immediate benefits for all four families.
Fig. 3. ITFG2 is a newly identified gene related to neurodevelopmental delay and ataxia.
Index and sister are homozygous for NM_018463.3:c.361C>T, p.Gln121* and parents are confirmed heterozygous carriers. Photographs of male index (12 years old) and female sibling (10 years old) showing mild dysmorphic features. Male index: thick hair, narrow forehead, bushy eyebrows with synophris, almond-shape eyes, long eyelashes, broad nasal bridge, short columella, short and marked philtrum with Cupid bow and small mouth. Sister: narrow forehead, bushy eyebrows with synophris, small eyes almond shaped, short palpebral fissure, long eyelashes, broad and tall nasal bridge, thin lips with small mouth. Corresponding IGV image in exon 4 of ITFG2 is shown (variant QS = 5165 and 4733 in index and sibling, respectively). Parents of the patients provided written informed consent for the use of their children images in scientific publication.
A similar example refers to a form of early onset cholestasis with hepatomegaly (USP53 gene). The male index presented at 8 months old with jaundice, itching, and pigmented stools. Elevated liver transaminases and bilirubin were detected in blood, and intrahepatic cholestasis was seen in a liver biopsy. Family history was positive for a similarly affected male sibling, suggesting an autosomal recessive or XL disease. A novel homozygous nonsense variant was detected in USP53, a gene not associated with any phenotype in OMIM (last accessed 8 January 2020). Recently, Maddirevula et al. reported the gene as a candidate for cholestatic liver disease based on a consanguineous family with two children having a homozygous frameshift variant in USP5310. In addition, five unrelated patients were identified in our data repository. These patients presented a similar phenotype, mainly including early onset intrahepatic cholestasis and presented five distinct homozygous likely LoF variants in USP53 NM_019050.2:c.169C>T, (p.Arg57*); NM_019050.2:c.475_476delCT, (p.Leu159fs); NM_019050.2:c.822+1delG, p:?; NM_019050.2: c.951delT, (p.Phe317fs); NM_019050.2:c.1214dupA, (p.Asn405fs). These results strongly indicate that biallelic loss of function variants in USP53 are causal for an autosomal recessive form of early onset cholestatic liver disease.
The third gene relates to a severe early onset form of dilated cardiomyopathy (DCM) and congenital heart defects (CAP2 gene). Family history was positive, with two deceased siblings with similar phenotype. A nonsense, homozygous variant was detected in CAP2. The gene was considered as a candidate because of the phenotype observed in Cap2-null mice with cardiomyopathy and cardiac conduction disorder11. Furthermore, Aspit et al. recently reported two children with DCM from a consanguineous Beduine family. Both patients had a homozygous variant affecting the donor splice site of CAP2 exon 7, which causes skipping of exons 6 and 7 in patient-derived fibroblasts12. Taking both studies into account, these findings strongly suggest a role of CAP2 in DCM and the importance of this gene for normal function of the human heart.
All referring clinicians were re-contacted to notify the new diagnoses established based on our data repository.
Secondary findings
Secondary findings were evaluated for the 59 actionable genes according to the latest recommendations of the ACMG13. A total of 337 families in this study were interested to know about secondary findings, and therefore provided signed informed consent. Only 12 families did not wish to be informed about such findings. Four heterozygous P/LP variants were reported in these actionable genes, namely MYBPC3, MYH6, KCNQ1, and BRCA1.
Discussion
In this cohort, we have shown the excellent diagnostic value of ES/GS as first-line testing in patients with suspected genetic diseases. In 61.3% of the patients, a genetic diagnosis was established (Fig. 1a).
Similar reports applying ES/GS as first-line testing in developing countries to achieve genetic diagnosis are scarce. Two recent studies describe their results after performing ES/GS in patients with limited or no previous access to genetic testing. The first study was developed in China and included 1323 patients for whom a ‘mini’ clinical exome was performed in a proband-only-based design. The ES included only 2742 diagnostic genes14. Hu et al. reported a diagnostic yield of 28.8%, which is considerable, but well below the 61.3% yield detected in our cohort. The limited number of assessed genes, singleton testing, and lack of genetic training among the doctors referring the patients may explain the lower diagnostic yield of the Chinese study14.
A second study included a smaller number of patients (60 cases) with suspected genetic diseases from Mexico15. Scocchia et al. reported a high diagnostic yield (68.3%) after GS was used as first-line diagnostic testing. Almost half of the patients presented relatively large CNVs (microdeletions), which could be expected based on the clinical selection with 76.7% of the patients presenting a phenotype consistent with malformation patterns. Notably, the genetic diagnosis influenced clinical management in 48.8% (n = 20) of the cases.
High diagnostic and clinical utility of ES/GS when used as first-line diagnostic test has been demonstrated also in studies from developed countries. French et al. and Stark et al. (United Kingdom and Australia) showed a diagnostic yield of 57.5% (80 patients with ES) and 21% (195 patients with GS), respectively. Similarly, both studies reported a relevant clinical utility (32.6% and up to 48%), concluding that ES/GS are valuable as first-line diagnostic tests16,17.
Several factors are known to influence ES/GS diagnostic yield. Sample size of the reported cohort, consanguinity, and design of testing (trio vs. singleton) are reported to significantly influence diagnostic yield8. The used technology (ES vs. GS), the training of genetic scientists evaluating the data, and establishing genotype–phenotype connections are other recognized factors directly related to the testing/analysis procedure. Finally, a relevant factor is the accuracy of the patients’ clinical evaluation as well as selection and precise communication of the clinical phenotype to the laboratory specialists evaluating the genetic data.
In this cohort of 349 index patients, diagnostic yield was higher in consanguineous families (60.1 vs. 39.5%). Similarly, Alfares et al., reported a diagnostic yield of 49% in patients with a wide range of clinical presentations (222/454). Diagnostic yield was 53% among consanguineous families vs. 39% in patients from non-consanguineous marriages18. Al-Dewik et al., established the genetic diagnosis in 48.3% patients with rare genetic disorders (246/509). Diagnostic yield was higher in consanguineous families (52.4 vs. 39.5%)19. Al-Shamsi et al. reported 50% ES diagnostic yield among 85 patients suspected to have inborn errors of metabolism20. All patients originated from the Middle Eastern region. These studies consistently reported high diagnostic yield among consanguineous families, yet, below the yield reported here.
We consider that the testing technology in combination with the detailed clinical assessment and the analysis of the family history, positively influenced the high diagnostic yield in this study. In addition, the combination of clinical, genetic, and biochemical data facilitated genetic diagnosis in many cases, especially in this cohort greatly composed by patients with metabolic diseases. Novel or very rare variants, which would otherwise be classified as VUS, were classified as pathogenic given the clear pathological biochemical results (enzyme and/or biomarker).
Sending biological samples for genetic testing across country borders is a complex procedure; complicated packaging is necessary to ensure safe handling of the samples during transport, with increased shipping costs. In this study, we have used filter cards (CentoCard®) for handling and transportation of biological material (dried blood spots, DBS) from Pakistan to Germany. The use of DBS makes genetic, enzymatic, and biomarker testing possible. This efficient system of sample collection and transportation can be easily applied in similar studies and it is even more relevant in times of pandemic outbreaks.
A known advantage of ES/GS is the possibility of unbiased assessment of genes. As a result, dual diagnoses can be established. These patients usually present with a wide clinical spectrum representing blended phenotypes. In this study, we identified dual diagnoses in 2.1% of the cases. Similarly, Monies et al., reported dual molecular diagnoses in 1.5% of cases from a highly consanguineous population21. We previously reported dual diagnoses in 1% of 1000 ES cases22. Dual molecular diagnoses were detected in 1.2% (148/11,877) according to the analysis performed by Balci et al., which included five different studies23.
Besides differences in study designs from Schoccia et al. and Hu et al., both groups reported high impact of test results on clinical management (48.8% and 45.1%, respectively), such as referral to other specialties for complementary care, avoiding further diagnostic interventions (e.g., biopsy), starting or discontinuing a therapy, and modifying (post-test) genetic counseling14,15.
In our study the genetic diagnosis had a larger impact on clinical management. Especially relevant were therapy related decisions, such as initiation of the appropriate treatment in a timely manner implemented in 51.9% of the patients. Clinical utility is even more important in developing countries with limited resources and access to health care. Having a specific diagnosis allows careful planning of the available resources. As previously reported, the use of ES as first-line testing is cost-effective, as compared with the standard diagnostic (stepwise) pathway24. Application of ES results in a considerable reduction of health care costs, even more so when applied earlier in the diagnostic trajectory25.
Results from this study could serve as the foundation for implementation of national or regional programs aiming to reduce the burden of severe genetic conditions, and to facilitate the medical care and timely implementation of appropriated therapies for these patients. We show that genomic testing is a valuable diagnostic strategy in developing countries with limited access to genetic testing, especially in populations with elevated consanguinity rate.
In conclusion, our results indicate the excellent diagnostic and clinical value of a genomic approach as first-line diagnostic testing when combined with careful clinical evaluation and patient selection. Specifically, for patients with suspected genetic diseases from resource-limited regions, ES can be considered as the test of choice to achieve genetic diagnosis. In this cohort, ES/GS allowed for genetic research and validation of new gene-phenotype associations, giving hope to previously undiagnosed patients.
Methods
Patients
Patients were selected by local physicians from the Children’s Hospital of Lahore (Pakistan) based on careful evaluation of the phenotype and strong suspicion of an underlying genetic disorder. The Children’s Hospital of Lahore is a tertiary referral center receiving patients from the Punjab province and the rest of the country. This diverse population comprises at least 8–9 different ethnic groups. Most patients were referred from the Gastroenterology department, other referrals included cases from Neurology, Cardiology, Haematology, Nephrology, Oncology, and Endocrinology. Families were invited to the Children’s Hospital of Lahore and provided with information related to genetic testing. After receiving genetic counseling and signing informed consent, families were invited for full anamnesis and clinical examination.
For the purpose of this research, all index cases and relatives tested during the period of 1 year (July 2018–July 2019) were included. Data from 1019 individuals, including 349 index cases and 670 relatives, were extracted from our database and individually curated.
Ethics section
Written informed consents included: consent for genetic test related to the disease(s) of the patient, for secondary findings (unrelated to the main concern but clinically relevant, ACMG gene list13) and research findings (related to the main concern, but implicating genes not yet associated to human diseases). The consent form can be found as Supplementary information. Parents/guardians or adult patients signed the written consents. Written informed consent for publication of clinical data and images was obtained from the patients’ parents.
All analyses were performed in concordance to the provisions of the German Gene Diagnostic Act (Gendiagnostikgesetz). Ethical approval was granted by the Institutional Review Board (IRB)—Ethical committee from the Children’s hospital and the Institute of Child Health (Lahore), to cover the research aspects of the project.
Genetic testing strategy
Blood samples were collected from the index and available relatives (dried blood spots on filter cards—CentoCard®), and sent to CENTOGENE laboratories (Rostock, Germany). Massive parallel sequencing was the most used method with ES being the most selected test, applied as first-line testing. In cases with clear phenotypes, the genetic testing strategy was based on clinical suspicion. Therefore, a relevant panel of genes was indicated if a specific disorder was suspected. Chromosomal microarray analysis (CMA) was mainly indicated in patients with multiple malformations. In cases with no genetic diagnosis, GS was performed as a reflex test. Additional tests, such as biochemical testing, MLPA, qPCR, and Sanger sequencing were performed to confirm initial sequencing findings where necessary. A summary of the genetic testing strategy is depicted in Supplementary Fig. 2.
Genetic testing was also offered to other close relatives, such as similarly affected siblings, or as part of predictive testing. DNA was extracted from dried blood spots on CentoCards® using standard, spin column-based methods.
ES and GS
ES was performed as previously described22. In short, the Nextera Rapid Capture Exome Kit (Illumina, San Diego, CA) or the SureSelect Human All Exon kit (Agilent, Santa Clara, CA) were used for enrichment, and a HiSeq4000 (Illumina) instrument for the actual sequencing with the average coverage targeted to at least 100×. An in-house bioinformatics pipeline, including read alignment to GRCh37/hg19 genome assembly, variant calling, annotation and comprehensive variant filtering is applied. Our ES bioinformatics pipeline is based on the 1000 Genomes Project (1000G) data analysis—data pipeline and GATK best practice recommendations and is composed from widely used open source software projects. First, raw-sequencing reads are converted to standard fastq format using Illumina bcl2fastq software. Then short-reads are aligned to the GRCh37 (hg19) build of the human reference genome using bwa software with the mem algorithm. The alignments are converted to binary bam file format, sorted on the fly and de-duplicated without intermediate input–output operations to temporary files to achieve maximal performance. Afterward variant calling is performed on the secondary alignment files using three different variant callers (GATK HaplotypeCaller26, FreeBayes and SAMtools27). For GS, genomic DNA was fragmented by sonication, and Illumina adapters were ligated to generated fragments for subsequent sequencing on the HiSeqX platform (Illumina) to yield an average coverage depth of at least 30×. Raw sequence data analysis, including base calling, de-multiplexing, alignment to the hg19 human reference genome (GRCh37), and variant calling, was performed using the HiSeq Analysis Software v2.0 pipelines (Illumina, Inc., San Diego, CA). The short-reads were aligned to the GRCh37 (hg19) build of the human reference genome using Isaac aligner algorithm28. Variant calling was performed on the alignment files for SNVs and insertion–deletions (indels) using Starling Small Variant Caller28. Canvas29 and Manta30 were used to detect structural variants and CNVs. Variants were annotated using SnpEff31 and in-house bioinformatics tools22.
Variant evaluation and classification
After variant annotations, filtering and prioritization were performed with an in-house developed tool. The system allows importing of the annotated variants and customized filtering taking into account variant and phenotype-related parameters (frequencies, zygosity, type of variant, HPO terms, mode of inheritance, among others). Trained scientists and human geneticists evaluated the clinical and genetic data. Relevant variants were considered based on compatibility with the suspected phenotype and disease mechanism. All provided clinical data, family history, consanguinity, disease onset/course, available test results, and clinical suspicions were considered. The clinical information was ‘translated’ into HPO terms, registered in our database, and applied for each analysis during variant filtration and prioritization since we previously detected relationship between the number of HPO terms and diagnostic results22. For the selected variants, mode of inheritance of the gene (OMIM®) and all relevant variant information were considered (zygosity, type of the variant, frequency in public databases – gnomAD, ExAc, and disease centered databases—HGMD32, CentoMD®33). For variants previously detected at CENTOGENE, clinical status and clinical information of the carrier individuals were evaluated as well, which aided in variant selection and final classification. Variant nomenclature followed standard recommendations34,35.
Selected candidate variants were classified according to published ACMG guidelines as pathogenic (P), likely pathogenic (LP), and variant of unknown significance (VUS)36,37. Likely benign and benign variants were excluded from reporting.
Interpretation of the findings was done in the clinical context, thus reports were issued as: (a) confirmed diagnosis, for P/LP variant(s) explaining the phenotype(s), (b) potential, for variants formally classified as VUS but with high evidence and compatible phenotype, and (c) unclear, for VUS compatible with the clinical phenotype.
Other methods
Sanger sequencing, MLPA, qPCR, or CMA were performed depending on clinical suspicion or to confirm other tests results. For example, if a large heterozygous deletion was suspected based on ES data, a confirmation by an orthogonal method (e.g. qPCR) was performed. Forward and reverse primers were used for Sanger sequencing, on a 3730xl sequencer (Thermo Fisher Scientific, Waltham, MA). Genome-wide copy number variation + SNP analysis was performed using CytoScan® 750K Array and CytoScan® HD Array according to the manufacturer’s protocols (Thermo Fisher Scientific Inc.). Results were analyzed with the Chromosome Analysis Suite software (ChAS, Affymetrix, Inc., Santa Clara, CA). Analysis thresholds were as set at follows according to our validation to exclude false positive calls: heterozygous deletions with a minimum of 25 markers and/or a size >50 kb; homozygous deletions with at least five aberrant markers and a size >1 kb; duplications >200 kb; regions with absence of heterozygosity >3 Mb. MLPA® analyses were performed with commercially available kits according to the manufacturer’s instructions (MRC-Holland, Amsterdam, The Netherlands). MLPA reactions were run on an ABI 3730xl/3130xl DNA Analyzers (Applied Biosystems). To confirm CNV when no commercially available MLPA kit was available, we performed quantitative PCR assays (qPCR). When possible, in-house designs targeting 2–3 exons within the copy number variant and 1–2 additional fragments outside the alteration were used. Products were run on a LightCycler 480 II (Roche). Segregation of the variant(s) was evaluated in available family members.
The activities of the following enzymes were determined by fluorimetry in dried blood spots: alpha mannosidase, beta-hexosaminidase subunit A, total hexosaminidases, alpha-N-acetylgalactosaminidase, beta-glucocerebrosidase. The activities of the following enzymes were determined by liquid chromatography coupled with mass spectrometry in dried blood spots: acid sphingomyelinase, beta-galactosidase, N-acetylgalactosamine 6-sulfatase. The following biomarkers were quantified in dried blood spots using mass spectrometry: Glucosylsphingosine (Lyso-Gb1), Lyso-SM-509, Lyso-SM-465. All tests were clinically validated according to ISO 15189 guidelines.
Clinical utility
Referring clinicians were retrospectively requested to fill a standard form to report on changes regarding clinical management after a genetic diagnosis was made based on: (i) new medical/surgical treatment implemented, (ii) changes in clinical management such as referral to other relevant medical specialties, changes in life style, avoidance of decompensating agents or special surveillance initiated, and (iii) prenatal diagnosis or predictive testing made possible.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We acknowledge the patients and families for their participation and the support of the staff of the Children’s Hospital of Lahore Hospital and CENTOGENE.
Author contributions
H.C., P.B., and A.R. designed and lead the study, consulted the patients, organized the data collection and diagnostic workflows, and critically evaluated the manuscript. A.M.B.-A supervised patients’ data extraction and curation, performed data analyses and wrote the manuscript. C.B. performed data analysis and contributed to the manuscript. M.N.A., N.S., A.S., and J.P.-L. consulted the patients. V.S. and M.H. organized and supervised sample collection and diagnostic workflows. M.E.R., S.A., C.P., N.A., I.P., and O.P. supervised and analyzed patient’s NGS data. C.C. supervised biochemical testing and data analysis. All authors evaluated the manuscript. H.C. and A.M.B.-A. are joint first authors of the manuscript.
Data availability
The dataset generated and/or analyzed during the current study are available from the corresponding author on reasonable request. All pathogenic and likely pathogenic variants reported are submitted to ClinVar repository and accession numbers are provided in Supplementary Data 1.
Competing interests
The authors A.M.B.-A., V.S., C.B., J.P.-L., M.E.R., S.A., C.P., M.H., N.A., I.P., O.P., C.C., P.B., A.R. are employees at CENTOGENE, AG. None of the other authors declared a potential competing interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Huma Cheema, Aida M. Bertoli-Avella.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41525-020-00150-z.
References
- 1.Schieppati A, Henter JI, Daina E, Aperia A. Why rare diseases are an important medical and social issue. Lancet. 2008;371:2039–2041. doi: 10.1016/S0140-6736(08)60872-7. [DOI] [PubMed] [Google Scholar]
- 2.Jinnah HA, et al. Treatable inherited rare movement disorders. Mov. Disord. 2018;33:21–35. doi: 10.1002/mds.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sedel F, Lyon-Caen O, Saudubray JM. Treatable hereditary neuro-metabolic diseases. Rev. Neurol. 2007;163:884–896. doi: 10.1016/S0035-3787(07)92631-4. [DOI] [PubMed] [Google Scholar]
- 4.van Karnebeek CD, Houben RF, Lafek M, Giannasi W, Stockler S. The treatable intellectual disability APP www.treatable-id.org: a digital tool to enhance diagnosis & care for rare diseases. Orphanet J. Rare Dis. 2012;7:47. doi: 10.1186/1750-1172-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Karnebeek CD, Stockler S. Treatable inborn errors of metabolism causing intellectual disability: a systematic literature review. Mol. Genet. Metab. 2012;105:368–381. doi: 10.1016/j.ymgme.2011.11.191. [DOI] [PubMed] [Google Scholar]
- 6.Wright CF, FitzPatrick DR, Firth HV. Paediatric genomics: diagnosing rare disease in children. Nat. Rev. Genet. 2018;19:253–268. doi: 10.1038/nrg.2017.116. [DOI] [PubMed] [Google Scholar]
- 7.Verma A. empowering the neurogenetic testing services in developing countries: use the basic skills with speed and scale. Ann. Neurosci. 2015;22:1–3. doi: 10.5214/ans.0972.7531.220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark MM, et al. Meta-analysis of the diagnostic and clinical utility of genome and exome sequencing and chromosomal microarray in children with suspected genetic diseases. NPJ Genom. Med. 2018;3:16. doi: 10.1038/s41525-018-0053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harripaul R, et al. Mapping autosomal recessive intellectual disability: combined microarray and exome sequencing identifies 26 novel candidate genes in 192 consanguineous families. Mol. Psychiatry. 2018;23:973–984. doi: 10.1038/mp.2017.60. [DOI] [PubMed] [Google Scholar]
- 10.Maddirevula S, et al. Identification of novel loci for pediatric cholestatic liver disease defined by KIF12, PPM1F, USP53, LSR, and WDR83OS pathogenic variants. Genet. Med. 2019;21:1164–1172. doi: 10.1038/s41436-018-0288-x. [DOI] [PubMed] [Google Scholar]
- 11.Field J, et al. CAP2 in cardiac conduction, sudden cardiac death and eye development. Sci. Rep. 2015;5:17256. doi: 10.1038/srep17256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aspit L, et al. CAP2 mutation leads to impaired actin dynamics and associates with supraventricular tachycardia and dilated cardiomyopathy. J. Med. Genet. 2019;56:228–235. doi: 10.1136/jmedgenet-2018-105498. [DOI] [PubMed] [Google Scholar]
- 13.Kalia SS, et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet. Med. 2017;19:249–255. doi: 10.1038/gim.2016.190. [DOI] [PubMed] [Google Scholar]
- 14.Hu X, et al. Proband-only medical exome sequencing as a cost-effective first-tier genetic diagnostic test for patients without prior molecular tests and clinical diagnosis in a developing country: the China experience. Genet. Med. 2018;20:1045–1053. doi: 10.1038/gim.2017.195. [DOI] [PubMed] [Google Scholar]
- 15.Scocchia A, et al. Clinical whole genome sequencing as a first-tier test at a resource-limited dysmorphology clinic in Mexico. NPJ Genom. Med. 2019;4:5. doi: 10.1038/s41525-018-0076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French CE, et al. Whole genome sequencing reveals that genetic conditions are frequent in intensively ill children. Intensive Care Med. 2019;45:627–636. doi: 10.1007/s00134-019-05552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stark Z, et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet. Med. 2016;18:1090–1096. doi: 10.1038/gim.2016.1. [DOI] [PubMed] [Google Scholar]
- 18.Alfares A, et al. A multicenter clinical exome study in unselected cohorts from a consanguineous population of Saudi Arabia demonstrated a high diagnostic yield. Mol. Genet. Metab. 2017;121:91–95. doi: 10.1016/j.ymgme.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Al-Dewik N, et al. Clinical exome sequencing in 509 Middle Eastern families with suspected Mendelian diseases: The Qatari experience. Am. J. Med. Genet. A. 2019;179:927–935. doi: 10.1002/ajmg.a.61126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Shamsi A, Hertecant JL, Souid AK, Al-Jasmi FA. Whole exome sequencing diagnosis of inborn errors of metabolism and other disorders in United Arab Emirates. Orphanet J. Rare Dis. 2016;11:94. doi: 10.1186/s13023-016-0474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monies D, et al. The landscape of genetic diseases in Saudi Arabia based on the first 1000 diagnostic panels and exomes. Hum. Genet. 2017;136:921–939. doi: 10.1007/s00439-017-1821-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trujillano D, et al. Clinical exome sequencing: results from 2819 samples reflecting 1000 families. Eur. J. Hum. Genet. 2017;25:176–182. doi: 10.1038/ejhg.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balci TB, et al. Debunking Occam’s razor: diagnosing multiple genetic diseases in families by whole-exome sequencing. Clin. Genet. 2017;92:281–289. doi: 10.1111/cge.12987. [DOI] [PubMed] [Google Scholar]
- 24.Tan HL, et al. Nonsynonymous variants in the SMAD6 gene predispose to congenital cardiovascular malformation. Hum. Mutat. 2012;33:720–727. doi: 10.1002/humu.22030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vrijenhoek T, et al. Whole-exome sequencing in intellectual disability; cost before and after a diagnosis. Eur. J. Hum. Genet. 2018;26:1566–1571. doi: 10.1038/s41431-018-0203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van der Auwera GA, et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013;43:11 10 11-11 10 33. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raczy C, et al. Isaac: ultra-fast whole-genome secondary analysis on Illumina sequencing platforms. Bioinformatics. 2013;29:2041–2043. doi: 10.1093/bioinformatics/btt314. [DOI] [PubMed] [Google Scholar]
- 29.Roller E, Ivakhno S, Lee S, Royce T, Tanner S. Canvas: versatile and scalable detection of copy number variants. Bioinformatics. 2016;32:2375–2377. doi: 10.1093/bioinformatics/btw163. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics. 2016;32:1220–1222. doi: 10.1093/bioinformatics/btv710. [DOI] [PubMed] [Google Scholar]
- 31.Cingolani P, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly. 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stenson PD, et al. Human Gene Mutation Database (HGMD): 2003 update. Hum. Mutat. 2003;21:577–581. doi: 10.1002/humu.10212. [DOI] [PubMed] [Google Scholar]
- 33.Trujillano D, et al. A comprehensive global genotype-phenotype database for rare diseases. Mol. Genet. Genom. Med. 2017;5:66–75. doi: 10.1002/mgg3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.den Dunnen JT, et al. HGVS recommendations for the description of sequence variants: 2016 update. Hum. Mutat. 2016;37:564–569. doi: 10.1002/humu.22981. [DOI] [PubMed] [Google Scholar]
- 35.Simons A, Shaffer LG, Hastings RJ. Cytogenetic nomenclature: changes in the ISCN 2013 compared to the 2009 edition. Cytogenet. Genome Res. 2013;141:1–6. doi: 10.1159/000353118. [DOI] [PubMed] [Google Scholar]
- 36.South ST, et al. ACMG Standards and Guidelines for constitutional cytogenomic microarray analysis, including postnatal and prenatal applications: revision 2013. Genet. Med. 2013;15:901–909. doi: 10.1038/gim.2013.129. [DOI] [PubMed] [Google Scholar]
- 37.Richards S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauer P, et al. Development of an evidence-based algorithm that optimizes sensitivity and specificity in ES-based diagnostics of a clinically heterogeneous patient population. Genet. Med. 2019;21:53–61. doi: 10.1038/s41436-018-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset generated and/or analyzed during the current study are available from the corresponding author on reasonable request. All pathogenic and likely pathogenic variants reported are submitted to ClinVar repository and accession numbers are provided in Supplementary Data 1.