Abstract
Retinal ganglion cells (RGCs) are known to be involved in several ocular disorders, including glaucoma and Leber hereditary optic neuropathy (LHON), and hence represent target cells for gene therapies directed towards these diseases. Restricting gene therapeutics to the target cell type in many situations may be preferable compared to ubiquitous transgene expression, stimulating researchers to identify RGC-specific promoters, particularly promoter sequences that may also be appropriate in size to fit readily into recombinant adeno associated viral (AAV) vectors, the vector of choice for many ocular gene therapies. In the current study we analysed EGFP expression driven by various sequences of the putative human NEFH promoter in order to define sequences required for preferential expression in RGCs. EGFP expression profiles from four different potential NEFH promoter constructs were compared in vivo in mice using retinal histology and mRNA expression analysis. Notably, two efficient promoter sequences, one comprising just 199 bp, are presented in the study.
Subject terms: Genetics, Molecular medicine
Introduction
Inherited ocular disorders have been at the forefront of the field of gene therapy; particularly given the recent market authorisation of Luxturna in the US and Europe, a recombinant adeno associated virus (AAV) 2 therapy for biallelic RPE65-linked inherited retinal degenerations1. To date there are approximately 30 clinical trials for recombinant AAV therapies for ocular indications (clinicaltrials.gov). Restricting expression of a gene therapy to the target tissue or target cell type, in principle, should provide significant potential safety benefit, as well as possibly increasing the efficacy of the therapy2,3. The use of well characterised AAV serotypes with specific cell tropisms, cell/tissue specific promoters as well as surgical procedures to deliver the therapy locally all represent ways of achieving this. For example, AAV2 is known to target retinal ganglion cells (RGCs) efficiently following intraocular injection4–7.
We previously demonstrated that a novel 2.2 kb murine Neurofilament heavy (Nefh) gene promoter preferentially and efficiently drove EGFP expression in RGCs and amacrine cells (ACs), compared to a ubiquitous CMV promoter8 when administered intravitreally in adult wild type mice using AAV2. Others too have investigated promoter sequences that express preferentially in RGCs. However, many promoter sequences of genes known to be RGC-specific, such as Brn3a, Thy1 and Rbpms, are too large to be incorporated into AAV vectors, the vector of choice for many ocular gene therapies. AAV has a maximum cargo capacity of approximately 4.7 kb8–13. One study demonstrated that the DCX promoter (3310 bp; doublecortin) can drive strong and specific RGC expression following AAV-QuadYF-mediated delivery14,15. In addition, Simpson et al.16 analysed various promoters with similar expression profiles and found the NEFL (2693 bp; neurofilament, light polypeptide) promoter to be the most specific to RGC in mice and NHPs. Following intravitreal (IV) delivery, high levels of gene expression from a 1 kb upstream sequence of the SNCG gene was shown to co-localise with Brn3A indicating preferential expression in RGCs. However this promoter was not further refined6.
Clearly, when treating a human condition, employing a human DNA sequence, rather than the murine Nefh promoter sequence, may be preferable in terms of utilising the human transcription and translation machinery. Thus in the current study we analysed upstream sequences of the human Neurofilament Heavy (NEFH) gene to establish regions that convey efficient tissue specificity. The focus was also on identifying a smaller ‘RGC promoter’ that as part of a gene therapy construct would readily fit within the 4.7 kb confines of the AAV packaging capacity. Since AAV2 is the vector of choice for targeting rodent RGCs17–19 and in addition is known from Luxturna and various clinical trials to be well tolerated in the human eye (Ref.1, clinicaltrials.gov), this serotype was utilised in the current study.
Results
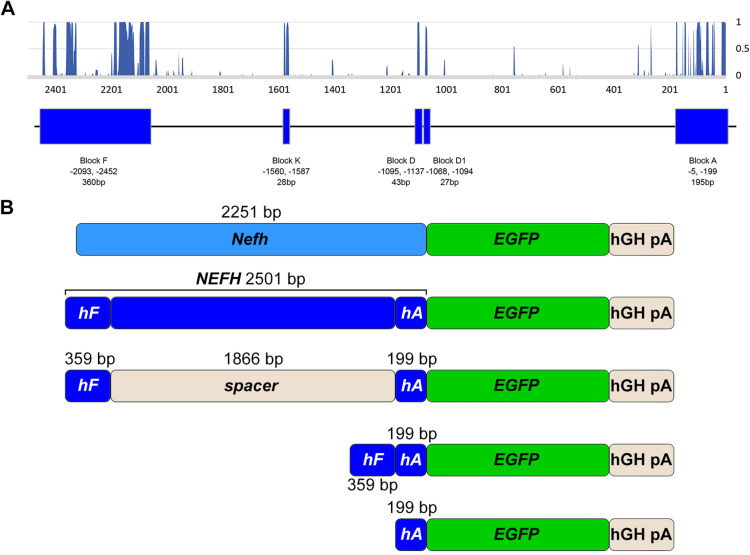
Significant advances have been made in the development of gene therapies for ocular disease; indeed the first in vivo AAV gene therapy to receive market authorisation was Luxturna for RPE65-linked recessive inherited retinal degenerations1. Clearly, using promoters that restrict transgene expression to the target cell or tissue type may represent advantages in terms of potential safety and efficacy in gene therapy approaches. Previous studies suggested that the murine Nefh mRNA is one of the most highly enriched transcripts in RGCs with an enrichment factor (EF) of 245-fold in the associated analysis8,10. We established that a 2.2 kb murine Nefh promoter (Nefh-P) driving an EGFP gene, not only provided high levels of transgene expression in the ganglion cell layer (GCL) but also enabled highly preferential expression in RGCs with only a minimal number of ACs expressing the EGFP transgene. The objective of the current study was the characterisation and in vivo evaluation of a human NEFH promoter for potential use in AAV-mediated gene therapies. In the current study a 2.5 kb region of upstream sequence of the human NEFH gene was compared between 40 placental mammalian species using an in silico pipeline, which was developed specifically to isolate basewise conservation data from UCSC. Conservation data ranged from 0 to 1 for a given base, where 0 represents no significant conservation between mammals and 1 indicates complete conservation. Results of this analysis were plotted to visualise conserved regions (Fig. 1A); the rational being that sequence conservation between mammalian species might indicate functionally important sequences. Employing this methodology, two highly conserved regions, termed Block F and Block A were identified. 2.5 kb of the NEFH upstream region, encompassing both Block F and Block A and the endogenous sequence in between were then cloned upstream of an EGFP cDNA (NEFH-P). Additionally, the Block F and Block A sequences were generated in a construct with a non-functional spacer sequence between the blocks and cloned upstream of the EGFP cDNA (hF-sp-hA-P). Notably this spacer sequence was designed to be the same length as the endogenous sequence. Blocks F and A were also engineered in one construct such that they were juxtaposed and upstream of the EGFP cDNA (hF-hA-P). Finally, Block A was cloned on its own upstream of the EGFP cDNA (hA-P; Fig. 1B). High titre preparations of AAV2/2 expressing NEFH-P, hF-sp-hA-P, hF-hA-P and hA-P were generated and EGFP expression profiles compared following subretinal (SR) or intravitreal (IV) injection into eyes of wild type mice. Full sequences of human NEFH-P, indicating conserved Block F and conserved Block A, and hA-P are provided in Supplemental Table 1.
Figure 1.
NEFH upstream sequence analysis and construct design. (A) Sequence conservation between 40 placental mammal species within the 2.5 kb region directly upstream of the human NEFH transcriptional start site, i.e. the putative human NEFH promoter region. Conservation level between 0 and 1 was assigned to each nucleotide. Units of conserved regions have been defined as Blocks F, K, D, D1 and (A,B) Schematic representation of human NEFH promoter constructs driving an EGFP cDNA. Conserved regions hF and hA consisting of Block F and Block A, and the spacer are indicated. The murine Nefh promoter is also given for reference. Promoter element sizes are given in base pairs (bp).
Histological analysis of promoter activity
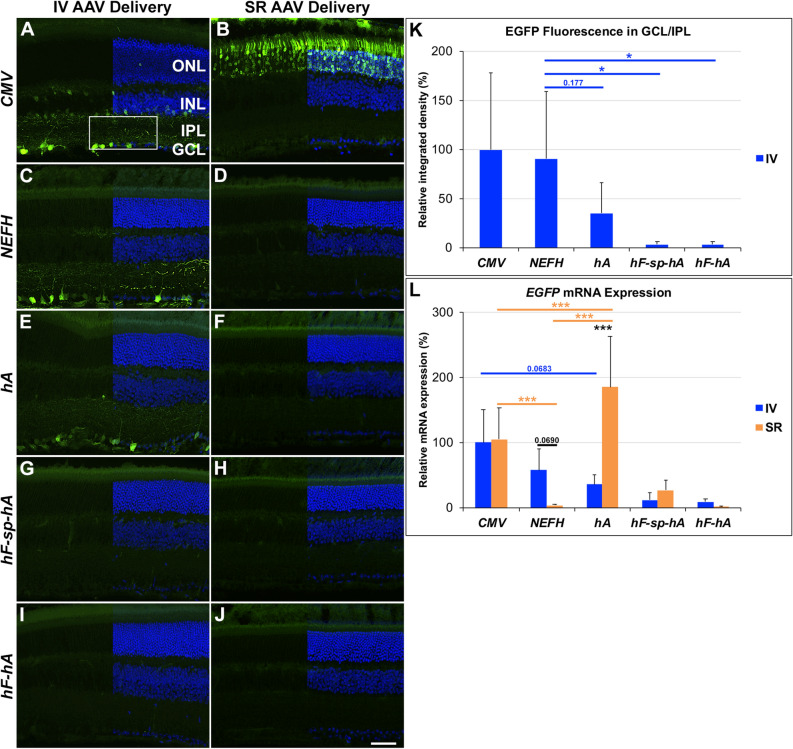
Following both IV and SR injection of various RGC promoter constructs, native EGFP-positive cells were assessed 4 weeks post AAV delivery. Native EGFP-positive cells were identified in the GCL and the inner plexiform layer (IPL), with NEFH-P, hA-P and CMV-P (Fig. 2A,C,E), but not with hF-sp-hA-P or hF-hA-P (Fig. 2G,I) when delivered IV, while photoreceptor cells were readily transduced with CMV-P when delivered SR and, to a lesser extent, EGFP-positive cells were found in the GCL, IPL and inner nuclear layer (IN; Fig. 2B). Notably, EGFP-positive cells were not detected with any of the NEFH promoter constructs following SR injection (Fig. 2D,F,H,J). Native EGFP fluorescence (normalised integrated fluorescence intensity) levels in the GCL and IPL following IV delivery of human NEFH-P, hA-P, hF-sp-hA-P and hF-hA-P promoter constructs were quantified and compared to EGFP fluorescence from CMV-P (100 ± 78.1%). Fluorescence levels were 90.9 ± 68.2%, 35.2 ± 30.8%, 3.4 ± 2.4% and 2.4 ± 2.4% respectively (Fig. 2K); Thus NEFH-P and CMV-P expressed at similar levels and the 199 bp hA-P provided approximately a third of CMV-P expression levels.
Figure 2.
Native EGFP expression following intravitreal (IV) and subretinal (SR) injection of AAV delivered NEFH promoter constructs. 1 × 109 vg of CMV-P, NEFH-P, hA, hF-sp-hA or hF-hA vectors were delivered by IV or SR injection to adult wild type mice and analysed 4 weeks post-injection. For histology, eyes were enucleated, fixed in 4% pfa, cryosectioned and stained with DAPI. Green and blue label correspond to native EGFP and DAPI fluorescence, respectively. Native EGFP fluorescence was detectable from CMV-P (A), NEFH-P (C) and hA-P (E) following IV injection. When delivering SR, native EGFP fluorescence was detectable from the CMV-P construct (B). ONL outer nuclear layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer. Scale bar (J) 50 μm. (K) Integrated fluorescence intensity of EGFP label was quantified in the GCL and IPL layers (as exemplified by the white rectangle in A) in the IV injected retinas (using cellSens); relative values are given as mean + SD in a bar chart (CMV-P was taken as 100%), n = 4–5, *p < 0.05 (t-test). (L) mRNA was purified from transduced whole retinas and EGFP expression determined using RT-qPCR; Actb was used as internal control. Relative EGFP mRNA expression levels from CMV-P, NEFH-P, hA-P, hF-sp-hA-P or hF-hA-P delivered IV (n = 11, 10, 12, 11, 5 respectively) were compared to expression levels following SR delivery (n = 10, 13, 10, 5 respectively) ; mean + SD [compared to CMV-P IV delivery (n = 5)] are given in a bar chart, n = 5–12, ***p < 0.001 (ANOVA); p values are not given for hF-sp-hA-P and hF-hA-P.
EGFP mRNA analysis of promoter activity in murine retina
Initially, the human NEFH-P vector was compared to the previously published murine Nefh-P vector8. 1 × 109 vg NEFH-P or Nefh-P were delivered by IV injection to eyes of wild type mice and EGFP mRNA expression compared 4 weeks post-injection using RT-qPCR in whole retinal RNA samples. The level of EGFP mRNA from human NEFH-P (39.3 ± 21.8%; n = 10), while still robust, was ~ 2.5-fold lower (p < 0.05) than from murine Nefh-P. Both promoter constructs were also injected SR into wild type mice and EGFP expression levels analysed by RT-qPCR 4 weeks post-injection. Levels of EGFP expression from both NEFH-P and Nefh-P were reduced significantly, by 93.4% and 92.5% respectively, when delivered SR compared to IV suggesting high level of specificity could be provided by the murine and human Nefh promoters (n = 10 and n = 5 respectively, p < 0.001).
Next, 1 × 109 vg of CMV-P, human NEFH-P, hF-sp-hA-P, hF-hA-P and hA-P were delivered either IV or SR into adult wild type mice and compared to mRNA expression from CMV-P 4 weeks post-injection (Fig. 2L). Levels of EGFP mRNA expression from IV CMV-P were taken as 100% and all other expression levels are given relative to this. Interestingly, levels of EGFP expression from CMV-P were not significantly different when delivered IV or SR (100 ± 51.0%, n = 5 versus 105 ± 49.0%, n = 11). Expression from NEFH-P was somewhat lower than from CMV-P when delivered IV, but this was not significant (57.0 ± 32.1%, n = 10). However, when delivered SR EGFP expression from NEFH-P was reduced to 3.8 ± 1.2% (n = 10) indicating high levels of specificity (p < 0.001 when compared to CMV-P SR).
AAV constructs with hA-P and human NEFH-P expressed similar levels of EGFP when 1 × 109 vg were delivered IV (35.6 ± 14.8% versus 57.0 ± 32.1% relative to CMV-P, n = 12), but expression from hA-P was somewhat less than from CMV-P IV (p < 0.05). In contrast, when delivered SR, hA-P expressed EGFP at significantly higher levels, i.e. 185.0 ± 77.8% than CMV-P delivered SR (n = 13, p < 0.05). Expression levels from hF-sp-hA-P and hF-hA-P IV were significantly lower at 12.3 ± 11.5%, n = 11 and 8.99.0 ± 4.82%, n = 5, respectively, than from CMV-P (p < 0.05). These constructs were also significantly lower than NEFH-P (p < 0.01) and hA-P (p < 0.001). After SR injection, these two constructs expressed 27.3 ± 14.6% (n = 5) and 1.63 ± 0.72% (n = 5), respectively, compared to CMV-P.
Immunocytochemistry analysis of promoter activity
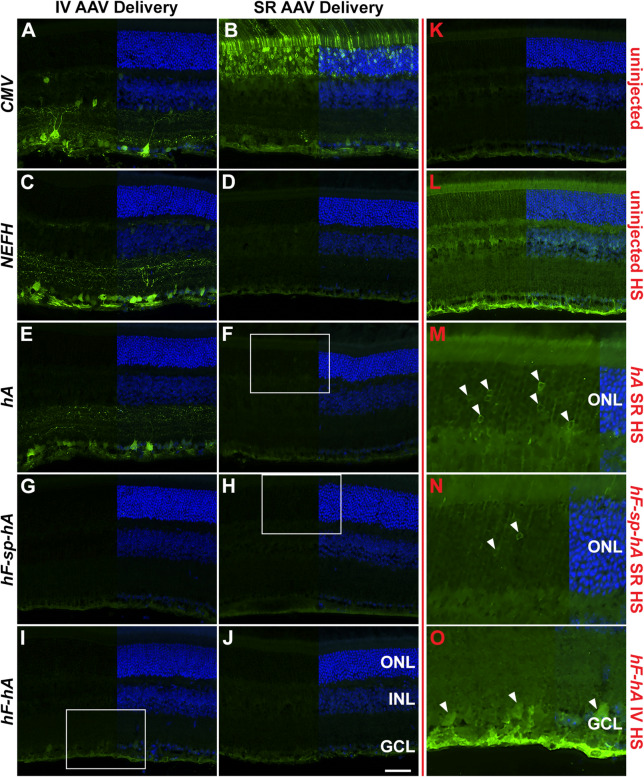
Distribution of native EGFP-positive cells in the retinal layers and mRNA expression analysis indicated that CMV-P, NEFH-P and hA-P were the most effective RGC promoters (Fig. 2). However, since high levels of EGFP mRNA were observed following SR injection of hA-P, EGFP protein expression from all promoter constructs was evaluated in more detail following both SR and IV delivery of 1 × 109 vg of AAV constructs, using EGFP immunocytochemistry. No EGFP expression was observed from hF-sp-hA-P following IV injection (Fig. 3G). Similar expression profiles were observed, with strong EGFP label detected in the GCL layer, from CMV-P, NEFH-P and hA-P following IV (Fig. 3A,C,E respectively). A small amount of expression from hF-hA-P was observed following IV delivery (Fig. 3I). Only CMV-P expressed high levels of EGFP in the outer nuclear (ONL) layer following SR delivery (Fig. 3B). Images were also analysed at high sensitivity (HS) conditions (with increased exposure) in order to identify cells with low EGFP expression. Minimal EGFP expression could be observed in the ONLs of retinas that had received hA-P and hF-sp-hA-P via SR delivery (Fig. 3M,F,H,N, respectively). HS analysis of images also identified minimal RGC expression from hF-hA-P following IV delivery (Fig. 3O). No expression was determined from NEFH-P or hF-hA-P following SR injection with HS analysis indicating that expression from these promoters is restricted (Fig. 3D,J). No label was detected in uninjected retinas at either standard or HS analysis (Fig. 3K,L). Thus, like CMV-P, NEFH-P and hA-P expressed high levels of EGFP in the GCL following IV injection (Fig. 3A,C,E), but in contrast to CMV-P, EGFP expression in the ONL was minimal following SR delivery (Fig. 3B,D,F).
Figure 3.
EGFP expression following intravitreal (IV) and subretinal (SR) injection of AAV delivered NEFH promoter constructs utilising EGFP immunocytochemistry. 1 × 109 vg of CMV-P, NEFH-P, hA-P, hF-sp-hA-P or hF-hA-P vectors were delivered by IV or SR injection to adult wild type mice and analysed 4 weeks post-injection. For histology, eyes were enucleated, fixed in 4% pfa and cryosectioned. EGFP immunocytochemistry was performed utilising chicken anti-EGFP primary (Abcam), Alexa-Fluor-488 conjugated secondary antibodies (green) and DAPI nuclear stain (blue). Strong label was detected with IV delivery for CMV-P (A), NEFH-P (C) and hA-P (E) and with SR delivery in CMV-P (B) samples; there was no label in the uninjected retinas (K). At higher sensitivity levels [HS in labels; (L–O)], minimal transduction of photoreceptor cells with SR delivery of hA-P (M) and hF-sp-hA-P (N) and retinal ganglion cell with IV delivery of hF-hA-P (O) constructs was detected (white arrowheads; white rectangles in F, H and I indicate the areas depicted at HS in (M), (N) and (O), respectively); note the high background in the HS images as demonstrated by the uninjected retina (L). ONL outer nuclear layer, INL inner nuclear layer, GCL ganglion cell layer. Scale bar (J) 50 μm.
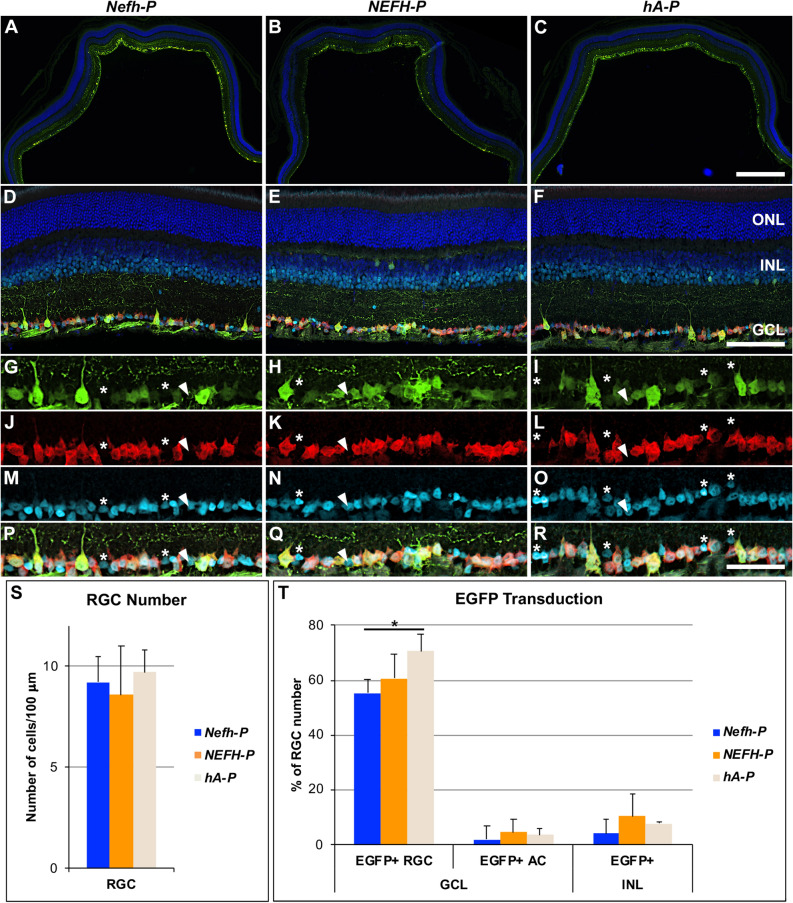
As the human NEFH-P and hA-P promoters were both efficient and highly preferential to RGCs we further analysed expression from these promoters in subsequent experiments. Specificity and efficacy of RGC targeting by NEFH-P and hA-P was determined following IV injection of 1 × 109 vg of NEFH-P and hA-P AAV vectors in wild type retinas (Fig. 4). The previously described murine Nefh-P vector8 was used as a control. Transduced sections from the central retina were stained using immunocytochemistry for EGFP, RBPMS and PAX6 (Fig. 4A–R) and positive cells quantified. The number of RGCs detected were similar between the analysed groups (Fig. 4S), i.e. 9.2 ± 1.3, 8.6 ± 2.4 and 9.7 ± 1.1, per 100 μm, for murine Nefh-P, human NEFH-P and hA-P transduced retinas, respectively (n = 4).The percentage of EGFP-positive RGCs (RBPMS positive/PAX6 positive) was 55.0 ± 7.8%, 61 ± 9% and 71 ± 6.0% for Nefh-P, NEFH-P and hA-P, respectively (n = 4, Fig. 4T). While NEFH-P and hA-P did not differ statistically significantly, a significantly greater number of EGFP-positive RGCs was observed with hA-P compared to Nefh-P (t-test). The percentage of EGFP-positive ACs in the GCL (RBPMS negative/PAX6 positive, Fig. 4T) was much lower compared to EGFP positive RGCs and did not differ significantly between the three AAV promoter vectors; 2.0 ± 1.0%, 5.0 ± 4.6% and 4.0 ± 2.2% for murine Nefh-P, human NEFH-P and hA-P, respectively (n = 4). The percentage of EGFP-positive cells in the INL was also much lower compared to EGFP positive RGCs; 4.2 ± 2.7%, 10.5 ± 8.2% and 7.8 ± 0.70%, respectively (n = 4, Fig. 4T) and did not differ statistically significantly. In the above results, the number of RGCs in the GCL was set as 100% and all other numbers are given relative to this. Cells were quantified automatically in cellSens, except for ACs, which were counted manually.
Figure 4.
EGFP expression following IV delivery of Nefh-P, NEFH-P or hA-P. Nefh-P, NEFH-P or hA-P was delivered via IV injection (1 × 109) to adult wild type mice (n = 4). Four weeks post-delivery, eyes were enucleated, fixed in 4% pfa and cryosectioned. Immunocytochemistry for EGFP (Alexa-Fluor-488 label, green), RBPMS (Cy3 label, red) and PAX6 (Cy5 label, light blue) was carried out. (A–C) Overview of EGFP-transduced retinas. (D–F) Overview of EGFP-, RBPMS- and PAX6-label in EGFP-transduced retinas. Distribution of EGFP (G–I), RBPMS (J–L), PAX6 (M–O) and their overlay (P–R) in the GCL is presented. Most cells were positive for the three markers demonstrating that they were EGFP-transduced RGCs (G–R). Arrowheads indicate amacrine cells (ACs, RBPMS negative/PAX6 positive) that expressed EGFP, while asterisk indicate ACs (RBPMS negative/PAX6 positive), which did not express EGFP. Bar chart representation of the number of RGCs in the transduced retinas (S) and the percentage of EGFP-positive RGCs and ACs in the GCL and the EGFP-positive cells in the INL (T); the number of RGCs was taken as 100%; bars represent mean + SD; *p < 0.05 (ANOVA). Automated (for RGC and INL cells) and manual (for ACs) quantification was carried out in cellSens. ONL outer nuclear layer, INL inner nuclear layer, GCL ganglion cell layer. Scale bars: 500 μm (C), 100 μm (F), 50 μm (R).
EGFP expression in RGCs was also analysed quantitatively in retinal wholemounts using the human constructs. NEFH-P and hA-P (1 × 109 vg) AAV vectors were IV injected into adult wild type mice and retinal wholemounts (Fig. 5A–D) and optic nerves (ONs; Fig. 5E,F) assessed 4 weeks post-delivery using immunocytochemistry for EGFP and BRN3A (RGC). A significant proportion of the retinas were highly transduced; EGFP-positive RGCs were quantified automatically in 6 transduced areas of each wholemount using cellSens. The percentage of EGFP-positive RGCs following IV delivery of NEFH-P and hA-P promoter constructs was 29.7 ± 7.1% and 41.5 ± 9.2%, respectively, but this difference was not significant (n = 6; Fig. 5G,H). Note that the number of analysed RGCs/area was similar between the two groups, 632.7 ± 94.0 and 682.7 ± 45.3, respectively (Fig. 5G). Some axons from RGCs were also positive for EGFP in both the wholemounts and ONs (Fig. 5C,D,E,F). The percent of EGFP-positive RGCs determined in the wholemounts had a similar trend but lower numbers compared to those determined in the retinal sections; note that the same AAV dosage was used in both experiments. A likely explanation for this is that the wholemounts have significantly higher EGFP background levels than 12 μm thick retinal sections, hampering detection of low EGFP signal intensity cells. Therefore the efficacy of EGFP detection is higher in sections than wholemounts, resulting in higher numbers of EGFP-positive cells. (Note that detection efficacy of RGCs either with RBPMS or BRN3A is likely to be close to 100% in both wholemounts and sections as the signal/noise ratio for these labels is much higher.
Figure 5.
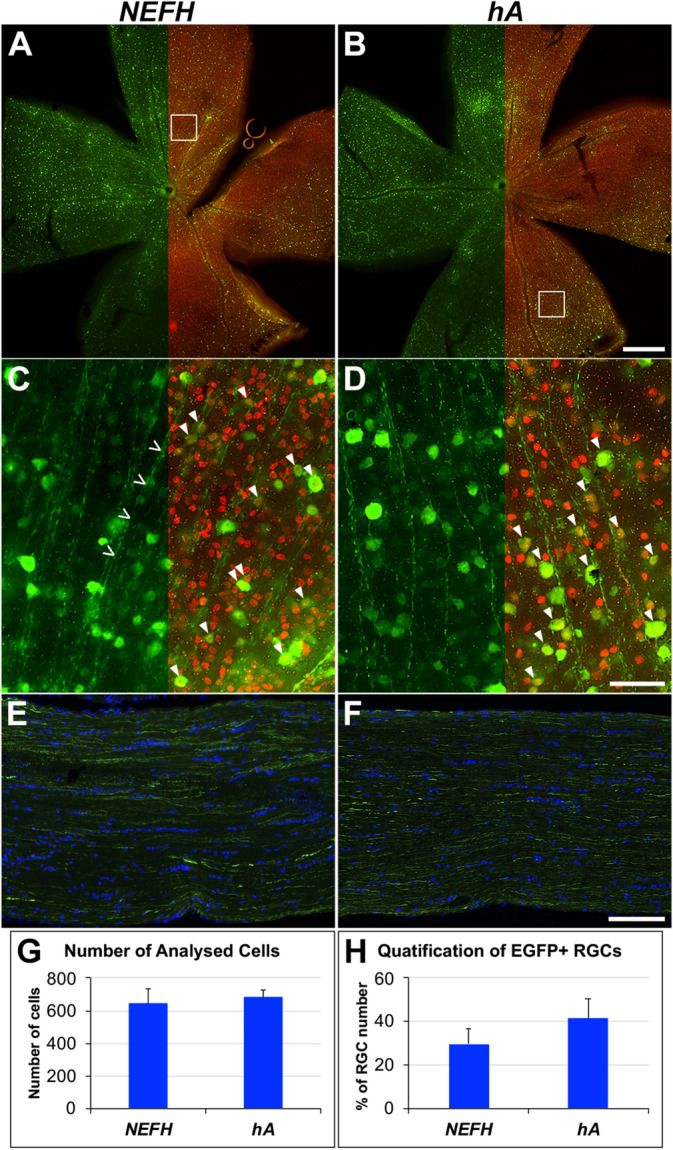
EGFP expression from NEFH promoter constructs in retinal wholemounts and optic nerves. 1 × 109 vg of NEFH-P or hA-P AAV were delivered by IV injection to adult wild type mice, and EGFP positive cells analysed 4 weeks post-injection. Eyes were enucleated and fixed in 4% pfa; retinas wholemounted and optic nerves (ONs) cryosectioned longitudinally. Retinas were immunostained for BRN3A (Cy3 label, red) and EGFP (Alexa-Fluor-488 label, green). ONs were immunostained for EGFP (Alexa-Fluor-488 label, green), and counterstained with DAPI. (A,B) Overview of wholemount retinas with EGFP signal and BRN3A label (retinal ganglion cell, RGC) overlaid on the right half of the images. (C,D) Magnified view of wholemount retinas with EGFP signal and BRN3A label overlaid on the right half of the images. Some BRN3A-positive cells (RGCs) were co-labelled with EGFP; closed arrowheads indicate some double positive cells. Open arrowheads delineate an example of an EGFP-positive RGC axon. (E,F) EGFP-positive RGC axons detected in ONs. Scale bars: 500 μm (B), 50 μm (D), 100 μm (F). EGFP-positive RGCs were counted in six representative areas imaged at ×20 magnification (such as C and D) in each wholemount transduced with either NEFH-P or hA-P AAV (n = 6) using automated quantification in cellSens. The number of RGCs in the analysed areas (G) and the percent of EGFP-positive RGCs in the transduced retinas (F) are given in bar charts; bars represent mean + SD. Statistical significance between NEFH-P and hA-P AAV-transduced retinas was carried out using a t-test.
Discussion
A key objective of the study was to generate a valuable human RGC promoter, which provides preferential transgene expression in RGCs, but is minimal in size, thereby optimising its utility in gene therapy vectors such as AAV. Of note, Kim et al., 2006 analysed gene expression in RGC and non-RGC cells from post-mortem human retinas. They showed the greatest (~ 245-fold) enrichment of NEFH mRNA in RGC8,10 followed closely by NEFL (~ 150-fold enrichment) and NEFM.
Given the potential utility of such a promoter for future human gene therapies targeted to RGCs the focus of the current study was to analyse the human NEFH gene upstream sequence. This analysis resulted in the identification of two conserved regions, blocks A and F, which were subsequently included in various configurations in AAV vectors to drive EGFP reporter gene expression (Fig. 1). Because the Thy1 promoter, which is known to drive RGC-specific expression, is only effective when a large ~ 6 kb spacer is included between the core promoter and enhancer elements11,12, we decided to include a spacer sequence between conserved regions F and A in one promoter construct. The spacer used was approximately the same length as the endogenous promoter sequence between conserved regions F and A.
In the current study we determined that the level of EGFP mRNA from the human NEFH-P, akin to the murine promoter, provided effective gene expression in murine RGCs. The data suggest that expression levels from the human NEFH-P both at the mRNA and protein level are in the same order of magnitude as levels provided by the CMV promoter (Fig. 2A,B,C,D,K,L) and similar to that seen for murine Nefh-P8. Compared to CMV-P, NEFH-P also provided high levels (57.8 ± 32.1%) of reporter gene expression in the GCL. Expression in the INL was minimal following IV injection into the murine eye. In contrast, levels of expression following SR injection of the AAV2/2 NEFH-P vector were barely detectable at the mRNA level (3.83 ± 1.24%) and were undetectable using histological analysis, highlighting the specificity of the NEFH-P for RGCs. Interestingly, the highly truncated NEFH promoter in the AAV2/2 hA-P vector (199 bp promoter) also enabled significant expression of EGFP following IV injection when evaluated by mRNA analysis (35.6 ± 14.8% compared to CMV-P IV), and the percentage of native EGFP positive cells in the GCL was 71.3 ± 3.13% compared to CMV-P IV. The 199 bp hA-P promoter was compared to the murine and human genome using BLAST. However, no more than 20% sequence identity was seen between it and other sequences, indicating that hA-P is unique to NEFH. It is of note that relatively high levels of EGFP mRNA expression were detected following SR injection of hA-P, yet only an insignificant amount of EGFP was found using EGFP immunocytochemistry (Fig. 3). This seems to indicate that the 199 bp hA-P promoter, while transcribed at high levels in the ONL, is not translated efficiently in these cells. Hence this very small promoter appears to convey RGC specificity by unknown post-transcriptional regulation.
Notably, a large proportion of cells in the transduced area of the GCL were EGFP-positive following IV injection of NEFH-P and hA-P (~ 50–70%, Fig. 4T). Only 2–5% of EGFP positive cells were ACs (EGFP positive/RBPMS negative/PAX6 positive; Fig. 4T). In addition, 5–10% of cells in the INL expressed EGFP. The identity of the EGFP-positive cells in the INL was not investigated but at least some of them appear to be ACs.
The preferential RGC expression exhibited by the promoter constructs described herein represent significant refinements for RGC gene therapies. Some promoters known to express specifically in RGCs have either not been expressed highly or are too large to be included in AAV vectors, which have a maximum cargo capacity of approximately 4.7 kb, e.g. Brn3a, Thy1 and Rbpms8–13. Notably, the size of both the human NEFH promoter (2.5 kb) and, particularly, the truncated hA-P promoter (199 bp) are ideal for application within AAV vectors and thereby represents a significant advantage over the larger RGC promoters published to date6,14–16.
AAV2 incorporating the human connexin 36 (CX36/GJD2) promoter (2.8 kb) transduced NHP foveal ganglion cells. However, when shortened to 1.8 kb, expression was only seen in damaged retinas5. Jüttner et al.20 identified several synthetic promoters that target NHP or human post-mortem RGCs specifically, however, transduction varied between 1 and 13% of the total RGC population following SR injection and utilising AAV2/8BP2. It would be of interest to see whether the transduction efficacy could be improved with IV delivery. Interestingly, the authors found that less than 1% of the 230 synthetic promoter sequences tested by SR in mice replicated the specificity of their source genes20. The study also clearly showed how expression profiles from many of the same promoters varied between species (mouse, NHP and human retina). In the current study we have demonstrated that the RGC specificity of both the murine Nefh8 and human NEFH promoters was similar in mice. Simpson et al.16 described a 2693 bp NEFL promoter, which appears to have a similar expression profile to NEFH-P and hA-P, though notably hA-P is ~ 85% smaller than the NEFL promoter. It will however be important to evaluate hA-P and NEFH-P in NHP or human retina to evaluate whether the specificity of hA-P and NEFH-P obtained following IV delivery in mouse, is reflected in higher species; particularly as some promoters which express in the GCL in mice have been shown only to express in damaged NHP retinas, for example, the 0.48 kb hSYN and the 1.8 kb XC36/GJD2 promoters5.
AAVs have been utilised extensively for ocular gene delivery to animal models and in human clinical trials as well as for two in vivo licensed medicinal products; Luxturna and Zolgensma. For example, AAV2 and AAV9 are known to transduced RGCs in the perifoveal ring and to a lesser extent the peripheral retina in NHPs5,16 and clinical trials for LHON have demonstrated that AAV2 transduces RGCs efficiently in humans and indeed provides beneficial effects7,21. Additionally, a plethora of newer AAV serotypes have been shown to infect primate or human RGCs with greater efficacy, demonstrating enhanced tropism for both the foveal centre and peripheral retina. These include AAV2BP822, Anc8023, AAV2/8 Y733F24, AAV2/2 quadYF25,26 and AAV7m822,26,27, among others.
RGC delivery may be enhanced through incorporation of novel peptide insertions into VP1. Modification of AAV2 VP1 with peptides (NNPTPSR or GLSPPTR), which are applicable to any serotype, resulted in greater EGFP expression than AAV7m828. Offering the potential for insertion of such peptides into VP1 of the most efficient RGC transducing serotypes to further boost expression. These novel serotypes, or a combination thereof, may provide the enhanced transduction required to effectively treat RGC-based disorders such as glaucoma, DOA, LHON, among others (reviewed in Ref.29), and may provide the breadth of delivery needed for the effective utilisation of novel technologies such as optogenetics, for example (reviewed in Ref.30). In such cases, the ability to restrict transgene expression to RGCs while still providing effective transgene expression, as demonstrated herein, would be highly advantageous to minimise potential off-target effects and could thereby improve safety and efficacy.
The current data suggest that NEFH-P drives highly specific expression in the RGC layer, with minimal expression observed in the INL or ONL, even following SR injection. In addition, when NEFH-P was reduced by over 90% (hA-P), the expression profile remained substantially the same. The 199 bp hA-P promoter therefore represents a very interesting and potentially versatile promoter that easily fits within the confines of the 4.7 kb transgene capacity of AAV vectors, thereby enabling delivery of larger genes while still providing efficient and highly preferential transgene expression in RGCs following IV injection.
Materials and methods
In silico analysis of the human NEFH promoter
To define a putative human NEFH promoter region, data from the University of California Santa Cruz (UCSC) genome browser (mm10 mouse mammalian conservation track31) were used to establish the conservation upstream of the transcriptional start site of NEFH gene. An in silico pipeline8, was used to isolate basewise conservation data (conservation data ranged from 0 to 1 for a given base, where 0 represents no significant conservation between mammals and 1 indicates complete conservation). Sequences from 40 placental mammal species (Supplementary Table S1, Ref.8) were used for conservation alignment and a graph plotted to visualise conserved regions of the human NEFH upstream sequence (Fig. 1A).
Constructs and viral production
A murine 2.2 kb Nefh promoter driving EGFP expression (Nefh-P) was generated as described previously8. To generate a human NEFH promoter equivalent a 1.9 kb fragment of human genomic DNA (was amplified using the following primer pair: 5′ AGATCATCTTAAGACGCGTTGCTGTCAGCTGCTTGTGA 3′ and 5′GAGGTACAGTGTTCTCCTAAC 3′). The purified PCR product was cloned into pcDNA3.1 + (Invitrogen); an additional fragment of custom synthesised DNA (GeneWiz, South Plainfield, USA), corresponding to bp − 5 to − 637 of the NEFH sequence, was cloned into the above 1.9 kb partial promoter to generated a full 2501 bp of contiguous NEFH upstream sequence (− 5 to − 2452 of NM_ 021076.3). The full 2501 bp NEFH, upstream sequence was then excised and cloned in place of the CMV promoter in pAAV-CMV-EGFP2 to create NEFH-P. To create hA-P (− 5 to − 199 of NM_ 021076.3), conserved region A was amplified from human genomic DNA using the following primer pair: 5′-ATCGATGACGCGTCTCTGACGCAGCGTCGATT-3′ and 5′-AGATCATGATATCGGCCTGAGCAGGTGCGCGA-3′ and the amplification product cloned in place of the CMV promoter in pAAV-CMV-EGFP2. To generate hF-hA-P (− 5 to − 199 and − 2093 to − 2452 of NM_021076.3), the sequence of the conserved regions was custom synthesised (GeneWiz, South Plainfield, USA) and cloned into pAAV.CMV-EGFP2 in place of the CMV promoter. hF-sp-hA-P (− 5 to − 199, 1866 bp of lambda, and − 2093 to − 2452 of NM_021076.3) was generated by amplifying 1866 bp of lambda DNA using the following primer pair (5′-ATCGATGTTTAAACTACTACCGATTCCGCCTAGT-3′ and 5′-ATGCATGTTTAAACAGGCATTTATACTCCGCTGG-3′) and cloning this amplification product between the conserved Blocks A and F in hF-hA-P. All EGFP genes also included an hGH poly A. Nucleotides included in constructs are indicated in Fig. 1 and Supplemental Table 1. Sanger sequencing was used to verify all plasmid constructs. For each candidate NEFH promoter construct, recombinant AAV2 viruses were generated. Genomic titres were determined, as described previously32,33.
Intravitreal and subretinal injection of mice
Wild type 129 S2/SvHsd mice (Harlan UK Ltd, Oxfordshire UK) were maintained under specific pathogen free conditions. Injections were performed in strict compliance with the European Union (Protection of Animals used for Scientific Purposes) Regulations 2012 (S.I. no. 543 of 2012) and the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals. Intravitreal injections were performed as described8,34 and subretinal injections were performed as described in Ref.33. 1.0 × 109 vg of virus was injected into eyes and RNA and histological analysis performed 4 weeks post-injection.
RNA analysis
Retinas were harvested 4 weeks post-injection and total RNA extracted as described35–37. In vivo expression levels of EGFP were determined by reverse transcription PCR (RT-qPCR) on a StepOne Real Time PCR System (Applied Biosystems, Foster City, CA, USA) using a one step QuantiTect SYBR Green RT-qPCR kit (Qiagen Ltd., Crawley, UK) and the following primer pair: 5′ TTCAAGAGGACGGCAACATCC 3′ and 5′ CACCTTGATGCCGTTCTTTCGC 3′. RT-qPCRs were performed twice in triplicate. Expression levels were normalised using the internal housekeeping gene β-actin (Actb) and the following primer pair: 5′ AGAGCAAGAGAGGCATCC 3′ and 5′ TCATTGTAGAAGGTGTGGTGC 3′. Standard curves of Actb were generated by serially diluting retinal RNA 5×. Standard curves of EGFP were generated by serially diluting plasmid DNA containing an EGFP gene 10×. A minimum of 4 points were used in all standard curves.
Immunohistochemistry and microscopy
Mice were sacrificed, eyes enucleated and optic nerves collected. Tissue samples were fixed in 4% paraformaldehyde in PBS overnight then washed in PBS. Some of the retinas and the optic nerves were cryoprotected in 10%, 20% and 30% sucrose in PBS, embedded in OCT (VWR), cryosectioned (12 μm) and thaw-mounted onto Polysine slides (Thermo Scientific). For wholemounting, fixed and PBS-washed retinas were removed from the eyecups and processed for immunocytochemistry immediately. Immunocytochemistry was performed as described before38. Sections/wholemounts were incubated with primary antibodies for EGFP (ab13970, Abcam; 1:1000 dilution), RBPMS (abn1376, Millipore; 1:400 dilution), BRN3A (sc-8429, Santa Cruz; 1:200) and CHX10 (sc-374151, Santa Cruz; 1:200 dilution) overnight for sections and for 3 days for wholemounts at 4 °C. Sections/wholemounts were then washed in PBS incubated with secondary antibodies conjugated with Alexa-Fluor-488, Cy3 and Alexa-Fluor-647 (Jackson ImmunoResearch Laboratories) in 1:400 dilution for 2 h for sections and 2 days for wholemounts and nuclei counterstained with DAPI. Samples were covered using Hydromount (National Diagnostics).
Fluorescent microscopy was carried out utilising an Olympus IX83 inverted motorised microscope (cellSens v1.9 software, Olympus) equipped with a SpectraX LED light source (Lumencor) and an Orca-Flash4.0 LT PLUS/sCMOS camera (Hamamatsu). Samples were imaged using a 10 × plan fluorite and a 20 × plan super apochromat objective utilising enhanced focal imaging (EFI) with typically 5–8 Z-slices (30 slices in 20 × images for colocalisation in wholemounts) using cellSens. Lateral frames were stitched together for pan-retinal sections and wholemounts in cellSens.
Representative sections from each eye were taken from the central retina (within 250 μm of the optic nerve) and analysis performed in the central part of the stitched sections utilising cellSens; same settings were applied to all samples. Integrated fluorescence intensity of EGFP (measured in the EGFP transduced areas of GCL and IPL, and normalised to length of the analysed area of the retina) was defined as the sum of fluorescence intensity of each pixel of the analysed area and measured in cellSens; 4 measurements/retina were taken; n = 4. Automated colocalisation of EGFP and RBPMS immunolabels and automated counting of EGFP positive cells in the INL were carried out using the Count and Measure module in cellSens and were normalised to the length of the measured area of the retina. All cells in two stitched frames/retina were counted; n = 4. Amacrine cells (RBPMS negative/PAX6 positive) that expressed EGFP were counted manually; n = 4. Automated colocalisation of BRN3A (RGCs) and EGFP labels in wholemounts were also carried out using the Count and Measure module in cellSens. Six representative areas of each wholemount were analysed and the average of these measurements calculated (n = 5). Images taken with different filter sets were post-processed in Photoshop CS6 and 2020 (Adobe) for the figures; the same settings/operations were applied for all images both in cellSens and Photoshop.
Statistical analysis
Student’s t-test and ANOVA (with Tukey’s multiple comparisons post-hoc test) were performed using Prism 8 (GraphPad) and p < 0.05 was considered statistically significant.
Ethical statement
Ethical approval for this work was provided by the Irish Medicines Board (now Health Products Regulatory Authority) pursuant to the European Union (Protection of Animals Used for Scientific Purposes) Regulations 2012 (SI 543/2012). All work was submitted to and approved by the Animal Research Ethics Committee (AREC) of Trinity College Dublin.
Supplementary information
Acknowledgements
We thank Charles Murray for technical assistance.
Author contributions
S.M.-W.: experimental design, experimentation, figures, writing and reviewing manuscript. N.C.: experimental design, experimentation, figures, writing and reviewing manuscript. M.B.: experimentation. L.K.F: experimental design, experimentation, reviewing manuscript. K.S.H.: experimentation. M.C.: experimentation. P.H.: writing and reviewing manuscript. P.F.K.: experimentation, writing and reviewing manuscript. A.P.: experimental design, experimentation, figures, figure design and artwork, writing and reviewing manuscript. G.J.F.: P.I., experimental design, writing and reviewing manuscript.
Funding
This research was supported by Science Foundation Ireland (SFI), Fighting Blindness Ireland (FB Irl), the Health Research Board Ireland (HRB), Irish Research Council (IRC) and Health Research Charities Ireland (HRCI).
Competing interests
SM-W, NC, AP, KSH, PFK and GJF have filed a patent describing aspects of retinal ganglion cell promoters. MB, LKF, MC, and PH declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sophia Millington-Ward, Naomi Chadderton, Arpad Palfi and G. Jane Farrar
Supplementary information
is available for this paper at 10.1038/s41598-020-73257-z.
References
- 1.Russell S, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palfi A, et al. Adeno-associated virus-mediated rhodopsin replacement provides therapeutic benefit in mice with a targeted disruption of the rhodopsin gene. Hum. Gene Ther. 2010;21:311–323. doi: 10.1089/hum.2009.119. [DOI] [PubMed] [Google Scholar]
- 3.Wolff S, et al. Endotoxin-induced gene expression differences in the brain and effects of iNOS inhibition and norepinephrine. Intensive Care Med. 2009;35(4):730–734. doi: 10.1007/s00134-009-1394-7. [DOI] [PubMed] [Google Scholar]
- 4.Vandenberghe LH, et al. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci. Transl. Med. 2011;3:88ra54. doi: 10.1126/scitranslmed.3002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin L, et al. Intravitreal injection of AAV2 transduces macaque inner retina. Investig. Ophthalmol. Vis. Sci. 2011;52(5):2775–2782. doi: 10.1167/iovs.10-6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaffiol A, et al. A new promoter allows optogenetic vision restoration with enhanced sensitivity in macaque retina. Mol. Ther. 2017;25:2546–2560. doi: 10.1016/j.ymthe.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vignal C, et al. Safety of rAAV2/2-ND4 gene therapy for Leber hereditary optic neuropathy. Ophthalmology. 2018;125:945–947. doi: 10.1016/j.ophtha.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 8.Hanlon KS, et al. A novel retinal ganglion cell promoter for utility in AAV vectors. Front. Neurosci. 2017;11:521. doi: 10.3389/fnins.2017.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadal-Nicolás FM, et al. Displaced retinal ganglion cells in albino and pigmented rats. Front. Neuroanat. 2014;8:99. doi: 10.3389/fnana.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim CY, Kuehn MH, Clark AF, Kwon YH. Gene expression profile of the adult human retinal ganglion cell layer. Mol. Vis. 2006;12:1640–1648. [PubMed] [Google Scholar]
- 11.Spanopoulou E, Giguere V, Grosveld F. The functional domains of the murine Thy-1 gene promoter. Mol. Cell. Biol. 1991;11:2216–2228. doi: 10.1128/MCB.11.4.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alić I, et al. Neural stem cells from mouse strain Thy1 YFP-16 are a valuable tool to monitor and evaluate neuronal differentiation and morphology. Neurosci. Lett. 2016;634:32–41. doi: 10.1016/j.neulet.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Ye L, Gu L, Caprioli J, Piri N. RNA-binding protein Rbpms is represented in human retinas by isoforms A and C and its transcriptional regulation involves Sp1-binding site. Mol. Genet. Genom. 2018;293:819–830. doi: 10.1007/s00438-018-1423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Leeuw CN, et al. Targeted CNS delivery using human minipromoters and demonstrated compatibility with adeno-associated viral vectors. Mol. Ther. Methods Clin. Dev. 2014;8:1–5. doi: 10.1038/mtm.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith CA, Chauhan BC. In vivo imaging of adeno-associated viral vector labelled retinal ganglion cells. Sci. Rep. 2018;8:1490. doi: 10.1038/s41598-018-19969-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson EM, et al. New minipromoter Ple345 (NEFL) drives strong and specific expression in retinal ganglion cells of mouse and primate retina. Hum. Gene Ther. 2019;30:257–272. doi: 10.1089/hum.2018.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali RR, Reichel MB, De Alwis M, Kanuga N, Kinnon C, Levinsky RJ, et al. Adeno-associated virus gene transfer to mouse retina. Hum. Gene Ther. 1998;9:81–86. doi: 10.1089/hum.1998.9.1-81. [DOI] [PubMed] [Google Scholar]
- 18.Hellström M, Ruitenberg M, Pollett M, et al. Cellular tropism and transduction properties of seven adeno-associated viral vector serotypes in adult retina after intravitreal injection. Gene Ther. 2009;16:521–532. doi: 10.1038/gt.2008.178. [DOI] [PubMed] [Google Scholar]
- 19.Wilson A, Di Polo A. Gene therapy for retinal ganglion cell neuroprotection in glaucoma. Gene Ther. 2012;19:127–136. doi: 10.1038/gt.2011.142. [DOI] [PubMed] [Google Scholar]
- 20.Jüttner J, et al. Targeting neuronal and glial cell types with synthetic promoter AAVs in mice, non-human primates and humans. Nat. Neurosci. 2019;22:1345–1356. doi: 10.1038/s41593-019-0431-2. [DOI] [PubMed] [Google Scholar]
- 21.Feuer WJ, et al. Gene therapy for leber hereditary optic neuropathy: Initial results. Ophthalmology. 2016;123:558–570. doi: 10.1016/j.ophtha.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramachandran PS, et al. Evaluation of dose and safety of AAV7m8 and AAV8BP2 in the non-human primate retina. Hum. Gene Ther. 2017;28:154–167. doi: 10.1089/hum.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carvalho LS, et al. Synthetic adeno-associated viral vector efficiently targets mouse and nonhuman primate retina in vivo. Hum. Gene Ther. 2018;29:771–784. doi: 10.1089/hum.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrs-Silva H, et al. High-efficiency transduction of the mouse reina by tyrosine-mutant AAV serotype vectors. Mol. Ther. 2009;17(3):463–471. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrs-Silva H, et al. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol. Ther. 2011;19(2):293–301. doi: 10.1038/mt.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalkara D, et al. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 2013;5(189):189ra76. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- 27.Hickey DG, et al. Tropsim of engineered and evolved recombinant AAV serotypes in the rd1 Mouse and ex vivo primate retina. Gene Ther. 2017;12:787–800. doi: 10.1038/gt.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michalakis, S & Biel M. WO/2019/076856 AAV Vectors (2019).
- 29.Farrar GJ, Chadderton N, Kenna PF, Millington-Ward S. Mitochondrial disorders: Aetiologies, models systems, and candidate therapies. Trends Genet. 2013;29:488–497. doi: 10.1016/j.tig.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Simunovic MP, et al. Optogenetic approaches to vision restoration. Exp. Eye Res. 2019;178:15–26. doi: 10.1016/j.exer.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Kent WJ, et al. The human genome browser at UCSC. Genome. Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohr UP, Wulf MA, Stahn S, Steidl U, Haas R, Kronenwett R. Fast and reliable titration of recombinant adeno-associated virus type-2 using quantitative real-time PCR. J. Virol. Methods. 2002;106:81–88. doi: 10.1016/S0166-0934(02)00138-6. [DOI] [PubMed] [Google Scholar]
- 33.O'Reilly M, et al. RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am. J. Hum. Genet. 2007;81:127–135. doi: 10.1086/519025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chadderton N, et al. Intravitreal delivery of AAV-NDI1 provides functional benefit in a murine model of Leber hereditary optic neuropathy. Eur. J. Hum. Genet. 2013;21:62–68. doi: 10.1038/ejhg.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Millington-Ward S, et al. Suppression and replacement gene therapy for autosomal dominant disease in a murine model of dominant retinitis pigmentosa. Mol. Ther. 2011;19:642–649. doi: 10.1038/mt.2010.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palfi A, et al. Efficient gene delivery to photoreceptors using AAV2/rh10 and rescue of the Rho−/− mouse. Mol. Ther. Methods Clin. Dev. 2015;2:15016. doi: 10.1038/mtm.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chadderton N, et al. Improved retinal function in a mouse model of dominant retinitis pigmentosa following AAV-delivered gene therapy. Mol. Ther. 2009;17:593–599. doi: 10.1038/mt.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palfi A, et al. microRNA regulatory circuits in a mouse model of inherited retinal degenerations. Sci. Rep. 2016;6:31431. doi: 10.1038/srep31431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.