Abstract
Aim:
In the presence of food allergies, especially egg allergies, primary physicians in Turkey avoid vaccine administration and refer children to a hospital setting. We aimed to evaluate children who had allergies or suspected allergies and were referred to our Well Child Clinic in a university hospital for vaccination.
Material and Methods:
Charts of all children referred to our clinic due to concerns for allergies in the last two years, were reviewed. Demographic data, laboratory evaluation and reactions after immunization were recorded.
Results:
A total of 122 children with or without a confirmed diagnosis of allergies were referred by primary physicians. In the history, 50 children (43.5%) had reactions with egg, 42 (36.5%) had reactions with multiple foods, nine (7.8%) had reactions with milk and seven (6.1%) had reactions with a previous vaccination. The most common reaction was rash (n=89, 86.4%). Nine children reported anaphylaxis. Skin testing or serum allergen specific IgE measurement revealed that 66 (54.1%) children had sensitization to egg white and 25 (20.5%) had sensitization to egg yolk. Most children (n=87, 71.9%) were referred for all the 12th-month vaccines, and 21 children were referred only for the measles-mumps-rubella vaccine (n=21, 17.4%). The median delay time in the administration of the measles-mumps-rubella vaccine was 20.0 (interquartile range: 8.7-41.2) days. No reaction was observed except for one child reporting a slight rash several hours after vaccination.
Conclusion:
Egg allergy was the most common barrier of vaccine administration in children referred from family physicians. Given the absence of any reactions, we support the administration of the measles-mumps-rubella vaccine in primary care settings to prevent delays in national vaccine schedule.
Keywords: Allergy, children, egg, immunization, measles-mumps-rubella
Öz
Amaç:
Besin alerjisi olan çocuklarda, aile hekimleri aşılama uygulamaktan kaçınmakta ve çocukları hastanelere yönlendirmektedir. Bu çalışmada Çocuk Sağlığı İzlem Polikliniği’mize alerjisi olup, aşılama için yönlendirilen çocukları değerlendirmeyi hedefledik.
Gereç ve Yöntemler:
Son iki yılda aşı yapılması için yönlendirilen alerjik çocukların dosyaları geriye dönük incelendi. Demografik ve laboratuvar verilerle, aşı sonrası reaksiyonlar değerlendirildi.
Bulgular:
Alerjisi olan ya da alerji şüphesiyle yönlendirilen 122 çocuk saptandı. Çocukların 50’sinde (%43,5) yumurta, 42’sinde (%36,5) çoklu besinler, dokuzunda (%7,8) süt, yedisinde (%6,1) daha önceki aşılamalar, öyküde etmen olarak bildirildi. En sık bildirilen reaksiyon cilt döküntüsüydü (n=89, %86,4). Sadece dokuz çocukta anafilaksi yakınması vardı. Cilt testi ve kanda alerjen spesifik IgE ölçümü ile hastaların 66’sında yumurta beyazı (%54,1) ve 25’inde (%20,5) yumurta sarısına duyarlanma saptandı. Çocukların çoğu (n=87, 71,9%) tüm 12. ay aşıları için, 21 (%17,4) çocuk sadece kızamık-kabakulak-kızamıkçık aşısı için yönlendirilmişti. Ortanca gecikme süresi kızamık-kabakulak-kızamıkçık aşısı için 20 gündü (8,7-41,2). Bir çocukta aşılamadan birkaç saat sonra ortaya çıkan döküntü dışında, diğer çocuklarda reaksiyon izlenmedi.
Çıkarımlar:
Yumurta alerjisi, aile hekimlerinden yönlendirilen çocukların aşılamasında en yaygın engel olarak bulundu. Yumurta ile ciddi reaksiyon tanımlayan çocuklarda dahi aşılama sonrası reaksiyon izlenmemiştir. Ciddi reaksiyon olmadığı düşünüldüğünde, ulusal aşı takviminde gecikmelerin önlenmesi için, kızamık-kabakulak-kızamıkçık aşısının aile hekimlerince uygulanmasında sakınca gözlenmemiştir.
Keywords: Alerji, aşılama, çocuk, kızamık-kabakulak-kızamıkçık, yumurta
Introduction
Vaccination is the most important public health measure in controlling some infectious diseases. Therefore, all vaccines included in the Ministry of Health National vaccination schedule should be administered to all children fully and timely. Sometimes problems may be confronted in the application of the vaccination schedule. One of the most common causes leading to these problems is a history of allergy. Hypersensitivity reactions caused by vaccines may be due to the preservatives included in the vaccine or the remnants of the culture media in which the vaccine was grown (1). Anaphylaxis following vaccination is a rare reaction, which is reported with a rate of 0.5–1 in one million doses (2). Death due to anaphylaxis developing after vaccination has been reported with a rate of one in 50 million doses (3).
Although fatal reactions are very rare, delays are experienced in vaccination in children with a history of allergy. Many family health centers (FHCs) avoid administering the measles-mumps-rubella (MMR) vaccine, which should be administered at the age of one year, especially in children who have or are thought to have food allergy, if the responsible nutrient is egg. Studies have shown that the MMR vaccine can be adminsitered safely even in children who had shown severe hypersensitivity reaction against egg (4–8). Therefore, the MMR vaccine can be administered even in children with severe egg allergy without performing a skin test priorly and at the full dose at one time, in contrast to previous recommendations (9–11).
Vaccination of children with egg allergy does not need to be performed in centers other than the those where other routine vaccines are administered, and taking additional precautions is not necessary (11). However, different applications are observed for these patients most of the time. Children with egg allergy are referred by FHCs to tertiary care health institutions where allergy centers are available for MMR vaccination. This leads to unnecessary delays in vaccination, an increase in workload in tertiary care health institutions for a healthcare service that can safely be performed in a primary care setting, economic loss due to the use of skin prick test (SPT) or allergen-specific immunoglobulin (Ig)-E measurements, and an increase in concerns about vaccination in families.
In this study, because of differences in clinical application related to vaccination observed in practice in children with allergy, we aimed to report the clinical findings, laboratory results, and reactions observed following vaccination in children who were not vaccinated and referred by FHCs to our Well Child Outpatient Clinic because they had a history of allergy.
Material and Methods
The files of patients who had a history of food allergy or described suspicious allergic symptoms with unknown cause, and therefore were referred to our Well Child Follow-up Outpatient Clinic without administering vaccination by FHC between September 2016 and September 2018, and patients who were followed up with a diagnosis of food allergy by the Division of Pediatric Allergy and referred to our outpatient clinic for vaccination were screened retrospectively. Ethics committee approval was obtained from our university’s committee (Date: 05.10.2018, Number: 09.2018.657). Informed consent was not obtained because the study, which was planned and performed in accordance with the Helsinki Declaration, was conducted retrospectively.
Demographic and clinical charcateristics
Socioeconomic data such as age and sex, and data including described allergic reactions, responsible allergen agent detected in tests, which vaccine the patient was referred for, tests performed before vaccination, which vaccine was administered, how the vaccine was administered and reactions following vaccination, were collected and reviewed. Children who had a suspicious history of allergy were evaluated using SPT or allergen-specific IgE in blood. If a history of drug use that could affect the SPT was present, the drug was discontinued 10 days before the test. Positive (histamine) controls and negative controls (serum saline) were applied on the volar surface of the forearm with milk, egg white, and egg yolk. The reaction was evaluated 15 minutes later and enduration of ≥3 mm in comparison with the negative control was considered a significant reaction. Food allergen-specific IgE was specified using the radioallergosorbent (RAST) test. In patients, in whom reaction with vaccination was described, an SPT with the vaccine was performed before vaccination.
Statistical Analysis
Analysis of the data was performed using the Statistical Package for the Social Sciences package program 20.0 (SPSS Inc., Armonk, NY: IBM Corp.). Among the descriptive statistics, continuous variables are expressed as median and interquartile range (IQR). Frequency analyses are expressed as number (n) and frequency (%).
Results
A total of 122 children with a previous history of allergy and referred to our outpatient clinic for vaccination were evaluated. The patients’ demographic and clinical characteristics are shown in Table 1. The most common allergic symptom described by the families was skin findings such as erythema or rash (n=89, 86.4%), a history of anaphylaxis was found only in nine patients (n=9, 8.7%). According to the descriptions of the families, egg was the most common responsible factor that caused hypersensitivity (n=50, 43.5%). Among the patients who described anaphylaxis, the responsible factor was specified as egg in four patients, vaccine in two patients (MMR in one patient, second-month vaccines in one patient), and milk in three patients.
Table 1.
The patients’ demographic and clinical characteristics
| Clinical and demographic characteristics | n | % |
|---|---|---|
| Female | 52 | 42.6 |
| Male | 70 | 57.4 |
| Age at the time of diagnosis, | ||
| median (IQR), (months) | 12 (12–13) | |
| Allergic complaint described | ||
| Skin symptoms | 89 | 86.4 |
| Gastrointestinal tract symptoms | 24 | 23.3 |
| Anaphylaxis | 9 | 8.7 |
| Responsible factors according to family report | ||
| Only egg | 50 | 43.5 |
| Reaction to multiple foods | 42 | 36.5 |
| Only milk | 9 | 7.8 |
| Vaccine | 7 | 6.1 |
| Drug | 1 | 0.9 |
| Unknown | 6 | 5.2 |
IQR: Interquartile range. Gastrointestinal tract findings were described by the families as vomiting, diarrhea, bloody stool or mucus in the stool
The factors found in patients in whom the responsible allergen was searched for by performing laboratory tests are shown in Figure 1. In some patients, both SPT was performed and allergen-specific IgE was tested in blood, whereas one of the two methods was preferred in some others. Accordingly, laboratory tests revealed milk allergy in 27 patients (22.1%), egg white allergy was found in 66 patients (54.1%), and egg yolk allergy was found in 25 patients (20.5%). In 30 patients (29.4%), multiple factors were found as allergens in the tests. No allergen could be detected in the tests of thirty patients.
Figure 1.
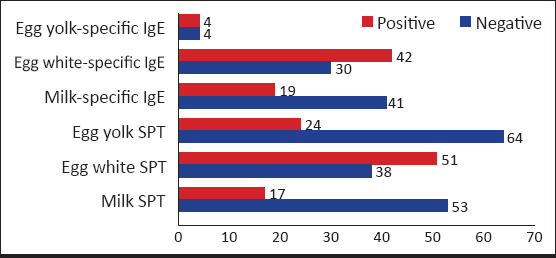
Allergens detected in the patients in whom laboratory test was performed
SPT: Skin prick test, numbers (n) express the number of patients
An SPT with the vaccine was performed before vaccination in 12 patients [MMR in seven patients, pneumococcus and combination vaccine (pentavalent) in five patients] and no reaction was observed. All 12th-month vaccines were administered in a great portion of the patients (n=87, 71.9%), and only MMR vaccine was administered to 21 (17.4%) patients, MMR and varicella vaccines were administered to five (4.1%) patients, MMR and conjugated pneumococcus vaccine (CPV) were administered to two (1.7%) patients, and various childhood vaccines were administered to the other patients. Vaccines were administered with incrementing doses (1/10 and 9/10) in divided doses in six patients. For MMR, which was the most commonly administered vaccine, the median delay time compared with normal was 20 days (IQR: 8.75–41.25, minimum: 0 days, maximum: 505 days). No severe allergic reaction was observed following vaccination in any patients. A mild eruption was reported a few hours after administration of MMR vaccine in one patient who was described by the family as having eruption with egg.
In our study, SPTs with the accused vaccines were performed in two children who were referred with a suspicion of anaphylaxis following vaccination. No reaction was observed following SPTs in any patients. Subsequently, the vaccine was administered to these patients in divided doses (10% of the vaccine was administered primarily, and 90% of the vaccine was administered half an hour later).
Discussion
Vaccination is one of the most efficient interventions for the prevention of contagious diseases in the community. Therefore, all children should be enabled to reach this service without delay. In recent years, the anti-vaccination movement has been proliferating in our country as well as throughout the world (12). Healthcare workers should have correct knowledge about vaccines and inform families correctly to counter the anti-vaccination movement. In our study, it was observed that vaccines could be safely administered to children with a history of allergy. No reaction following MMR vaccination was observed in children with egg allergy, which is one of the issues that lead to confusion, and even in children with a history of egg anaphylaxis. In light of these findings, MMR vaccination should be prevented from becoming a source of anxiety for families that have children with egg allergy.
Milk and egg allergies are the most common food allergies observed in children aged between 0 and 5 years in Turkey. Considering the prevalence, referral of families to hospitals outside FHCs for MMR vaccination of all children with egg allergy or request of evaluation by an allergist would cause an unnecessary workload and delay in vaccination. In our study, a history of anaphylaxis was present only in 8.9% of patients who were referred to our center. The remaining patients had been referred because of mild allergic symptoms. In addition, the diagnosis of egg allergy was not clear in a significant portion of these patients, and vaccination was delayed and the child was referred to an allergist only because of suspicion. In a study conducted by Ainsworth et al., (13) specific IgE measurement or SPT was priorly performed in terms of egg allergy in only 29% of the children who were referred to hospital for MMR vaccination. In case of clinical suspicion, evaluation by pediatric allergists is needed to clarify the diagnosis of food allergy. However, there is no need for delaying vaccination or administering vaccine in the hospital setting. The MMR vaccine contains live attenuated measles, mumps, and rubella viruses. It is grown in cultures of chicken embryo fibroblasts. Therefore, it contains only a negligible level of ovalbumin, which is egg white protein (0–1 ng/mL). In light of this information, our study also showed that children with egg yolk reaction or hypersensitivity were also referred for further evaluation in the same context, and vaccinations were delayed, although they had no increased risk in terms of a vaccine reaction. In previous studies, skin test with a vaccine (5, 8) was recommended before MMR vaccination in children with egg allergy, but currently, this is abandoned. The MMR vaccine can be administered to children with egg allergy by taking routine precautions, even if there is a history of anaphylaxis, and there is no need to perform a skin test before vaccination (2, 4, 7, 10).
It has been predicted that vaccination against measles throughout the world prevented 21.2 million deaths between 2000 and 2017 (14). In Turkey, the prevalence of measles shows a wavy course; no case was reported in 2008, 7405 cases were reported in 2013, nine cases were reported in 2016 and 716 cases were reported in 2018 (15). Increases in the number of cases are in parallel with the changes in vaccination rates (Fig. 2). The number of vaccine refusals reached 23 000 by 2018. The possibility of epidemic increases in case the number of anti-vaxxers reaches 50 000 (12). Considering that anti-vaccine movement is proliferating also in Turkey, delays because of a cause that is not a real contraindication such as egg allergy in MMR vaccination have put unvaccinated children in jeopardy. Being allergic to neomycin or gelatin, pregnancy, and immunosupression are among the real contraindications for the MMR vaccine (16, 17). Egg allergy is not among the contraindications for this vaccine. Anaphylaxis following vaccination has been rarely reported (one in 1.8–14.4 million doses), and it was shown that it occurred against the other components of the vaccine (gelatin or neomycin) rather than egg (17–20). Independent of a history of egg allergy or anaphylaxis, healthcare personnel and equipment competent to perform intervention when anaphylaxis develops, should be available at the time of all vaccinations (21).
Figure 2.

Number of cases of measles reported in Turkey by years
World Health Organization http://apps.who.int/immunization_monitoring/globalsummary/incidences?c=TUR) and annual MMR vaccination rate (Health statistics annual 2017 https://dosyasb.saglik.gov.tr/Eklenti/31096,turkcesiydijiv1pdf.pdf?0
In the extended immunization program circular issued by the Ministry of Health in 2008, anaphylactic or anaphylactoid reactions against egg were specified among the definite contraindications for the MMR vaccine, but it was emphasized that egg allergies excluding anaphylaxis did not constitute an obstacle for vaccination (22). According to the American Academy of Pediatrics (AAP) Infectious Diseases Committee, the MMR vaccine can be administered to children with egg allergy without taking any special precautions, even if they report a history of severe allergic reactions, because the risk of anaphylaxis is very low following vaccination (9). Skin test with the vaccine before vaccination may not predict allergic reaction against the vaccine, and most hypersensitivity reactions following the MMR vaccine are observed against other components of the vaccine such as gelatin, rather than against egg protein (9, 10). According to the recommendation of the British Society for Allergy and Clinical Immunology, all children with egg allergy should be immunized with the MMR vaccine routinely by family physicians and this information should be transmitted to families at the time of diagnosis. Allergists should be consulted only if anaphylaxis following vaccination has been reported (23). However, anaphylaxis with egg is not an absolute contraindication for MMR vaccination in these resources, in contrast to the Ministry of Health circular (9, 10, 23). In our country, these data and recent studies should be considered when updating the Ministry of Health’s vaccination recommendations.
Studies have shown that children with egg allergy are frequently referred to higher level centers for MMR vaccinations by family physicians (11, 13). In a study conducted by Cronin et al. (11), the authors reported that 69.5% of the patients referred for vaccination to their center were referred for MMR vaccination. In addition to egg allergy, being allergic to other foods, antibiotic allergies, eczema, the presence of family history of vaccine reaction, and nonspecific reactions against vaccines, are among the reasons of referral of patients referred for measles-mumps-rubella vaccination. However, mild minor reactions in the form of eruptions were observed in only six of 310 patients who were administered MMR vaccines (11). Allergy to foods other than egg also emerged as a reason for the referral of patients to hospital for MMR vaccination in other studies (13). Similarly, in our study, administration of MMR was avoided in FHCs because families reported reaction with foods other than egg or just reported suspicion of a reaction. In a study conducted by Goodyear-Smith et al. (24), the family physicians of children who were attempted to be referred to a special immunization clinic because they had egg allergy were informed that MMR vaccination was not disadvantageous, it was recommended that the vaccines of these children should be administered in primary healthcare centers, and no reactions were observed in any of the children. However, a delay in vaccination was experienced in 69% of the patients. In a study by Ainsworth et al. (13), no severe reaction was observed following vaccination administered at hospital setting in any of 110 children who were referred to hospital by family physicians for MMR vaccination, but it was observed that the primary dose was delayed more than 30 days in 81% of children. In our country, actualizing an appropriate referral system and continuous education of primary healthcare workers will provide a reduction in the number of inappropriate referrals and unnecessary use of resources. In addition, unnecessary delays in vaccination can be prevented by accurately informing primary care physicians who are primarily responsible for appropriate vaccination. In this study, the median delay time in MMR vaccination was found as 20 days and the reason for the delay time not to be very long was the fact that these children were rapidly evaluated in our center and their vaccinations were prioritized.
One of the results found in our study was that the other 12th-month vaccines (conjugated pneumococcus, Varicella) administered in association with MMR vaccine were also not administered and delayed, despite not containing egg antigen. As a result, children were put under risk in terms of infectious diseases that could be prevented through vaccination. Considering that even vaccines containing egg protein do not lead to severe systemic reactions, avoiding the 12th-month vaccines that should be administered in association with the MMR vaccine and do not contain egg protein has no scientific basis. This may result from insufficient knowledge of primary healthcare workers about vaccination or their safety hesitations.
It is not possible to predict the possibility of developing a severe allergic reaction to any vaccine. However, a patient who shows a severe allergic reaction to a specific vaccine is under risk at subsequent vaccinations with the same vaccine. In our study, one patient was referred to us following MMR vaccination and one patient was referred following the second-month vaccination with suspicion of anaphylaxis. Because of deficient documentation, it may not be possible to determine if these patients presenting in this way meet anaphylaxis criteria (25). Non-specific systemic reactions such as vasovagal reaction, fever, generalized skin eruption, malaise, muscle pain, diarrhea, and syncope following vaccination are considered as anaphylaxis, and patients are erroneously stigmatized as being “allergic to vaccines” (21). In our study, the SPT was performed with the accused vaccines and found to be negative in two children who were referred with a suspicion of anaphylaxis following vaccination; vaccination was performed in divided doses without any problems. The sensitivity and specificity of skin tests performed with vaccines are not clear and may not always predict post-vaccination reaction, but these tests are recommended because they give information related to sensitization (21). Vaccination with a less allergenic vaccine (if available) is recommended for children whose SPTs result in a reaction and who have a history of anaphylaxis with vaccine. If this is not possible, vaccination with gradually increasing doses with 15–30-minute intervals under conditions allowing emergency action if anaphylaxis develops, and subsequent observation for at least 30 minutes is recommended (10, 21).
In conclusion, no severe adverse events following vaccination were observed in any of the patients whose vaccinations were not performed by primary healthcare physicians because of a suspicion of allergy and were referred to us. As the possibility of observing severe hypersensitivity following vaccination is very low, immunization of these children without delay will prevent families’ concerns about vaccination. It is not considered harmful to administer MMR vaccine to children who have egg allergy or are suspected of having egg allergy at primary healthcare institutions. It should be targeted to inform primary care healthcare workers who are responsible for vaccination about this issue. If anaphylaxis following vaccination is suspected and vaccination with the same vaccine should be administered, it would be appropriate to administer the vaccine after evaluation by an allergist. Ensuring available personnel and equipment competent to intervene for anaphylaxis during all vaccinations should not be ignored.
Footnotes
Ethics Committee Approval: Ethics committee approval for our study was obtained from our univeristy’s committee (date: 05.10.2018, number: 09.2018.657).
Informed Consent: Patient consent was not obtained due to the retrospective design of the study. However, informed consent was obtained before applying SPT to the patients.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - P.B., E.K.A.; Design - P.B., E.K.A, A.K., S.B., H.E.B., A.Ö.; Supervision - P.B., E.K.A., S.B., A.K., A.Ö.; Materials - H.E.B., P.B., E.K.A., S.B., A.K., A.Ö.; Data Collection and/or Processing - H.E.B., P.B., E.K.A.; Analysis and/or Interpretation - H.E.B., P.B., E.K.A.; Literature Review - H.E.B., P.B., E.K.A.; Writing - H.E.B., P.B., E.K.A.; Critical Review - P.B., E.K.A., S.B., A.K., A.Ö.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
Etik Kurul Onayı: Çalışmamız için etik kurul onayı üniversitemiz kurulundan 05.10.2018 tarih ve 09.2018.657 numarası ile alındı.
Hasta Onamı: Çalışmanın geriye dönük tasarımından dolayı hasta onamı alınmamıştır. Ancak hastalara DDT uygulanırken onam alınmıştır.
Hakem Değerlendirmesi: Dış bağımsız.
Yazar Katkıları: Fikir - P.B., E.K.A.; Tasarım - P.B., E.K.A, A.K., S.B., H.E.B., A.Ö.; Denetleme - P.B., E.K.A., S.B., A.K., A.Ö.; Malzemeler - H.E.B., P.B., E.K.A., S.B., A.K., A.Ö.; Veri Toplanması ve/veya İşlemesi - H.E.B., P.B., E.K.A.; Analiz ve/veya Yorum - H.E.B., P.B., E.K.A.; Literatür Taraması - H.E.B., P.B., E.K.A.; Yazıyı Yazan - H.E.B., P.B., E.K.A.; Eleştirel İnceleme - P.B., E.K.A., S.B., A.K., A.Ö.
Çıkar Çatışması: Yazarlar çıkar çatışması bildirmemişlerdir.
Mali Destek: Yazarlar bu çalışma için mali destek almadıklarını beyan etmişlerdir.
References
- 1.Caubet JC, Ponvert C. Vaccine allergy. Immunol Allergy Clin North Am. 2014;34:597–613. doi: 10.1016/j.iac.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Echeverría-Zudaire LA, Ortigosa-del Castillo L, Alonso-Lebrero E, et al. Consensus document on the approach to children with allergic reactions after vaccination or allergy to vaccine components. Allergol Immunopathol (Madr) 2015;43:304–25. doi: 10.1016/j.aller.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Zhou W, Pool V, Iskander JK, et al. Surveillance for safety after immunization:Vaccine Adverse Event Reporting System (VAERS)--United States 1991-2001. MMWR Surveill Summ. 2003;52:1–24. [PubMed] [Google Scholar]
- 4.Fasano MB, Wood RA, Cooke SK, Sampson HA. Egg hypersensitivity and adverse reactions to measles, mumps, and rubella vaccine. J Pediatr. 1992;120:878–81. doi: 10.1016/s0022-3476(05)81953-5. [DOI] [PubMed] [Google Scholar]
- 5.Herman JJ, Radin R, Schneiderman R. Allergic reactions to measles (rubeola) vaccine in patients hypersensitive to egg protein. J Pediatr. 1983;102:196–9. doi: 10.1016/s0022-3476(83)80519-8. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien TC, Maloney CJ, Tauraso NM. Quantitation of residual host protein in chicken embryo-derived vaccines by radial immunodiffusion. Appl Microbiol. 1971;21:780–2. doi: 10.1128/am.21.4.780-782.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James JM, Burks AW, Roberson PK, Sampson HA. Safe administration of the measles vaccine to children allergic to eggs. N Engl J Med. 1995;332:1262–6. doi: 10.1056/NEJM199505113321904. [DOI] [PubMed] [Google Scholar]
- 8.Baxter DN. Measles immunization in children with a history of egg allergy. Vaccine. 1996;14:131–4. doi: 10.1016/0264-410x(95)00154-s. [DOI] [PubMed] [Google Scholar]
- 9.Kimberlin DW. 31th edition. Itaska (IL): American Academy of Pediatrics; 2018. Red Book—2018-2021 Report of the Committee on Infectious Diseases; p. 53. [Google Scholar]
- 10.Kelso JM, Greenhawt MJ, Li JT, et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130:25–43. doi: 10.1016/j.jaci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Cronin J, Scorr A, Russell S, McCoy S, Walsh S, O'Sullivan R. A review of a paediatric emergency department vaccination programme for patients at risk of allergy/anaphylaxis. Acta Paediatr. 2012;101:941–5. doi: 10.1111/j.1651-2227.2012.02737.x. [DOI] [PubMed] [Google Scholar]
- 12.Gür E. Vaccine hesitancy - vaccine refusal. Turk Pediatri Ars. 2019;54:1–2. doi: 10.14744/TurkPediatriArs.2019.79990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ainsworth E, Debenham P, Carrol ED, Riordan FA. Referrals for MMR immunisation in hospital. Arch Dis Child. 2010;95:639–41. doi: 10.1136/adc.2009.162487. [DOI] [PubMed] [Google Scholar]
- 14.Dabbagh A, Laws RL, Steulet C, et al. Progress Toward Regional Measles Elimination - Worldwide 2000-2017. MMWR Morb Mortal Wkly Rep. 2018;67:1323–9. doi: 10.15585/mmwr.mm6747a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. WHO vaccine-preventable diseases:monitoring system. 2020 global summary. [Accessed July 18 2019]. Available from:http://apps.who.int/immunization_monitoring/globalsummary/incidences?c=TUR.
- 16.Kowalzik F, Faber J, Knuf M. MMR and MMRV vaccines. Vaccine. 2018;36:5402–7. doi: 10.1016/j.vaccine.2017.07.051. [DOI] [PubMed] [Google Scholar]
- 17.McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS. Prevention of measles, rubella, congenital rubella syndrome, and mumps 2013:summary recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2013;62:1. [PubMed] [Google Scholar]
- 18.Kelso JM, Jones RT, Yunginger JW. Anaphylaxis to measles, mumps, and rubella vaccine mediated by IgE to gelatin. J Allergy Clin Immunol. 1993;91:867–72. doi: 10.1016/0091-6749(93)90344-f. [DOI] [PubMed] [Google Scholar]
- 19.Sakaguchi M, Ogura H, Inouye S. IgE antibody to gelatin in children with immediate-type reactions to measles and mumps vaccines. J Allergy Clin Immunol. 1995;96:563–5. doi: 10.1016/s0091-6749(95)70304-7. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi M, Nakayama T, Inouye S. Food allergy to gelatin in children with systemic immediate-type reactions, including anaphylaxis, to vaccines. J Allergy Clin Immunol. 1996;98:1058–61. doi: 10.1016/s0091-6749(96)80191-6. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson L, Brockow K, Alm J, et al. Vaccination and allergy:EAACI position paper, practical aspects. Pediatr Allergy Immunol. 2017;28:628–40. doi: 10.1111/pai.12762. [DOI] [PubMed] [Google Scholar]
- 22.T.C. Sağlık Bakanlığı, Temel Sağlık Hizmetleri Genel Müdürlüğü, GenişletilmişBağışıklama ProgramıGenelgesi, 25.02.2008. [Accessed July 17 2019]. Available from:https://dosyasb.saglik.gov.tr/Eklenti/1117,gbpgenelge2008pdf.pdf .
- 23.Clark AT, Skypala I, Leech SC, et al. British Society for Allergy and Clinical Immunology guidelines for the management of egg allergy. Clin Exp Allergy. 2010;40:1116–29. doi: 10.1111/j.1365-2222.2010.03557.x. [DOI] [PubMed] [Google Scholar]
- 24.Goodyear-Smith F, Wong F, Petousis-Harris H, Wilson E, Turner N. Follow-up of MMR Vaccination Status in Children Referred to a Pediatric Immunization Clinic on Account of Egg Allergy. Hum Vaccin. 2005;1:118–22. doi: 10.4161/hv.1.3.1888. [DOI] [PubMed] [Google Scholar]
- 25.Muraro A, Roberts G, Worm M, et al. Anaphylaxis:guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026–45. doi: 10.1111/all.12437. [DOI] [PubMed] [Google Scholar]


