Abstract
Hepatic sinusoidal obstruction syndrome (HSOS) can be caused by the intake of pyrrolizidine alkaloids (PAs). The disease has a high mortality rate, a poor prognosis and limited treatment options. Managing pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome (PA-HSOS) is a significant challenge for hepatologists. This case report describes five patients with PA-HSOS that were treated with low molecular weight heparin (LMWH) between 2014 and 2019. All five patients had a history of taking PA-containing herbal preparations before the onset of the disease. They all met the Nanjing diagnostic criteria and were diagnosed with PA-HSOS. Symptomatic treatment was administered to all five patients. In addition to symptomatic treatment, all five patients were treated with LMWH for approximately 8–21 days. After treatment, their ascites disappeared, symptoms improved and the hepatic venous blood flow had improved compared with before treatment. There was no obvious discomfort during the 6 months of follow-up. LMWH may play a useful role in the early treatment of PA-HSOS. Therefore, for patients in the early stages of PA-HSOS, in addition to symptomatic treatment, early anticoagulant intervention can be attempted to improve patient prognosis under close monitoring of coagulation.
Keywords: Low molecular weight heparin, early anticoagulant intervention, hepatic sinusoidal obstruction syndrome
Introduction
Hepatic sinusoidal obstruction syndrome (HSOS) is also known as hepatic venous occlusive disease. HSOS is a hepatic vascular disease that is histologically characterized by oedema, necrosis, detachment of endothelial cells in the small sinusoidal hepatic and interlobular veins and intrahepatic congestion, which leads to portal hypertension and liver dysfunction.1 In the Western world, most patients with HSOS receive myeloablative pretreatment during the course of haematopoietic stem cell transplantation (HSCT).2–4 However, in China, HSOS is mainly associated with plants containing pyrrolidine alkaloids (PAs), such as the herb chrysanthemum-like groundsel (known as Gynura segetum in China).2–4 The disease is not only rare in the clinic, but also has a high mortality rate, a poor prognosis and limited treatment options.5 This article reports on five patients with HSOS caused by PAs who were treated with low molecular weight heparin (LMWH) between 2014 and 2019. In addition, the current report reviews the available literature to describe the clinical characteristics of HSOS and raise awareness of the disease. The treatments outlined in this report may provide clinical suggestions for treating the disease.
Case reports
This was a retrospective review of five cases with pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome (PA-HSOS) that were admitted to the Department of Hepatology, First Hospital of Jilin University, Changchun, Jilin Province, China between 1 January 2014 and 31 December 2019. Each patient provided verbal informed consent for publication of their case. One of these patients was male and four were female (age range, 62–68 years). None of the five patients had a history of hepatitis or cirrhosis, but all patients had a history of Gynura segetum (a plant containing PAs) intake before the onset of the disease. One of the patients had taken Gynura segetum for varicose veins of the lower limbs and the other four has taken Gynura segetum for joint-related trauma. The exposure time to PAs ranged from 8 days to 3 months. In this current report, all five patients underwent computed tomography (CT) examination and two underwent liver biopsy. The infection and autoimmune indicators of all five patients were within normal limits. The clinical manifestations, imaging and pathological data for the five patients are presented below. The laboratory test results for the five patients are shown in Table 1.
Table 1.
Laboratory test results upon readmission of five patients diagnosed with pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome.
| Test parameter | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Alanine aminotransferase, U/l | 75 | 116 | 1154 | 26 | 39 |
| Aspartate aminotransferase, U/l | 98 | 93 | 965 | 68 | 38 |
| Glutamyl transpeptidase, U/l | 170.6 | 37.3 | 67.2 | 133.4 | 111.1 |
| Albumin, g/l | 33.5 | 29.9 | 36.1 | 26.9 | 26.9 |
| Total bilirubin, µmol/l | 47.3 | 22.1 | 87.1 | 41.2 | 22.1 |
| Prothrombin, sec | 15.7 | 14.9 | 19.8 | 14.6 | 15.4 |
| Prothrombin time activity, % | 61 | 65 | 46 | 71 | 63 |
Case 1
A 62-year-old male was admitted to hospital for abdominal pain, abdominal distension and jaundice on 2 September 2018. His physical examination revealed jaundice, hepatomegaly, and slow and positive migration. A contrast-enhanced abdominal CT showed hepatic congestion and invisible hepatic veins, which was thought to be caused by hepatic vena obliterans. Vascular ultrasound showed that hepatic vein stenosis was reduced, as was the portal vein flow rate. As a result, HSOS was strongly suspected. A detailed examination of the patient's medical history revealed that he had taken Gynura segetum for varicose veins of the lower limbs. A liver biopsy showed mild fibrosis around the central vein of the hepatic lobules, with III and II band sinus dilatation and congestion. In addition, the surrounding hepatocytes had atrophied and disappeared. Individual manifold areas were dilated and mildly fibrotic, with limited lymphocyte infiltration in the interstitial area. Based on the patient's clinical data, he was diagnosed with PA-HSOS.
Case 2
A 66-year-old female who had taken Gynura segetum for 8 days for joint trauma was admitted to hospital on 21 April 2017 after 2 weeks with symptoms of abdominal distension, abdominal pain and nausea. She had varicose veins in the abdominal wall, while other physical examinations were normal. A hepatic vein ultrasound suggested reduced blood flow velocity in the hepatic vein system, while an abdominal Doppler colour ultrasound indicated ascites. Her abdominal CT showed patchy liver enhancement, and, considering congestive changes, hepatic venous occlusion was considered possible. The patient underwent a liver biopsy, and the results showed oedema and fibrosis of the central venous wall, with significant dilation and congestion of the hepatic sinusoids. The venous lumen of the manifold area was significantly dilated, and lymphocytes and foam cells had infiltrated the interstitial area. Based on the patient's clinical data, she was diagnosed with PA-HSOS.
Case 3
A 67-year-old female had taken approximately 500 g of Gynura segetum for 15 days after femoral head replacement surgery. After 7 days, the patient developed abdominal distension, nausea and emesis and was admitted to hospital on 8 September 2016 for further treatment. Physical examination showed no obvious abnormalities. Abdominal ultrasonography suggested fluid accumulation in the peritoneum. Contrast-enhanced CT of the abdomen indicated hepatic congestion, and hepatic segments of the inferior vena cava and hepatic veins did not exclude Budd–Chiari syndrome or hepatic vein occlusion. Furthermore, ‘map-like’ density with uneven changes were observed on CT images. Although the patient had no liver biopsy, she was diagnosed with PA-HSOS due to her history of exposure to PAs, clinical features and auxiliary examination.
Case 4
A 62-year-old female took Gynura segetum for 3 months after spinal trauma, although the specific dose was unknown. The patient visited hospital for intermittent abdominal distension on 20 November 2019. A physical examination revealed varicose abdominal veins, enlarged liver and ascites. A hepatic vein ultrasound suggested that the hepatic vein system was unclear. Moreover, a contrast-enhanced abdominal CT indicated diffuse liver heterogeneity and HSOS was not excluded. Although the patient did not undergo a liver biopsy, PA-HSOS was diagnosed based on the clinical symptoms and auxiliary examination.
Case 5
A 68-year-old female was admitted to hospital on 5 August 2014 for abdominal distension after taking Gynura segetum for 8 days due for a waist sprain. An examination confirmed positive shifting dullness and swollen bilateral lower limbs. An abdominal ultrasound suggested fluid in the abdominal cavity. A contrast-enhanced CT showed an uneven, ‘map-like’ enhancement after an enhanced scan, focal oedema scattered in the liver tissue and unclear observation of the hepatic vein. The patient was diagnosed with PA-HSOS based on the clinical symptoms and auxiliary examination.
Symptomatic treatment was administered to all five patients, including discontinued exposure to PAs, liver protection and ascites management. None of the patients underwent liver transplantation. In addition to symptomatic treatment, all five patients received early anticoagulant intervention with LMWH (40 mg enoxaparin sodium by subcutaneous injection twice a day) for approximately 8–21 days. Patients were closely monitored for coagulation routine and bleeding during anticoagulant therapy. None of the patients experienced severe side-effects, such as bleeding, during treatment. After treatment, the ascites disappeared, symptoms improved, and the hepatic venous blood flow had improved compared with before treatment. Although the disease still remained, the patients' symptoms improved significantly and there was no obvious discomfort during 6 months of follow-up.
Discussion
Hepatic sinusoidal obstruction syndrome is caused by toxic injury to the endothelial cells of small hepatic blood vessels, particularly the sinusoidal endothelium in zone 3 of the liver acinus.6 Damaged sinusoids lead to sloughing and downstream occlusion of the terminal hepatic venules, which leads to intrahepatic congestion, hepatic injury and portal hypertension.3 In developed countries, HSOS often occurs in patients that have received cytoreductive therapy prior to HSCT or oxaliplatin-containing chemotherapy for colorectal carcinoma.2–4 The main cause of HSOS in China is ingestion of PA-containing herbal preparations or dietary supplements.5 The main clinical features of PA-HSOS are ascites, hepatomegaly, jaundice, abdominal distention and oedema.5 Since the early clinical picture is not obvious, it can be difficult to diagnose PA-HSOS. In this current report, abdominal distention and unexplained ascites were the main manifestations in all five patients. In clinical practice, for patients with ascites and hepatic distension without underlying disease, HSOS should be considered a possibility. For patients with suspected HSOS, a detailed medical history should be collected, imaging examinations should be conducted as soon as possible and a liver biopsy should be performed if necessary.
Pyrrolizidine alkaloid-containing plants are widely distributed and more than 300 PAs have been found in over 6000 plants.7 Although the exact mechanism of PA-HSOS is unclear, some studies have shown that PAs produce dehydropyrrolidinidine alkaloids and dehydroretronecine (DHR) through cytochrome-p450-mediated activation.8,9 DHR further interacts with glutathione or proteins to form pyrrole-glutathione conjugates or pyrrole-protein adducts (PPAs), respectively.8,9 PPAs are considered the leading cause of liver damage.8,9 In addition, glutathione depletion, reduced NO, increased expression of matrix metalloproteinases and vascular endothelial growth factor, and coagulation system abnormalities may contribute to the occurrence of PA-HSOS.10,11 Chinese people have the habit of taking herbal preparations, some of which contain PAs. The long-term use of herbs that contain PAs increasing the risk of the disease.
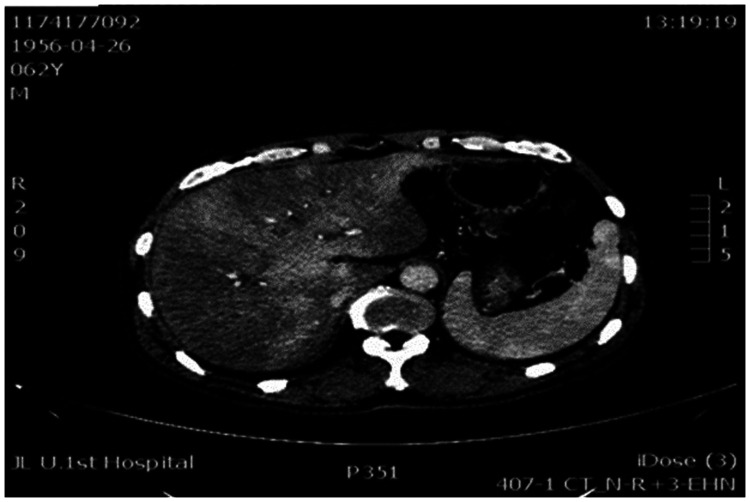
The diagnosis of PA-HSOS relies on clinical history, clinical features, laboratory examination, imaging, biomarkers and liver histopathology. To date, most of the studies on the diagnostic criteria of HSOS have focused on HSCT-related HSOS.12 However, in China, the main cause of HSOS is ingestion of PA-containing herbals.13 Therefore, the Chinese Society of Gastroenterology Committee of Hepatobiliary has formulated specific diagnostic criteria (Nanjing Criteria) for this disease.13 Together with a history of PA exposure, the patients must meet the following three conditions: (i) signs or symptoms of abdominal pain, abdominal distension, hepatomegaly and ascites; (ii) elevated total bilirubin or other liver function abnormalities; and (iii) imaging examination showing typical contrast-enhanced CT or magnetic resonance imaging manifestations, that is, the liver has ‘plaque-like’ and ‘map-like’ uneven density changes. In addition, patchy liver enhancement and heterogeneous hypoattenuation are valuable signs of PA-HSOS. In the current report, imaging studies from the five patients showed typical ‘map-like’ uneven density changes, which are the characteristic imaging manifestations of the disease (Figure 1). Additionally, intrahepatic vascular colour Doppler ultrasound can show intrahepatic blood vessels and blood flow and can evaluate treatment effectiveness by evaluating the blood flow in the liver after treatment. In addition, pathological examination is still the gold standard for disease diagnosis. Typical pathological manifestations are congested liver tissue, liver sinus dilation, hepatic vein wall thickening, fibrosis, lumen narrowing and even occlusion.8 In the current report, all patients had a history of taking PA-containing herbal preparations, as well as typical clinical features and imaging manifestations. In addition, the pathological findings of two patients (cases 1 and 2) suggested HSOS.
Figure 1.
Representative abdominal computed tomography scan showing typical ‘map-like’ uneven density changes in the liver, which are the characteristic imaging manifestations of pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome.
Managing PA-HSOS is a challenge for hepatologists as there is no definitive treatment for PA intoxication. The therapeutic strategies for PA-HSOS include PA exposure termination, symptomatic treatment and liver transplantation.12 HSOS is essentially a liver microcirculatory disorder, and, since there may be abnormal activation of the coagulation pathway in the early stage of disease, anticoagulant therapy may improve the prognosis of patients in the early stage of clinical disease. Therefore, in the early stage of HSOS, the focus should be on liver microcirculation and anticoagulant therapy in addition to symptomatic treatment. Defibrotide is a type of polydisperse oligonucleotide with anticoagulant, antithrombotic and anti-inflammatory effects.14 Defibrotide is the only approved drug for severe HSCT-related HSOS in Western countries.14 Since defibrotide is not yet approved in China, its effectiveness remains unknown in patients with PA-HSOS. It should also be noted that defibrotide is very expensive, so many patients in China would not be able to afford the treatment. LMWH also has anticoagulant, antithrombotic and anti-inflammatory effects; and is effective in treating venous thrombosis with a low risk of bleeding.15 Therefore, in our opinion there are some similarities in the pharmacology of LMWH and defibrotide. The findings of a previous study suggested that LMWH could effectively prevent hepatocyte necrosis and apoptosis caused by early liver injury.16 A Chinese retrospective study reported that anticoagulant therapy (LMWH combined with warfarin) significantly improved the response rates of patients with PA-HSOS compared with the non-anticoagulant group (60% versus 27%, respectively).17 In the current case report, all five patients were treated with LMWH; and as a result, their symptoms and liver blood flow improved significantly, and their ascites disappeared after treatment. These findings suggest that LMWH may play a useful role in the early treatment of PA-HSOS. Therefore, for patients in the early stages of the disease, in addition to symptomatic treatment, early anticoagulant intervention can be attempted to improve patient prognosis under close monitoring of coagulation. However, anticoagulation therapy in the late stage of the disease may increase the risk of bleeding, which may also be related to coagulation dysfunction, thus limiting its application in the late stage of HSOS.
In conclusion, this current case report describes the improvement in symptoms of five patients with PA-HSOS after treatment with LMWH. Therefore, early anticoagulant intervention may be attempted to improve patient prognosis under close monitoring of coagulation. Although treatment with LMWH improves the patient's liver blood flow and delays the development of disease, there is currently no specific disease treatment. With the development of the disease, liver transplantation may still be the only way to cure PA-HSOS.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This study was sponsored by the National Science and Technology Major Project (no. 2017ZX10202202, no. 2018ZX10302206), National key research plan ‘precision medicine research’ key project (no. 2017YFC0908103), the National Natural Science Foundation of Jilin Province (no. 20160101097JC), Program for JLU Science and Technology Innovative Research Team (no. 2017TD-08) and the Fundamental Research Funds for the Central Universities.
ORCID iD
Pujun Gao https://orcid.org/0000-0002-9592-119X
References
- 1.Gao H, Li N, Wang JY, et al. Definitive diagnosis of hepatic sinusoidal obstruction syndrome induced by pyrrolizidine alkaloids. J Dig Dis 2012; 13: 33–39. [DOI] [PubMed] [Google Scholar]
- 2.Dignan FL, Wynn RF, Hadzic N, et al. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol 2013; 163: 444–457. [DOI] [PubMed] [Google Scholar]
- 3.Morine Y, Shimada M, Utsunomiya T. Evaluation and management of hepatic injury induced by oxaliplatin-based chemotherapy in patients with hepatic resection for colorectal liver metastasis. Hepatol Res 2014; 44: 59–69. [DOI] [PubMed] [Google Scholar]
- 4.Robinson SM, Mann J, Vasilaki A, et al. Pathogenesis of FOLFOX induced sinusoidal obstruction syndrome in a murine chemotherapy model. J Hepatol 2013; 59: 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiaofeng D, Quanchu W. Successful treatment of one case of hepatic veno-occlusive syndrome caused by Gynura segetum. Chin J Gastroenterol and Hepatol 2018; 27: 478–480. [Google Scholar]
- 6.Valla DC, Cazals-Hatem D. Sinusoidal obstruction syndrome. Clin Res Hepatol Gastroenterol 2016; 40: 378–385. [DOI] [PubMed] [Google Scholar]
- 7.Chojkier M. Hepatic sinusoidal-obstruction syndrome: toxicity of pyrrolizidine alkaloids. J Hepatol 2003; 39: 437–446. [DOI] [PubMed] [Google Scholar]
- 8.Lin G, Cui YY, Hawes EM. Characterization of rat liver microsomal metabolites of clivorine, an hepatotoxic otonecine-type pyrrolizidine alkaloid. Drug Metab Dispos 2000; 28: 1475–1483. [PubMed] [Google Scholar]
- 9.Mattocks AR. Toxicity of pyrrolizidine alkaloids. Nature 1968; 217: 723–728. [DOI] [PubMed] [Google Scholar]
- 10.Yang M, Ruan J, Gao H, et al. First evidence of pyrrolizidine alkaloid N-oxide-induced hepatic sinusoidal obstruction syndrome in humans. Arch Toxicol 2017; 91: 3913–3925. [DOI] [PubMed] [Google Scholar]
- 11.Yang M, Ruan J, Fu PP, et al. Cytotoxicity of pyrrolizidine alkaloid in human hepatic parenchymal and sinusoidal endothelial cells: firm evidence for the reactive metabolites mediated pyrrolizidine alkaloid-induced hepatotoxicity. Chem Biol Interact 2016; 243: 119–126. [DOI] [PubMed] [Google Scholar]
- 12.Yang XQ, Ye J, Li X, et al. Pyrrolizidine alkaloids-induced hepatic sinusoidal obstruction syndrome: pathogenesis, clinical manifestations, diagnosis, treatment, and outcomes. World J Gastroenterol 2019; 25: 3753–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhuge Y, Liu Y, Xie W, et al. Expert consensus on the clinical management of pyrrolizidine alkaloid induced hepatic sinusoidal obstruction syndrome. J Gastroenterol Hepatol 2019; 34: 634–642. [DOI] [PubMed] [Google Scholar]
- 14.Richardson PG, Triplett BM, Ho VT, et al. Defibrotide sodium for the treatment of hepatic venoocclusive disease/sinusoidal obstruction syndrome. Expert Rev Clin Pharmacol 2018; 11: 113–124. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Shengli J, Fengshan W. Research progress of the pharmacological effects and clinical applications of low molecular weight heparins. Pharm Biotechnol 2014; 21: 573–578. [Google Scholar]
- 16.Kukner A, Tore F, Firat T, et al. The preventive effect of low molecular weight heparin on CCL4-induced necrosis and apoptosis in rat liver. Ann Hepatol 2010; 9: 445–454. [PubMed] [Google Scholar]
- 17.Zhuge YZ, Wang Y, Zhang F, et al. Clinical characteristics and treatment of pyrrolizidine alkaloid-related hepatic vein occlusive disease. Liver Int 2018; 38: 1867–1874. [DOI] [PubMed] [Google Scholar]