Abstract
Objective
CD4+ T lymphocyte count remains the most common biomarker of immune status and disease progression in human immunodeficiency virus (HIV)-positive individuals. VISITECT®CD4 is an instrument-free, low-cost point-of-care CD4 test with a cut-off of 350 CD4 cells/μL. This study aimed to evaluate VISITECT®CD4 test's diagnostic accuracy.
Methods
Two hundred HIV-positive patients attending a tertiary HIV centre in South India were recruited. Patients provided venous blood for reference and VISITECT®CD4 tests. An additional finger-prick blood sample was obtained for VISITECT®CD4. VISITECT®CD4's diagnostic performance in identifying individuals with CD4 counts ≤350 cells/μL was assessed by calculating sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) taking flow cytometry as the reference.
Results
The overall agreement between VISITECT®CD4 and flow cytometry was 89.5% using venous blood and 81.5% using finger-prick blood. VISITECT®CD4 showed better performance using venous blood [sensitivity: 96.6% (95% confidence interval: 92.1%–98.9%), specificity: 70.9% (57.1%–82.4%), PPV: 89.7% (83.9%–94.0%) and NPV: 88.6% (75.4%–96.2%)] than using finger-prick blood [sensitivity: 84.8% (77.9%–90.2%), specificity: 72.7% (59.0%–83.9%), PPV: 89.1% (82.7%–93.8%) and NPV: 64.5% (51.3%–76.3%)].
Conclusion
VISITECT®CD4 performed well using venous blood, demonstrating its potential utility in decentralization of CD4 testing services in resource-constrained settings.
Keywords: VISITECT®CD4 test, point-of-care diagnostics, CD4+ count, HIV monitoring assay, HIV/AIDS, resource-limited settings
Introduction
CD4+ T cell count is an indicator of immune function and remains an important tool to assess human immunodeficiency virus (HIV) disease stage, progression, and prognosis. HIV infection leads to depletion of CD4+ T cells in gut-associated lymphoid tissue with subsequent reductions of circulating CD4+ lymphocytes in the peripheral blood.1 Current WHO guidelines recommend lifelong antiretroviral therapy (ART) regardless of CD4+ cell count, with analysis of viral load as the preferred monitoring approach.2 However, assessment of CD4+ count at diagnosis can improve early ART initiation and retention.3 For patients with higher CD4+ counts (>350 cells/μL), longer term ART adherence may be enhanced if the patient is prepared for a few weeks through ART HIV education and counselling until ART readiness is confirmed. Previous studies have shown that access to CD4+ testing following diagnosis could improve ART initiation and retention4,5 and predict treatment outcomes: low CD4+ counts (<350 cells/µL) were associated with increased risk of non-adherence.6 In resourced-constrained setting where universal treatment is not feasible, ART initiation should be prioritised in HIV-positive individuals with advanced disease or CD4+ counts of less than 350 cells/µL2. Standard laboratory based CD4+ cell measurement techniques require an initial investment in flow cytometric technology, infrastructure requirements and associated reagent; these can be unaffordable or unavailable in resource-constrained settings, limiting patient access to CD4+ testing.7 Point-of-care (POC) CD4+ testing has the potential to overcome challenges of traditional laboratory based approaches and can provide reliable results under field conditions.8
The lateral flow-based VISITECT®CD4 rapid test (Omega Diagnostics, UK) is a promising tool to guide treatment decisions at the POC without extensive training or sophisticated equipment. This test provides a semi-quantitative determination of CD4 counts with a cut-off of 350 cells/µL. The schematic and testing procedures for VISITECT®CD4 have been reported elsewhere.9
This study aimed to evaluate the diagnostic accuracy of VISITECT®CD4 in detecting CD4+ counts less than 350 cells/µL compared with the gold standard, flow cytometry.
Materials and methods
This was a prospective diagnostic accuracy study. HIV-positive patients attending the HIV clinic at the YRG Centre for AIDS Research Education (YRG CARE), Chennai, India, were consecutively recruited between May and September 2017. No inclusion or exclusion criteria were applied beyond confirmation of HIV infection. All study participants aged 15 years and older provided written informed consent prior to enrolment. The study was approved by the YRG CARE institutional ethics committee. All procedures were conducted in accordance with the principles laid out in the 1964 Declaration of Helsinki and its later amendments
An expected sensitivity of 60% was reported in earlier studies.9,10 CD4+ cells were enumerated from all specimens using both VISITECT®CD4 and flow cytometry. Staff were trained to perform the VISITECT®CD4 test by experienced operators from Omega Diagnostics.
Limited sociodemographic information was collected using previously developed data collection forms to capture participants’ age and gender. A unique study ID was assigned to each participant to link sociodemographic information with diagnostic test results. A trained phlebotomist collected a finger-prick blood sample with a lancet and the venous whole blood specimens were collected using a 2-mL vacutainer tube (Becton Dickinson, Franklin Lakes, NJ, USA) containing ethylenediaminetetraacetic acid.
For each participant, four CD4+ tests were performed in parallel at the YRG CARE laboratory by trained lab technicians: (i) VISITECT®CD4 from finger-prick blood (performed and documented by one lab technician); (ii) VISITECT®CD4 from venous blood (two tests by two lab technicians); and (iii) a reference flow cytometric CD4+ count using FlowCARE PLG CD4+ reagent and a NAVIOS flow cytometer (Beckman Coulter, Brea, CA, USA) performed using the same venous sample used for VISITECT®CD4. The output in the VISITECT®CD4 test was either above reference (AR; CD4+ > 350 cells/µL) or below reference (BR; CD4+ cells < 350 cells/µL). Finger-prick testing was performed at the POC and the results were recorded immediately. Venous specimens were transported to the laboratory in gel-packed containers and maintained at 20–25°C before testing. The testing laboratory was in the second level of the same block as the specimen collection centre. All CD4+ assessments were performed within 6 hours of sample collection. VISITECT®CD4 tests were performed and results were recorded independently and blinded from the results of other testing procedures. The flow cytometry results were used for patient management and the VISITECT®CD4 test results did not influence clinical management of the patients participating in this study.
The diagnostic performance of VISITECT®CD4 in identifying samples with CD4+ counts less than 350 cells/µL was assessed by calculating sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). Analyses were performed using STATA statistical software version 15.0 (Stata Corp, College Station, TX, USA). Findings were reported according to the STARD reporting guidelines.11
Ethical approval and informed consent
All procedures were in accordance with the ethical standards of the YRG CAREs institutional review board, as well as the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from all participants included in the study.
Results
Demographic characteristics of study participants
Among the 200 HIV-positive patients recruited to the study, 123 were men (61.5%) and 77 were women (38.5%). The median age was 40 years (interquartile range [IQR]: 36–47.5 years; range: 15–68 years). CD4 counts ranged from 3 to 1264 cells/μL with a median of 222 cells/μL (IQR: 86.5–375 cells/μL). One hundred and forty-five patients (72.5%) had CD4 counts of ≤350 cells/μL and 55 (27.5%) had CD4 counts of >350 cells/μL according to the reference test (flow cytometry). Twenty-four (12%) participants had CD4+ counts between 300 and 400 cells/μL, of whom 14 and 10 had CD4 counts of 300–350 and 351–400 cells/μL, respectively. One participant had a CD4 count of exactly 350 cells/μL. There were no inconclusive reference or VISITECT®CD4 test results.
VISITECT®CD4 from finger-prick blood
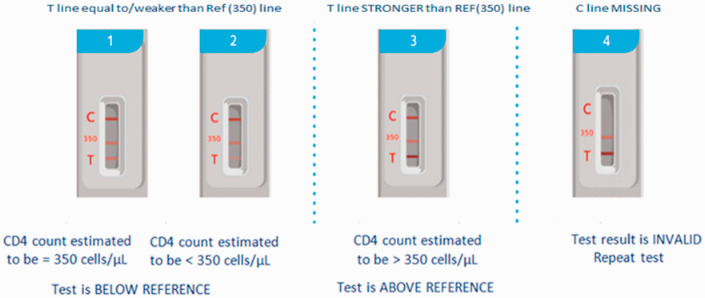
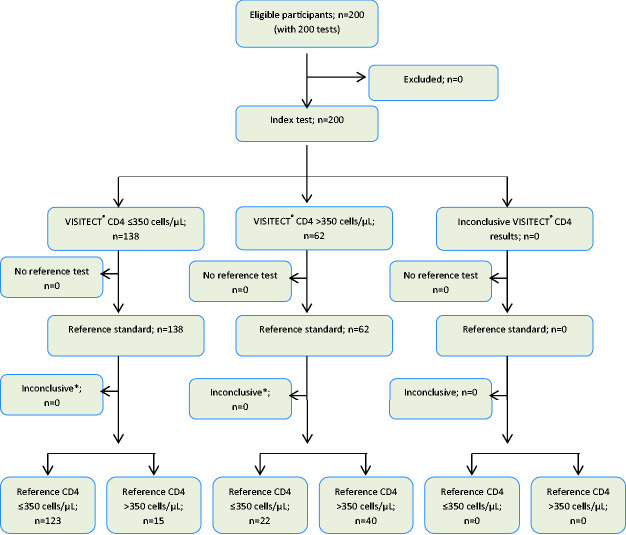
The disposable VISITECT®CD4 test uses 30 µL of whole blood and provides semi-quantitative results at a 350 cells/µL cut-off within 40 minutes (Figure 1). The visual interpretation of AR and BR for finger-prick blood samples using the VISITECT®CD4 test was compared for agreement with the quantitative results of flow cytometry. The overall agreement was 81.5% (163/200; Figure 2). The sensitivity, specificity, PPV and NPV of VISITECT®CD4 from finger-prick blood were 84.8% [95% confidence interval (CI): 77.9% to 90.2%], 72.7% (59.0% to 83.9%), 89.1% (82.7% to 93.8%) and 64.5% (51.3% to 76.3%), respectively (Table 1A). The performance characteristics of VISITECT®CD4 excluding the 24 specimens with CD4+ counts between 300 and 400 cells/μL are given in Table 1B for both finger-prick and venous blood samples.
Figure 1.
Interpretation of VISITECT®CD4 test results.
Figure 2.
Flow diagram of 200 VISITECT® CD4 tests performed using 200 finger-prick blood samples reported as per the STARD statement.11 *Results of the second (duplicate) VISITECT® CD4 test using venous blood.
Table 1A.
Performance characteristics of the VISITECT® CD4 Test.
| Flow cytometry |
VISITECT®CD4 test (visual reading) |
|||
|---|---|---|---|---|
|
Venous blood |
Finger-prick blood |
|||
| ≤350 cells/µL | >350 cells/µL | ≤350 cells/µL | >350 cells/µL | |
| ≤350 cells/µL (145) | 140 | 5 | 123 | 22 |
| >350 cells/µL (55) | 16* | 39* | 15 | 40 |
| Sensitivity, % (95%CI) | 96.6% (92.1% to 98.9%) | 84.8% (77.9% to 90.2%) | ||
| Specificity, % (95%CI) | 70.9% (57.1% to 82.4%) | 72.7% (59.0% to 83.9%) | ||
| PPV, % (95%CI) | 89.7% (83.9% to 94.0%) | 89.1% (82.7% to 93.8%) | ||
| NPV, % (95%CI) | 88.6% (75.4% to 96.2%) | 64.5% (51.3% to 76.3%) | ||
*The results of the second (duplicate) VISITECT®CD4 test using venous blood were 17 and 38 participants with ≤350 and >350 cells/μL, respectively, giving a sensitivity of 96.6% (92.1% to 98.9%), a specificity of 69.1% (55.2% to 80.9%), a PPV of 89.2% (83.2% to 93.6%) and a NPV of 88.4% (74.9% to 96.1%).
Table 1B.
Performance characteristics of the VISITECT®CD4 test excluding borderline specimens (n = 24).
| Flow cytometry |
VISITECT®CD4 test (visual reading) |
|||
|---|---|---|---|---|
|
Venous blood |
Finger-prick blood |
|||
| ≤350 cells/µL | >350 cells/µL | ≤350 cells/µL | >350 cells/µL | |
| <300 cells/µL (131) | 128 | 3 | 115 | 16 |
| >400 cells/µL (45) | 8 | 37 | 11 | 34 |
| Sensitivity, % (95%CI) | 97.7% (93.5% to 99.5%) | 87% (80.9% to 92.9%) | ||
| Specificity, % (95%CI) | 82.2% (67.9% to 92.0%) | 75.6% (60.5% to 87.1%) | ||
| PPV, % (95%CI) | 94.1% (89.5% to 96.8%) | 91.3% (86.2% to 94.6%) | ||
| NPV, % (95%CI) | 92.5% (80.0% to 97.4%) | 68.0% (56.6% to 77.6%). | ||
VISITECT®CD4 from venous blood
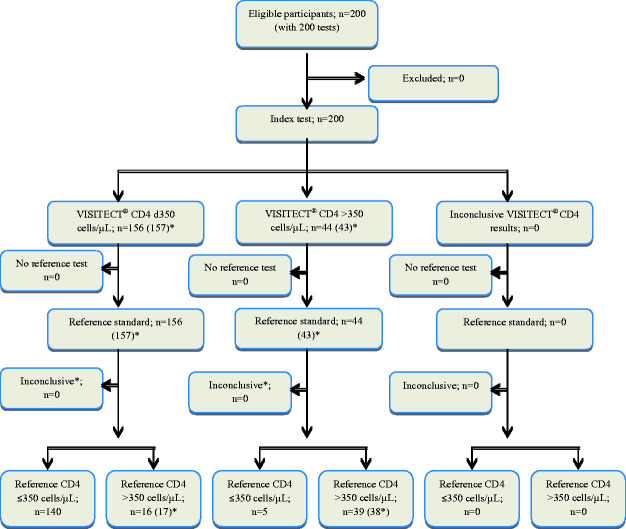
The overall agreement between VISITECT®CD4 from venous blood and flow cytometry was 89.5% (179/200; Figure 3). The sensitivity, specificity, PPV and NPV of VISITECT®CD4 were 96.6% (95% CI: 92.1% to 98.9%), 70.9% (95% CI: 57.1% to 82.4%), 89.7% (95% CI: 83.9% to 94.0%) and 88.6% (95% CI: 75.4% to 96.2%), respectively (Table 1A). A second (duplicate) VISITECT®CD4 test from venous blood gave similar results (Figure 3 & Table 1A). One specimen result was invalid because of a missed control line and this was not included in the analysis.
Figure 3.
Flow diagram of 200 VISITECT® CD4 tests performed using 200 venous blood samples reported as per the STARD statement.11 *Results of the second (duplicate) VISITECT®CD4 test using venous blood.
Discussion
In this study, we assessed the diagnostic performance of VISITECT®CD4, an affordable, equipment-free test for semi-quantitative measurement of CD4 counts at the POC. We compared the results of the VISITECT®CD4 test using both finger-prick and venous blood samples with those of the gold standard (flow cytometry) using a cut-off of 350 cells/µL. We found that the results of the VISITECT®CD4 test agreed well with those of the reference test, with an overall agreement of 81.5% for finger-prick blood samples and 89.5% for venous blood samples.
We observed better performance of the VISITECT®CD4 test using venous blood compared with finger-prick blood samples. This was reflected in a higher sensitivity in detecting samples with ≤350 cells/µL [96.6% (95% CI: 92.1% to 98.9%) vs 84.8% (95% CI: 77.9% to 90.2%)]. This result corroborates those of a study conducted in South Africa showing superior performance of VISITECT®CD4 using venous blood (sensitivity 81.7%, 95% CI: 72.3% to 91.1%) compared with finger-prick blood (sensitivity 60.7%, 95% CI: 45.0% to 76.3%).9 It is notable, however, that the sensitivity of VISITECT®CD4 reported here was significantly higher than that reported in the South African study using both sample types (96.6% vs 81.7% for venous blood and 84.8% vs 60.7% for finger-prick blood). This improved performance could result in part from the laboratory-based design and minor improvements to the test kit used in the present study. Another potential explanation for this difference was the distribution of CD4+ counts in our study population. A moderate proportion (24.5%) of patients had CD4+ counts between 250 and 450 cells/µL and the majority of samples (75.5%) fell outside this range. There may also be differences in the frequency of low CD4+ counts (<350 cells/µL) between Indian and African populations. Our findings further confirm that the VISITECT®CD4 test using venous blood could serve as a reliable alternative for measurement of CD4 counts, enabling accurate, timely clinical decision making at the POC.
The option to use finger-prick blood samples makes the VISITECT®CD4 test particularly suitable for health care workers in settings where venipuncture may not be feasible. Whilst the high sensitivity (and PPV) of VISITECT®CD4 using a 350-cells/µL cut-off and venous blood is encouraging, the lower specificity (and NPV) using finger-prick samples could be considered a limitation. Up to 35% (22/62) of patients had false negative results (CD4 count >350 cells/µL by VISITECT®CD4 using finger-prick blood but were demonstrated to have ≤350 CD4 cells/µL by flow cytometry). These patients (22/145 or 15% of patients with CD4 counts ≤350 cells/μL by flow cytometry) would not be given appropriate care if clinical decision relating to their immunological status and disease progression stage was based on VISITECT®CD4 test results from finger-prick blood.
Differences in the diagnostic performance of POC CD4 tests using finger-prick compared with venous blood have been previously reported.12,13 In spite of the laboratory challenges in resource-poor settings14 studies have shown that absolute CD4 counts and CD4 percentages obtained from capillary and venous blood are generally in agreement.15,16 Thus, researchers have speculated that test operator errors17 or variation in sample volume collection18 may be the causes of sub-optimal diagnostic performance of POC CD4 tests using finger-prick or capillary blood.
In this study, the VISITECT®CD4 test was performed by trained lab technicians using provided capillary tubes for sample collection in a clinical laboratory setting. This design minimises the likelihood of test operator errors and/or sample collection errors as contributing factors to suboptimal performance of the test using finger-prick blood. The results of duplicate testing of venous blood samples by different test operators confirmed that the VISITECT®CD4 test performed reliably using venous blood. However, the single finger-prick test by a third test operator did not allow head-to-head comparison between the venous and finger-prick tests results. Thus, test operator variation could not be ruled out as a source of differences in the diagnostic performance of VISITECT®CD4 test for different sample types. Research into the potential causes of the differential accuracy of the VISITECT®CD4 test using finger-prick blood samples is needed to guide implementation strategies, especially because finger-prick blood sampling would be the preferred method for POC CD4 testing.19
Conclusions
CD4 testing remains an important part of HIV treatment and care. The strong performance of VISITECT®CD4 using venous blood confirms its potential for decentralisation of CD4 testing services in resource-constrained settings. Further improvements in the diagnostic performance of VISITECT®CD4 using finger-prick blood are needed to maximise the potential impact of this test when deployed in the field.
Acknowledgements
The authors would like to acknowledge the clinical and laboratory staff who assisted in this study as well as the men and women who agreed to participate in the study.
Authors’ contributions
PB, SSS, DAA, CMM and SMC conceived and designed the analysis. VV, KK and PN collected the data. EV, JF, VV and SI performed data analysis. VV, SI, PB, SL and MDP wrote the paper. All authors critically reviewed the manuscript and approved the final version.
Declaration of conflicting interest
The study was funded by Omega Diagnostics. SMC and DAA developed the VISITECT® CD4 device at the Burnet Institute in Australia, which owns the intellectual property associated with the VISITECT®CD4 test. SL and MDP are employed by the Burnet Institute but were not involved in test development. JF and CMM are employees of Omega Diagnostics, which is the manufacturer of the VISITECT®CD4 test. They were not involved in the study processes and implementation other than in study design and training of the test operators.
Funding
This study was supported by Omega Diagnostics (Asia) Pvt, Ltd., Mumbai, India. Omega Diagnostics provided the VISITECT®CD4 tests free of charge for study purposes. The authors gratefully acknowledge the support of the Victorian Operational Infrastructure Support Program received by the Burnet Institute. Funding was provided by the National Health and Medical Research Council of Australia (NHMRC) through a Career Development Fellowship to SL.
ORCID iD
Pachamuthu Balakrishnan https://orcid.org/0000-0002-1710-9155
References
- 1.Ford N, Meintjes G, Pozniak A, et al. The future role of CD4 cell count for monitoring antiretroviral therapy. Lancet Infect Dis 2015; 15: 241–247. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, https://apps.who.int/iris/handle/10665/208825 (2016, accessed 16 July 2019). [PubMed]
- 3.Govindasamy D, Meghij J, Kebede Negussi E, et al. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low- and middle-income settings–a systematic review. J Int AIDS Soc 2014; 17: 19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai MA, Okal DO, Rose CE, et al. Effect of point-of-care CD4 cell count results on linkage to care and antiretroviral initiation during a home-based HIV testing campaign: a non-blinded, cluster-randomised trial. Lancet HIV 2017; 4: e393–e401. [DOI] [PubMed] [Google Scholar]
- 5.Wynberg E, Cooke G, Shroufi A, et al. Impact of point-of-care CD4 testing on linkage to HIV care: a systematic review. J Int AIDS Soc 2014; 17: 18809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonsah JY, Njamnshi AK, Kouanfack C, et al. Adherence to antiretroviral therapy (ART) in Yaoundé-Cameroon: Association with opportunistic infections, depression, ART regimen and side effects. PLoS One 2017; 12: e0170893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zachariah R, Reid SD, Chaillet P, et al. Viewpoint: Why do we need a point-of-care CD4 test for low-income countries? Trop Med Int Health 2011; 16: 37–41. [DOI] [PubMed] [Google Scholar]
- 8.Pham MD, Agius PA, Romero L, et al. Performance of point-of-care CD4 testing technologies in resource-constrained settings: A systematic review and meta-analysis. BMC Infect Dis 2016; 16: 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luchters S, Technau K, Mohamed Y, et al. Field performance and diagnostic accuracy of a Low-cost instrument-free point-of-care CD4 test (VISITECT®CD4) performed by different health worker cadres among pregnant women. J Clin Microbiol 2019; 57: e01277–18. Epub ahead of print February 2019. DOI: 10.1128/JCM.01277-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajian-Tilaki K. Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform 2014; 48: 193–204. [DOI] [PubMed] [Google Scholar]
- 11.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015; 351: h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pham MD, Agius PA, Romero L, et al. Acceptability and feasibility of point-of-care CD4 testing on HIV continuum of care in low and middle income countries: a systematic review. BMC Health Serv Res 2016; 16: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaw PA, Daneau G, Coly AA, et al. Multisite evaluation of a point-of-care instrument for CD4(+) T-cell enumeration using venous and finger-prick blood: the PIMA CD4. J Acquir Immune Defic Syndr 2011; 58: e103–e111. [DOI] [PubMed] [Google Scholar]
- 14.Birx D, De Souza M, Nkengasong JN. Laboratory challenges in the scaling up of HIV, TB, and malaria programs: The interaction of health and laboratory systems, clinical research, and service delivery. Am J Clin Pathol 2009; 131: 849–851. [DOI] [PubMed] [Google Scholar]
- 15.MacLennan CA, Van Oosterhout JJG, White SA, et al. Finger-prick blood samples can be used interchangeably with venous samples for CD4 cell counting indicating their potential for use in CD4 rapid tests. AIDS 2007; 21: 1643–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sitoe N, Luecke E, Tembe N, et al. Absolute and percent CD4+ T-cell enumeration by flow cytometry using capillary blood. J Immunol Methods 2011; 372: 1–6. [DOI] [PubMed] [Google Scholar]
- 17.Fajardo E, Metcalf C, Piriou E, et al. Errors generated by a point-of-care CD4+ T-lymphocyte analyser: a retrospective observational study in nine countries. Bull World Health Organ 2015; 93: 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bond MM, Richards-Kortum RR. Drop-to-drop variation in the cellular components of fingerprick blood: implications for point-of-care diagnostic development. Am J Clin Pathol 2015; 144: 885–894. [DOI] [PubMed] [Google Scholar]
- 19.Mtapuri-Zinyowera S, Chiyaka ET, Mushayi W, et al. PIMA point of care CD4+ cell count machines in remote MNCH settings: Lessons learned from seven districts in Zimbabwe. Infect Dis (Auckl) 2013; 6: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]