Abstract
Vasopressin local infiltration is useful in gynecological surgery because it can reduce hemorrhage. Depending on the activities of the sympathetic system and the renin–angiotensin system, reactions to vasopressin may differ and predicting its systemic effects is difficult. Because life-threatening complications can occur, infiltration with vasopressin should be administered with caution. A 42-year-old female patient was diagnosed with uterine leiomyomas. During a robot-assisted laparoscopic myomectomy, 50 U of vasopressin, which is ten-times the recommended dose, was accidentally infiltrated. Subsequently, bradycardia with a heart rate of 25 bpm occurred, which recovered within 3 minutes. Peripheral perfusion indices and the diameter of the radial and brachial arteries also decreased markedly and recovered within 1 hour. The surgery was concluded without additional events. The patient was discharged 2 days later with no abnormal findings. Because vasopressin infiltration can cause life-threatening complications, it is necessary to determine the extent of patient reactions to vasopressin using measures such as the peripheral perfusion index or radial and brachial artery diameters. These measures may also help to predict the occurrence of complications.
Keywords: Vasopressin, gynecologic surgical procedures, uterine myomectomy, vasoconstriction, baroreflex, bradycardia, perfusion index
Introduction
Vasopressin causes vascular smooth muscle constriction and myometrial contraction.1,2 Consequently, vasopressin local infiltration can reduce hemorrhage (which is the most common complication of gynecological surgery), shorten the duration of surgery, and prevent additional complications such as infection.3 However, cautionary measures should be taken against life-threatening complications, such as pulmonary edema and cardiac arrest, which are associated with vasopressin.1
This is a report of a case of severe bradycardia and vasoconstriction following accidental intramyometrial infiltration of 50 U of vasopressin (1 U/mL) during a robot-assisted laparoscopic myomectomy. The novel aspect of this report is that it traces changes in vital signs and other parameters such as the diameters of arteries, which may be important in selecting the appropriate anesthetic management for patients who are undergoing vasopressin infiltration.
Case report
A 42-year-old female patient underwent transvaginal ultrasonography and magnetic resonance imaging to address menorrhagia. She was diagnosed with one 4-cm intramural and two 2-cm subserosal uterine leiomyomas, and she was expected to undergo robot-assisted laparoscopic myomectomy. The patient’s height and weight were 152.2 cm and 55.7 kg, respectively. Her medical history was unremarkable and laboratory test results, chest radiograph, and electrocardiogram (ECG) results showed no abnormality, and her American Society of Anesthesiologists physical status classification was 1.
In the surgical theater, three-lead continuous electrocardiography, noninvasive blood pressure, pulse oximetry, and bispectral index were preferentially performed. Before anesthesia induction, the patient’s blood pressure (BP) was 128/68 mmHg, heart rate (HR) was 54 beats per minute (bpm), body temperature was 36.6°C, and respiratory rate was 16 breaths per minute. Total intravenous anesthesia was performed using propofol and remifentanil. Target-controlled infusion of propofol and remifentanil was performed using the Schnider and Minto models, respectively. Anesthesia was maintained with 50% oxygen and nitrous oxide 50%, propofol with an effect-site concentration (Ce) of 2.2 mg/mL, and remifentanil with a Ce of 3.0 ng/mL. Subsequently, a rectus sheath block was performed on both sides for postoperative pain control, and continuous arterial line monitoring was set up through cannulation into the right radial artery. The pulse oximeter was attached to the left fourth finger. Plasma solution-A® (CJ HealthCare, Seoul, Korea) was administered at a rate of 300 mL/hour as a maintenance fluid.
A trocar was inserted through a skin incision. Myomas were found at a 30° Trendelenburg position. Intramyometrial infiltration of the prepared 0.1 U/mL vasopressin was performed through a 15-gauge injection cannula at 1 hour and 20 minutes after anesthesia induction. Right before the infiltration, vital signs were as follows: BP, 148/87 mmHg; HR, 54 bpm; peripheral oxygen saturation (SpO2), 100%; and peripheral perfusion index (PPI), 1.0.
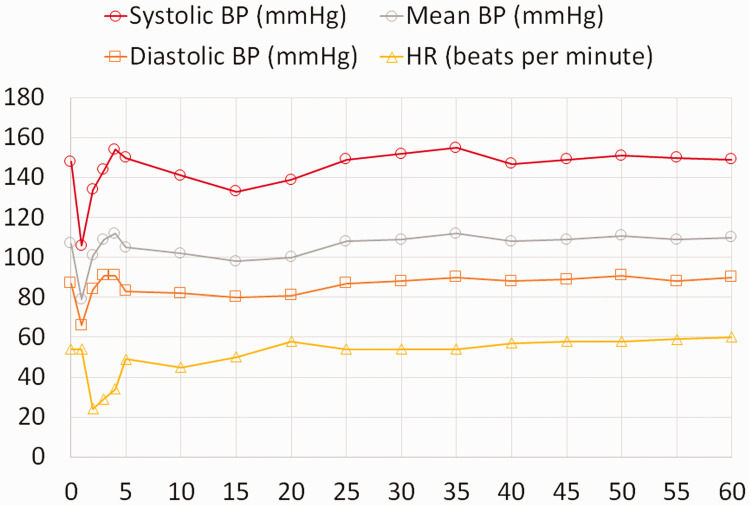
The duration of infiltration was 2 minutes, and 50 mL of vasopressin was infiltrated. Immediately after infiltration began, BP decreased to 106/66 mmHg for a minute. There was no significant change in HR. One minute into infiltration, BP began to increase to 134/84 mmHg 1 minute later. HR kept decreasing to 24 bpm at the 2-minute mark. The infiltration was discontinued and atropine was prepared. The inspired oxygen concentration was set to 100% to compensate for the inability to measure SpO2 because of a sharp decrease in the peripheral perfusion index. The patient’s lip color remained bright red. Subsequently, HR slowly increased and the prepared atropine was not administered. A systolic BP of 130 to 150 mmHg and a diastolic BP of 80 to 90 mmHg were maintained and no additional treatment was administered. HR kept increasing gradually to resolve bradycardia within 3 minutes of its occurrence. Five minutes after infiltration, BP was 150/83 mmHg and HR was 50 bpm (Figure 1).
Figure 1.
Changes in vital signs over time (minutes) after vasopressin infiltration. The x-axis represents time.
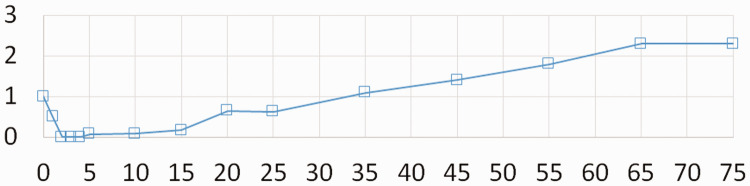
The BPs that were measured immediately before the infiltration and 10 minutes after the infiltration were similar. When HR was stabilized and SpO2 was maintained at 100%, the surgery was resumed and it was realized that the concentration of infiltrated vasopressin had been 1 U/mL instead of 0.1 U/mL, which corresponded to a total infiltrated dose of 50 U. PPI decreased as soon as bradycardia occurred and could not be measured until bradycardia subsided. The lowest PPI measurement was 0.07 and it gradually increased and reached 2.0 1 hour after infiltration (Figure 2).
Figure 2.
Change in peripheral perfusion index over time (minutes) after vasopressin infiltration. The x-axis represents time. The value of 0 indicates the case where the index cannot be measured.
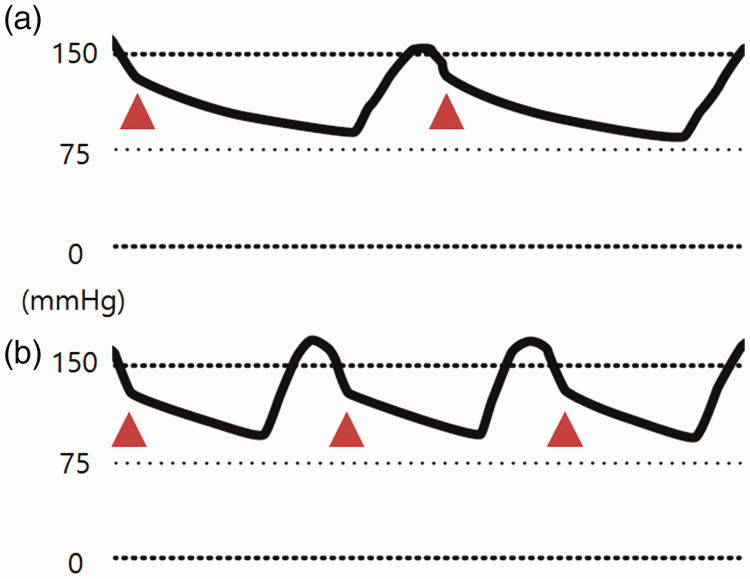
The arterial waveform at 5 minutes after infiltration had a relatively high dicrotic notch position, which decreased after 1 hour (Figure 3).
Figure 3.
The arterial waveform at 5 minutes after vasopressin infiltration (a) and 1 hour later (b). The y-axis represents arterial blood pressure. ▲ indicates the positions of dicrotic notches.
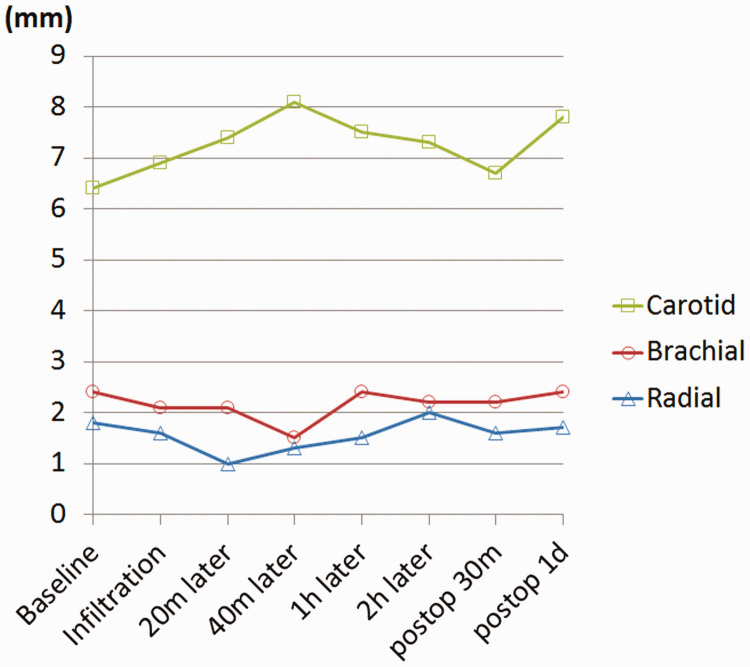
The diameters of the left radial artery at the proximal wrist crease level, the left brachial artery at the medial epicondyle level of the humerus, and the right carotid artery in the upper 1-cm portion of the sternoclavicular joint were periodically measured from identical locations before and after the infiltration. Before infiltration, the diameters were 1.6 mm, 2.1 mm, and 6.9 mm, respectively. They changed to 1.0 mm, 2.1 mm, and 7.4 mm, respectively, at 20 minutes after infiltration; 1.3 mm, 1.5 mm, and 8.1 mm, respectively, at 40 minutes after infiltration; and 1.5 mm, 2.4 mm, and 7.5 mm, respectively, at 60 minutes after infiltration (Figure 4).
Figure 4.
Changes in the diameter of the carotid, brachial, and radial arteries over time after vasopressin infiltration. “Postoperative 30 minutes” equals 3 hours and 15 minutes after infiltration.
Arterial blood gas analysis (ABGA) was performed 15 minutes before and 10 minutes after the infiltration. The hemoglobin concentration (Hbc) increased from 9.9 to 10.9 g/dL. No significant event other than intramyometrial vasopressin infiltration occurred within these 25 minutes. Subsequently, Hbc was sustained at 10.9 g/dL until the surgery was completed. There was no urination within the first hour after infiltration; however, 130 mL of urine was passed by the patient during the following 2 hours.
The surgery was completed without additional events. The total duration of anesthesia was 4 hours and 15 minutes. The total duration of surgery was 3 hours and 5 minutes. The anesthesia lasted for 2 hours and 45 minutes starting from the beginning of infiltration to the end of surgery. The estimated blood loss was 50 mL. The patient was sent to the ward after 30 minutes of thorough examinations in the post-anesthetic care unit. The vital signs that were measured on the ward were also stable. No abnormality was found on post-surgical tests. The patient was discharged 2 days post-surgery.
This case report was approved by the institutional review board of Eulji University Medical Center and was conducted in compliance with the EQUATOR Network guidelines. Written informed consent for publication was obtained from the patient.
Discussion
Vasopressin elicits constriction of the vascular smooth muscle and contraction of the myometrium through V1, a vasopressin receptor subtype, and antidiuretic effect in the kidney collecting duct cells through V2.1,2 When infiltrated, vasopressin reduces hemorrhage, which is the most common complication during gynecological surgery, through its vasoconstrictive and uterine contraction effects, shortens the duration of surgery, and prevents additional complications such as infection.3 However, cautionary measures should be taken against life-threatening complications such as pulmonary edema and cardiac arrest,1 and it is recommended that the total dosage of vasopressin does not exceed 4 to 6 U. Vasopressin should also be diluted because the risk of complications increases with inadvertent intravascular injection. The regular concentration is 0.2 U/mL, but the minimum concentration with therapeutic effect has been reported to be 0.05 U/mL.3
This report describes the case of a patient with bradycardia and vasoconstriction that occurred after an intramyometrial vasopressin infiltration. Vasopressin for infiltration was supposed to be diluted to 0.1 U/mL. Because of a miscommunication, 100 mL of vasopressin with ten-times the intended concentration of 1 U/mL was prepared, and 50 mL of this high-dose vasopressin was administered during infiltration. This means that 50 U of vasopressin had been injected, which is ten-times the recommended dose. The duration of infiltration was 2 minutes, and infiltration was discontinued when bradycardia with a HR of 25 bpm was observed. Vasopressin acts on the area postrema to augment baroreflex and inhibit efferent sympathetic nerve activity.1,2 High-dose vasopressin infiltration augments the baroreflex in excess. As the sympathetic outflow decreases, BP decreases. Subsequently, vasoconstriction occurs, BP increases, and the high augmentation of the baroreflex causes bradycardia. Plasma concentration decreases with vasopressin distribution and bradycardia gradually subsides. It was previously reported that bradycardia was followed by cardiac arrest in a patient who was undergoing an intramyometrial vasopressin infiltration, and a strategy for preventing bradycardia is to use a drug that suppresses the baroreflex. Typical drugs include propofol, anticholinergics such as glycopyrrolate and atropine, sevoflurane, isoflurane, and desflurane.4–7 Because fentanyl suppresses the baroreflex at high doses,8 using opioids such as fentanyl and remifentanil at high doses can be helpful.
Severe vasoconstriction, which manifested as more than a 50% decrease in the diameters of the radial and brachial arteries on echograms, was reported to follow vasopressin infiltration.9 Because our hospital had a case of cardiac arrest after vasopressin infiltration in 2017,10 all robotic gynecological surgeries that include vasopressin infiltration, together with monitoring the PPI, monitor the diameter changes of radial and brachial arteries as indices of the patient’s reaction to vasopressin. For the current case, the diameters of the radial and brachial arteries initially decreased after vasopressin infiltration but recovered 1 hour later. PPI is a value that was obtained from the photoelectric plethysmographic signal of pulse oximetry, and it reflects real-time peripheral blood flow. Thus, when peripheral blood flow decreases with vasoconstriction, its value also decreases.11 In this case, the PPI was 1.0 immediately before vasopressin infiltration, but it started decreasing when infiltration was started. Five minutes after infiltration, the PPI was 0.07. It gradually increased above 2.0 after 1 hour.
A dicrotic notch is a unique shape that is observed at points that represent the closure of the aortic valve during the cardiac cycle of the arterial waveform. Because the closure of the aortic valve occurs faster with higher values of systemic vascular resistance (SVR), there is a superior shift of the dicrotic notch when SVR increases.12 The arterial waveform immediately after infiltration is shown in Figure 3a with a superiorly shifted dicrotic notch, which reflects an increased SVR. One hour later, it changed as shown in Figure 3b, with an inferiorly shifted dicrotic notch reflecting a relatively decreased SVR. There was no urination within the first hour after infiltration, but urination occurred at a rate of 65 mL/hour thereafter. It is fairly difficult to predict the plasma concentration of vasopressin. The plasma half-life of vasopressin is between 4 and 20 minutes2 and the exact rate of systemic absorption through the myometrium during an infiltration cannot be determined. However, it can be estimated that the systemic effects of vasopressin significantly subside after 1 hour of infiltration based on these findings. The vasopressin system, the sympathetic nervous system, and the renin–angiotensin system are crucial for controlling blood pressure. When one of the two latter systems is intact, the vasopressin system only plays a minor role in controlling blood pressure. However, when neither of the two latter systems are intact, the vasopressin system takes on a major role, and even a small increase in the plasma vasopressin concentration can increase systemic vascular resistance.1 It is difficult to determine the activities of an individual’s sympathetic nervous system and renin–angiotensin system, and it is equally difficult to predict how an individual would react to vasopressin without vasopressin infiltration. Given such difficulties, PPI and the diameter changes in arteries are parameters that can quantitatively represent reactions to vasopressin and facilitate a better understanding of the magnitude and duration of its systemic effects in patients who are undergoing vasopressin infiltration. This will help to improve anesthetic management.
Another unique observation was the sudden increase of Hbc. While the ABGA that was conducted before infiltration showed Hbc of 9.9 g/dL, this increased by 1.0 g/dL in 25 minutes to 10.9 g/dL without any attributable event other than the vasopressin infiltration. Authors suggest that this is caused by severe vasoconstriction following the infiltration; as vasoconstriction increases the central blood volume, it also increases the pressure within the venules.13 In accordance with the Starling equation, such a change causes an increase in net flow from the capillaries to the interstitial space (i.e. an increase in extravascular fluid shift of the plasma),14 and similarly, severe vasoconstriction must have caused hemoconcentration. Authors suggest the following two causes for pulmonary edema, which was reported as a complication of vasopressin infiltration: the first is the myocardial ischemia that results from the reduced cardiac oxygen delivery that was observed when using vasopressin at high doses;2 and the second is, as mentioned above, the extravascular fluid shift that is caused by the vasopressin-induced severe vasoconstriction. Thus, the possibility that pulmonary edema can occur without heart failure must be taken into account.
In conclusion, this case assessed the hemodynamic changes that occurred after a high-dose intramyometrial vasopressin infiltration. Depending on the activities of the sympathetic nervous system and the renin–angiotensin system, the reaction to vasopressin may differ; even a small dose of vasopressin infiltration can result in fatal hemodynamic changes. Observing PPI or the diameters of arteries provides a quantitative assessment of changes in the patient’s condition after vasopressin infiltration. It can also facilitate predictions of the time it takes for changes to peak and/or return to baseline. PPI may have significant clinical usefulness because it can be easily measured. Gynecological surgeons and anesthesiologists should understand the life-threatening complications of vasopressin infiltration. Specifically, anesthesiologists should establish sufficient understanding of vasopressin’s systemic effects and provide anesthetic management that can minimize complications and ensure the safety of patients. They should also be prepared at all times to take appropriate measures against possible complications.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Goo Kim https://orcid.org/0000-0002-9402-5537
Tae Woo Kim https://orcid.org/0000-0002-2115-6448
Dong Ho Park https://orcid.org/0000-0002-6587-3756
References
- 1.Park KS, Yoo KY. Role of vasopressin in current anesthetic practice. Korean J Anesthesiol 2017; 70: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treschan TA, Peters J. The vasopressin system: physiology and clinical strategies. Anesthesiology 2006; 105: 599–612. [DOI] [PubMed] [Google Scholar]
- 3.Chudnoff S, Glazer S, Levie M. Review of vasopressin use in gynecologic surgery. J Minim Invasive Gynecol 2012; 19: 422–433. [DOI] [PubMed] [Google Scholar]
- 4.Sato M, Tanaka M, Umehara S, et al. Baroreflex control of heart rate during and after propofol infusion in humans. Br J Anaesth 2005; 94: 577–581. [DOI] [PubMed] [Google Scholar]
- 5.Parlow JL, Van Vlymen JM, Odell MJ. The duration of impairment of autonomic control after anticholinergic drug administration in humans. Anesth Analg 1997; 84: 155–159. [DOI] [PubMed] [Google Scholar]
- 6.Umehara S, Tanaka M, Nishikawa T. Effects of sevoflurane anesthesia on carotid-cardiac baroreflex responses in humans. Anesth Analg 2006; 102: 38–44. [DOI] [PubMed] [Google Scholar]
- 7.Muzi M, Ebert TJ. A comparison of baroreflex sensitivity during isoflurane and desflurane anesthesia in humans. Anesthesiology 1995; 82: 919–925. [DOI] [PubMed] [Google Scholar]
- 8.Lennander O, Henriksson BA, Martner J, et al. Effects of fentanyl, nitrous oxide, or both, on baroreceptor reflex regulation in the cat. Br J Anaesth 1996; 77: 399–403. [DOI] [PubMed] [Google Scholar]
- 9.Riess ML, Ulrichs JG, Pagel PS, et al. Case report: severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg 2011; 113: 1103–1105. [DOI] [PubMed] [Google Scholar]
- 10.Lee GG, Baek SY, Kim TW, et al. Cardiac arrest caused by intramyometrial injection of vasopressin during a robotic-assisted laparoscopic myomectomy. J Int Med Res 2018; 46: 5303–5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lima A, Bakker J. Noninvasive monitoring of peripheral perfusion. Intensive Care Med 2005; 31: 1316–1326. [DOI] [PubMed] [Google Scholar]
- 12.Nirmalan M, Dark PM. Broader applications of arterial pressure wave form analysis. Contin Educ Anaesth Crit Care Pain 2014; 14: 285–290. [Google Scholar]
- 13.Peters J, Mack GW, Lister G. The importance of the peripheral circulation in critical illnesses. Intensive Care Med 2001; 27: 1446–1458. [DOI] [PubMed] [Google Scholar]
- 14.Gropper MA, Miller RD, Cohen N, et al. Perioperative fluid and electrolyte therapy In: Miller RD. (ed) Miller’s anesthesia. 9th ed Philadelphia: Elsevier, 2020, pp.1480–1523. [Google Scholar]