Abstract
Objective
The therapeutic efficacy of apigenin in PC12 cells and rats remains uncertain. The aim of this study was to investigate the neuroprotective effects of apigenin against cerebral ischemia/reperfusion injury, both in vitro and in vivo.
Methods
We first treated PC12 cells with cobalt chloride (CoCl2) to create a model of oxidative stress injury. Cell viability was then determined using a multifunctional microplate reader. In addition, reactive oxygen species (ROS) levels, apoptosis, and mitochondrial membrane potentials (MMPs) were examined using high-content cytometer analysis. The efficacy of apigenin treatment was also analyzed in a rat middle cerebral artery occlusion (MCAO) model using TTC staining and neurological deficit scores.
Results
The half-inhibitory concentration of CoCl2 was 1.2 mM. Pretreatment with 10 µg ⋅ mL−1 apigenin significantly enhanced cell viability, reduced ROS levels, alleviated apoptosis, and improved MMP in PC12 cells with CoCl2-induced injury in vitro. In addition, apigenin treatment in vivo significantly improved neurological deficit scores and reduced infarct areas in MCAO rats. These results suggest that the neuroprotective mechanisms of apigenin may be related to mitochondrial activation.
Conclusions
Apigenin had excellent neuroprotective effects for the treatment of cerebral ischemia/reperfusion injury in vitro and in vivo.
Keywords: Cerebral ischemia/reperfusion, neuropharmacology, apigenin, flavonoids, PC12 cell model, oxidative stress, mitochondria, middle cerebral artery occlusion rat model
Introduction
Ischemia stroke (IS, also known as cerebral ischemia/reperfusion) makes up more than 80% of all stroke, and is a primary cause of human disability and death.1 Mechanisms of IS injury include energy metabolism disorder, glutamate excitotoxicity, oxidative stress, apoptosis, ion balance disorder, and inflammation, because of the blocked blood supply.2 Oxidative stress, which occurs because of an imbalance between antioxidants and pro-oxidants, plays a major role in IS events. Neuronal cells are highly sensitive to injuries induced by oxidative stress,3 which can result from the suppression of mitochondrial metabolic function. The main manifestation of oxidative stress is the production of free radicals.4 Increasing evidence has revealed that apoptosis, changes in mitochondrial membrane potential (MMP), and oxidative stress are related to IS, and are connected by the mitochondrial pathway.5,6
Reactive oxygen species (ROS) are the main products of cellular processes, and in PD12 cells they are primarily generated in the mitochondria.7,8 Approximately 2% of mitochondrial oxygen consumption is used to generate ROS.9 ROS have been reported to serve a critical role in the release of proapoptotic proteins and cytochrome c, which induces apoptosis in neuronal cells.10 Furthermore, ROS accumulation during hypoxia-induced lipid peroxidation results in altered brain functions.11 It has been reported that cobalt chloride (CoCl2)-induced hypoxia causes oxidative stress by lowering the activities of antioxidative enzymes.12 Therefore, the inhibition of ROS-induced oxidative stress is considered a promising therapeutic strategy for the treatment of IS.13
Thrombolytic agents have been adopted for the clinical treatment of IS, and work by converting inactive plasminogen to plasmin, thus improving cerebral blood flow. Nevertheless, their efficacy is limited because of their narrow therapeutic time window and a number of side effects.14 Hence, the development of novel, effective monomer compounds for the treatment of IS are urgently needed. Apigenin (chemical name: 4',5,7-trihydroxyflavone, PubChem number: 329756029, molecular weight: 270.24) is found in many fruits, vegetables, and herbs, such as oranges, onions, and parsley.15 It is one of the main monomeric flavonoids, can be extracted from celery, and has strong antioxidant activity.16 Apigenin has been reported to prevent UVB-induced oxidative stress and DNA damage in adult human dermal fibroblasts.17,18 Apigenin can also ameliorate injury caused by cerebral ischemia/reperfusion in the hearts and brains of rats, and alleviates drug-induced nephrotoxicity in human renal proximal tubular epithelial cells in vitro.19,20 In addition, the treatment effects of flavonoids on oxidative DNA damage have been widely researched, and flavonoids have been demonstrated to reduce damage by eliminating ROS generation.21
In the present study, we aimed to investigate the neuroprotective effects of apigenin on CoCl2-induced oxidative stress injury and apoptosis in PC12 cells in vitro, and to identify the therapeutic effects of apigenin on cerebral ischemia/reperfusion injury in rats. The results demonstrated that apigenin has significant efficacy against cerebral ischemia/reperfusion injury, both in vitro and in vivo.
Materials and methods
Materials
Apigenin (purity >97%, batch number: 17011304) was purchased from Pufei Biotechnology Co., Ltd. (Chengdu, China). The CoCl2 (batch number: 20180724), ROS Kit (2′,7′-dichlorofluorescin diacetate, DCFH-DA), and MMP Kit were purchased from Beyotime Biotechnology Co., Ltd. (Shanghai, China). Annexin V-FITC and propidium iodide (PI) were purchased from BioVision Inc. (Milpitas, CA, USA). PC12 cells were purchased from the Cell Bank at Chinese Academy of Sciences (Shanghai, China). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, batch number: 1015D0510), Dulbecco’s Modified Eagle’s medium (DMEM), and fetal bovine serum (FBS) were purchased from Sijiqing Biological Engineering Materials Co., Ltd. (Hangzhou, China). All other materials were sourced from the Hunan University of Chinese Medicine. Adult male Sprague Dawley rats (250–300 g) were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China). The animal experiment protocol was approved by the Animal Experimentation Ethics Committee of Hunan University of Chinese Medicine.
Cell cultures
The PC12 cell line is a rat adrenal pheochromocytoma cell line provided by ATCC (Manassas, VI, USA). After differentiation, PC12 cells resemble neurons with synapses and angular morphology. Differentiated PC12 cells were grown on polystyrene tissue culture dishes in DMEM containing 10% FBS at 37°C in a humidified atmosphere containing 95% air/5% CO2. The culture medium was exchanged twice per week.22
Cytotoxicity of apigenin
To investigate the toxicity of apigenin in PC12 cells, an MTT assay was carried out according to the manufacturer’s protocol.
Apigenin was dissolved in dimethyl sulfoxide (DMSO) and diluted to the final concentration in phosphate-buffered saline (PBS). The final proportion of DMSO was no more than 1%. The PC12 cells were treated with apigenin (0, 1, 10, 100, 200, 300, 400, 500, 600, 700, 800, 900, and 1,000 µg · mL−1) for 24 hours. After the incubation, MTT (2 mg · mL−1) solution was added and co-incubated for 4 hours. Next, the supernatant solution was suctioned off and 150 µL of DMSO was added to each well to produce formazan dissolution. The absorbance (optical density [OD] value) of cells was detected at 570 nm. Cell viability was calculated using the following equation: cell viability (%) = mean ODexperimental group/mean ODcontrol group × 100%.
Cell injury model and treatment
The cell injury model was established by adding CoCl2 to PC12 cells.23 Briefly, CoCl2 was dissolved in distilled H2O (10 mM), and was freshly prepared immediately before use. Next, stock solutions were diluted to the final concentrations in DMEM (without FBS). Logarithmically growing PC12 cells were seeded at a density of 3 × 103 cells/mL. After adherence, the CoCl2 solution at different concentrations (0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.5, 2.0, and 3.0 mM) was added directly to the cells and incubated for 24 hours. A culture of untreated PC12 cells was also maintained in DMEM for the same duration, under normoxic conditions. Thus, the half inhibitory concentration (IC50) of CoCl2 in PC12 cells was obtained.
For the apigenin experiments, cells incubated with 1.2 mM CoCl2 for 24 hours served as the control group, while the untreated cells served as the normal group. To detect the most effective concentration of apigenin, the PC12 cells were pretreated with various concentrations of apigenin (1, 5, 10, 20, 40, 60, 80, 100, and 200 µg · mL−1) for 1 hour before being treated with 1.2 mM CoCl2 for 24 hours. Cell viability was determined by MTT assay, and the follow-up steps were the same as in the cytotoxicity of apigenin experiments.
Cell viability
The MTT assay was used to determine cellular mitochondrial dehydrogenase activity, reflecting cell viability. PC12 cells were plated in 96-well plates and treated as described in the cytotoxicity of apigenin methods. Cell viability was determined by conventional MTT reduction assay. The dark blue formazan solids that formed in intact cells were solubilized with DMSO. The OD values were measured using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) at 570 nm.
Determination of ROS levels
Intercellular ROS levels were measured using a ROS Assay Kit according to the manufacturer’s protocol. In brief, the dichlorodihydrofluorescein diacetate (DCFH-DA; 10 mM) supplied in the kit was diluted to 10 µM in serum-free medium. Subsequently, PC12 cells were pretreated with apigenin (10 µg · mL−1) for 1 hour at a density of 3 × 103/96-well. Next, 1.2 mM CoCl2 solution was added and co-incubated for 24 hours. The supernatant was then replaced with serum-free medium. The DCFH-DA solution (10 µM) was added to the cells for 30 minutes at 37°C, and they were then resuspended with PBS (0.1 mM) after washing twice with the indicated inducers. Fluorescence intensity was detected using a high-content cytometer (PerkinElmer, Shanghai, China) to determine ROS levels.
Cell apoptosis
The annexin V-FITC/PI assay was performed to analyze cell apoptosis. PC12 cells were treated with apigenin (10 µg · mL−1) and 1.2 mM CoCl2 solution for 24 hours, as described in the previous paragraph. Next, PC12 cells were washed with PBS and resuspended in binding buffer (500 µL) before being incubated with annexin V-FITC (5 µL) and PI (10 µL) for 15 minutes in the dark. After washing twice with PBS, 100 µL PBS was added to each well. Cell apoptosis was analyzed using a high-content cytometer at 488 nm and 525 nm. In this assay, annexin V-FITC represents early apoptosis, while PI represents late apoptosis.
Detection of MMP
To evaluate mitochondrial function, mitochondrial transmembrane potential was evaluated according to the manufacturer's instructions. The MMP was measured using a specific cyanine dye, JC-1. Briefly, JC-1 was diluted in JC-1 buffer at a ratio of 1:4. PC12 cells were then treated with apigenin (10 µg · mL−1) and 1.2 mM CoCl2 solution for 24 hours, as outlined in the section describing the determination of ROS levels. Cells were then resuspended in 500 µL incubation buffer containing JC-1 at 37°C for 20 minutes, washed twice with PBS, and resuspended in 1× incubation buffer. Adherent cells were detected and the fluorescence intensity was quantified using a high-content cytometer at 514 nm.
Establishment of the middle cerebral artery occlusion (MCAO) model in rats
To investigate the neuroprotective effects of apigenin in vivo, an MCAO model was established. In brief, the rats (250–300 g) were anesthetized with 10% chloral hydrate (approximately 1.2 mL) administered by intraperitoneal (i.p.) injection. The common carotid artery (CCA) was separated from the adjacent muscles and nerves to expose the external carotid artery (ECA). The CCA and ECA were then ligated, and the internal carotid artery (ICA) was clamped and a small opening was cut on one side using ophthalmic scissors.24 A poly-L-lysine-coated monofilament nylon thread was inserted into the ICA at a depth of approximately 17 to 18 mm. Next, the nylon thread was slowly withdrawn about 1 mm, until it encountered resistance, and the nylon thread was fixed. After 1.5 hours, the whole monofilament nylon thread was slowly withdrawn to allow reperfusion.22 Finally, 1 mL of saline was administered via i.p. injection.
Triphenyltetrazolium chloride (TTC) staining
The rats were randomly divided into the following groups: sham group, control group (MCAO), and apigenin group (MCAO + apigenin). The sham group received the same surgery as the other rats, except nylon monofilaments were not inserted. At 2 hours post-reperfusion, the rats were administrated either saline or apigenin. For the control and sham groups, 1 mL saline was administered. For the apigenin group, the dose of apigenin was 25 mg·kg−1, which was dissolved in distilled water and administered by i.p. injection (1 mL) once every 24 hours for 7 days. Neurological deficit scores were evaluated in the rats.25 The scores were assessed as follows: 0, no motor disability (normal); 1, limb weakness and limbs cannot fully extend (mild); 2, circling to one side (moderate); 3, leaning to one side (severe); and 4, unconscious or unable to ambulate spontaneously (critical).
After 24 hours, the hearts of the rats were perfused with saline. Their brains were immediately sliced into sections of 2 mm thickness. Brain sections were stained with 1% TTC solution for 20 minutes at 37°C, and were then fixed with 4% paraformaldehyde buffer for 10 minutes at room temperature.22 The total infarction area was displayed using ImageJ software and the infarction rate was calculated using the following formula: white area/whole brain area × 100%,26 where the white parts of the brain represented the infarct area and the red parts of the brain were normal.
Statistical analysis
The results were analyzed using GraphPad software (GraphPad Software Inc., La Jolla, CA, USA). Statistical differences were evaluated using a t-test for the comparison of two groups and a one-way analysis of variance for multiple-group comparisons. Data are expressed as the mean ± standard deviation (SD), and P < 0.05 was taken to indicate a significant difference.
Results
Effects of CoCl2 concentrations on PC12 cell viability and morphology
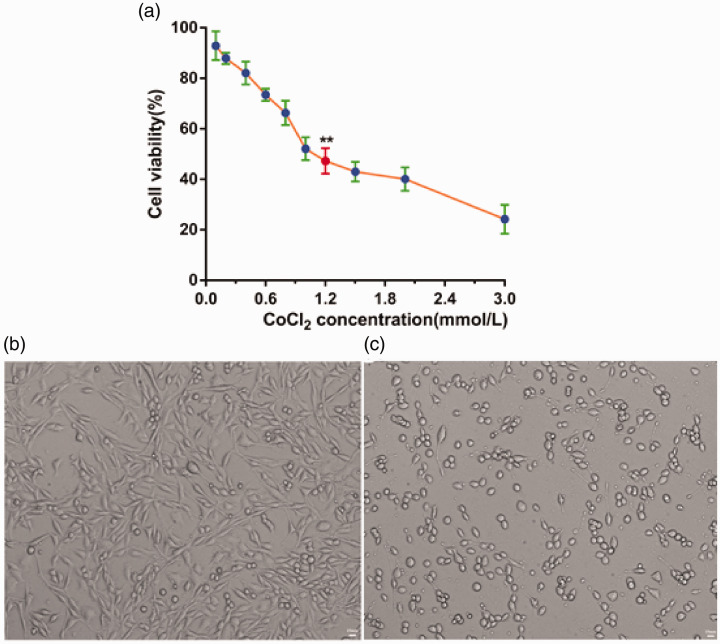
PC12 cell viability decreased in a concentration-dependent manner with increasing concentrations of CoCl2 for 24 hours (Figure 1a). The results from the MTT assay demonstrated that the IC50 of CoCl2 for PC12 cells was 1.2 mM. That is, the viability of PC12 cells was close to 50% (47.3 ± 5.04%) when the concentration of CoCl2 was set as 1.2 mM; thus, this concentration was adopted for the following studies.
Figure 1.
Viability of PC12 cells treated with different concentrations (0.1, 0.2, 0.4, 0.6, 0.8, 1, 1.2, 1.5, 2, and 3 mM) of CoCl2 for 24 hours. Cell viability was assessed using the MTT assay. Data are expressed as mean ± SD. **P < 0.01 vs. 0 mM concentration of CoCl2 (n = 6). (a) PC12 cells were treated with 1.2 mM CoCl2 for 24 hours (200× magnification) (b) Blank PC12 cell group. (c) 1.2 mM CoCl2 group.
To further characterize the effects of CoCl2 on PC12 cells, morphological changes were observed in differentiated cells under an optical microscope (bright field). The morphology of normal PC12 cells and CoCl2-treated PC12 cells contrasted sharply (Figure 1b,c). Normal cells had round cell bodies with fine dendritic networks similar to those of neurons, whereas CoCl2-treated cells were small and proliferated to form cell clusters without neuronal characteristics. We observed pyknosis and a loss of angular morphology in the PC12 cells treated with 1.2 mM CoCl2 for 24 hours. Thus, CoCl2 treatment decreased PC12 cell proliferation and significantly influenced the survival of PC12 cells via CoCl2-induced injury. Together, these results suggest that CoCl2 can be used to simulate free radical oxidative damage in vitro.
Apigenin increased cell viability in PC12 cells with CoCl2-induced injury
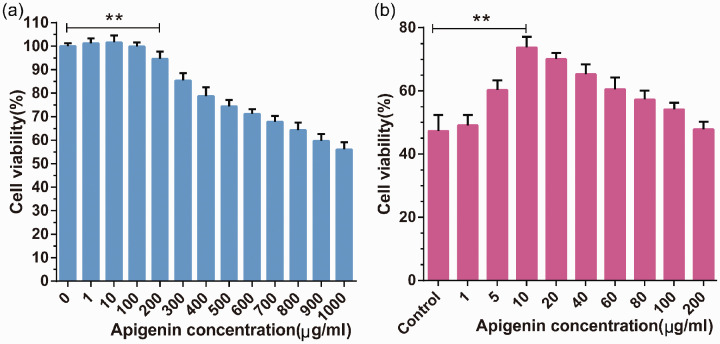
To investigate the optimal effective concentration of apigenin, we first determined the range of non-toxic concentrations of apigenin in PC12 cells. The MTT results showed that, when the concentration of apigenin was below 200 µg · mL−1, PC12 cell viability was higher than 90% and was not significantly different from that of untreated PC12 cells (Figure 2a). This result indicates that apigenin is not cytotoxic below 200 µg/mL in these cells. Therefore, we used an apigenin concentration range of 0 to 200 µg/mL for further experiments.
Figure 2.
Effects of apigenin on PC12 cell viability. (a) PC12 cells were treated with different concentrations of apigenin (0, 1, 10, 100, 200, 300, 400, 500, 600, 700, 800, 900, and 1000 µg/mL) for 24 hours to investigate its effects on cell viability. (b) The viability of PC12 cells pretreated with various concentrations of apigenin (1, 5, 10, 20, 40, 60, 80, 100, 200 µg/mL) for 1 hour before injury was induced by CoCl2. Data are expressed as the mean ± SD (n = 6), **P < 0.01 vs. 0 µg/mL concentration of apigenin.
Before PC12 cells were treated with CoCl2, they were pretreated with apigenin at a series of concentrations (1, 5, 10, 20, 40, 60, 80, 100, and 200 µg/mL) for 1 hour. As shown in Figure 2b, the viability of PC12 cells was enhanced to 73.78 ± 3.35% (P < 0.01) with an apigenin concentration of 10 µg/mL, and there was a statistically significant difference in cell viability between the control group and the 10 µg/mL apigenin group. Furthermore, cell viability in the 10 µg/mL apigenin group was significantly higher than with other concentrations. Based on these findings, an apigenin concentration of 10 µg/mL was used in all subsequent experiments. Moreover, these results demonstrated that apigenin has neuroprotective effects against neuronal cell injury.
Apigenin reduced CoCl2-induced intracellular ROS levels in PC12 cells
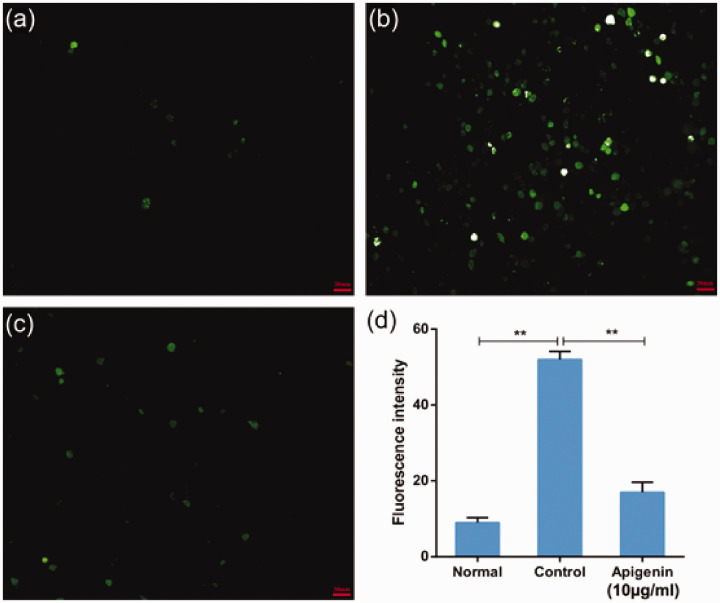
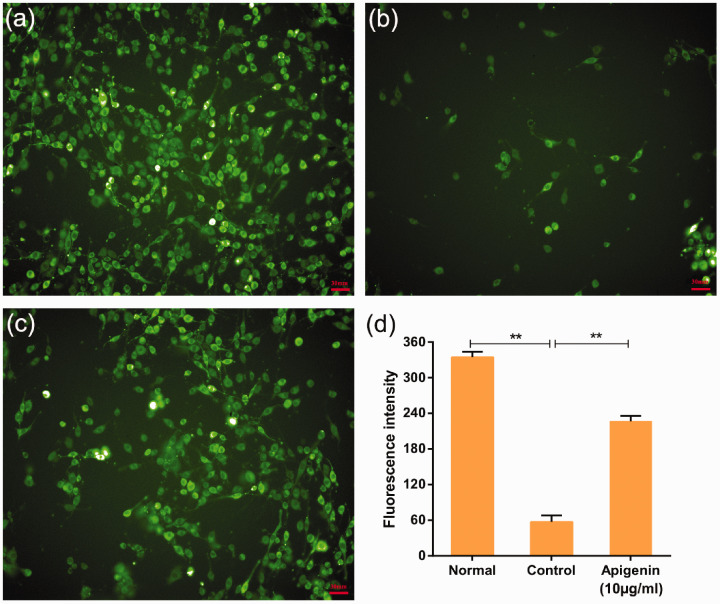
Mitochondria are considered to be the main site of ROS production, and increased intracellular ROS levels reflect mitochondrial dysfunction. Thus, we further evaluated the effects of apigenin on CoCl2-induced ROS generation using DCFH-DA, which freely crosses the cell membrane. The mean fluorescence intensity of this marker represents ROS accumulation. The mean (±SD) fluorescence intensities in the untreated (normal group) and CoCl2-treated (control group) cells were 9.0 (±1.3) and 52.1 (±2.1), respectively (Figure 3a,b). The green fluorescence of the control group was markedly stronger than that of the normal group. Compared with the control group, the green fluorescence intensity was weaker in the apigenin group (mean ± SD, 17.0 ± 2.6) (Figure 3c). Thus, CoCl2 treatment in PC12 cells significantly elevated intracellular ROS levels (P < 0.01, Figure 3d) compared with untreated cells. Compared with the control group, pre-treatment with 10 µg/mL apigenin significantly decreased CoCl2-induced ROS production (P < 0.01).
Figure 3.
Protective effects of apigenin on CoCl2-induced intracellular reactive oxygen species (ROS) accumulation. Intracellular ROS levels were determined based on dichlorofluorescin (DCF) fluorescence, as described in the materials and methods section. Representative images from the untreated (normal) group (a), 1.2 mM CoCl2 (control) group (b), and 10 µg/mL apigenin pretreatment + 1.2 mM CoCl2 (apigenin) group (c). (d) The bar chart shows the quantitative analysis of the median fluorescence intensity. CoCl2 is commonly used to identify and test ROS inducers, and to induce ROS. Data are expressed as the mean ± SD (n = 3), **P < 0.01.
Apigenin protected PC12 cells against CoCl2-induced apoptosis
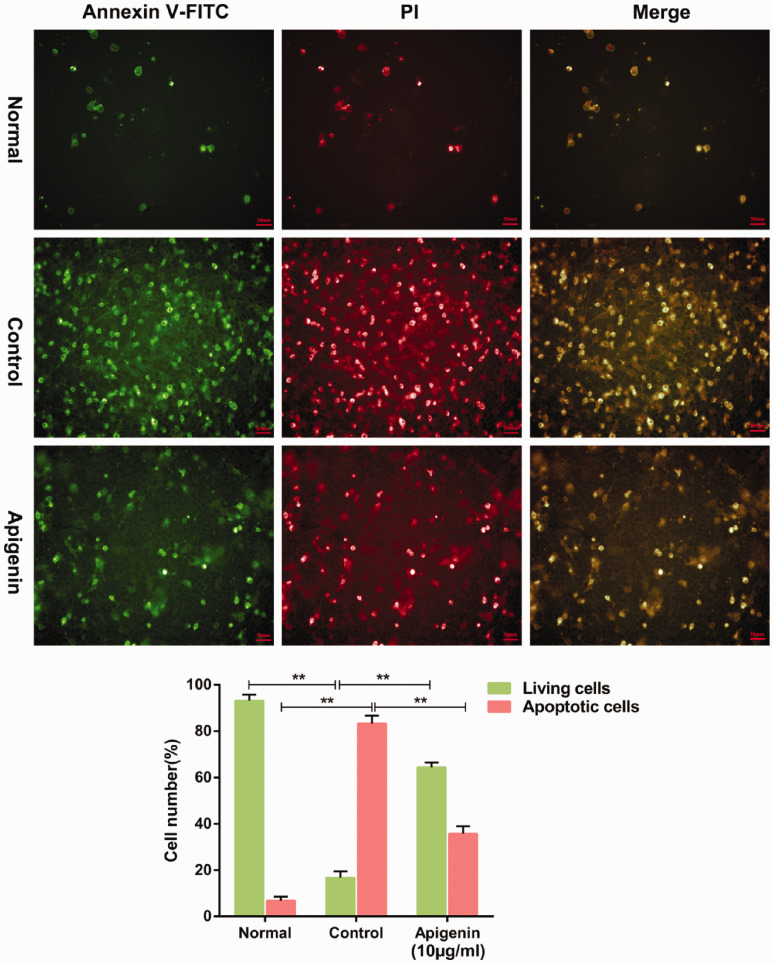
The annexin V-FITC/PI assay was performed to evaluate the effects of apigenin on CoCl2-induced cell apoptosis. The annexin V-FITC probe was used to detect the early apoptosis of PC12 cells (green fluorescence), while PI was used to detect the late apoptosis of PC12 cells (red fluorescence). The median fluorescence intensity of cells incubated with CoCl2 (control group) was markedly stronger than that of untreated cells (normal group), and pretreatment with apigenin reduced the median fluorescence intensity (Figure 4). Quantitative analysis revealed that the control group had a significantly higher apoptosis rate compared with the normal group (83.3% ± 3.4% vs. 6.8% ± 1.7%, respectively, P < 0.01). Apigenin pretreatment significantly lowered the apoptosis rate (35.8% ± 3.1%) compared with the control group (P < 0.01). Under the same conditions, the rates of viable cells showed equivalent trends. Together, these results indicate that apigenin treatment suppresses CoCl2-induced apoptosis in PC12 cells.
Figure 4.
Apigenin decreased apoptosis and increased the number of live cells in CoCl2-treated PC12 cells. To analyze the role of apigenin in PC12 cells, cells were exposed to phosphate-buffered saline (normal group), 1.2 mM CoCl2 (control group), or pretreatment with apigenin + 1.2 mM CoCl2 (apigenin group). The cells were then stained with annexin V-FITC/propidium iodide (PI). The images are from a representative experiment. Data are expressed as the mean ± SD (n = 3), ** P < 0.01.
Apigenin protected PC12 cells against CoCl2-induced MMP depolarization
When PC12 cells were exposed to CoCl2, MMPs rapidly depolarized, as shown by the reduced green fluorescence in the control group compared with the untreated (normal) group (Figure 5a,b). Pretreatment with apigenin increased the MMPs, as indicated by the enhanced green fluorescence (Figure 5c). Compared with the normal group (334.5 ± 9.3), the control group had significantly less mean fluorescence (57.2 ± 10.7, P < 0.01) in the quantitative analysis. The mean fluorescence value in the group with apigenin pretreatment (226.6 ± 8.1) was significantly higher than that of the control group (P < 0.01) (Figure 5d). Thus, these results demonstrate a protective role of apigenin pretreatment on CoCl2-induced MMP reduction.
Figure 5.
Effects of apigenin on the mitochondrial membrane potential. High-content analysis images of JC-1 fluorescence (as an indicator of mitochondrial membrane potential) in PC12 cells with no treatment (normal group) (a), after 12 hours exposure to CoCl2 alone (control group) (b), or after exposure to CoCl2 in combination with 10 μg/mL apigenin (apigenin group) (c). (d) The bar chart shows the quantitative analysis of the green average fluorescence value. Data are expressed as the mean ± SD (n = 3), ** P < 0.01.
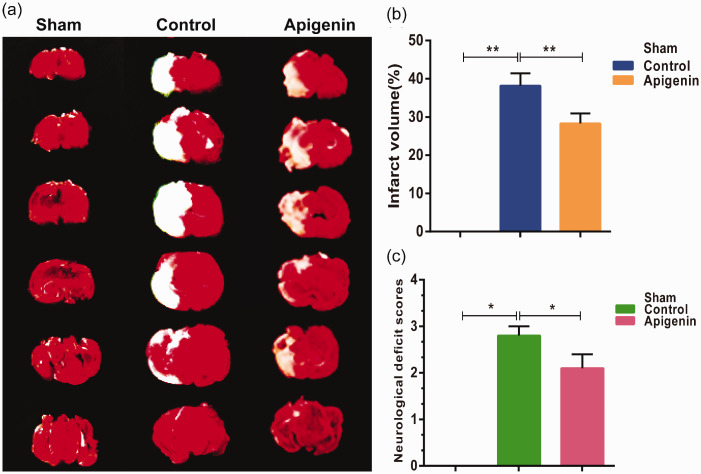
Apigenin decreased the volume of cerebral infarction in rats
The TTC-stained rat brain slices from the different groups (n = 6 per group) are shown in Figure 6a. This figure shows that the apigenin group had markedly smaller infarct volumes compared with the control group. The infarct area was then quantified using ImageJ. The infarct volume percentage was significantly lower in the apigenin group (MCAO rats with apigenin treatment; approximately 28.3 ± 2.6%) than in the control group (MCAO rats without any treatment; approximately 37.0 ± 2.3%; P < 0.01) (Figure 6b). Figure 6c shows the neurological deficit scores of the rats in each group. The control group had significantly higher scores compared with the sham group, indicating that the model was successful. Moreover, scores in the apigenin group were significantly lower than in the control group (P < 0.05). Together, these results suggest that apigenin has a strong neuroprotective effect in vivo.
Figure 6.
Effects of apigenin on infarct volume and neurological functional outcome in rats (n = 6). (a) The white areas indicate infarcted brain tissue (TTC staining). (b) The percentages of cerebral infarction volume were calculated as described in the materials and methods section. (c) Neurological deficit scores. *P < 0.05; **P < 0.01.
Discussion
Traditional Chinese medicine monomers are widely used to prevent and treat IS based on their reliable therapeutic effects and low toxicities. One such monomer is apigenin, which is found in vegetables, fruit, and Chinese medicine, and is a type of flavonoid. Apigenin has antioxidative, antitumor, and antimicrobial activities; offers cerebrovascular protection; and has other biological activities. Previous studies have observed that apigenin can attenuate doxorubicin-induced cardiotoxicity by reducing oxidative stress and apoptosis in male rats.27 Recently, our group reported that apigenin has a potential neuroprotective role in PC12 cells.28,29 However, the underlying mechanisms of the effects of apigenin on CoCl2-induced injury in PC12 cells have not yet been elucidated. The present study revealed that apigenin had neuroprotective effects against CoCl2-induced neuronal oxidative stress and damage in PC12 cells. These effects mainly included marked improvements in morphological changes (such as cell shrinkage and condensed nuclei), cell viability, ROS levels, apoptosis, and MMP.
CoCl2-treated PC12 cells are a commonly used model for investigating cerebral ischemia/reperfusion injury in vitro.30 It is also a common method for evaluating the antioxidant efficiency or oxidative stress susceptibility of cells that are susceptible to oxidative injury.31 CoCl2 can induce neuronal cell injury and lead to the generation of intracellular ROS, apoptosis, changes in MMP, and transcriptional changes in some genes (e.g., genes encoding HIF-1α and p53) and plasmid DNA.32,33 These substances accelerate PC12 cell death. It has been reported that CoCl2 can mimic oxidative stress injury conditions at the cellular level, including the production of ROS in neuronal cells.34,35 PC12 cells are similar to neuronal cells in morphology, structure, and function, and have typical characteristics of neuronal cells.36 Moreover, as a model to study exocytosis for neurosecretion, PC12 cells have some advantages, including over chromaffin cells.37 Therefore, we used CoCl2-induced PC12 cells to study cell viability, ROS levels, cell apoptosis, and MMP. In the present study, we thus established an in vitro model of CoCl2-induced oxidative injury in PC12 cells.
Mitochondria have previously been described as the most important sensors of oxidative stress.38 However, mitochondria are also the major ROS-producing organelles and the target of a number of ROS-related diseases. ROS function as messengers in multiple intracellular signaling pathways and serve as mediators of oxidative injury.3 Previous studies suggest that overexpression of protein deglycase DJ-1 improves mitochondrial function via a mechanism involving protein kinase B phosphorylation on threonine 308.39 Apoptotic signals initially lead to enhanced mitochondrial permeability and a loss of mitochondrial transmembrane potential.40,41 Alterations in MMP, cell apoptosis, increased ROS, mitochondria swelling, and decreased superoxide dismutase are all characteristics of mitochondrial dysfunction.42 In the present study, we demonstrated that oxidative stress led to the inhibition of cell apoptosis, the generation of ROS, and the reduction of MMP via mitochondrial-dependent pathways in vitro.
Recent studies have reported that apigenin protects the brain against cerebral ischemia/reperfusion injury via caveolin-1/VEGF both in vitro and in vivo.43 Apigenin also has protective effects against 1-methyl-4-phenylpyridinium-induced neurotoxicity in PC12 cells.28 Moreover, several investigations have suggested that apigenin has antioxidative and anti-apoptotic effects on the numbers of viable and apoptotic blastomeres in mice.44 The results of the current study suggest that apigenin alleviates CoCl2-induced oxidative stress injury and apoptosis in PC12 cells via mitochondrial pathways. Our findings consistently demonstrated that apigenin ameliorated CoCl2-induced ROS generation, apoptosis, and MMP reduction in PC12 cells by inhibiting mitochondrial dysfunction. Thus, the present study provides evidence for the possible neuroprotective application of apigenin as an antioxidant flavonoid.
Conclusion
Apigenin protected against CoCl2-induced oxidative stress injury and apoptosis in PC12 cells. The IC50 value of CoCl2 in PC12 cells was 1.2 mM, and the optimal effective concentration of apigenin was 10 µg · mL−1. This treatment concentration resulted in reduced ROS generation, the inhibition of cell apoptosis, increased numbers of live cells, and improved MMP in PC12 cells with CoCl2-induced injury. We also demonstrated that apigenin significantly improved neurological deficit scores and reduced the infarct area in rats. Together, these results suggest that apigenin has great potential as a clinical therapeutic drug and might be useful for treating oxidative stress injury in IS.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the domestic first-class construction discipline of Chinese Medicine in Hunan University of Chinese Medicine, and the open foundation for first-level discipline of Chinese Medicine in Hunan University of Chinese Medicine (2018ZYX48).
ORCID iDs
Yuhong Wang https://orcid.org/0000-0002-1411-2201
Dan Huang https://orcid.org/0000-0003-4221-574X
References
- 1.Zhang Q, Zhao YH. Therapeutic angiogenesis after ischemic stroke: Chinese medicines, bone marrow stromal cells (BMSCs) and their combinational treatment. Am J Chin Med 2014; 42: 61–77. [DOI] [PubMed] [Google Scholar]
- 2.Sahota P, Savitz SI. Investigational therapies for ischemic stroke: neuroprotection and neurorecovery. Neurotherapeutics 2011; 8: 434–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naseem M, Parvez S. Role of melatonin in traumatic brain injury and spinal cord injury. ScientificWorldJournal 2014; 2014: 586270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol 2000; 62: 649–671. [DOI] [PubMed] [Google Scholar]
- 5.Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab 2004; 24: 133–150. [DOI] [PubMed] [Google Scholar]
- 6.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 2004; 3: 205–214. [DOI] [PubMed] [Google Scholar]
- 7.Widlansky ME, Gutterman DD. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid Redox Signal 2011; 15: 1517–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhai H, Chen QJ, Gao XM, et al. Inhibition of the NF-κB pathway by R65 ribozyme gene via adeno-associated virus serotype 9 ameliorated oxidized LDL induced human umbilical vein endothelial cell injury. Int J Clin Exp Pathol 2015; 8: 9912–9921. [PMC free article] [PubMed] [Google Scholar]
- 9.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 2000; 29: 222–230. [DOI] [PubMed] [Google Scholar]
- 10.Ott M, Gogvadze V, Orrenius S, et al. Mitochondria, oxidative stress and cell death. Apoptosis 2007; 12: 913–922. [DOI] [PubMed] [Google Scholar]
- 11.Biswal S, Sharma D, Kumar K, et al. Global hypoxia induced impairment in learning and spatial memory is associated with precocious hippocampal aging. Neurobiol Learn Mem 2016; 133: 157–170. [DOI] [PubMed] [Google Scholar]
- 12.Nair AR, DeGheselle O, Smeets K, et al. Cadmium-induced pathologies: where is the oxidative balance lost (or not)?. Int J Mol Sci 2013; 14: 6116–6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia J, Zhang L, Shi X, et al. SOD2 mediates amifostine-induced protection against glutamate in PC12 cells. Oxid Med Cell Longev 2016; 2016: 4202437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattiasson G, Shamloo M, Gido G, et al. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med 2003; 9: 1062–1068. [DOI] [PubMed] [Google Scholar]
- 15.Malik S, Suchal K, Khan SI, et al. Apigenin ameliorates streptozotocin-induced diabetic nephropathy in rats via MAPK/NF-kappaB/TNF-alpha and TGF-beta1/MAPK/fibronectin pathways. Am J Physiol Renal Physiol 2017; 313: F414–F422. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Marzo N, Perez-Sanchez A, Ruiz-Torres V, et al. Antioxidant and photoprotective activity of apigenin and its potassium salt derivative in human keratinocytes and absorption in Caco-2 cell monolayers. Int J Mol Sci 2019; 20: E2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang SP, Hu YY, Tan W, et al. Compatibility art of traditional Chinese medicine: from the perspective of herb pairs. J Ethnopharmacol 2012; 143: 412–423. [DOI] [PubMed] [Google Scholar]
- 18.MaryBritto S, Shanthakumaric D, Agiland B, et al. Apigenin prevents ultraviolet-B radiation induced oxidative stress and DNA damage in human dermal fibroblasts. Mutat Res Genet Toxicol Environ Mutagen 2017; 821: 28–35. [DOI] [PubMed] [Google Scholar]
- 19.Tsalkidou EG, Tsaroucha AK, Chatzaki E, et al. The effects of apigenin on the expression of Fas/FasL apoptotic pathway in warm liver ischemia-reperfusion injury in rats. Biomed Res Int 2014; 2014: 157216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C, He H, Luo Y, et al. Involvement of Bcl-2 signal pathway in the protective effects of apigenin on anoxia/reoxygenation-induced myocardium injury. J Cardiovasc Pharmacol 2016; 67: 152–163. [DOI] [PubMed] [Google Scholar]
- 21.Begum N, Prasad NR. Apigenin, a dietary antioxidant, modulates gamma radiation-induced oxidative damages in human peripheral blood lymphocytes. Biomed Prev Nutr 2012; 2: 16–24. [Google Scholar]
- 22.Ling C, Liang J, Zhang C, et al. Synergistic effects of salvianolic acid B and puerarin on cerebral ischemia reperfusion injury. Molecules 2018; 23: 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Raimondo D, Tuttolomondo A, Butta C, et al. Effects of ACE-inhibitors and angiotensin receptor blockers on inflammation. Curr Pharm Des 2012; 18: 4385–4413. [DOI] [PubMed] [Google Scholar]
- 24.Kou DQ, Jiang YL, Qin JH, et al. Magnolol attenuates the inflammation and apoptosis through the activation of SIRT1 in experimental stroke rats. Pharmacol Rep 2017; 69: 642–647. [DOI] [PubMed] [Google Scholar]
- 25.Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989; 20: 84–91. [DOI] [PubMed] [Google Scholar]
- 26.Belayev L, Khoutorova L, Zhao W, et al. Neuroprotective effect of darbepoetin alfa, a novel recombinant erythropoietic protein, in focal cerebral ischemia in rats. Stroke 2005; 36: 1071–1076. [DOI] [PubMed] [Google Scholar]
- 27.Zare MFR, Rakhshan K, Aboutaleb N, et al. Apigenin attenuates doxorubicin induced cardiotoxicity via reducing oxidative stress and apoptosis in male rats. Life Sci 2019; 232: 116623. [DOI] [PubMed] [Google Scholar]
- 28.Liu W, Kong S, Xie Q, et al. Protective effects of apigenin against 1-methyl-4-phenylpyridinium ion-induced neurotoxicity in PC12 cells. Int J Mol Med 2015; 35: 739–746. [DOI] [PubMed] [Google Scholar]
- 29.Guo H, Kong S, Chen W, et al. Apigenin mediated protection of OGD-evoked neuron-like injury in differentiated PC12 cells. Neurochem Res 2014; 39: 2197–2210. [DOI] [PubMed] [Google Scholar]
- 30.Jin A, Li X, Zhu YY, et al. Four new compounds from the bulbs of Lycoris aurea with neuroprotective effects against CoCl2 and H2O2-induced SH-SY5Y cell injuries. Arch Pharm Res 2014; 37: 315–323. [DOI] [PubMed] [Google Scholar]
- 31.Iloki-Assanga SB, Lewis-Lujan LM, Fernandez-Angulo D, et al. Retino-protective effect of Bucida buceras against oxidative stress induced by H2O2 in human retinal pigment epithelial cells line. BMC Complement Altern Med 2015; 15: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandel NS, Maltepe E, Goldwasser E, et al. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 1998; 95: 11715–11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung JY, Kim WJ. Involvement of mitochondrial and Fas-mediated dual mechanism in CoCl2 induced apoptosis of rat PC12 cells. Neurosci Lett 2004; 371: 85–90. [DOI] [PubMed] [Google Scholar]
- 34.Sandner P, Wolf K, Bergmaier U, et al. Hypoxia and cobalt stimulate vascular endothelial growth factor receptor gene expression in rats. Pflugers Arch 1997; 433: 803–808. [DOI] [PubMed] [Google Scholar]
- 35.Zou W, Yan M, Xu W, et al. Cobalt chloride induces PC12 cells apoptosis through reactive oxygen species and accompanied by AP-1 activation. J Neurosci Res 2001; 64: 646–653. [DOI] [PubMed] [Google Scholar]
- 36.Guo Z, Yuan Y, Guo Y, et al. Nischarin attenuates apoptosis induced by oxidative stress in PC12 cells. Exp Ther Med 2019; 17: 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westerink RHS, Ewing AG. The PC12 cell as model for neurosecretion. Acta Physiol (Oxf) 2008; 192: 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo LX, Liu JH, Xia ZN. Geniposide inhibits CoCl2 -induced PC12 cells death via the mitochondrial pathway. Chin Med J (Engl) 2009; 122: 2886–2892. [PubMed] [Google Scholar]
- 39.Zhang Y, Gong XG, Wang ZZ, et al. Overexpression of DJ-1/PARK7, the Parkinson’s disease-related protein, improves mitochondrial function via Akt phosphorylation on threonine 308 in dopaminergic neuron-like cells. Eur J Neurosci 2016; 43: 1379–1388. [DOI] [PubMed] [Google Scholar]
- 40.Bak DH, Kim HD, Kim YO, et al. Neuroprotective effects of 20(S)-protopanaxadiol against glutamate-induced mitochondrial dysfunction in PC12 cells. Int J Mol Med 2016; 37: 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halestrap AP, Doran E, Gillespie JP, et al. Mitochondria and cell death. Biochem Soc Trans 2000; 28: 170–177. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, Peng M, Yang Y, et al. Protective effects of salidroside on mitochondrial functions against exertional heat stroke-induced organ damage in the rat. Evid Based Complement Alternat Med 2015; 2015: 504567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pang Q, Zhao Y, Chen X, et al. Apigenin protects the brain against ischemia/reperfusion injury via caveolin-1/VEGF in vitro and in vivo. Oxid Med Cell Longev 2018; 2018: 7017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Safari M, Parsaie H, Sameni HR, et al. Anti-oxidative and anti-apoptotic effects of apigenin on number of viable and apoptotic blastomeres, zona pellucida thickness and hatching rate of mouse embryos. Int J Fertil Steril 2018; 12: 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]