Abstract
Objective
To evaluate serum microRNA (miR)-29a/b expression in gestational diabetes mellitus (GDM) and its influence on neonatal prognosis.
Methods
This was a retrospective study including 68 pregnant women with GDM (GDM group) and 55 healthy pregnant women of similar age range and gestation period (healthy group).
Results
The area under the curve was 0.829 for the diagnosis of GDM using serum miR-29a expression, 0.857 for diagnosis using serum miR-29b expression, and 0.944 for combined diagnosis (using both miR-29a and miR-29b). The fasting insulin (FINS) level of the GDM group was significantly lower than that of the healthy group; levels of fasting plasma glucose (FPG), 2-h plasma glucose (2hPG), and glycated hemoglobin (HbA1c) were significantly higher in the GDM group than in the healthy group. Both miR-29a and miR-29b were positively correlated with FINS levels and negatively correlated with FPG, 2hPG, and HbA1c levels. Serum miR-29a/b expression in pregnant women with GDM was not correlated with neonatal weight, premature delivery, or asphyxia but was correlated with pathologic jaundice.
Conclusions
Serum miR-29a/b expression was downregulated in pregnant women with GDM and correlated with neonatal pathologic jaundice, showing good individual (miR-29a or miR-29b) diagnostic value and excellent combined (miR-29a and miR-29b) diagnostic value.
Keywords: Expression, gestational diabetes mellitus, miR-29a, miR-29b, prognosis, pathologic jaundice
Introduction
Gestational diabetes mellitus (GDM), a common gestational disease, refers to a moderate glucose intolerance in pregnant women.1 Studies have shown that 1% to 14% of pregnant women suffer from GDM, and nearly one-third of pregnant women with GDM suffer from postpartum depression.2,3 The incidence of GDM has increased, and GDM may pose high risk of long-term complications, including obesity, glucose metabolism disorders, and cardiovascular diseases in mothers and their newborns, resulting in negative economic and health effects.4,5 Pregnant women at high risk of GDM in the early stage or with diabetes mellitus (DM) may experience adverse pregnancy outcomes even if they have undergone a glucose tolerance test and received early treatment.6 Although the treatment guidelines on GDM have been used clinically for a long time to reduce poor perinatal outcomes and decrease the incidence of type 2 DM, hospitalization costs have greatly increased.7 Hence, further research for effective treatment methods with lower costs and new diagnostic tools is important for improving pregnancy outcomes and reducing associated costs.
The pathogenesis of GDM includes islet β-cell secretion deficiency, insulin resistance (IR), inflammatory reaction, and glucolipid metabolism imbalance.8,9 MicroRNAs (miRNAs) are noncoding RNA sequences that regulate gene expression at the posttranscriptional level and induce a regulating effect on cytobiological function and human diseases.10 The miR-29 family comprises miR-29a, miR-29b, and miR-29c; of these, miR-29a and miR-29b are transcribed from chromosome 7q32.3. Moreover, the pathological significance of their direct or indirect targets involves glucose transport, islet β-cells, and diabetic nephropathy (DN), among others.11 miR-29a is abnormally expressed not only in the serum of patients with type 2 DM but also in 3T3-L1 adipocytes cultured in medium containing high insulin and high glucose, indicating that miR-29a plays an important role in metabolic diseases.12 Zong et al.13 reported that miR-29b can negatively regulate the blood glucose content of rats with GDM and reduce their oxidative stress by targeting PI3K/Akt signal transduction. miR-29a and miR-29b derived from macrophages in adipose tissue or from bone marrow mesenchymal stem cells exhibit an IR function to regulate obesity or senility and are crucial regulators for glucose metabolism and lipid oxidation related to insulin stimulation.14–16
There are few studies on serum miR-29a and miR-29b expression in women with GDM or the evaluation of prognosis in newborns. This study aimed to determine serum miR-29a/b expression in pregnant women with GDM and thus explore miR-29a/b as a potential diagnostic marker and therapeutic target.
Material and methods
General data
This retrospective study included 68 pregnant women with GDM (age range, 20–40 years; mean age, 32.65 ± 4.63 years) admitted to our hospital from June 2018 to June 2019; these patients were categorized as the GDM group. The inclusion criterion was as follows: patients who met the standards formulated for GDM by the American Diabetes Association in 2012.17 On the basis of the results of 75-g glucose tolerance test conducted between 24 and 28 weeks of gestation, patients were diagnosed with GDM if fasting plasma glucose (FPG) was >5.1 mmol/L, 1-hour plasma glucose was >10.0 mmol/L, or 2-hour plasma glucose (2hPG) was >8.5 mmol/L. Exclusion criteria were as follows: patients with communication disorders or severe mental diseases; those with malignant tumors or severe cardiac, pulmonary, liver, renal, and other dysfunctions; and those with hypertension or endocrine and metabolic diseases before pregnancy. Fifty-five pregnant women without GDM of similar age range (20–40 years; mean age, 31.27 ± 4.01 years) and gestation were included and categorized as the healthy group. This study was approved by the ethics committee of the Second Affiliated Hospital of Guangxi Medical University (Nanning, Guangxi Province). Participants were informed about the study procedures before agreeing to participate and signing the consent form.
Plasma glucose markers and serum expression of miR-29a/b
In the morning, 5 mL of fasting venous blood was collected from the cubital vein of participants into a vacuum blood collection tube without anticoagulants and centrifuged at 3000 ×g for 10 minutes at 4°C. The supernatant was separated and stored at −70°C for later use. An automatic biochemical analyzer (PUZS-300, Dibosi Biological Technology Co. Ltd., Shanghai, China) was used to measure fasting insulin (FINS), FPG, 2hPG, and glycated hemoglobin (HbA1c) levels in the samples, and reverse transcription-PCR was used to determine the relative expression quantity of miR-29a/b in serum. Expression of serum miR-29a/b was detected by reverse transcription-PCR. Trizol Total RNA Isolation Reagent (R21086, Yuanye Bio-Technology Co. Ltd., Shanghai, China) was first used to extract the total RNA from serum; then, the concentration and purity of RNA were evaluated using a spectrophotometer (XY-388-07351, Xiyuan Biotechnology Co. Ltd., Shanghai, China), and the Mir-X miRNA qRT-PCR SYBR kit (638314, Stratagene Biotechnology Co. Ltd., Shanghai, China) was used to prepare cDNA, with reverse transcription reaction conditions of 37°C for 60 minutes and 85°C for 5 minutes. cDNA, the product of reverse transcription, was stored in a refrigerator at −20°C for later use. The U6 gene was taken as the reference gene, with primer sequences shown in Table 1. The primer sequence was provided by Daixuan Biotechnology Co. Ltd. (Shanghai, China). The reaction system was 9.5 μL of deionized, distilled H2O, 12.5 μL of 2× SYBR Advantage Premix, 0.5 μL of 50× ROX Dye, 0.5 μL of mRQ3′ primer, and 2.0 μL of cDNA; the total volume was 25 μL; and the PCR reaction conditions were 90°C for 5 minutes, 90°C for 15 s, and 60°C for 1 minute, with a total of 40 cycles. A SYBR real-time fluorescent quantitative PCR (4351104, Ai Yan Shanghai Biological Technology Co. Ltd., Shanghai, China) was used for PCR amplification, and the 2−△CT method was used to calculate the REQ of miR-29a/b.
Table 1.
Primer sequences used to detect expression of miR-29a and miR-29b.
| Gene | Upstream primer sequence | Downstream primer sequence |
|---|---|---|
| miR-29a | 5′-CGTGTGACCGGCGGCAT ACTACACCATTTTCTATCA-3′ |
5′-ATTATCGATAAGCTGCCAGGAG TGTTTCTAGGTATCCG-3′ |
| miR-29b | 5′-GACACGGATCCAATGTAA GCCTCGTGCTCACTG-3′ |
5′-GACTTCTCGAGTCGACAAGGTC ATGTGCACTGGGAAG-3′ |
| U6 | 5′-ATTGGAACGATACAGAGAAGATT-3′ | 5′-GGAACGCTTCACGAATTTG-3′ |
Observation indicators
To analyze the relationship between serum miR-29a/b and neonatal prognosis in pregnant women with GDM, neonatal weight and incidences of premature delivery, asphyxia, and pathological jaundice were recorded in neonates.
Statistical analysis
The statistical analysis software SPSS version 19.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis, and GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA) was used to illustrate the data. Enumeration data were represented by number of cases and percentages ([n (%)]), and the chi-square test was used for between-group comparisons of enumeration data. Measurement data were presented as mean ± standard deviation, and Wilcoxon test was used for between-group comparisons of measurement data. Receiver operating characteristic (ROC) curves were used to analyze the diagnostic value of miR-29a/b expression in pregnant women with GDM, and the Pearson correlation coefficient (PCC) was used to determine the correlation of miR-29a/b with FINS, FPG, 2hPG, and HbA1c. P < 0.05 indicated significance.
Results
Baseline data
There were no significant between-group differences in age, gestational week, body mass index (BMI), height, placental weight, increased body mass during gestation period, abdominal girth, systolic and diastolic blood pressures, DM history, hyperlipidemia, drinking history, smoking history, place of residence, or other baseline data (Table 2).
Table 2.
Comparison of baseline data (mean ± SD) of healthy participants and those with GDM.
| Category | Healthy (n = 55) | GDM (n = 68) | χ2/t-value | P-value |
|---|---|---|---|---|
| Age (years) | 31.27 ± 4.01 | 32.65 ± 4.63 | 1.744 | 0.084 |
| Gestational weeks | 38.98 ± 1.05 | 39.09 ± 1.11 | 0.560 | 0.577 |
| BMI (kg/m2) | 21.88 ± 2.93 | 23.06 ± 3.56 | 1.975 | 0.051 |
| Height (cm) | 161.54 ± 5.23 | 162.01 ± 5.12 | 0.637 | 0.526 |
| Placental weight (kg) | 0.55 ± 0.13 | 0.57 ± 0.14 | 0.813 | 0.418 |
| BMI during gestation (kg) | 13.79 ± 4.31 | 15.12 ± 4.73 | 1.613 | 0.109 |
| Abdominal girth (cm) | 99.86 ± 6.24 | 101.35 ± 6.54 | 1.282 | 0.202 |
| Systolic blood pressure (mmHg) | 114.26 ± 9.13 | 115.62 ± 9.29 | 0.813 | 0.418 |
| Diastolic blood pressure (mmHg) | 72.99 ± 7.12 | 74.08 ± 6.92 | 0.857 | 0.393 |
| History of diabetes mellitus | 3.578 | 0.059 | ||
| Yes | 13 (23.64) | 27 (39.71) | ||
| No | 42 (76.36) | 41 (60.29) | ||
| Hyperlipidemia | 2.594 | 0.107 | ||
| Yes | 8 (14.55) | 18 (26.47) | ||
| No | 47 (85.45) | 50 (73.53) | ||
| Drinking history | 0.191 | 0.662 | ||
| Yes | 15 (27.27) | 21 (30.88) | ||
| No | 40 (72.73) | 47 (69.12) | ||
| Smoking history | 0.699 | 0.403 | ||
| Yes | 14 (25.45) | 22 (32.35) | ||
| No | 41 (74.55) | 46 (67.65) | ||
| Residence place | 0.548 | 0.459 | ||
| Rural | 23 (41.82) | 24 (35.29) | ||
| Urban | 32 (58.18) | 44 (64.71) |
GDM, gestational diabetes mellitus; BMI, body mass index.
Serum miR-29a/b expression
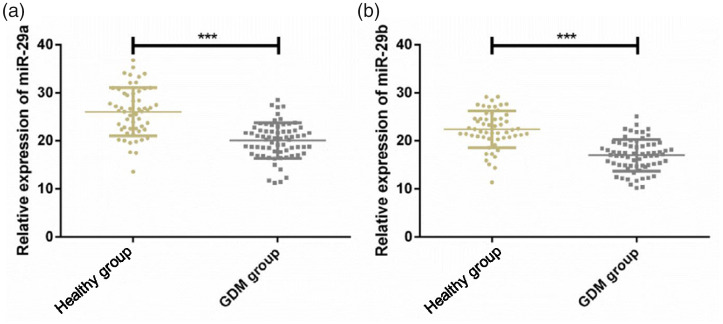
Relative expression quantities of serum miR-29a were 25.92 ± 5.01 and 20.13 ± 3.4 and those of serum miR-29b were 22.49 ± 3.46 and 17.28 ± 3.24 in the healthy and GDM groups, respectively. Serum expression of miR-29a and miR-29b was significantly downregulated in pregnant women with GDM compared with healthy women (P < 0.05) (Figure 1).
Figure 1.
Serum miR-29a/b expression in pregnant women with gestational diabetes mellitus (GDM). Expression of (A) serum miR-29a, and (B) serum miR-29b was downregulated in pregnant women with GDM relative to that in the healthy group. ***P < 0.001.
Diagnostic value of serum miR-29a/b for GDM
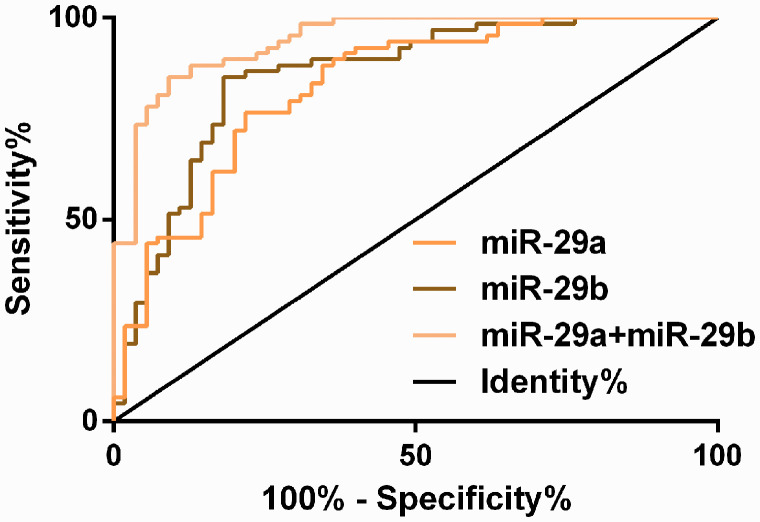
In the ROC curve for the diagnosis of GDM using serum miR-29a and miR-29b expression separately, the area under the curve (AUC) was 0.829 (95% CI, 0.755–0.903) for diagnosis using miR-29a, with a censored value of 22.29, sensitivity of 76.47%, and specificity of 78.18%; the AUC was 0.857 (95% CI, 0.787–0.926) for diagnosis using miR-29b, with a censored value of 20.23, sensitivity of 85.29%, and specificity of 81.82%. A ROC curve for the diagnosis of GDM was then created for miR-29a and miR-29b expression combined, and a logistic regression model was built using miR-29a and miR-29b as independent variables, with Logit (Pjoint detection) = 19.455 + 3.543 miR-29a + 30.148 miR-29b. The AUC of the ROC curve for the combined diagnosis using miR-29a and miR-29b expression was fitted through the probability value in the model. The AUC was 0.944 (95% CI, 0.907–0.982) for the combined diagnosis, with a censored value of 0.65, sensitivity of 86.76%, and specificity of 90.91% (Figure 2 and Tables 3 and 4).
Figure 2.
Receiver operating characteristic (ROC) curve for diagnosis of gestational diabetes mellitus (GDM) using serum miR-29a/b expression.
Table 3.
Results of binary logistic regression analysis.
| Variable | B | SE | Wald test | P-value | OR | 95% CI |
|---|---|---|---|---|---|---|
| miR-29a | −0.407 | 0.093 | 19.193 | <0.001 | 0.665 | 0.554–0.798 |
| miR-29b | −0.506 | 0.106 | 22.833 | <0.001 | 0.603 | 0.490–0.742 |
| Constant | 19.455 | 3.543 | 30.148 | <0.001 | 2.814E8 | — |
OR, odds ratio; CI, confidence interval.
Table 4.
Receiver operating characteristic parameters for GDM diagnosis using serum miR-29a/b expression.
| Group | AUC | 95% CI | SE | Cut-off | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| miR-29a | 0.829 | 0.755–0.903 | 0.038 | 22.29 | 76.47 | 78.18 |
| miR-29b | 0.857 | 0.787–0.926 | 0.035 | 20.23 | 85.29 | 81.82 |
| miR-29a+miR-29b | 0.944 | 0.907–0.982 | 0.019 | 0.65 | 86.76 | 90.91 |
GDM, gestational diabetes mellitus; AUC, area under the ROC curve; CI, confidence interval.
Plasma glucose indices
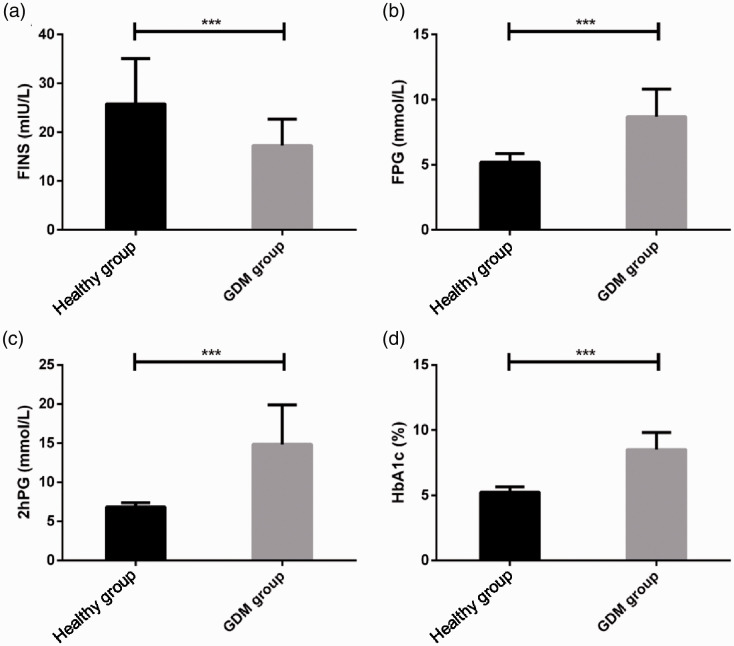
The FINS level was significantly lower and FPG, 2hPG, and HbA1c levels were significantly higher in the GDM group than in the healthy group, with a significant between-group difference (P < 0.05) (Figure 3).
Figure 3.
Results of indices related to plasma glucose (PG) of pregnant women with gestational diabetes mellitus (GDM). (A) Fasting insulin (FINS) level in the GDM group were considerably lower than those in the healthy group; (B) fasting PG (FPG) levels in the GDM group were considerably higher than those in the healthy group; (C) 2-hour (2hPG) levels in the GDM group were considerably higher than those in the healthy group; and (D) glycated hemoglobin (HbA1c) levels in the GDM group were considerably higher than those in the healthy group. ***P < 0.001.2.
Serum miR-29a/b expression and plasma glucose indices
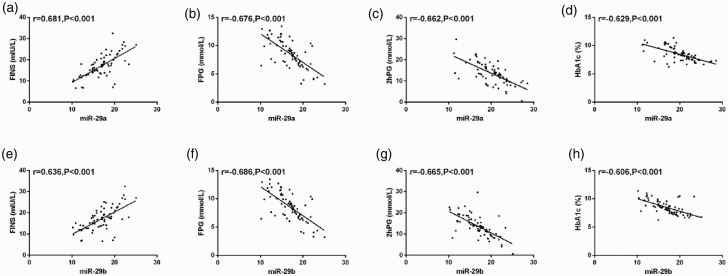
The correlations between miR-29a/b and plasma glucose indices of pregnant women with GDM were analyzed using PCC. We found that miR-29a was positively correlated with FINS level (r = 0.681, P < 0.001) and negatively correlated with FPG, 2hPG, and HbA1c levels (r = −0.676, P < 0.001; r = −0.662, P < 0.001; and r = −0.629, P < 0.001, respectively). Furthermore, miR-29b was positively correlated with FINS levels (r = 0.636, P < 0.001) and negatively correlated with FPG, 2hPG, and HbA1c (r = −0.686, P < 0.001; r = −0.665, P < 0.001; and r = −0.606, P < 0.001, respectively) (Figure 4).
Figure 4.
Results of the correlation between miR-29a/b and indices related to plasma glucose (PG) of pregnant women with gestational diabetes mellitus. (A–D) Serum expression of miR-29a showed a significant positive correlation with fasting insulin (FINS) level (r = 0.681, P < 0.001) and a significant negative correlation with fasting PG (FPG), 2-hour PG (2hPG), and glycated hemoglobin (HbA1c) levels (r = −0.676, P < 0.001; r = −0.662, P < 0.001; and r = −0.629, P < 0.001, respectively). (E, F) miR-29b had a significant positive correlation with FINS levels (r = 0.636, P < 0.001) and significant negative correlations with FPG, 2hPG, and HbA1c levels (r = −0.686, P < 0.001; r = −0.665, P < 0.001; and r = −0.606, P < 0.001, respectively).
Serum miR-29a/b expression and neonatal prognosis
Serum miR-29a/b expression in pregnant women with GDM was not correlated with neonatal weight, premature delivery, or asphyxia but was significantly correlated with pathologic jaundice in newborns (P = 0.002) (Table 5).
Table 5.
Relationship between serum miR-29a/b expression in GDM pregnant women and neonatal prognosis.
| Category | n | miR-29a expression |
χ2 | P-value | miR-29b expression |
χ2 | P-value | ||
|---|---|---|---|---|---|---|---|---|---|
| High (n = 31) |
Low (n = 37) |
High (n = 29) |
Low (n = 39) |
||||||
| Neonatal weight | 1.634 | 0.201 | 2.119 | 0.146 | |||||
| ≤4.0 kg | 45 | 23 (74.19) | 22 (59.46) | 22 (75.86) | 23 (58.97) | ||||
| >4.0 kg | 23 | 8 (25.81) | 15 (40.54) | 7 (24.14) | 16 (41.03) | ||||
| Premature delivery | 0.982 | 0.322 | 1.443 | 0.230 | |||||
| No | 58 | 25 (80.65) | 33 (89.19) | 23 (79.31) | 35 (89.74) | ||||
| Yes | 10 | 6 (19.35) | 4 (10.81) | 6 (20.69) | 4 (10.26) | ||||
| Asphyxia | 0.033 | 0.855 | 0.541 | 0.462 | |||||
| No | 64 | 29 (93.55) | 35 (94.59) | 28 (96.55) | 36 (92.31) | ||||
| Yes | 4 | 2 (6.45) | 2 (5.41) | 1 (3.45) | 3 (7.69) | ||||
| PJ | 8.628 | 0.003 | 9.992 | 0.002 | |||||
| No | 47 | 27 (87.10) | 20 (54.05) | 26 (89.66) | 21 (53.85) | ||||
| Yes | 21 | 4 (12.90) | 17 (45.95) | 3 (10.34) | 18 (46.15) | ||||
GDM, gestational diabetes mellitus.
Discussion
An increasing number of researchers have studied the metabolic regulatory mechanisms of miR-29a/b. Pullen et al.18 reported that mRNA levels of the plasma membrane monocarboxylate transporter 1 (MCT1) are enhanced by inhibiting miR-29a expression in the pancreatic islets of primary cultured rats, implying that miR-29a/b has a supplementary effect on the specific silencing of β-cells in MCT1 transport protein and may influence the secretion of pancreatic β-cells. Tung et al.19 found that miR-29a is a potential negative regulator of cannabinoid cb1 receptor and inhibits the expression of proinflammatory and profibrogenic mediators, preventing glomeruli damage caused by fibrosis in patients with DM. Mafi et al.20 reported that miR-29b may regulate cytokine-mediated inflammatory processes via the Sp1/NF-κB/miR-29b axis and thus participate in progression of DN. The study by Song et al.21 on miR-29 family members, in 3T3-L1 adipocyte showed that these miR-29 members may play the role of negative regulators in glucose metabolism through miR-29/SPARC (secreted protein acidic and rich in cysteine) signal transduction pathway. However, the regulatory mechanism related to miR-29a/b in pregnant women with GDM remains unclear, and our results should be helpful in clarifying the pathogenesis of GDM through miR-29a/b.
In this study, serum expression of miR-29a and miR-29b was found to be considerably downregulated in the GDM group compared with the healthy group, suggesting that miR-29a and miR-29b participate in the occurrence and development of GDM. We drew a ROC curve to determine the diagnostic efficiency of miR-29a/b; the AUCs were 0.829 and 0.857 for individual diagnosis using miR-29a and miR-29b, respectively, and 0.944 for combined diagnosis. These results imply that serum miR-29a or miR-29b has favorable diagnostic value as a marker for GDM, and serum miR-29a+miR-29b has excellent value for diagnosis of GDM. Furthermore, we performed a correlation analysis for plasma glucose indices in the two groups. The results showed that miR-29a/b was positively correlated with FINS levels and negatively correlated with FPG, 2hPG, and HbA1c levels, implying that low miR-29a/b expression may be related to IR and that miR-29a/b is a negative regulator of plasma glucose metabolism. Finally, we evaluated the effect of serum miR-29a/b expression on neonatal prognosis and found no correlation with neonatal weight, premature delivery, or asphyxia but a significant correlation with PJ, implying that low serum miR-29a/b expression was significantly correlated with PJ in neonates of women with GDM. Zhao et al.22 found that miR-29a is silenced to increase the expression level of a gene (INSIG1) that may participate in glucose homeostasis and enhance the levels of the key gluconeogenesis enzyme phosphoenolpyruvate carboxykinase 2 (PCK2) to increase glucose levels. Moreover, serum miR-29a expression began to decrease before plasma glucose levels increased; thus, it was deduced that miR-29a is a negative regulator of plasma glucose, which is consistent with our results. On the basis of the research on abnormal glucose metabolism in GDM pregnant women after delivery, Ma et al.23 reported that 2hPG, HbA1c, 1-h insulin, and 2-h insulin levels are independent risk factors for postoperative IR in pregnant women with GDM, implying that a treatment modality that decreases BMI and blood lipid levels after delivery may be helpful in the recovery of normal glucose metabolism in women with GDM. As shown in a previous study, PJ refers to the jaundice occurring in newborns within 24 hours of birth; 15.9% of mothers of newborns with PJ had GDM, suggesting that a small proportion of newborns of GDM mothers are affected by PJ.24
This study evaluated low serum miR-29a/b expression in pregnant women with GDM, which was related to neonatal PJ and showed excellent value for combined diagnosis, but there is still room for improvement. First, the basic experiments may be supplemented to further understand the specific regulatory mechanisms of miR-29a/b in GDM. Second, the sample size of GDM pregnant women could be increased to enhance the accuracy of results. More research may be performed on the catalyst for miR-29a/b secretion or expression, and thus, new methods may be explored to prevent GDM occurrence through miR-29a/b accelerant. The novelty of this study lies in exploring the diagnostic value of serum miR-29a/b as a combined indicator in GDM and its relationship with neonatal prognosis; miR-29a/b could be of great importance as an additional biological indicator of GDM.
In conclusion, serum miR-29a/b expression was significantly downregulated in pregnant women with GDM and significantly correlated with neonatal PJ. Thus, serum miR-29a/b can be considered a useful diagnostic tool in pregnant women with GDM.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
References
- 1.Damm P, Houshmand-Oeregaard A, Kelstrup L, et al. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia 2016; 59: 1396–1399. [DOI] [PubMed] [Google Scholar]
- 2.Poulsen H, Meurman JH, Kautiainen H, et al. Oral health in women with a history of high gestational diabetes risk. Dent J (Basel) 2019; 7: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert L, Gross J, Lanzi S, et al. How diet, physical activity and psychosocial well-being interact in women with gestational diabetes mellitus: an integrative review. BMC Pregnancy Childbirth 2019; 19: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiefari E, Arcidiacono B, Foti D, et al. Gestational diabetes mellitus: an updated overview. J Endocrinol Invest 2017; 40: 899–909. [DOI] [PubMed] [Google Scholar]
- 5.McIntyre HD, Catalano P, Zhang C, et al. Gestational diabetes mellitus. Nat Rev Dis Primers 2019; 5: 47. [DOI] [PubMed] [Google Scholar]
- 6.Sweeting AN, Ross GP, Hyett J, et al. Gestational diabetes mellitus in early pregnancy: evidence for poor pregnancy outcomes despite treatment. Diabetes Care 2016; 39: 75–81. [DOI] [PubMed] [Google Scholar]
- 7.He Z, Xie H, Liang S, et al. Influence of different diagnostic criteria on gestational diabetes mellitus incidence and medical expenditures in China. J Diabetes Investig 2019; 10: 1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powe CE, Allard C, Battista MC, et al. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care 2016; 39: 1052–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez TL, Van Pelt RE, Anderson MA, et al. Women with gestational diabetes mellitus randomized to a higher–complex carbohydrate/low-fat diet manifest lower adipose tissue insulin resistance, inflammation, glucose, and free fatty acids: a pilot study. Diabetes Care 2016; 39: 39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy KB. MicroRNA (miRNA) in cancer. Cancer Cell Int 2015; 15: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slusarz A, Pulakat L. The two faces of miR-29. J Cardiovasc Med (Hagerstown) 2015; 16: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li ZH, Xiong QY, Xu L, et al. miR-29a regulated ER-positive breast cancer cell growth and invasion and is involved in the insulin signaling pathway. Oncotarget 2017; 8: 32566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zong H, Wang E, Han Y, et al. Effect of miR-29b on rats with gestational diabetes mellitus by targeting PI3K/Akt signal. Eur Rev Med Pharmacol Sci 2019; 23: 2325–2331. [DOI] [PubMed] [Google Scholar]
- 14.Liu T, Sun YC, Cheng P, et al. Adipose tissue macrophage-derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem Biophys Res Commun 2019; 515: 352–358. [DOI] [PubMed] [Google Scholar]
- 15.Su T, Xiao Y, Xiao Y, et al. Bone marrow mesenchymal stem cells-derived exosomal miR-29b-3p regulates aging-associated insulin resistance. ACS Nano 2019; 13: 2450–2462. [DOI] [PubMed] [Google Scholar]
- 16.Massart J, Sjögren RJ, Lundell LS, et al. Altered miR-29 expression in type 2 diabetes influences glucose and lipid metabolism in skeletal muscle. Diabetes 2017; 66: 1807–1818. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association . 2. Classification and diagnosis of diabetes. Diabetes Care 2017; 40: S11–S24. [DOI] [PubMed] [Google Scholar]
- 18.Pullen TJ, Da Silva Xavier G, Kelsey G, et al. miR-29a and miR-29b contribute to pancreatic β-cell-specific silencing of monocarboxylate transporter 1 (Mct1). Mol Cell Biol 2011; 31: 3182–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tung CW, Ho C, Hsu YC, et al. MicroRNA-29a attenuates diabetic glomerular injury through modulating cannabinoid receptor 1 signaling. Molecules 2019; 24: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mafi A, Aghadavod E, Mirhosseini N, et al. The effects of expression of different microRNAs on insulin secretion and diabetic nephropathy progression. J Cell Physiol 2019; 234: 42–50. [DOI] [PubMed] [Google Scholar]
- 21.Song H, Ding L, Zhang S, et al. MiR-29 family members interact with SPARC to regulate glucose metabolism. Biochem Biophys Res Commun 2018; 497: 667–674. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C, Dong J, Jiang T, et al. Early second-trimester serum miRNA profiling predicts gestational diabetes mellitus. PLoS One 2011; 6: e23925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y, Wang N, Gu L, et al. Postpartum assessment of the beta cell function and insulin resistance for Chinese women with previous gestational diabetes mellitus. Gynecol Endocrinol 2019; 35: 174–178. [DOI] [PubMed] [Google Scholar]
- 24.Saadat SH, Naderi S, Zare S, et al. Epidemiologic study of jaundice in newborns with jaundice in the first 24 hours of birth in Children's Hospital and Shariati Hospital of Bandar Abbas in 2010-2014. J Res Med Dent Sci 2018; 6: 113–117. [Google Scholar]