Graphical abstract
Keywords: Lipid rafts, Drug discovery, Scaffold proteins, Cancer, Allodynia, Infectious diseases, Anesthesia
Highlights
-
•
The stomatin/prohibitin/flotillin/HflK/C (SPFH) domain is present in archaea, bacteria and eukaryotes.
-
•
Mammalian prohibitins are the most studied SPFH proteins.
-
•
Prohibitin ligands exhibit anticancer, cardioprotective, anti-inflammatory, antiviral, and antiosteoporotic activities.
-
•
Other SPFH proteins are targeted by volatile anesthetics, anti-allodynia, anticancer, and anti-inflammatory agents.
-
•
SPFH proteins represent emerging therapeutic targets against a wide variety of diseases.
Abstract
The stomatin/prohibitin/flotillin/HflK/HflC (SPFH) domain is present in an evolutionarily conserved family of proteins that regulate a myriad of signaling pathways in archaea, bacteria and eukaryotes. The most studied SPFH proteins, prohibitins, have already been targeted by different families of small molecules to induce anticancer, cardioprotective, anti-inflammatory, antiviral, and antiosteoporotic activities. Ligands of other SPFH proteins have also been identified and shown to act as anesthetics, anti-allodynia, anticancer, and anti-inflammatory agents. These findings indicate that modulators of human or bacterial SPFH proteins can be developed to treat a wide variety of human disorders.
Introduction
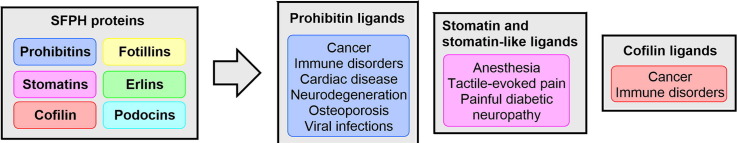
The stomatin/prohibitin/flotillin/HflK/HflC (SPFH) domain, also known as the prohibitin homology (PHB) domain or Band-7 domain, is composed of an approximatively 200-amino acid motif of ancient origin. This domain is found in archaeal, bacterial and eukaryotic proteins.1, 2 Based on their homology, these proteins are classified as prohibitins, flotillins (or Reggie), flotillin-likes, stomatins, erlins, podocins, HflKs, and HflCs. Most of the SPFH proteins shuttle between several cellular compartments based on their post-translational modification. They usually regulate a myriad of signaling proteins, including kinases, membrane receptors, channel complexes, AAA proteases, transcription factors, transcriptional coactivators and inhibitors, actin, myosin, tubulins and small GTPases.3, 4, 5, 6
Prohibitins (PHBs) are highly conserved proteins present in archaea, bacteria and every eukaryotes. Phylogenetic analyses indicate that PHBs can be divided into two types based on their relation with yeast PHB1 and PHB2. In animals and the cyanobacteria Synechocystis sp. for instance, only two isoforms, PHB1 and PHB2, are present, whereas the plants Arabidopsis thaliana and Oryza sativa contain respectively seven and four PHB isoforms. Several classes of mammalian PHB ligands have been discovered and shown to display diverse profiles of pharmacological activities, suggesting that they stabilize different conformations of PHBs.7 The most studied class of PHB ligands are natural compounds called flavaglines found in trees used in traditional Chinese medicine. Most of the other types of PHBs ligands are small synthetic molecules identified in phenotypic screenings.
Considering that the genetic inactivation of PHBs induce mitochondrial dysfunctions leading to apoptosis, PHB ligands represent invaluable tools to unravel the role of PHBs in physiology in addition to their therapeutic potential.
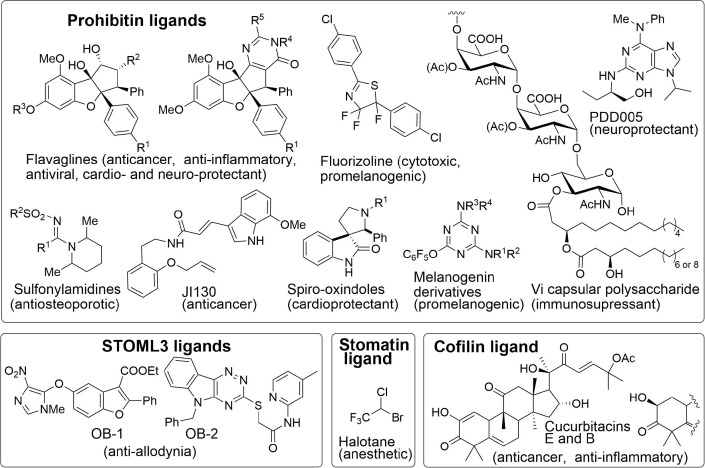
Prohibitins-1 or 2 (PHB1/2) are targeted by several classes of compounds, but some drugs targeting flotillins, STOML3, and unc-1 have also been discovered (Fig. 1 ).
Fig. 1.
Representative examples of SPFH proteins ligands.
Prohibitins and their ligands
Overview of the function of prohibitins
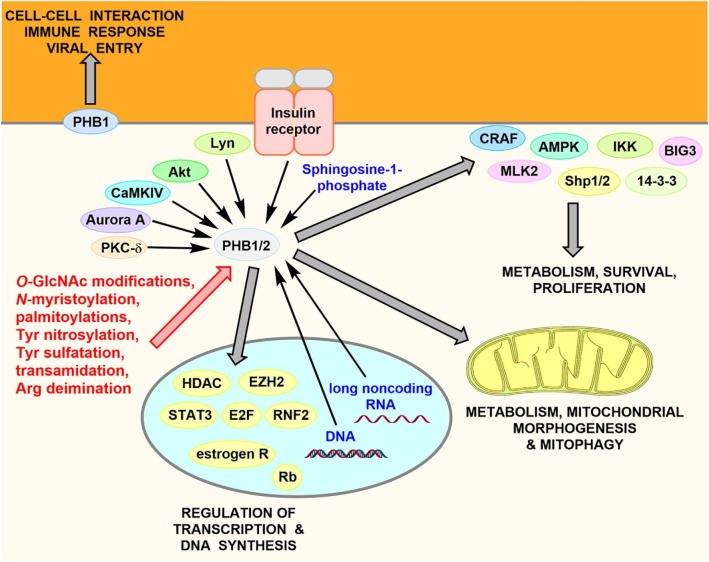
PHB1 and PHB2 undergo more than 70 different post-translational modifications that regulate the interaction with their partner proteins and their cellular localization in the plasma membrane, mitochondria, endoplasmic reticulum, peroxisomes, the cytosol or the nucleus.8 PHBs are also directly regulated by two second messengers, phosphatidylinositol 3,4,5-triphosphate (PIP3) and sphingosine-1-phosphate.9, 10 In addition, PHBs can also bind to DNA8, 11 and long non-coding RNA.12
PHBs interact with a multitude of signaling proteins, such as the kinases C-RAF (RAF1), Akt, IKK, MLK2, AMPK, the phosphatase Shp1/2, the scaffold proteins 14-3-3 and BIG3, histone deacetylases, the histone N-methyltransferase EZH2, the transcription factors STAT3, E2F, p53, HES1, RNF2, Rb, and the estrogen receptor to cite few examples.7
Flavaglines as PHB ligands
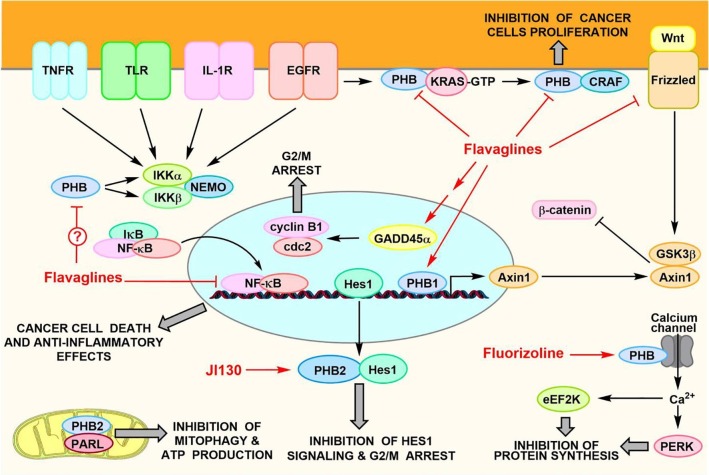
The most studied PHB ligands are complex natural ompounds called flavaglines (Fig. 1)13 that both induce the death of cancer cells and promote the survival of non-cancer cells toward various types of stresses. In addition to their effect on PHB signaling, flavaglines also inhibit the initiation factor of translation eIF4A to induce anticancer, antiviral and neuroprotectant effects.14 Therein, we will comment only on the effects that were demonstrated to be due to an action on PHBs. These anticancer drugs block the PHB-dependent activation of both C-RAF (Raf-1) and KRAS downstream of tyrosine kinase receptors.15, 16, 17 This effect is determinant to their in vivo antitumor efficacy in several murine models of cancers (Fig. 3). Flavaglines also directly inhibit the interaction between PHB2 and the protease PARL, leading to an inhibition of mitophagy in cancer cells.18 They also inhibit the signaling of Wnt and NF-κB,19, 20, 21 and activate the signaling of p38 MAP kinase, the TRPM6 channel and GADD45α to promote the death of cancer cells.22, 23, 24
Fig. 3.
Overview of the mechanism of action of PHB ligands in cancer cells.
In addition to their potent anticancer effects, flavaglines protect the heart of mice against the severe toxicity of widely used chemotherapeutic agents, such as doxorubicin, through the activation of mitochondrial STAT3 and the small heat shock protein Hsp27 in cardiac cells.25, 26 Flavaglines also reduce the inflammation and, at the same time, protect the intestinal epithelial cells against the inflammatory stress in a mouse model of inflammatory bowel disease.27 In line with these studies, a flavagline developed by the pharmaceutical company Bayer exhibits anti-inflammatory and neuroprotectant properties in mouse models of Parkinson's disease and traumatic brain injury.28
The viruses responsible for chronic hepatitis C, Chikungunya, hand-foot-and-mouth disease and probably COVID-19 use PHBs for viral replication and/or internalization. Noteworthy, these events have been shown to be blocked by flavaglines.29, 30, 31, 32, 33
Other families of PHB ligands
Fluorizoline, a PHB ligand with a very different structure, was shown to be cytotoxic to cancer cells by inducing a fragmentation of mitochondria,34 inhibiting RAS-induced C-RAF activation,16 and triggering a calcium influx leading to the phosphorylation of two key factors regulating protein synthesis, initiation factor 2 (eIF2) and elongation factor 2 (eEF2).35 Unfortunately, this compound is not active in vivo, probably because of a poor bioavailability.36
Melanogenin derivatives are perfluorophenoxytriazines that bind to PHBs to induce the biosynthesis of melanin through a pathway that involves PHBs, the autophagic factor LC3, the kinase ERK and the transcription factor MITF.37
Chang and collaborators have identified some sulfonyl- and phosphoryl-amidines that block in vitro and in vivo the differentiation of pre-osteoclasts into osteoclasts for the treatment of osteoporosis. They identified PHB1 as the molecular target of these compounds. Similarly to flavaglines these compounds are also able to block the PHB-dependent entry of Chikungunya virus into microglial cells,31 indicating that they may display some pharmacological effects unrelated to osteoclastogenesis.
Qadri and collaborators discovered that the Vi capsular polysaccharide (Vi) of Salmonella typhi suppresses the immune response during the initial stages of typhoid fever infection through its binding to cell surface PHBs. This binding inhibits ERK activity signaling and interleukin-8 secretion in human intestinal epithelial cells.38 Vi also blocks the GTPases Rac1 and Cdc42, NF-κB signaling, ERK pathways and actin cytoskeletal rearrangements in monocytes and T-cells to suppress the immune response.39
K. Rajalingam and collaborators took advantage of this immunosuppressive activity to propose a new therapeutic strategy against multiple sclerosis.40 Indeed, they found that Vi alleviates symptoms in a mouse model of this disease. They also showed that Vi blocks C-RAF activation in T helper 17 cells that express PHB1 at the cell surface.
Spirooxindoles are another class of PHB ligands that can protect cardiomyocytes against the adverse effects of the anticancer medicine doxorubicin.41 Similarly to flavaglines, they induce the phosphorylation of STAT3 and its translocation to mitochondria to promote cell survival.
The adenine derivative PDD005 is a PHB ligand that rescues cognitive deficits associated with aging in mice.42 It also alleviates neurogenesis deficiency and inhibits neuro-inflammation, confirming PHBs as emerging therapeutic targets against neurodegenerative diseases.
Flotillins
Similarly to PHBs, flotillins exist as 2 isoforms, flotillin-1 and flotillin-2, which are ubiquitously expressed scaffold proteins involved in various aspects of cell signaling, cytoskeleton remodeling, endocytosis, release of extracellular vesicles and protein trafficking.43 To fulfill these functions, they directly interact with several proteins, including the EGF receptor, the kinases Lyn, Fyn, Lck, cRAF, MEK1, ERK2, cRAF, MEK1/2, ERK1/2, KSR1 the small GTPases Gαq, Rab11, β-catenin, γ-catenin, E-cadherin, N-cadherin, the adaptor protein ArgBP2, the adapter protein SORBS1 /CAP/Ponsin, the E3 ubiquitin-protein ligase Cbl, α-actinin or F-actin. As far as we know, no small molecules targeting flotillins has been discovered yet, but such agents could display interesting effects to treat several types of diseases, including cancers, immunological, cardiac and neurodegenerative disorders.
Cofilin and their ligands, cucurbitacins
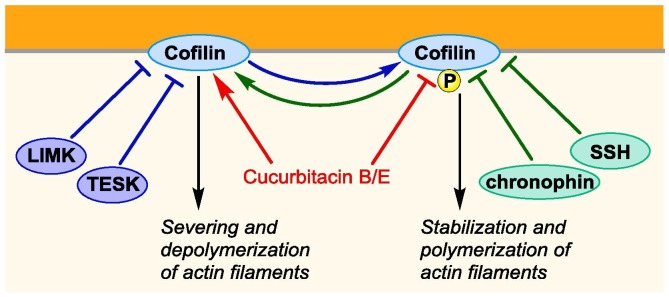
Cofilin promotes actin filament turnover by stimulating depolymerization and severance of actin filaments.44 As such, it is involved in cancer cell survival, migration, and invasion. This scaffold protein is inactivated by LIM-kinases (LIMKs) and testicular protein kinases (TESKs), which are downstream of receptor tyrosine kinases, G protein-coupled receptors integrins and cadherin signaling pathways (Figure 4). Cofilin is reactivated by dephosphorylation by the slingshot (SSH) family of protein phosphatases and chronophin.
Fig. 4.
Activation of unphoshorylated cofilin by cucurbitacinB/E leading to a severing and depolymerisation of actin filaments.
Cucurbitacin E is a tetracyclic triterpenoid cytotoxic in several cancer cell lines. Using an affinity chromatography approach, Yoshikawa and coworkers identified cofilin as the molecular target of this natural compound.45 Furthermore, Cucurbitacin E was shown to inhibit the phosphorylation of cofilin and the depolymerization of actin (Fig. 2 B). Thereafter, Olsen and collaborators found that cucurbitacin E irreversibly alkylates cysteine residues of cofilin-1 as well as other proteins.46
Fig. 2.
Overview of PHB signaling. Post-translational modifications and endogenous ligands (sphingosine-1-phosphate, DNA and long noncoding RNA) control the localization of PHBs and their interactions with signaling proteins.
Cucurbitacin B, an analogue of cucurbitacin E, which also induces a rapid dephosphorylation of cofilin, was shown to rapidly translocate to mitochondria to induce apoptosis.47 Importantly, cucurbitacin B inhibited tumor growth in a murine model of multiple myeloma, without any apparent toxicity, suggesting that cofilin could represent a valuable target in oncology.
Stomatin, stomatin-like proteins and their ligands
The best characterized functions of the mammalian members of the stomatin family (stomatin, stomatin-like proteins and podocin) are related to the modulation of ion channel function. For instance, stomatin directly regulates the activity the glucose transporter type 1 (GLUT1) and members of the acid-sensing ion channel (ASIC) family.
To characterize the mechanism of action of volatile anesthetics, such as halothane, Sedensky and Morgan screened C. elegans mutants that display an anomalous behavior to volatile anesthetics.48, 49 Worms carrying a mutation of the stomatin homologues unc-1 and unc-24 displayed abnormal locomotion and an impaired sensitivity to volatile anesthetics. Interestingly, these mutations affect residues localized in the SPFH domain, suggesting that volatile anesthetics act via stomatin in lipid rafts.
Unc-1 plays a central role in the regulation of the anesthetic response, while unc-24 controls the correct localization of unc-1. Subsequently, it has been shown that unc-1 physically interacts with a homolog of degenerin, which is a component of Acid-sensing ion channels (ASICs) and the degenerin/epithelial sodium channel (DEG/ENaC) in human cells.50 Altogether, these studies suggest that the binding of volatile anesthetics to stomatin affects the activity of ASICs and DEG/ENaCs to induce their anesthetic effect.
Similarly to PHBs, stomatin-like protein 3 (STOML3, also called SLP3) binds to cholesterol in lipid rafts where it is organized as oligomers. In sensory cells specialized to detect small mechanical changes, STOML3 facilitates force transfer and regulates the sensitivity of mechano-gated channels Piezo 1 and 2 (Fig. 5 ).51 Recently, Gary Lewin and collaborators screened a library of small molecules for their ability to inhibit STOML3 self-association. They identified two compounds, OB-1 and OB-2 that reduce the sensitivity of mechanically gated currents in sensory neurons and block mechanoreceptors in vivo. Tactile allodynia is a neurological condition in which extreme pain can occur with a simple touch. Notably, OB-1 was able to completely abolish the tactile-evoked pain in a mouse model of allodynia. This drug also alleviated painful diabetic neuropathy induced by streptozotocin in mice. Thus, this impressive study paved the road for the development of new treatments against painful diabetic neuropathy and tactile allodynia.
Fig. 5.
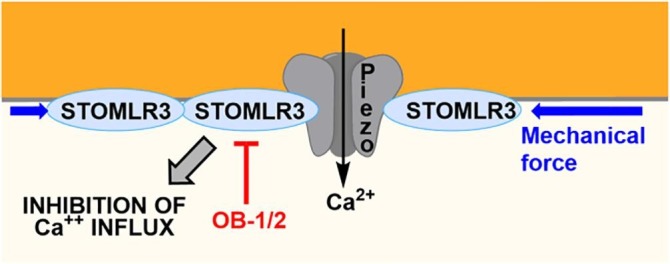
Inhibition of STOML3 oligomerization by OB-1/2 to inhibit Piezo channels and consequently to alleviate tactile allodynia.
SPFH proteins as therapeutic targets against infectious diseases
Flotillins are present in bacteria, archaea, and eukaryotes. Lopez and collaborators found that zaragozic acid, an inhibitor of squalene oxidase that affects the composition of lipid rafts, inhibits the oligomerization of the flotillin-homolog protein FloA, which is necessary for assembly of protein complexes involved in Staphylococcus aureus virulence. Importantly, zaragozic acid could limit infections by multidrug-resistant S. aureus in mice.52 In line with this study, Manoil and collaborators found that mutation of the SPFH proteins HflK and HflC increases the sensitivity of Pseudomonas aeruginosa toward the aminoglycoside tobramycin,53 confirming that SPFH proteins represent potential targets to develop novel antibiotics against pathogenic multidrug-resistant bacteria.
Matz Kooji and collaborators discovered an unusual prohibitin-like protein (PHBL) in the murine malaria model parasite Plasmodium berghei. 54 They demonstrated that this protein is essential for parasite colonization of the mosquito vector, suggesting that it may also offer some opportunities for the development of a novel class of antimalarial medicines.
Conclusion
SPFH proteins form a large family of ubiquitous proteins that regulate a myriad of signaling pathways, not only in humans and animals but also in infectious bacteria. Up to now, most of the drug targeting an SPFH protein act on PHB1 and PHB2, which are the most studied SPFH proteins. These compounds display some potential for the treatment of cancers, osteoporosis, inflammatory, cardiac, and neurodegenerative diseases. However, the recent discovery of ligands for other SPFH protein indicates that modulators of other SPFH proteins can be used to treat a wide variety of diseases, including bacterial infections and malaria.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Financial support from the National Natural Science Foundation of China (No. 81673296) and the start-up Foundation from Tianjin University of Science and Technology is gratefully acknowledged.
References
- 1.Rivera-Milla E., Stuermer C.A.O., Málaga-Trillo E. Ancient origin of reggie (flotillin), reggie-like, and other lipid-raft proteins: convergent evolution of the SPFH domain. Cell Mol Life Sci. 2006;63(3):343–357. doi: 10.1007/s00018-005-5434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinderhofer M., Walker C.A., Friemel A., Stuermer C.A.O., Möller H.M., Reuter A. Evolution of prokaryotic SPFH proteins. BMC Evol Biol. 2009;9(1):10. doi: 10.1186/1471-2148-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browman D.T., Hoegg M.B., Robbins S.M. The SPFH domain-containing proteins: more than lipid raft markers. Trends Cell Biol. 2007;17(8):394–402. doi: 10.1016/j.tcb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Danek M., Valentova O., Martinec J. Flotillins, Erlins, and HIRs: From Animal Base Camp to Plant New Horizons. Crit Rev Plant Sci. 2016;35(4):191–214. [Google Scholar]
- 5.Morrow I.C., Parton R.G. Flotillins and the PHB domain protein family: Rafts, worms and anesthetics. Traffic. 2005;6(9):725–740. doi: 10.1111/j.1600-0854.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 6.Thuaud F., Ribeiro N., Nebigil C.G., Désaubry L. Prohibitin ligands in cell death and survival: mode of action and therapeutic potential. Chem Biol. 2013;20(3):316–331. doi: 10.1016/j.chembiol.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Tabti R., Elderwish S. Prohibitin ligands: a growing armamentarium to tackle cancers, osteoporosis, inflammatory, cardiac and neurological diseases. Cell Mol Life Sci. 2020 doi: 10.1007/s00018-020-03475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sripathi S.R., Sylvester O., He W.L. Prohibitin as the molecular binding switch in the retinal pigment epithelium. Protein J. 2016;35(1):1–16. doi: 10.1007/s10930-015-9641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ande S.R., Mishra S. Prohibitin interacts with phosphatidylinositol 3,4,5-triphosphate (PIP3) and modulates insulin signaling. Biochem Biophys Res Commun. 2009;390(3):1023–1028. doi: 10.1016/j.bbrc.2009.10.101. [DOI] [PubMed] [Google Scholar]
- 10.Gomez L., Paillard M., Price M. A novel role for mitochondrial sphingosine-1-phosphate produced by sphingosine kinase-2 in PTP-mediated cell survival during cardioprotection. Basic Res Cardiol. 2011;106(6):1341–1353. doi: 10.1007/s00395-011-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan X., Liu Z., Wang L., Johnson D.G., Wei Q. Identification of prohibitin and prohibiton as novel factors binding to the p53 induced gene 3 (PIG3) promoter (TGYCC)(15) motif. Biochem Biophys Res Commun. 2014;443(4):1239–1244. doi: 10.1016/j.bbrc.2013.12.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Wang L.-N., Lin Y.-N. The novel long noncoding RNA LOC283070 is involved in the transition of LNCaP cells into androgen-independent cells via its interaction with PHB2. Asian J Androl. 2018;20(5):511–517. doi: 10.4103/aja.aja_36_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Q., Abou-Hamdan H., Désaubry L. Recent advances in the synthesis of flavaglines, a family of potent bioactive natural compounds originating from traditional chinese medicine. Eur J Org Chem. 2016;2016(36):5908–5916. [Google Scholar]
- 14.Malka-Mahieu H., Newman M., Désaubry L., Robert C., Vagner S. Molecular pathways: the eIF4F translation initiation complex—new opportunities for cancer treatment. Clin Cancer Res. 2017;23(1):21. doi: 10.1158/1078-0432.CCR-14-2362. [DOI] [PubMed] [Google Scholar]
- 15.Polier G., Neumann J., Thuaud F. The natural anticancer compounds rocaglamides inhibit the Raf-MEK-ERK pathway by targeting prohibitin 1 and 2. Chem Biol. 2012;19(9):1093–1104. doi: 10.1016/j.chembiol.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Yurugi H., Marini F., Weber C. Targeting prohibitins with chemical ligands inhibits KRAS-mediated lung tumours. Oncogene. 2017;36(33):4778–4789. doi: 10.1038/onc.2017.93. [DOI] [PubMed] [Google Scholar]
- 17.Yurugi H., Zhuang Y., Siddiqui F.A. A subset of flavaglines inhibits KRAS nanoclustering and activation. J Cell Sci. 2020;133(12):jcs244111. doi: 10.1242/jcs.244111. [DOI] [PubMed] [Google Scholar]
- 18.Yan C., Gong L., Chen L. PHB2 (prohibitin 2) promotes PINK1-PRKN/Parkin-dependent mitophagy by the PARL-PGAM5-PINK1 axis. Autophagy. 2020;16(3):419–434. doi: 10.1080/15548627.2019.1628520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arai M.A., Kofuji Y., Tanaka Y. Synthesis of rocaglamide derivatives and evaluation of their Wnt signal inhibitory activities. Org Biomol Chem. 2016;14(11):3061–3068. doi: 10.1039/c5ob02537k. [DOI] [PubMed] [Google Scholar]
- 20.Ho M.Y., Liang C.M., Liang S.M. MIG-7 and phosphorylated prohibitin coordinately regulate lung cancer invasion/metastasis. Oncotarget. 2015;6(1):381–393. doi: 10.18632/oncotarget.2804. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Jackson DN, Alula KM, Delgado-Deida Y, et al. The synthetic small molecule FL3 combats intestinal tumorigenesis via Axin1 inhibition of Wnt/β-catenin signaling. Cancer Res. 2020;80: in press. [DOI] [PMC free article] [PubMed]
- 22.Emhemmed F., Ali Azouaou S., Thuaud F. Selective anticancer effects of a synthetic flavagline on human Oct4-expressing cancer stem-like cells via a p38 MAPK-dependent caspase-3-dependent pathway. Biochem Pharmacol. 2014;89(2):185–196. doi: 10.1016/j.bcp.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Blanchard M.G., de Baaij J.H., Verkaart S.A. Flavaglines stimulate transient receptor potential melastatin type 6 (TRPM6) channel activity. PLoS ONE. 2015;10(3) doi: 10.1371/journal.pone.0119028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumann B., Bohnenstengel F., Siegmund D. Rocaglamide derivatives are potent inhibitors of NF-kappa B activation in T-cells. J Biol Chem. 2002;277(47):44791–44800. doi: 10.1074/jbc.M208003200. [DOI] [PubMed] [Google Scholar]
- 25.Bernard Y., Ribeiro N., Thuaud F. Flavaglines alleviate doxorubicin cardiotoxicity: implication of Hsp27. PLoS ONE. 2011;6(10) doi: 10.1371/journal.pone.0025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qureshi R., Yildirim O., Gasser A. FL3, a synthetic flavagline and ligand of prohibitins, protects cardiomyocytes via STAT3 from doxorubicin toxicity. PLoS ONE. 2015;10(11) doi: 10.1371/journal.pone.0141826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han J., Zhao Q., Basmadjian C., Désaubry L., Theiss A.L. Flavaglines ameliorate experimental colitis and protect against intestinal epithelial cell apoptosis and mitochondrial dysfunction. Inflamm Bowel Dis. 2016;22(1):55–67. doi: 10.1097/MIB.0000000000000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fahrig T., Gerlach I., Horvath E. A synthetic derivative of the natural product rocaglaol is a potent inhibitor of cytokine-mediated signaling and shows neuroprotective activity in vitro and in animal models of Parkinson's disease and traumatic brain injury. Mol Pharmacol. 2005;67(5):1544–1555. doi: 10.1124/mol.104.008177. [DOI] [PubMed] [Google Scholar]
- 29.Liu S., Wang W., Brown L.E. A novel class of small molecule compounds that inhibit hepatitis C virus infection by targeting the prohibitin-CRaf Pathway. EBioMedicine. 2015;2(11):1600–1606. doi: 10.1016/j.ebiom.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W., Liu S., Maiga R.I. Chemical synthesis enables structural reengineering of aglaroxin c leading to inhibition bias for hcv infection. J Am Chem Soc. 2018;141(3):1312–1323. doi: 10.1021/jacs.8b11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wintachai P., Thuaud F., Basmadjian C. Assessment of flavaglines as potential chikungunya virus entry inhibitors. Microbiol Immunol. 2015;59(3):129–141. doi: 10.1111/1348-0421.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Too I.H.K., Bonne I., Tan E.L., Chu J.J.H., Alonso S. Prohibitin plays a critical role in Enterovirus 71 neuropathogenesis. PLoS Pathog. 2018;14(1) doi: 10.1371/journal.ppat.1006778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nebigil C.G., Moog C., Vagner S., Benkirane-Jessel N., Smith D.R., Désaubry L. Flavaglines as natural products targeting eIF4A and prohibitins: From traditional Chinese medicine to antiviral activity against coronaviruses. Eur J Med Chem. 2020;203 doi: 10.1016/j.ejmech.2020.112653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moncunill-Massaguer C., Saura-Esteller J., Perez-Perarnau A. A novel prohibitin-binding compound induces the mitochondrial apoptotic pathway through NOXA and BIM upregulation. Oncotarget. 2015;6(39):41750–41765. doi: 10.18632/oncotarget.6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin X., Xie J., Zabolocki M. The prohibitin-binding compound fluorizoline affects multiple components of the translational machinery and inhibits protein synthesis. J Biol Chem. 2020 doi: 10.1074/jbc.RA120.012979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wierz M., Pierson S., Chouha N. The prohibitin-binding compound fluorizoline induces apoptosis in chronic lymphocytic leukemia cells ex vivo but fails to prevent leukemia development in a murine model. Haematologica. 2018;103 doi: 10.3324/haematol.2017.175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Djehal A., Krayem M., Najem A. Targeting prohibitin with small molecules to promote melanogenesis and apoptosis in melanoma cells. Eur J Med Chem. 2018;155:880–888. doi: 10.1016/j.ejmech.2018.06.052. [DOI] [PubMed] [Google Scholar]
- 38.Sharma A., Qadri A. Vi polysaccharide of Salmonella typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proc Natl Acad Sci USA. 2004;101(50):17492–17497. doi: 10.1073/pnas.0407536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santhanam S.K., Dutta D., Parween F., Qadri A. The virulence polysaccharide Vi released by salmonella typhi targets membrane prohibitin to inhibit T-cell activation. J Infect Dis. 2014;210(1):79–88. doi: 10.1093/infdis/jiu064. [DOI] [PubMed] [Google Scholar]
- 40.Buehler U., Schulenburg K., Yurugi H. Targeting prohibitins at the cell surface prevents Th17-mediated autoimmunity. EMBO J. 2018;37 doi: 10.15252/embj.201899429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elderwish S., Audebrand A., Nebigil C.G., Désaubry L. Discovery of 3,3’-pyrrolidinyl-spirooxindoles as cardioprotectant prohibitin ligands. Eur J Med Chem. 2020;186 doi: 10.1016/j.ejmech.2019.111859. [DOI] [PubMed] [Google Scholar]
- 42.Guyot A.-C., Leuxe C., Disdier C. A small compound targeting prohibitin with potential interest for cognitive deficit rescue in aging mice and tau pathology treatment. Sci Rep. 2020;10(1):1143. doi: 10.1038/s41598-020-57560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwiatkowska K., Matveichuk O.V., Fronk J., Flotillins C.A. At the intersection of protein S-palmitoylation and lipid-mediated signaling. Int J Mol Sci. 2020;21(7):2283. doi: 10.3390/ijms21072283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee M.-H., Kundu J.K., Chae J.-I., Shim J.-H. Targeting ROCK/LIMK/cofilin signaling pathway in cancer. Arch Pharm Res. 2019;42(6):481–491. doi: 10.1007/s12272-019-01153-w. [DOI] [PubMed] [Google Scholar]
- 45.Nakashima S., Matsuda H., Kurume A. Cucurbitacin E as a new inhibitor of cofilin phosphorylation in human leukemia U937 cells. Bioorg Med Chem Lett. 2010;20(9):2994–2997. doi: 10.1016/j.bmcl.2010.02.062. [DOI] [PubMed] [Google Scholar]
- 46.Gabrielsen M., Schuldt M., Munro J. Cucurbitacin covalent bonding to cysteine thiols: the filamentous-actin severing protein Cofilin1 as an exemplary target. Cell Commun Signal. 2013;11(1):58. doi: 10.1186/1478-811X-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang T., Liu J., Yang M. Cucurbitacin B exerts anti-cancer activities in human multiple myeloma cells in vitro and in vivo by modulating multiple cellular pathways. Oncotargetics. 2016;8(4) doi: 10.18632/oncotarget.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan P.G., Sedensky M.M. Mutations conferring new patterns of sensitivity to volatile anesthetics in Caenorhabditis elegans. Anesthesiology. 1994;81(4):888–898. doi: 10.1097/00000542-199410000-00016. [DOI] [PubMed] [Google Scholar]
- 49.Rajaram S., Sedensky M.M., Morgan P.G. Unc-1: a stomatin homologue controls sensitivity to volatile anesthetics in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1998;95(15):8761–8766. doi: 10.1073/pnas.95.15.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rajaram S., Spangler T.L., Sedensky M.M., Morgan P.G. A stomatin and a degenerin interact to control anesthetic sensitivity in Caenorhabditis elegans. Genetics. 1999;153(4):1673–1682. doi: 10.1093/genetics/153.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi Y., Andolfi L., Frattini F., Mayer F., Lazzarino M., Hu J. Membrane stiffening by STOML3 facilitates mechanosensation in sensory neurons. Nat Commun. 2015;6(1):8512. doi: 10.1038/ncomms9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koch G., Wermser C., Acosta I.C. Attenuating staphylococcus aureus virulence by targeting flotillin protein scaffold activity. Cell Chem Biol. 2017;24(7):845–857.e846. doi: 10.1016/j.chembiol.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hinz A., Lee S., Jacoby K., Manoil C. Membrane proteases and aminoglycoside antibiotic resistance. J Bacteriol. 2011;193(18):4790–4797. doi: 10.1128/JB.05133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matz J.M., Goosmann C., Matuschewski K., Kooij T.W.A. An unusual prohibitin regulates malaria parasite mitochondrial membrane potential. Cell Rep. 2018;23(3):756–767. doi: 10.1016/j.celrep.2018.03.088. [DOI] [PubMed] [Google Scholar]